Abstract
Diagnosis of tuberculosis (TB) in children by the tuberculin skin test (TST) poses a diagnostic challenge for physicians due to its low specificity and cross-reactivity with nontuberculous mycobacteria and bacille Calmette-Guerin (BCG). Although interferon-gamma release assays (IGRAs) have been shown as novel TST alternatives for diagnosis of latent TB infection (LTBI) in adults, their effectiveness is less clear in children. The present study examined QuantiFERON-TB Gold (QFT-G) responses and IFN-gamma production capacity of TST-positive children, younger children ≤5 years. A total of 517 children of whom 434 were TST positive ranging in age from 1 month to 18 years were evaluated by the QFT-G. Of the 517 children, 434 (84%) were TST positive, 25 (5.8%) of whom were found to be QFT-G positive and 25 (5.4%) with an indeterminate response. Of the 517 children, 355 (68.7%) were previously BCG immunized and 310/355 (87.3%) were TST positive including 18/27 (66.7%) QFT-positive children. Adequate IFN-gamma production by purified TB peptides or mitogen was observed in 92.8% of children, 29.6% of whom were <5 years. This study shows that the QFT-G assay is useful for diagnosis of LTBI. The finding of 5.8% positive QFT-G in 434 TST-positive children underscores the superior specificity of the QFT-G than the TST and its greater cost effectiveness in preventing unnecessary and potentially toxic treatment in children. The study suggests that the majority of positive TST in children represent false-positive reactions and supports the use of IGRAs for diagnosis of LTBI in children, including those <5 years of age.
Keywords: Latent tuberculosis, Mycobacterium tuberculosis, tuberculin skin test, BCG, quantiferon TB gold test, interferon gamma release assay, ESAT, CFP-10, mitogen, children
The tuberculin skin test (TST) was until recently the only means of diagnosing latent tuberculosis (TB) infection (LTBI). Recent advances in genomics and immunology have led to the development of two new interferon-γ (IFN-γ) release assays (IGRAs) for the diagnosis of TB that utilize highly specific peptides for Mycobacterium tuberculosis absent from bacille Calmette-Guerin (BCG) vaccine and most nontuberculous mycobacteria.1–13 The QuantiFERON-TB Gold (QFT-G) and QuantiFERON-TB Gold In-Tube (QFT-GIT) (Cellestis; Carnegie, Australia) assay11 measures IFN-γ by an enzyme-linked immunosorbent assay (ELISA) after stimulation by two antigens, i.e., the early secretory antigenic target-6 (ESAT-6) and the culture filtrate protein-10 (CFP-10) peptides. The enzyme-linked immunospot (ELISpot) (T-SPOT.TB; Oxford Immunotec, Oxford, UK) enumerates IFN-γ-secreting T cells.9,14–16 Several studies performed primarily in adults indicate that both tests are more specific than the TST and are not confounded by previous BCG vaccination or by infection with most nontuberculous bacteria.9,17–31 Powell et al.30 recommend that with unusual IFN-γ measurements, not only should the QFT-G and QFT-GIT be repeated with another blood sample to avoid erroneous diagnosis of M. tuberculosis infection and interpreted with caution if they recur but also encourage more meticulous recognition of these unusual IFN-γ measurements that may be a predictive marker of inaccurate results. In the study by Pareek et al.,24 the use of screening by QFT-GIT in the UK for latent TB in immigrant populations was found to be cost-effective, thereby preventing substantial numbers of future cases of active TB.
In their recommendations for use of the test, the Centers for Disease Control and Prevention (CDC) has suggested the need for additional studies in young children, especially those <5 years, both to establish the validity of the IGRAs and to make comparisons of these tests with the TST.12,13 Targeted TST testing is currently recommended by the American Academy of Pediatrics (AAP) Red Book Committee for the diagnosis of TB for children at high risk of TB infection or with other TB-predisposing medical conditions, e.g., HIV infection. The availability of these additional data would also assist in the formulation of new recommendations for TB testing in the pediatric age group by the AAP Red Book Committee.
The primary purpose of the present study was to examine the in vitro QFT-G responses of children at risk to TB in children in the United States and to compare age-related variations in IFN-γ production with QFT-G responses in the younger age groups. A secondary goal of this study is to alert the allergist-immunologist to the importance and usefulness of the IGRAs in the optimal management of patients who present with suspected TB infection that may be confounded by an ambiguous positive TST.
MATERIALS AND METHODS
Study Design
The study was approved by the Georgetown University Institutional Review Board under protocol 2003-080. Over a 7-year period from 2004 to 2011, 517 healthy children from the United States were recruited in the study. The study participants ranged in age from 1 month to 18 years and were referred mainly by pediatricians from the private and public health clinics in the greater Washington, DC, area who provide care for U.S. and foreign-born children. All children were enrolled in the study primarily because of an ambiguous diagnostically interpretable TST after their parents or guardians were informed of the availability of a new in vitro QFT-G test that could improve the diagnostic accuracy of the currently employed TST and after they had provided informed consent (Fig. 1). Detailed information was obtained by questionnaire on demographic and TB risk factors, including country of birth, travel, history of exposure to patients with active TB, and any previous history and date of BCG immunization. This group included a relatively larger minority population of Asians and Latinos, the majority of which had received BCG in countries where active TB infection is quite prevalent and where the use of BCG immunization of children is the norm. The majority of these children had their TST performed as part of international adoption policy and were being referred because of the uncertainty of interpretation of their positive TST responses, which could have resulted from their previous BCG immunization or TB infection. All children were healthy with no underlying conditions and who were not receiving any antiinflammatory or immunosuppressive therapy that might have affected the interpretation of test results.
Figure 1.
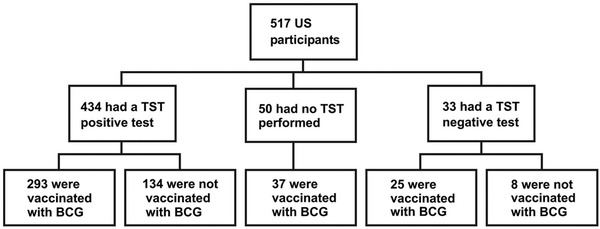
Chart showing the TST reactivity of total study group in comparison with their BCG immunization status.
The basis for inclusion of relatively younger children in the study was to permit the evaluation of the effects of the stage of maturation of the immune system on the QFT-G test results because of the known lowered immune responsiveness of the small infant. One of the major goals of the study was to determine whether there were any age-related variations in IFN-γ production, particularly in children <5 years of age that might preclude the future use of the QTB-G assay for the diagnosis of active TB or LTBI.
Written parental informed consent was obtained, and a verbal assent was obtained from older children (aged 12 years or more).
TST Procedures
The TST procedures used methods currently described in the classification and interpretation guidelines for TST reactions recommended by the Division of Tuberculosis Elimination, CDC,31 and the AAP Committee on Infectious Diseases.33,36,37 All TST procedures were performed by the referring healthcare providers and were not repeated during the study.
QuantiFERON-TB Gold
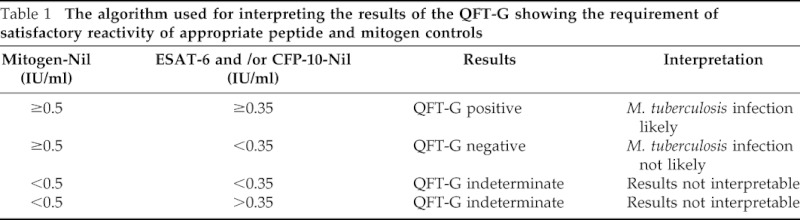
The QFT-G (Cellestis Ltd., Carnegie, Victoria, Australia) assay was used for the study of all children and was performed according to the manufacturer's instructions.11 The test kit is supplied with peptides simulating ESAT-6 and CFP-10 proteins, mitogen (phytohemagglutinin), and nil control, which are used to stimulate T cells in whole heparinized blood samples during incubation. The test uses a two-stage assay system; the first stage consists of incubation of whole blood with antigens, mitogen, and nil control, and the second involves measurement of IFN-γ production by ELISA in harvested plasma. Briefly, ∼5 ml of blood was obtained from each subject and placed in a 10-ml heparinized BD Vacutainer tube. After evenly mixing the blood by gentle rotation of the tube, a 1.0-ml aliquot was dispensed into individual wells for test antigens, mitogen, and nil control. After the addition of three drops (125 μl) of each antigen, mitogen, or nil control into each respective blood-containing well, the plate was covered, mixed thoroughly, and incubated in a humidified 37°C incubator for 16–24 hours. After incubation, plasma was removed from each well and the amount of IFN-γ measured by ELISA. The method used for interpretation of QFT-G test results consisted of an algorithm requiring the satisfactory reactivity of appropriate peptide and mitogen controls as shown in Table 1.
Table 1.
The algorithm used for interpreting the results of the QFT-G showing the requirement of satisfactory reactivity of appropriate peptide and mitogen controls
The information obtained from the QFT-G was not used by the investigators with an intent-to-treat purpose but may have been used in this way by the referring healthcare providers after receiving the test results.
Statistical Analysis
Data were analyzed by one-way analysis of variance or Kruskal-Wallis analysis of variance on ranks followed by a Holm-Sidak's multiple-comparisons test (when data were not normally distributed or variance was different between groups) using SigmaStat software (Chicago, IL). The unpaired t test was used to determine statistical differences between two groups.
RESULTS
Characteristics of Children
The age and gender distribution of children is shown in Table 2 and Fig. 2. Among the 517 children, 211were male and 306 female who ranged in age from 1 month to 18 years and stratified into the following five subgroups: 1 month to 1 years (61), 2–4 years (110), 5–9 years (125), 10–14 years (122), and 15–18 years (99). Of the 517 children, 434 were TST positive and 33 TST negative (Fig. 1). A total of 355 children had received the BCG vaccine; of these, 310 (87.3%) were TST positive and 26 (7.3%) TST negative. Shown in Table 3 is the ethnic distribution of the population in comparison with the reported ethnic distribution on the greater Washington, DC, area. The ethnic distribution of the study population was slightly higher in the Asian and Latino populations and lower in the African-American population.
Table 2.
Characteristics of U.S. children
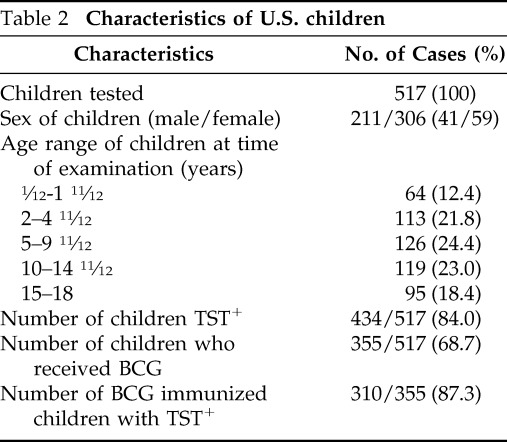
Figure 2.
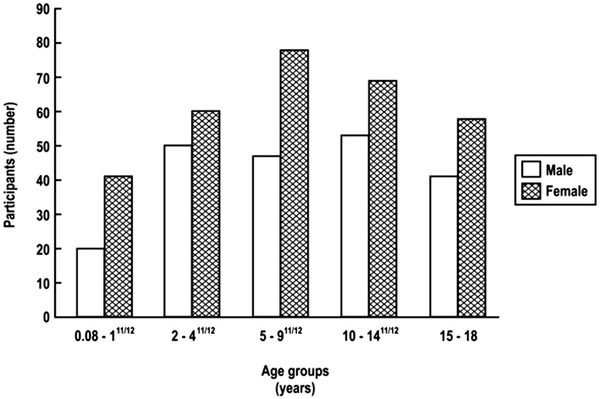
Age and gender distribution of children.
Table 3.
Ethnic distribution of the population
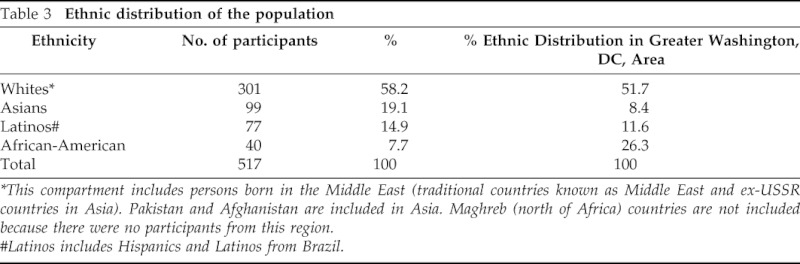
*This compartment includes persons born in the Middle East (traditional countries known as Middle East and ex-USSR countries in Asia). Pakistan and Afghanistan are included in Asia. Maghreb (north of Africa) countries are not included because there were no participants from this region.
#Latinos includes Hispanics and Latinos from Brazil.
Results of the QFT-G Assay Performed in Children
Shown in Table 4 is a summary comparison of the results of the QFT-G determinations performed in children with their clinical and x-ray findings. Only one child had a previous history of active TB diagnosed by chest x-ray and sputum culture after which he had been treated for a 6-month period with anti-TB medications. Of the 517 children studied, 27 (5.2%) showed positive QFT-G responses, and 37 (7.15%) children showed indeterminate results. When the data from the 27 QFT-G-positive children were analyzed more closely with respect to BCG immunization and TB exposure history, several comparisons could be made (Table 5). The ages of the 27 QFT-G-positive children ranged from 9 months to 18 years. It can be seen that 20 of the 27 subjects had received previous BCG immunization, 2 gave a history of previous exposure to TB, and 1 had positive chest x-ray.
Table 4.
Comparison of the clinical and QFT findings in 517 U.S. children
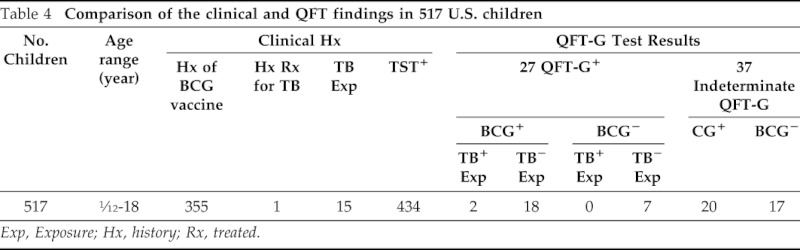
Exp, Exposure; Hx, history; Rx, treated.
Table 5.
Comparison of the BCG immunization status and TB exposure history in the 27 QFT-G-positive children
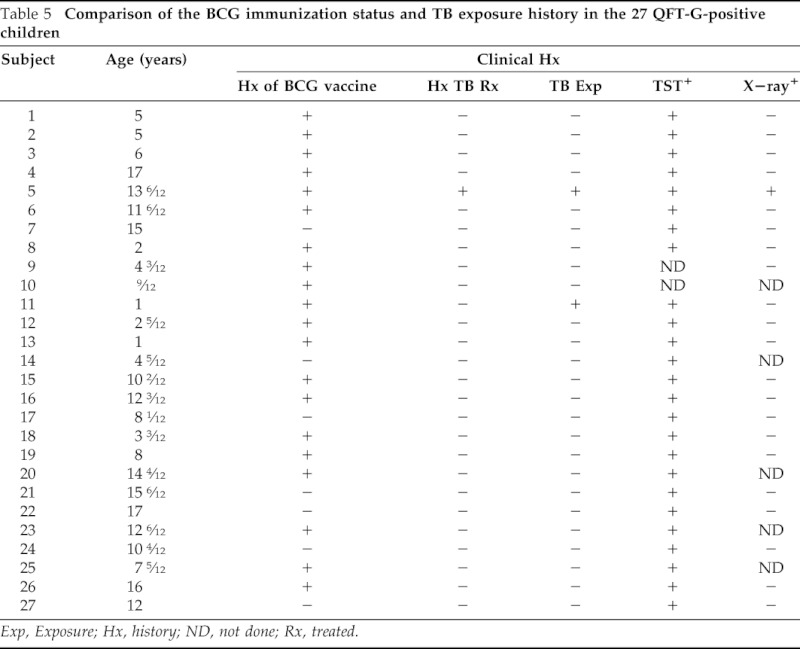
Exp, Exposure; Hx, history; ND, not done; Rx, treated.
Results of Studies of Age-Related Capacity of IFN-γ Production
Because one of the major goals of this research was to study variations in age-related immune capacity of the child to determine the validity of the use of the QFT-G assay in the younger age group particularly in children <5 years of age, the capacity of IFN-γ production was measured in various age groups of children in response to specific TB peptides as well as to mitogen. The results of these studies are shown in Figs. 3 and 4, respectively.
Figure 3.
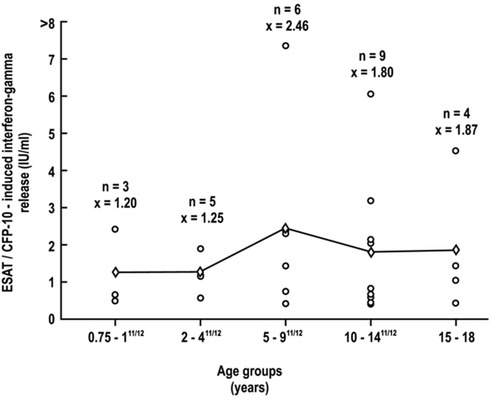
Age-related changes in IFN-γ production to specific peptides in 27 QFT-G-positive children between 0.75 and 18 years.
Figure 4.
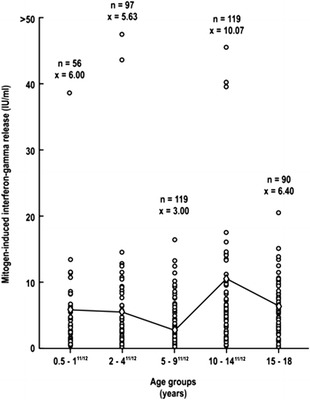
Age-related changes in IFN-γ production to mitogen in children between 0.5 and 18 years.
The IFN-γ production in response to specific peptides in the 27 QFT-G-positive children could be evaluated only in children in the 9 months to 18 years range, the group where the only positive QFT-G responses were observed (Fig. 3). Within this age range, no statistically significant differences in mean values were observed between the various subgroups that comprised this age range (p = 0.776). In contrast, IFN-γ production in response to mitogen could be measured in all 481 subjects within the age range of 6 months to 18 years (Fig. 4). In this age range, adequate IFN-γ responses were seen, and the only statistically significant difference in mean values among the various subgroups was seen between the 5–9 year and the 10–14 year groups (p < 0.05). In children <5 years of age, comparable mean values of IFN-γ production in response to mitogen was found, i.e., 5.8 IU/ml.
Of particular importance was the finding of adequate IFN-γ responses even in the youngest age groups <5 years with mean values of 5.9 IU/ml in the 0.5–2 years group and 5.6 IU/ml in the 2–4 years group.
DISCUSSION
The use of the traditional TST for diagnosis of TB poses a perplexing challenge for the healthcare provider entrusted to the care of children due to its low specificity and its cross-reactivity with nontuberculous mycobacteria or previous BCG immunization.13 The ambiguity of TST findings is of particular concern to the allergist-immunologist who often includes TB infection in the differential diagnosis of children suspected to have allergic disease possibly complicated by TB. Despite its shortcomings, routine TST testing of infants and children was the norm until the 1960s and 1970s, when rates of tuberculosis infection were high in the United States and universal screening for TB was required for all children.30–32 In 1996, the AAP's Committee on Infectious Diseases recommended the use of targeted TST in children of high-risk groups, such as Blacks, Hispanics, the socioeconomically disadvantaged, and children living in neighborhoods with higher average than the national average rates of TB,34 and discouraged universal testing of children who lacked these risk factors.34–36 The most recent recommendations of the AAP's Committee on Infectious Diseases37 are consistent with those of the CDC13 and recommend judicious use of the TST and the IGRAs, particularly in children <5 years of age.
In recent years, the availability of IGRAs has led to proposals for their use as alternatives to the TST.1,3–5,11–13 Their accessibility has also prompted the National Institute of Health and Clinical Excellence in the United Kingdom to recommend the use of the IGRAs to discriminate between a true- or false-positive reaction.38,39 In the original guidelines for using the QFT-G in detecting M. tuberculosis infection,12 the CDC cautiously recommended the need for additional studies regarding the use of QFT-G in young children, especially those aged <5 years, both to establish the validity of the assay in this younger age group and to make comparisons of the test with the TST. The basis for this guarded recommendation was based on the known lowered immune responsiveness of the young host, particularly children <5 years of age that might preclude the future use of the QTB-G assay for the diagnosis of either LTBI or active TB disease.33 One of the major goals of the present study, therefore, was to determine whether there were any age-related variations in IFN-γ production in the younger child.
The results of the present study confirm and extend published observations supporting the validity of QFT IFN release assays in two groups of children. In a group of 517 U.S. children referred by healthcare providers and pediatricians, 434 (84%) were TST positive. Of these 434 TST-positive children, 25 (5.8%) were found to be QFT-G positive and would be considered LTBI; because the vast majority of TST-positive reactive children, i.e. 434 (84%), appeared not to be infected with TB, they would not have required antituberculous treatment. In the absence of the availability of the IGRAs, therefore, these children would have been treated unnecessarily for LTBI. Of the 517 children, 355 were previously immunized with BCG; of these 355 children, 310 (87.3%) were TST positive, which included 18/27 (66.7%) children who were QFT positive. Therefore, of the 27 QFT-G-positive responses, 7 were seen in non-BCG-immunized children and 20 in children who had also received the BCG vaccine. The simultaneous occurrence in the 18 TST-positive children who had received BCG could have contributed to a further diagnostic dilemma of whether their skin reactivity was due either to previous immunization or TB infection, a problem that was resolved by the use of the QFT-G. The finding that only 18 TST-positive children were actually TB infected and would require antituberculous therapy obviated the unnecessary use of antituberculous medication in the remaining 292 children with TST-positive responses that were related to previous BCG immunization. Moreover, the unnecessary use of TB medication resulting from a lack of specificity of TST may have resulted in an increased risk of significant drug toxicity that was also avoided by the use of the QFT-G.
One of the most interesting results of the study is the finding of adequate IFN-γ production by the young child. For ease of discussion, IFN-γ is classically a T-cell-derived cytokine produced by Th1 CD4+ lymphocytes44 as part of the IL-12/IFN-γ pathway (Fig. 5). This pathway is critical in determining which of the three TB/macrophage outcomes will occur; these include 1) successful killing of the TB by the macrophage, 2) killing of the macrophage by the TB overwhelming the microbicidal capacity, and 3) establishment of chronic infection where both the TB and macrophage survive as in chronic infection seen in LTBI. Shown in Fig. 6 is a more detailed representation of the sequential IL-12/IL-2/IFN-γ/tumor necrosis factor-α (TNF-α) cytokine cascade involved in the macrophage/Th1 interaction resulting in successful killing. In the present study, adequate levels of IFN-γ were found in response to either ESAT-6 or CFP-10 peptides in all 27 QFT-G-positive children, which included 12 children as young as 9 months of age. The measurement of adequate IFN-γ production in response to mitogen was also documented in all 517 U.S. children but particularly in the 56 children as young as 6 month to 1 years and 97 in the 2–4 years group or a total of 153/517 (229.6%) in the group <5 years of age. Moreover, the levels of IFN-γ in the <5 year age group were comparable to those seen in the children >5 yrs of age.
Figure 5.
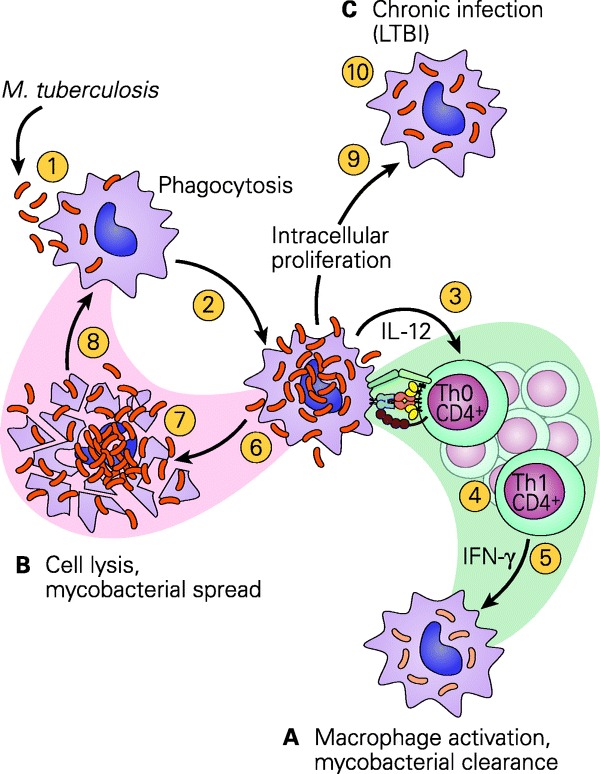
Schematic representation of the three TB/macrophage outcomes after the interaction of the tubercle bacillus with a macrophage. (A) The macrophage kills the organism; (B) the organism kills the macrophage; (C) the organism takes up residence in the macrophage and establishes chronic infection, as seen in latent TB infection (LTBI). [Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 201240].
Figure 6.
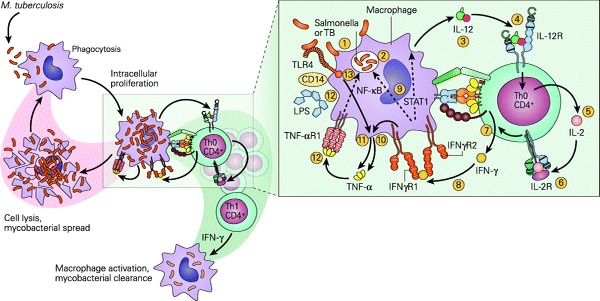
Schematic representation of the IL-12/IFN-γ pathway illustrating various activation pathways associated with the killing of intracellular bacterial organisms such as M. tuberculosis (TB) and Salmonella. After the uptake of the TB by the macrophage (1) into a phagosome (2), the macrophage is stimulated to produce IL-12 (3). The binding of IL-12 to its IL-12R on a Th CD4+ cell (4) induces the synthesis of IL-2 (5), and after autocrine binding of IL-2 to its receptor IL-2R (6) leads to the synthesis of IFN-γ (7). The binding of IFN-γ to its receptor IFNγR (8) activates both the signal transducer and activator of transcription 1 (STAT1) and nuclear factor (NF)-κB pathways (9) to kill the TB and to further enhance IL-12 production; the IFN- γ-activated macrophage (10) and Toll-like receptor 4 (TLR4) activation by the TB (11) induce TNF-α production that, after binding to its receptor (12), leads to further enhancement of intracellular killing of the TB (13). [Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 201240].
The results of the present study suggest that there may be adequate immunologic maturity with respect to T-cell-driven IFN-γ cytokine production by the lymphocytes of children younger than 5 years to justify the use of the QFT-G and other IGRAs for use in diagnosis of TB in this younger age group.
A number of unanswered questions remain concerning the use of IGRAs for the diagnosis of M. tuberculosis in children. With the availability of both ELISA and ELISpot assays now available in the US, how will the two compare with sensitivity? Will the measurement of other proinflammatory, e.g. IL-1, IL-6, and IL-17, or immunoregulatory, e.g. IL-10 and TGF-β, cytokines by IGRAs be helpful in further unraveling the diagnostic dilemma of TB in children?41,42,43 In the study reported by Krummel et al.,41 a combination of a M. tuberculosis-specific IFN-γ ELISpot with a M. tuberculosis-specific IL-2 ELISpot was shown to significantly improve the identification of individuals with the highest risk of recent M. tuberculosis infection and offers a promising method that should be explored to target the treatment of LTBI. Another promising diagnostic tool addressing the need for additional assays to improve diagnosis of M. tuberculosis infection in children was reported by Ruhwald et al.43 This study evaluated the performance of an IFN-γ-inducible protein 10 (IP-10)- and IL-2-based test for the diagnosis of M. tuberculosis infection in recently exposed children from Nigeria. After stimulation of lymphocytes in whole blood preparations with M. tuberculosis-specific antigens and mitogen, the high-risk children expressed significantly higher levels predominantly of IP-10 and lesser elevations of IL-2 than low-risk groups. These findings suggest that IP-10 and possibly IL-2 could be alternative or adjunct markers to IFN-γ in the diagnosis infection with M. tuberculosis.
Another practical application of IGRA tests is their use in screening patients before the use of corticosteroids and the recently developed biologic response modifiers, e.g. anti-TNF-α agents known to exacerbate TB infection.44 The observation that anti-TNF-α therapy is associated with reactivation of LTBI has led to clinical guidelines that recommend screening patients for previous evidence of TB infection before commencing anti-TNF-α treatment.40 Of particular concern for the allergist-immunologist is the known modifying effect of corticosteroids commonly used in the treatment of allergic disease on the TST, resulting in misleading false-negative results, thus exposing undiagnosed patients to the risk of TB reactivation when treated with these agents.
In conclusion, the results of the present study are in keeping with those of other studies and show that the QFT-G and QFT-GIT offer distinct advantages over the TST. They also provide new evidence that the young infant <5 years of age has sufficient immunologic capacity for IFN-γ production to allow the test to be performed in younger populations. It would seem prudent to follow the National Institute of Health and Clinical Excellence guidelines38,39 and perform the TST as an initial screening tool and carry out IFN-γ testing in patients with positive TST results.
Footnotes
Presented as a poster at the Annual 2011 Meeting of the American College of Allergy, Asthma and Immunology (ACAAI) in Boston, MA
Supported by research grants from National Institutes of Health (RO3AI060856), the Potts Foundation (AWD4285501), and the MedStar Research Institute (FY2007IRGA-04)
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Stout JE, Menzies D. Predicting tuberculosis: does the IGRA tell the tale? Am J Respir Crit Care Med 177:1055–1057, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Starke JR. Interferon-gamma release assays for diagnosis of tuberculosis infection in children. Pediatr Infect Dis J 25:941–942, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-γ release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 12:45–55, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pavić I, Topić RZ, Raos M, et al. Interferon-γ release assay for the diagnosis of latent tuberculosis in children younger than 5 years of age. Pediatr Infect Dis J 30:866–870, 2011. [DOI] [PubMed] [Google Scholar]
- 5. Machingaidze S, Wiysonge CS, Gonzalez-Angulo Y, et al. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta-analysis. Pediatr Infect Dis J 30:694–700, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Ling DI, Zwerling AA, Steingart KR, et al. Immune-based diagnostics for TB in children: what is the evidence? Paediatr Respir Rev 12:9–15, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Zhang S, Shao L, Mo L, et al. Evaluation of gamma interferon release assays using Mycobacterium tuberculosis antigens for diagnosis of latent and active tuberculosis in Mycobacterium bovis BCG-vaccinated populations. Clin Vaccine Immunol 17:1985–1990, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lalvani A, Pareek M. Interferon gamma release assays: principles and practice. Enferm Infecc Microbiol Clin 28:245–252, 2010. [DOI] [PubMed] [Google Scholar]
- 9. Connell TG, Ritz N, Paxton GA, et al. A three-way comparison of tuberculin skin testing, QuantiFERON-TB gold and T-SPOT. TB in children. PLoS One 3:e2624, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dewan PK, Grinsdale J, Liska S, et al. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis 6:47, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cellestis Web page, QuantiFERON-TB Gold In-Tube Test Manufacturer's instructions. Available at: www.cellestis.com; accessed June 11, 2007.
- 12. Mazurek GH, Jereb J, Lobue P, et al. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005. December 16;54(RR-15):49–55. Erratum in: MMWR Morb Mortal Wkly Rep. 54(50):1288, December 23, 2005. [PubMed] [Google Scholar]
- 13. Mazurek GH, Jereb J, Vernon A, et al. IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection: United States, 2010. MMWR Recomm Rep. 59(RR-5):1–25, 2010. [PubMed] [Google Scholar]
- 14. T-SPOT.TB T-Spot.TB is a type of ELISPOT assay, manufactured by Oxford Immunotec Ltd. in the UK, that counts the number of effector T cells that produce gamma interferon stimulated by proteins produced by the bacteria that cause tuberculosis. www.oxfordimmunotec.com. [Google Scholar]
- 15. Cruz AT, Geltemeyer AM, Starke JR, et al. Comparing the tuberculin skin test and T-SPOT. TB blood test in children. Pediatrics 127:e31–e8, 2011. [DOI] [PubMed] [Google Scholar]
- 16. Diel R, Loddenkemper R, Meywald-Walter K, et al. Comparative performance of tuberculin skin test, QuantiFERON-TB-Gold In Tube, and T-Spot. TB test in contact investigations for tuberculosis. Chest 135:1010–1018, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Bua A, Molicotti P, Delogu G, et al. QuantiFERON TB Gold: a new method for latent tuberculosis infection. New Microbiol 30:477–480, 2007. [PubMed] [Google Scholar]
- 18. Diel R, Loddenkemper R, Meywald-Walter K, et al. Predictive value of a whole blood IFN-γ assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med 177:1164–1170, 2008. [DOI] [PubMed] [Google Scholar]
- 19. Mori T, Sakatani M, Yamagishi F, et al. Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med 170:59–64, 2004. [DOI] [PubMed] [Google Scholar]
- 20. Kang YA, Lee HW, Yoon HI, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA 293:2756–2761, 2005. [DOI] [PubMed] [Google Scholar]
- 21. Brock I, Munk ME, Kok-Jensen A, et al. Performance of whole blood IFN-γ test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int J Tuberc Lung Dis 5:462–467, 2001. [PubMed] [Google Scholar]
- 22. Brock I, Weldingh K, Lillebaek T, et al. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med 170:65–69, 2004. [DOI] [PubMed] [Google Scholar]
- 23. Diel R, Ernst M, Döscher G, et al. Avoiding the effect of BCG vaccination in detecting Mycobacterium tuberculosis infection with a blood test. Eur Respir J 28:6–23, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Pareek M, Watson JP, Ormerod LP, et al. Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infect Dis 11:435–444, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang SH, Powell DA, Nagaraja HN, et al. Evaluation of a modified interferon-gamma release assay for the diagnosis of latent tuberculosis infection in adult and paediatric populations that enables delayed processing. Scand J Infect Dis 42:845–850, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lienhardt C, Fielding K, Hane AA, et al. Evaluation of the prognostic value of IFN-gamma release assay and tuberculin skin test in household contacts of infectious tuberculosis cases in Senegal. PLoS One 5:e10508, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dyrhol-Riise AM, Gran G, Wentzel-Larsen T, et al. Diagnosis and follow-up of treatment of latent tuberculosis; the utility of the QuantiFERON-TB Gold in-tube assay in outpatients from a tuberculosis low-endemic country. BMC Infect Dis 10:57, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Machado A, Jr., Emodi K, Takenami I, et al. Analysis of discordance between the tuberculin skin test and the interferon-gamma release assay. Int J Tuberc Lung Dis 13:446–453, 2009. [PubMed] [Google Scholar]
- 29. Nienhaus A, Schablon A, Diel R. Interferon-gamma release assay for the diagnosis of latent TB infection: analysis of discordant results, when compared to the tuberculin skin test. PLoS One 3:e2665, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Powell RD, III, Whitworth WC, Bernardo J, et al. Unusual interferon gamma measurements with QuanitFERON-TB Gold and QuantiFERON-TB In-Tube tests. PLoS ONE 6:1–6, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Centers for Disease Control. Tuberculosis in the United States: 1987. Atlanta, GA: Centers for Disease Control, US Department of Health and Human Services publication (CDC) 89-8322, 1989. [Google Scholar]
- 32. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med 161(4 Pt 1):1376–1395, 2000. [DOI] [PubMed] [Google Scholar]
- 33. American Academy of Pediatrics, Committee on Infectious Diseases. Update on tuberculosis skin testing of children. Pediatrics 97:282–284, 1996. [PubMed] [Google Scholar]
- 34. Starke JR, Jacobs RF, Jereb J. Resurgence of tuberculosis in children. J Pediatr 120:839–855, 1992. [DOI] [PubMed] [Google Scholar]
- 35. Cantwell MF, Snider DE, Jr, Cauthen GM, et al. Epidemiology of tuberculosis in the United States, 1985 through 1992. JAMA 272:535–539, 1994. [PubMed] [Google Scholar]
- 36. American Academy of Pediatrics, Committee on Infectious Diseases. Tuberculosis. In Report of the Committee on Infectious Diseases, 1991 22nd ed Elk Grove Village, IL: American Academy of Pediatrics, 492–493, 1991. [Google Scholar]
- 37. American Academy of Pediatrics. Tuberculosis. In Red book: 2009 report of the Committee on Infectious Disease, 28th ed Pickering LK, Baker CJ, Kimberlin DW, Long SS. (Eds). Elk Grove Village, IL: American Academy of Pediatrics, 680–701, 2009. [Google Scholar]
- 38. NICE Guidelines. CG33 Tuberculosis: Full Guideline. 22 March 2006. [Google Scholar]
- 39. Taylor RE, Cant AJ, Clark JE. Potential effect of NICE tuberculosis guidelines on paediatric tuberculosis screening. Arch Dis Child 93:200–203, 2008. [DOI] [PubMed] [Google Scholar]
- 40. Holland SM, Freeman AF, Bellanti JA. Immunity to bacteria. In Immunology IV. Clinical applications in health and disease. Bellanti JA. (Ed). Bethesda: I Care Press, 429–457, 2012. [Google Scholar]
- 41. Krummel B, Strassburg A, Ernst M, et al. Potential role for IL-2 ELISpot in differentiating recent and remote infection in tuberculosis contact tracing. PLoS One 5:e11670, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lighter J, Rigaud M, Huie M, et al. Chemokine IP-10: an adjunct marker for latent tuberculosis infection in children. Int J Tuberc Lung Dis 1:731–736, 2009. [PubMed] [Google Scholar]
- 43. Ruhwald M, Petersen J, Kofoed K, et al. Improving T-cell assays for the diagnosis of latent TB infection: potential of a diagnostic test based on IP-10. PLoS One 3:e2858, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology. America College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 59:762–784, 2008. [DOI] [PubMed] [Google Scholar]


