Abstract
This study assesses the relationship between otitis media and atopic conditions in children by comparing the incidence of tympanostomy tube placement between children with and without atopic conditions: asthma, allergic rhinitis, and atopic dermatitis. Study subjects were a cohort of 323 healthy children who participated in a study of vaccine response. All episodes of tympanostomy tube placement and physician diagnoses of allergic rhinitis and atopic dermatitis were collected through comprehensive medical record review. Asthma status was ascertained through application of established criteria. We compared incidence rates of tympanostomy tube placement between children with and without atopic conditions. We fitted data to a Poisson regression model to calculate relative risk ratios (RRs) and their corresponding 95% confidence intervals (95% CI). Three subjects were excluded who did not have parental authorization for using records for research. Of the remaining 320 subjects, 170 (53%) were male subjects, 268 (94%) were white, 124 (39%) were asthmatic patients, and 20 (6%) had tympanostomy tube placement. Children with asthma before the index date of tympanostomy tube placement were more likely to have tympanostomy tube placement compared with those without asthma (RR, 19.33; 95% CI, 11.41; 32.75; p < 0.001). We found a similar association between asthma ever (before or after index date) and the incidence of tympanostomy tube placement (RR, 1.53; 95% CI, 0.93–2.53; p = 0.095). This was true for children with allergic rhinitis compared with those without allergic rhinitis (RR, 1.70; 95% CI, 1.01–2.86; p = 0.007). Atopic dermatitis was not associated with the incidence of tympanostomy tube placement. Asthma or allergic rhinitis may be unrecognized risk factors for recurrent or persistent otitis media. However, given the small sample size of the study, a cohort study with a larger sample size is necessary.
Keywords: Allergic rhinitis, asthma, atopic dermatitis, atopy, child, infection, otitis media, pediatrics, tympanostomy tube
Asthma is the most common chronic disease of childhood in the United States. The 2007 National Health Interview Survey conducted by the National Center for Health Statistics reported historically high levels of asthma prevalence in 2007, estimating 6.7 million or 9.1% of children in the United States with current asthma.1,2 Other atopic conditions share a similar trend. Studies have reported a significant increase in the worldwide prevalence of atopic dermatitis.3,4 In the United States, the prevalence of atopic dermatitis is 10–19%,5–7 affecting nearly 17.8–31.6 million people depending on the eczema diagnostic criteria.5 Likewise, allergic rhinitis affects 26–33% of the U.S. population, ∼60 million Americans.5–7 Despite a significant proportion of people affected by asthma and other atopic conditions in the United States, the impact of atopy on the risk of common microbial infections is still poorly understood. Two epidemiological studies recently showed that asthmatic patients have a significantly increased risk of invasive pneumococcal disease,8,9 and, now, adults with asthma are recommended to receive a single dose of 23-valent pneumococcal polysaccharide vaccine.10 We recently reported that individuals with other atopic conditions such as atopic dermatitis and allergic rhinitis also have an increased risk of serious pneumococcal disease than those without such conditions.11
Despite this significant association between these atopic conditions and an increased risk of serious pneumococcal disease, little is known about whether this is true for common upper respiratory infections such as otitis media caused predominately by Streptococcus pneumoniae. Otitis media is a common infection of childhood and is a leading reason for physician office visits and antibiotic prescriptions among preschoolers in the United States.12–14 Frequent and persistent otitis media leads to insertion of tympanostomy tubes to prevent adverse outcomes related to frequent and persistent ear infections.15–17 Currently, more than 1 million tympanostomy tube placement procedures are performed annually in North America.18 Therefore, given a significant proportion of children affected by atopic conditions, assessing whether atopic conditions are a significant risk factor for recurrent or chronic otitis media has important clinical and public health implications.
In addressing this concern, previous studies that reported the association between otitis media and asthma have been limited. They were primarily cross-sectional study designs and both exposure (atopic conditions) and outcomes (otitis media) were based on self-report.19–32 Furthermore, few cohort studies have been conducted to assess the relationship between atopic conditions and otitis media using reliable ascertainment criteria for asthma and tympanostomy tube placement as a marker for frequent and persistent otitis media. Thus, this study sought to investigate the relationship between tympanostomy tube placement and atopic conditions in children.
METHODS
Study Design and Setting
This was a retrospective cohort study conducted in Olmsted County, MN. During the study period, characteristics of the Olmsted County population were similar to those of the U.S. white population with the exception of a higher proportion of the working population employed in the health care industry.33,34 Olmsted County, MN, is an excellent setting to conduct a population-based epidemiological study because medical care is virtually self-contained within the community. The Rochester Epidemiology Project35 has been continuously funded and maintained since 1960 and each patient is assigned a unique identifier. All clinical diagnoses are electronically indexed, and information from every episode of care is contained within detailed patient-based medical records.
Study Subjects
The institutional review boards at both Mayo Clinic and Olmsted Medical Center approved the study protocol. The details of the study subjects have been previously reported.36–38 Briefly, study subjects were 323 healthy children aged 12–18 years who participated in a previous study and were recruited from Olmsted County, MN, using a population-based stratified sampling by age and gender. Exclusion criteria included lack of research authorization for using medical records for research and residency outside Olmsted County, MN.
Dependent Variable
The dependent variable was the frequency (i.e., incidence) of tympanostomy tube placement including multiple episodes during the first 18 years of life. The entire medical record for each subject was reviewed to collect data on any episodes of tympanostomy tube placement. We used tympanostomy tube placement as a surrogate marker for either persistent or recurrent otitis media during childhood.
Independent Variable
The independent variables were asthma status and other atopic conditions, including atopic dermatitis and allergic rhinitis. Asthma status was ascertained by predetermined criteria (Table 1). Through review of the entire medical record, study subjects were considered to have definite or probable asthma. Ascertainment of asthma status was completed in a previous study38; therefore, it was independent of data collection for outcome measure—tympanostomy tube placement. Because most subjects in a previous study with probable asthma by the criteria became definite asthmatic patients over time, both definite and probable asthmatic patients were considered asthmatic in this study.39 The index date of asthma was defined as time when one met the criteria for asthma. We also collected the diagnosis date of asthma when a physician diagnosis of asthma was documented in medical records. The definitions of atopic dermatitis or allergic rhinitis were based on a physician diagnosis of atopic dermatitis or eczema and allergic rhinitis or hay fever documented in medical records.
Table 1.
The criteria for asthma
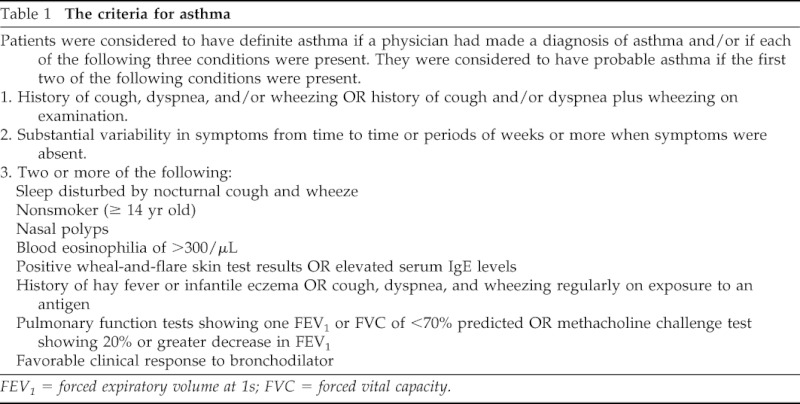
FEV1 = forced expiratory volume at 1s; FVC = forced vital capacity.
Data Analysis
The primary outcome measure was the incidence of tympanostomy tube placement that we analyzed three different ways in relation to timing of atopic conditions: (1) comparison of subsequent incidence of all tympanostomy tube placements between children with and without atopic conditions (i.e., atopic conditions before the first episode of tympanostomy tube placement), (2) comparison of the incidence of all tympanostomy tube placements between children with atopic conditions ever and those without such conditions during the first 18 years of life or last follow-up date (whichever came first), and (3) comparison of the incidence of all tympanostomy tube placements between children with atopic conditions ever and those without such conditions during the first 12 years of life only when tympanostomy tube placement occurred. The incidence rates of tympanostomy tube placement were calculated by dividing the number of tympanostomy tube placements by the total person-years based on the follow-up duration from the first clinic registration date to the last follow-up date or an end point of interest (whichever came first) as described previously.
The incidence rates of tympanostomy tube placement were assumed to follow the Poisson distribution; thus, we fitted data to a Poisson regression model to calculate risk ratios (RRs) adjusting for pertinent covariates and confounders. A univariate Poisson regression was fit to identify factors associated with tympanostomy tube placement. A multivariate Poisson regression was then fit to determine the independent impact of asthma status and other atopic conditions controlling for pertinent covariates. In addition, to assess the potential detection bias (i.e., parents of asthmatic patients might be more likely to seek medical care for ear infections and tube placement than those without asthma), we compared the incidence of tube placement before and after a physician diagnosis of asthma.
RESULTS
Study Subjects
Of the initial 323 subjects, 3 subjects were excluded due to lack of parental authorization for records research. Of the remaining 320 subjects, 170 (53%) were boys, 268 (94%) were white, 124 (39%) were asthmatic patients, and 20 (6%) had tympanostomy tube placement. The mean age at first tympanostomy tube placement in the cohort was 4.16 (SD, 2.78 years) years. Among asthmatic and nonasthmatic children, the mean ages at first tympanostomy tube placement were 4.98 (SD, 3.09 years) years and 3.33 (SD, 2.29 years) years, respectively. Characteristics of study subjects are summarized in Table 2.
Table 2.
Characteristics of study subjects and factors associated with the incidence of tympanostomy tube placement based on a univariate Poisson regression model
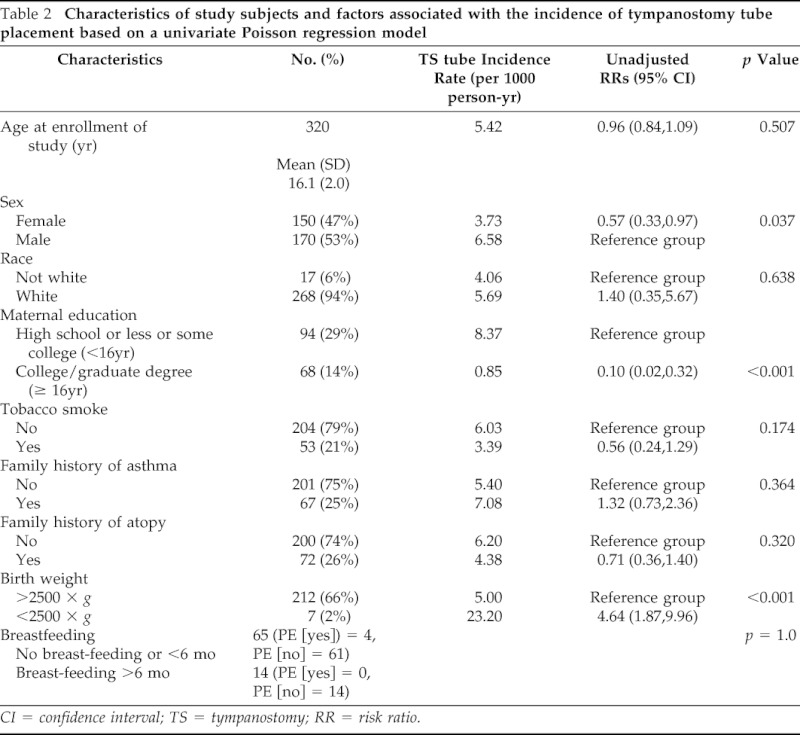
CI = confidence interval; TS = tympanostomy; RR = risk ratio.
Asthma and Tympanostomy Tube Placement
The results on the association between atopic conditions and tympanostomy tube placement are summarized in Table 2. Children with asthma were more likely to have tympanostomy tube placement compared with those without asthma (RR,19.3; 95% confidence interval [CI], 11.4–32.8; p < 0.001). When we assessed the incidence rates of tympanostomy tube placement without regard to index date of asthma during the first 18 years of life, asthmatic patients had a trend toward a higher incidence of tympanostomy tube placement when compared with nonasthmatic patients (6.69 versus 4.36 per 1000 person-years, respectively; RR, 1.53; 95% CI, 0.93–2.53; p = 0.095). Similar trends were observed when we limited analysis to the first 12 years of life only when tympanostomy tube placement occurred (10.88 versus 7.32 per 1000 person-years, respectively), but the results did not reach statistical significance (RR, 1.49; 95% CI, 0.88–2.50; p = 0.136).
Other Atopic Conditions and Tympanostomy Tube Placement
Children with atopic dermatitis and/or allergic rhinitis were more likely to have tympanostomy tube placement compared with those without such conditions (RR,19.3; 95% CI, 11.4–32.8; p < 0.001). The incidence rates of tympanostomy tube placement in children with and without a history of physician diagnosis of atopic dermatitis and allergic rhinitis were 7.34 and 4.66 per 1000 person-years, respectively (RR, 1.70; 95% CI, 1.01–2.86; p = 0.0460). Individuals with atopic dermatitis or allergic rhinitis in the first 12 years of life had a significantly increased incidence of tympanostomy tube placement compared with nonatopic individuals (13.39 versus 7.32 per 1000 person-years, respectively; RR, 1.97; 95% C,: 1.15–3.38; p = 0.014). When we analyzed separately between atopic dermatitis and allergic rhinitis, atopic dermatitis did not influence risk of otitis media but allergic rhinitis increased risk of tympanostomy tube placement compared with those without allergic rhinitis.
Tympanostomy Tube Placement before and after a Physician Diagnosis of Asthma
To assess potential detection bias, the incidence rate of tympanostomy tube placement was determined before and after a physician diagnosis of asthma. The results are summarized in Table 3. There was no difference in the incidence of tympanostomy tube placement before and after a physician diagnosis of asthma (8.03 versus 7.19 per 1000 person-year, respectively; unadjusted RR, 0.89; 95% CI, 0.49–1.67; p = 0.7255; Table 4). In addition, this observation was true for the incidence of tympanostomy tube placement before and after the index date of asthma.
Table 3.
Comparison of the incidence of tympanostomy tube placement between children with atopic conditions and those without atopic conditions
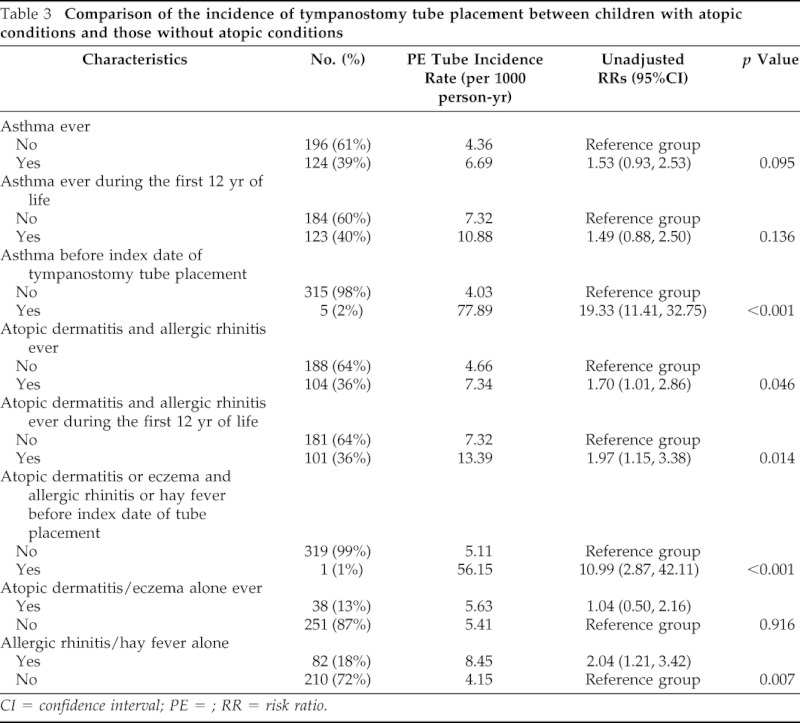
CI = confidence interval; PE = ; RR = risk ratio.
Table 4.
Incidence of TS tube placement before and after index date and physician diagnosis of asthma
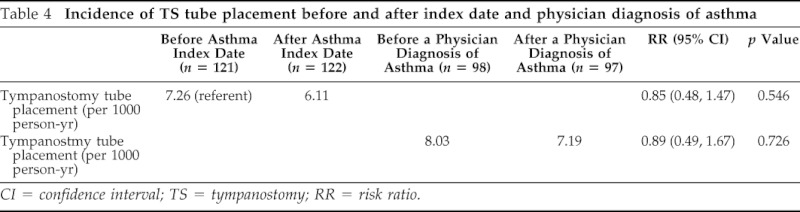
CI = confidence interval; TS = tympanostomy; RR = risk ratio.
DISCUSSION
More than one million tympanostomy tube placement procedures take place per year in North America for children with recurrent or chronic otitis media, and about 7% of children in the United States undergo this procedure during early childhood.39 Similarly, in our study ∼6% of children had undergone tympanostomy tube placement. Thus, given the significant number of children affected by atopic conditions in the United States, assessing atopic conditions as a risk factor for frequent and persistent ear infections is worthwhile.
In our study, children with asthma had a higher incidence rate of tympanostomy tube placement compared with those without asthma. This was also true for those with allergic rhinitis. This association could be caused by a detection bias because parents of children with asthma might be more likely to seek medical evaluations for upper respiratory infections than those without asthma. However, this association did not change before and after a physician diagnosis of asthma, suggesting a physician diagnosis did not seem to influence this association. In addition, we previously reported similar health care use between asthmatic and nonasthmatic patients and no differences in risk of outcome events between asthmatic patients with and without a physician diagnosis of asthma in our study setting.40–42
In support of our study findings, the literature suggests a potential association between atopic conditions and risk of otitis media. A cross-sectional study by Eldeirawi et al. using data from >7000 children between 2 and 11 years of age found that the lifetime prevalence of asthma was significantly associated with increased risk of otitis media.14 Also, Bentdal et al. reported a significant cross-sectional association between allergic diseases and atopic diseases, especially asthma, among 2,600 children aged 10 years in Oslo.22 Moreover, using data from the International Study of Asthma and Allergies in Childhood, Chen et al. found a significantly higher prevalence of infectious diseases including otitis media in children with asthma, allergic rhinitis, or atopic dermatitis.24
The biological mechanisms underlying this association between atopic disease and otitis media are unknown. We can postulate a few plausible mechanisms at both the structural and the functional levels. At a structural level, perhaps children with asthma or allergic rhinitis might be more likely to have allergic inflammation on upper respiratory tract, which might result in Eustachian tube dysfunction leading to otitis media.43 At a functional level, recent studies suggest a potential role of bacterial infections in development of asthma or bronchial hyperresponsiveness44,45 and the benefit from antibiotic treatment for asthma symptoms or bronchial reactivity.45,46 Our study results might not exclude this possibility of reverse causality (i.e., microbial infections or colonization might provoke airway symptoms and development of asthma).
However, given the reported increased risk of serious pneumococcal disease in atopics8,9and impaired innate47–50 and adaptive immune functions,51–54 we believe that patients with atopic conditions might be intrinsically susceptible to microbial infections or colonization due to impaired immune functions because microbial infections or colonization are unlikely to impair immune functions. Specific to pneumococcal immune functions in individuals with atopy, we recently reported a lower anti-pneumococcal polysaccharide antibody levels in asthmatic patients compared with nonasthmatic patients.55 Therefore, with the reported impairment in other innate and adaptive immune functions, the increased risk of otitis media, which is primarily caused by pneumococci, among children with asthma might be immunologically plausible.
One noteworthy finding of our study is the absence of a significant difference in the risk of recurrent or persistent otitis media (tympanostomy tube placement) before and after the index date of asthma. These results may potentially suggest that immunogenetic predisposition to asthma alone might be an important risk factor for recurrent or persistent otitis media. We reported similar findings on the increased risk of Streptococcus pyogenes infection before the onset of clinically defined asthma or other atopic conditions among children with asthma or other atopic conditions.42,56 Therefore, we postulate that susceptibility to microbial infections in individuals with atopic conditions is likely to be associated with the immunologic underpinnings of atopic conditions, and susceptibility to microbial infections and atopic status might be different outcomes of the same underlying biological factor. One example of such a biological factor associated with risk of atopic dermatitis and increased risk of skin infections is filaggrin, a crucial antimicrobial peptide in skin barrier.57,58 Recent studies suggest that filaggrin mutations, indeed, affect risks of asthma and allergic rhinitis as well.57,59,60 Thus, atopic patients with increased risk of microbial infections could be a phenotypic characteristic of filaggrin mutations and our study findings potentially support this notion.
The strengths of our study include study design as a cohort study, epidemiological advantages of study setting (self-contained health care environment with a unified medical record system for research), and predetermined criteria for asthma and actual incidence of events (tympanostomy tube placement as a marker for frequent and persistent ear infections) instead of self-report. However, our study has inherent limitations as a retrospective study. There were a significant number of missing data points for certain variables limiting our ability to adjust our study results for, but missing data points are likely to be subject to nondifferential misclassification. Along these lines, some pertinent variables were not available (e.g., allergic sensitization status or crowdedness in household). Our study was based on a small sample size. In addition, given the higher proportion of white population and people working in health care industry in our study setting, whether our study findings are generalizable to other settings needs to be interpreted carefully. For example, whether referral processes for tympanostomy tube placement or ear, nose, and throat practices in Olmsted County, MN, might differ from other settings is unknown and was not addressed. In addition, asthma prevalence in our study was relatively high because of the inclusive nature of asthma criteria and overrepresentation of children with asthma in our study subjects who participated in a previous vaccine study. However, underdetection or diagnosis of asthma has been also suggested. For example, Bisgaard and Szefler reported that of the 9,490 children from the United States and Europe, 32% of children were reported to suffer from recurrent symptoms of cough, wheeze, or breathlessness but they were not properly diagnosed and treated for asthma.61 Our study results need to be interpreted in these contexts.
In conclusion, asthma or allergic rhinitis may be unrecognized risk factors for recurrent or persistent otitis media. Immunogenetic predisposition to asthma itself might be a risk factor for recurrent and persistent otitis media. However, given the small sample size of the study, a cohort study with a larger sample size is necessary.
ACKNOWLEDGMENTS
The authors thank the staff of the Pediatric Asthma Epidemiology Research Unit who made this study possible. They thank Elizabeth Krusemark for administrative assistance and Dr. Gregory Poland and his staff for making the data set available and supporting the study.
Footnotes
Presented at the meeting of the Pediatric Academic Societies, Baltimore, Maryland, May 2009
Funded by the Scholarly Clinician Award from the Department of Pediatric and Adolescent Medicine Research Award, Mayo Clinic, Rochester, Minnesota, and was made possible by the Rochester Epidemiology Project (R01-AG034676) from the National Institute on Aging
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Moorman JE, Rudd RA, Johnson CA, et al. National surveillance for asthma—United States, 1980–2004. MMWR Surveill Summ 56:1–54, 2007. [PubMed] [Google Scholar]
- 2. Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics 123(suppl 3):S131–S145, 2009. [DOI] [PubMed] [Google Scholar]
- 3. Schultz Larsen F, Diepgen T, Svensson A. The occurrence of atopic dermatitis in north Europe: An international questionnaire study. J Am Acad Dermatol 34:760–764, 1996. [DOI] [PubMed] [Google Scholar]
- 4. Williams HC. Is the prevalence of atopic dermatitis increasing? Clin Exp Dermatol 17:385–391, 1992. [DOI] [PubMed] [Google Scholar]
- 5. Hanifin JM, Reed ML. A population-based survey of eczema prevalence in the United States. Dermatitis 18:82–91, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Nathan RA, Meltzer EO, Derebery J, et al. The prevalence of nasal symptoms attributed to allergies in the United States: Findings from the burden of rhinitis in an America survey. Allergy Asthma Proc 29:600–608, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Meltzer EO, Blaiss MS, Derebery MJ, et al. Burden of allergic rhinitis: Results from the Pediatric Allergies in America survey. J Allergy Clin Immunol 124(suppl):S43–S70, 2009. [DOI] [PubMed] [Google Scholar]
- 8. Juhn YJ, Kita H, Yawn BP, et al. Increased risk of serious pneumococcal disease in patients with asthma. J Allergy Clin Immunol 122:719–723, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Talbot TR, Hartert TV, Mitchel E, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med 352:2082–2090, 2005. [DOI] [PubMed] [Google Scholar]
- 10. The Advisory Committee on Immunization Practices (2008). ACIP Provisional Recommendations for Use of Pneumococcal Vaccines. Retrieved January 5, 2009, 2009, from www.cdc.gov/vaccines/recs/provisional/downloads/pneumo-Oct-2008-508.pdf.
- 11. Jung JA, Kita H, Yawn BP, et al. Increased risk of serious pneumococcal disease in patients with atopic conditions other than asthma. J Allergy Clin Immunol 125:217–221, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Auinger PLB, Kalkwarf HJ, Mansour ME. Trends in otitis media among children in the United States. Pediatrics 112:514–520, 2003. [DOI] [PubMed] [Google Scholar]
- 13. Zhou FSA, Kong Y, Nuorti JP. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997–2004. Pediatrics 121:253–260, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Teele DW, KJ, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: A prospective, cohort study. J Infect Dis 160:83–94, 1989. [DOI] [PubMed] [Google Scholar]
- 15. Keyhani S, Kleinman LC, Rothschild M, et al. Clinical characteristics of New York City children who received tympanostomy tubes in 2002. Pediatrics. 121:e24–e33, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Keyhani S, Kleinman LC, Rothschild M, et al. Overuse of tympanostomy tubes in New York metropolitan area: Evidence from five hospital cohort. BMJ 337:a1607, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singleton RJ, HR, Plant R, Yorita KL, et al. Trends in otitis media and myringtomy with tube placement among American Indian/Alaska native children and the US general population of children. Pediatr Infect Dis J 28:102–107, 2009. [DOI] [PubMed] [Google Scholar]
- 18. Kogan MD, Overpeck MD, Hoffman HJ, Casselbrant ML. Factors associated with tympanostomy tube insertion among preschool-aged children in the United States. Am J Public Health 90:245–250, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rystedt ISI, Strannegard O. Recurrent viral infections in patients with past or present atopic dermatitis. Br J Dermatol 114:575–582, 1986. [DOI] [PubMed] [Google Scholar]
- 20. Alles R, Parikh A, Hawk L, et al. The prevalence of atopic disorders in children with chronic otitis media with effusion. Pediatr Allergy Immunol 12:102–106, 2001. [DOI] [PubMed] [Google Scholar]
- 21. Anderson HR, Bland JM, Patel S, Peckham C. The natural history of asthma in childhood. J Epidemiol Community Health 40:121–129, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bentdal YE, Nafstad P, Karevold G, Kvaerner KJ. Acute otitis media in schoolchildren: Allergic diseases and skin prick test positivity. Acta Otolaryngol 127:480–485, 2007. [DOI] [PubMed] [Google Scholar]
- 23. Chantzi FM, Kafetzis DA, Bairamis T, et al. IgE sensitization, respiratory allergy symptoms, and heritability independently increase the risk of otitis media with effusion. Allergy 61:332–336, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Chen CF, Wu KG, Hsu MC, Tang RB. Prevalence and relationship between allergic diseases and infectious diseases. J Microbiol Immunol Infect 34:57–62, 2001. [PubMed] [Google Scholar]
- 25. Daly KA, Hoffman HJ, Kvaerner KJ, et al. Epidemiology, natural history, and risk factors: panel report from the Ninth International Research Conference on Otitis Media. Int J Pediatr Otorhinolaryngol 74:231–240, 2010. [DOI] [PubMed] [Google Scholar]
- 26. Eldeirawi K, Persky VW. History of ear infections and prevalence of asthma in a national sample of children aged 2 to 11 years: The Third National Health and Nutrition Examination Survey, 1988 to 1994. Chest 125:1685–1692, 2004. [DOI] [PubMed] [Google Scholar]
- 27. Hak E, Rovers MM, Sachs AP, et al. Is asthma in 2–12 year-old children associated with physician-attended recurrent upper respiratory tract infections? Eur J Epidemiol 18:899–902, 2003. [DOI] [PubMed] [Google Scholar]
- 28. Jones A. Asymptomatic bronchial hyperreactivity and the development of asthma and other respiratory tract illnesses in children. Thorax 49:757–761, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nafstad P, Magnus P, Jaakkola JJ. Early respiratory infections and childhood asthma. Pediatrics 106:E38, 2000. [DOI] [PubMed] [Google Scholar]
- 30. Souter MA, Mills NA, Mahadevan M, et al. The prevalence of atopic symptoms in children with otitis media with effusion. Otolaryngol Head Neck Surg 141:104–107, 2009. [DOI] [PubMed] [Google Scholar]
- 31. Todd NW, Feldman CM. Allergic airway disease and otitis media in children. Int J Pediatr Otorhinolaryngol Oct 10:27–35, 1985. [DOI] [PubMed] [Google Scholar]
- 32. Umapathy D, Alles R, Scadding GK. A community based questionnaire study on the association between symptoms suggestive of otitis media with effusion, rhinitis and asthma in primary school children. Int J Pediatr Otorhinolaryngol 71:705–712, 2007. [DOI] [PubMed] [Google Scholar]
- 33. Yunginger JW, Reed CE, O'Connell EJ, et al. A community-based study of the epidemiology of asthma. Incidence rates, 1964–1983. Am Rev Respir Dis 146:888–894, 1992. [DOI] [PubMed] [Google Scholar]
- 34. Katusic SK, Colligan RC, Barbaresi WJ, et al. Potential influence of migration bias in birth cohort studies. Mayo Clin Proc 73:1053–1061, 1998. [DOI] [PubMed] [Google Scholar]
- 35. Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am 245:54–63, 1981. [DOI] [PubMed] [Google Scholar]
- 36. Hanchard NA, Jacobson RM, Poland GA, Juhn YJ. An assessment of the association between childhood asthma and HLA DRB1*03 using extended haplotype analysis. Tissue Antigens 76:491–494, 2010. [DOI] [PubMed] [Google Scholar]
- 37. Ovsyannikova IG, Jacobson RM, Vierkant RA, et al. Human leukocyte antigen class II alleles and rubella-specific humoral and cell-mediated immunity following measles-mumps-rubella-II vaccination. J Infect Dis 191:515–519, 2005. [DOI] [PubMed] [Google Scholar]
- 38. Yoo KH, Agarwal K, Butterfield M, et al. Assessment of humoral and cell-mediated immune response to measles-mumps-rubella vaccine viruses among patients with asthma. Allergy Asthma Proc 31:499–506, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yunginger JW, Reed CE, O'Connell EJ, et al. A community-based study of the epidemiology of asthma. Incidence rates, 1964–1983. Am Rev Respir Dis 146:888–894, 1992. [DOI] [PubMed] [Google Scholar]
- 40. Juhn YJ, Johnson SK, Hashikawa AH, et al. The potential biases in studying the relationship between asthma and microbial infection. J Asthma 44:827–832, 2007. [DOI] [PubMed] [Google Scholar]
- 41. Lynch BA, Fenta Y, Jacobson RM, et al. Impact of delay in asthma diagnosis on chest x-ray and antibiotic utilization by clinicians. J Asthma 49:23–28, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frey D, Jacobson R, Poland G, et al. Assessment of the association between pediatric asthma and Streptococcus pyogenes upper respiratory infection. Allergy Asthma Proc 30:540–545, 2009. [DOI] [PubMed] [Google Scholar]
- 43. Skoner AR, Skoner KR, Skoner DP. Allergic rhinitis, histamine, and otitis media. Allergy Asthma Proc 30:470–481, 2009. [DOI] [PubMed] [Google Scholar]
- 44. Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 357:1487–1495, 2007. [DOI] [PubMed] [Google Scholar]
- 45. Huang YJ, Nelson CE, Brodie EL, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol 127:372–381, e371–373, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johnston S, Blasi F, Black P, et al. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med 354:1589–1600, 2006. [DOI] [PubMed] [Google Scholar]
- 47. Ingo M, Tamara K, Anja B, et al. An interaction between filaggrin mutations and early food sensitization improves the prediction of childhood asthma. J Allergy Clin Immunol 123:911–916, 2009. [DOI] [PubMed] [Google Scholar]
- 48. Weidinger S, O'Sullivan M, Illig T, et al. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J Allergy Clin Immunol 121:1203–1209.e1201, 2008. [DOI] [PubMed] [Google Scholar]
- 49. Contoli M, Message SD, Laza-Stanca V, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med 12:1023–1026, 2006. [DOI] [PubMed] [Google Scholar]
- 50. Wark PA, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 201:937–947, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Strannegard IL, Lindholm L, Strannegard O. T lymphocytes in atopic children. Int Arch Allergy Appl Immunol 50:684–692, 1976. [DOI] [PubMed] [Google Scholar]
- 52. Grove DI, Burston TO, Wellby ML, et al. Humoral and cellular immunity in asthma. J Allergy Clin Immunol 55:152–163, 1975. [DOI] [PubMed] [Google Scholar]
- 53. Grove DI, Reid JG, Forbes IJ. Humoral and cellular immunity in atopic eczema. Br J Dermatol 92:611–618, 1975. [DOI] [PubMed] [Google Scholar]
- 54. Schneider LC, Weinberg A, Boguniewicz M, et al. Abnormal immune response to varicella vaccine in subjects with atopic dermatitis compared to non-atopic controls. J Allergy Clin Immunol 121(suppl 1):S272–S273, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jung J, Kita H, Nahm M, et al. Influence of asthma status on serotype specific antibody pneumococcal antibody levels. Postrad Med 122:116–124, 2010. [DOI] [PubMed] [Google Scholar]
- 56. Juhn YJ, Frey D, Li X, Jacobson R. Streptococcus pyogenes upper respiratory infection and atopic conditions other than asthma: a retrospective cohort study. Prim Care Respir J doi: 10.4104/pcrj.2011.00110. [Epub ahead of print] January 23, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weidinger S, O'Sullivan M, Illig T, et al. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J Allergy Clin Immunol 121:1203–1209, e1201, 2008. [DOI] [PubMed] [Google Scholar]
- 58. Gao P-S, Rafaels NM, Hand T, et al. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J Allergy Clin Immunol 124:507–513, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li M, Chen X, Chen R, et al. Filaggrin gene mutations are associated with independent atopic asthma in Chinese patients. Allergy 66:1616–1617, 2011. [DOI] [PubMed] [Google Scholar]
- 60. van den Oord RA, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: Systematic review and meta-analysis. BMJ 339:b2433, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bisgaard H, Szefler S. Prevalence of asthma-like symptoms in young children. Pediatr Pulmonol 42:723–728, 2007. [DOI] [PubMed] [Google Scholar]


