Abstract
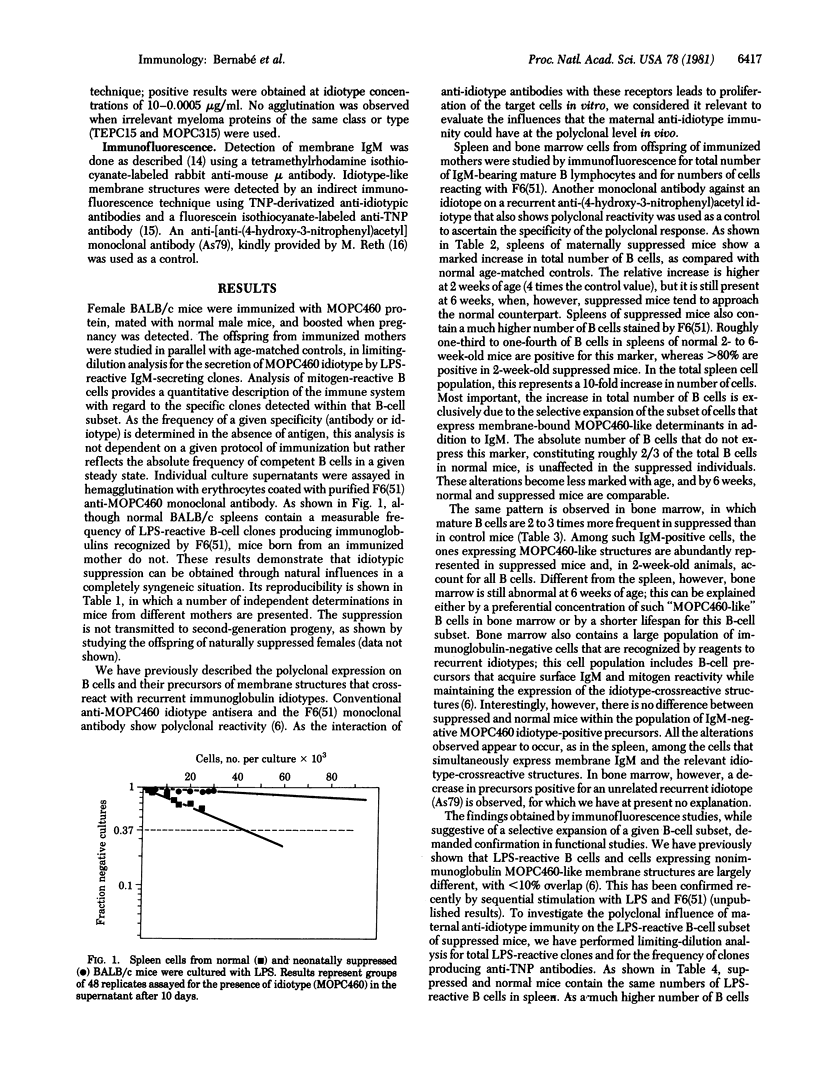
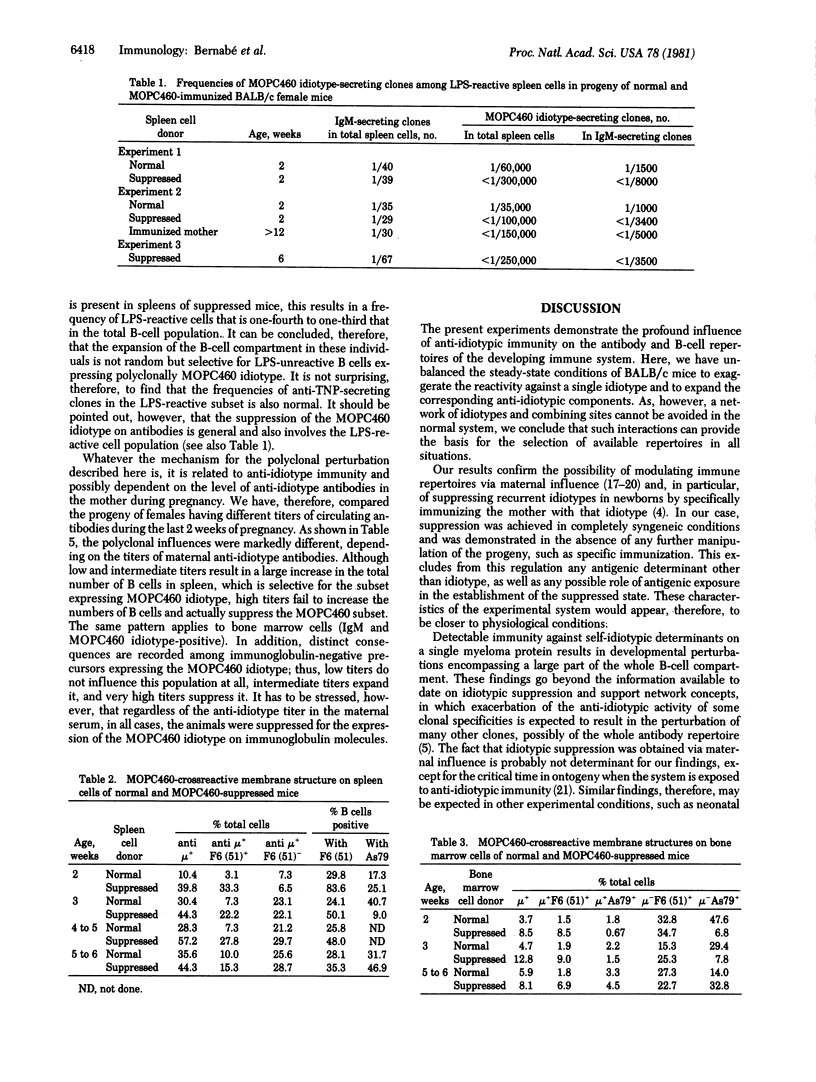
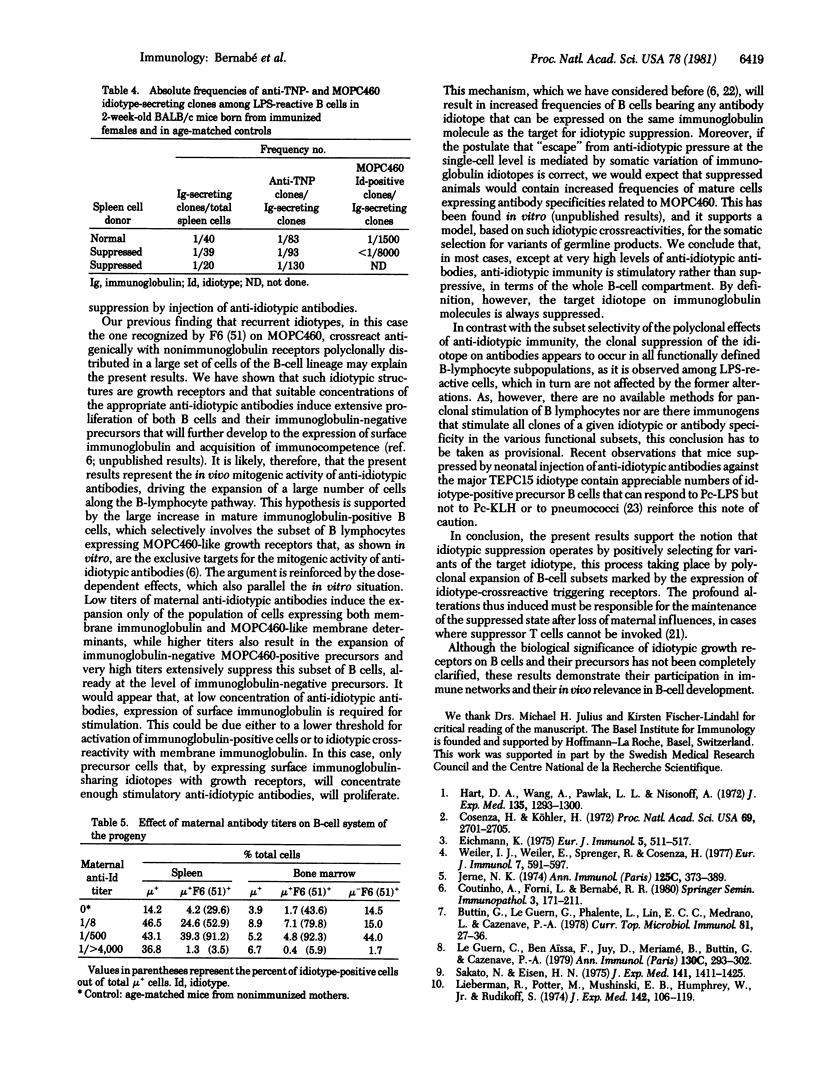
The progeny of BALB/c female mice actively immunized with the trinitrophenyl-binding myeloma protein MOPC460 and producing anti-idiotypic antibodies during pregnancy were compared with mice born of normal mothers for several characteristics of B lymphocytes and their precursors. In all cases, maternal anti-idiotypic immunity resulted in the suppression of the expression of that idiotype by immunocompetent cells in the progeny, as shown by limiting-dilution analysis in single clones of mitogen-reactive IgM-secreting cells. At critical concentrations of circulating maternal antibodies, suppression of the antibody idiotype was found to be accompanied by a large increase in the total number of mature small B lymphocytes. This increase can be accounted for by the selective expansion of B cells bearing nonimmunoglobulin surface structures crossreactive with a MOPC460 idiotope recognized by a monoclonal antibody. In addition, the large majority of newly formed mature B lymphocytes, as well as a large fraction of immunoglobulin-negative cells in the bone marrow of suppressed mice, bear such nonimmunoglobulin MOPC460 crossreactive determinant(s). These results suggest that the suppression of a given "recurrent" idiotype has profound consequences for a large part of the immune system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Coutinho A., Melchers F. Frequencies of mitogen-reactive B cells in the mouse. I. Distribution in different lymphoid organs from different inbred strains of mice at different ages. J Exp Med. 1977 Jun 1;145(6):1511–1519. doi: 10.1084/jem.145.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach R., Clark S. Immunological tolerance: transmission from mother to offspring. Science. 1975 Sep 5;189(4205):811–813. doi: 10.1126/science.1162355. [DOI] [PubMed] [Google Scholar]
- Buttin G., LeGuern G., Phalente L., Lin E. C., Medrano L., Cazenave P. A. Production of hybrid lines secreting monoclonal anti-idiotypic antibodies by cell fusion on membrane filters. Curr Top Microbiol Immunol. 1978;81:27–36. doi: 10.1007/978-3-642-67448-8_4. [DOI] [PubMed] [Google Scholar]
- Cosenza H., Julius M. H., Augustin A. A. Idiotypes as variable region markers: analogies between receptors on phosphorylcholine-specific T and B lymphocytes. Immunol Rev. 1977;34:3–33. doi: 10.1111/j.1600-065x.1977.tb00366.x. [DOI] [PubMed] [Google Scholar]
- Cosenza H., Köhler H. Specific suppression of the antibody response by antibodies to receptors. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2701–2705. doi: 10.1073/pnas.69.9.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Forni L., Bernabé R. R. The polyclonal expression of immunoglobulin variable region determinants on the membrane of B cells and their precursors. Springer Semin Immunopathol. 1980 Aug;3(2):171–211. doi: 10.1007/BF02053975. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Forni L., Blomberg B. Shared antigenic determinants by mitogen receptors and antibody molecules to the same thymus-independent antigen. J Exp Med. 1978 Oct 1;148(4):862–870. doi: 10.1084/jem.148.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A. The self-nonself discrimination and the nature and acquisition of the antibody repertoire. Ann Immunol (Paris) 1980 Nov-Dec;131D(3):235–253. [PubMed] [Google Scholar]
- Eichmann K. Idiotype suppression. II. Amplification of a suppressor T cell with anti-idiotypic activity. Eur J Immunol. 1975 Aug;5(8):511–517. doi: 10.1002/eji.1830050802. [DOI] [PubMed] [Google Scholar]
- Fung J., Köhler H. Mechanism of neonatal idiotype suppression. I. State of the suppressed B cells. J Immunol. 1980 Nov;125(5):1998–2003. [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Hart D. A., Wang A. L., Pawlak L. L., Nisonoff A. Suppression of idiotypic specificities in adult mice by administration of antiidiotypic antibody. J Exp Med. 1972 Jun 1;135(6):1293–1300. doi: 10.1084/jem.135.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson E. B., Herzenberg L. A. Active suppression of immunoglobulin allotype synthesis. I. Chronic suppression after perinatal exposure to maternal antibody to paternal allotype in (SJL x BALB-c)F 1 mice. J Exp Med. 1972 May 1;135(5):1151–1162. doi: 10.1084/jem.135.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Kindred B., Roelants G. E. Restricted clonal response to DNP in adult offspring of immunized mice: a maternal effect. J Immunol. 1974 Aug;113(2):445–448. [PubMed] [Google Scholar]
- Le Guern C., Ben Aïssa F., Juy D., Mariamé B., Buttin G., Cazenave P. A. Expression and induction of MOPC-460 idiotopes in different strains of mice. Ann Immunol (Paris) 1979 Mar-Apr;130(2):293–302. [PubMed] [Google Scholar]
- Lieberman R., Potter M., Humphrey W., Jr, Mushinski E. B., Vrana M. Multiple individual and cross-specific indiotypes on 13 levan-binding myeloma proteins of BALB/c mice. J Exp Med. 1975 Jul 1;142(1):106–119. doi: 10.1084/jem.142.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M., Imanishi-Kari T., Rajewsky K. Analysis of the repertoire of anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in C 57 BL/6 mice by cell fusion. II. Characterization of idiotopes by monoclonal anti-idiotope antibodies. Eur J Immunol. 1979 Dec;9(12):1004–1013. doi: 10.1002/eji.1830091216. [DOI] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Sakato N., Eisen H. N. Antibodies to idiotypes of isologous immunoglobulins. J Exp Med. 1975 Jun 1;141(6):1411–1426. doi: 10.1084/jem.141.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler I. J., Weiler E., Sprenger R., Cosenza H. Idiotype suppression by maternal influence. Eur J Immunol. 1977 Sep;7(9):591–597. doi: 10.1002/eji.1830070903. [DOI] [PubMed] [Google Scholar]
- Wikler M., Demeur C., Dewasme G., Urbain J. Immunoregulatory role of maternal idiotypes. Ontogeny of immune networks. J Exp Med. 1980 Oct 1;152(4):1024–1035. doi: 10.1084/jem.152.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]



