Abstract
Psoriasis is a common, chronic, inflammatory skin disorder. A number of genetic loci have been shown to confer risk for psoriasis. Collectively, these offer an integrated model for the inherited basis for susceptibility to psoriasis that combines altered skin barrier function together with the dysregulation of innate immune pathogen sensing and adaptive immunity. The major histocompatibility complex (MHC) harbours the psoriasis susceptibility region which exhibits the largest effect size, driven in part by variation contained on the HLA-Cw*0602 allele. However, the resolution of the number and genomic location of potential independent risk loci are hampered by extensive linkage disequilibrium across the region. We leveraged the power of large psoriasis case and control data sets and the statistical approach of conditional analysis to identify potential further association signals distributed across the MHC. In addition to the major loci at HLA-C (P = 2.20 × 10−236), we observed and replicated four additional independent signals for disease association, three of which are novel. We detected evidence for association at SNPs rs2507971 (P = 6.73 × 10−14), rs9260313 (P = 7.93 × 10−09), rs66609536 (P = 3.54 × 10−07) and rs380924 (P = 6.24 × 10−06), located within the class I region of the MHC, with each observation replicated in an independent sample (P ≤ 0.01). The previously identified locus is close to MICA, the other three lie near MICB, HLA-A and HCG9 (a non-coding RNA gene). The identification of disease associations with both MICA and MICB is particularly intriguing, since each encodes an MHC class I-related protein with potent immunological function.
INTRODUCTION
Psoriasis is a common, chronic, inflammatory skin disease characterized by painful, red, indurated scaly plaques that vary in extent yet can cover the entire skin surface. The underlying cutaneous pathological features are epidermal proliferation, increased vascularity and inflammation. Chronic psoriasis is associated with several important co-morbidities that include the development of a poly-articular arthritis and an increased predisposition to cardiovascular disease (1).
Recent progress has been made towards elucidating the genetic architecture of psoriasis (2). Both Linkage and association studies have provided robust evidence for a major disease susceptibility locus within the major histocompatibility complex (MHC) termed PSORS1 (psoriasis susceptibility locus 1), corroborating early serological studies which consistently showed an association between psoriasis and the HLA-Cw6 serotype. PSORS1 has been estimated to account for between 35 and 50% of heritability (3). Mapping studies have identified a 250 kb critical interval within the class I MHC region, containing at least nine genes, including HLA-C. Identification of the causal DNA variation within PSORS1 has been compounded, at least in part, by the extensive long-range linkage disequilibrium (LD) present across the MHC region of the short arm of chromosome 6. Substantial genetic evidence now points to sequence variation, across the locus harbouring the Cw*0602 allele, as the main causal determinant of psoriasis susceptibility in this region. For example, a combined sequencing and haplotype mapping analysis and two family-based association studies have found that only HLA-C contained coding variants unique to the risk haplotype (4–6). Several genome-wide association studies (GWAS) for psoriasis have now been reported (7–16). These studies have confirmed that by far the most significant association signal across the genome is for SNPs in strong LD with HLA*Cw0602. Our own recent observation (13) demonstrated a genetic interaction between ERAP-1 and a SNP tagging HLA-Cw*0602 providing an independent link for a role for HLA-C, as ERAP-1 is involved in peptide trimming prior to presentation by class I HLA proteins.
In addition to the HLA-C locus, fine mapping of the MHC region has provided evidence of two further independent association signals as well as evidence of the involvement of other HLA classical alleles (17). Here we sought to determine whether further loci could be revealed through the analysis of a data set some 2-fold larger, and with a greatly increased SNP density, than hereto analysed (13). We sought via this approach to provide insight into loci with multiple causal variants within a gene or region, as seen in a number of common complex disorders, and to inform ongoing studies of the pathobiology of psoriasis.
RESULTS
We used data from our previously reported GWAS, composed of 2178 psoriasis cases and 5175 geographically matched controls, as a discovery analysis set (13). Analysis was performed on all SNPs passing quality control within the extended MHC region, defined as the ∼8 Mb interval between SCGN and RPL12P1 (chr6: 25 652 429–33 368 333). The classical MHC region lies between C6orf40 and HCG24 (chr6: 29 640 147–33 115 544) and is split into class I (chr6: 29 640 147–31 478 898), class II (chr6: 31 478 898–32 191 844) and class III (chr6: 32 191 844–33 115 544) (18). (Chromosome positions are in Genome Reference Consortium Build 37.) After the imputation of classical alleles and SNPs and the removal of redundant SNPs (r2 = 1 with another SNP), the data set contained 58 015 SNPs (of which 2191 had been experimentally genotyped) together with 48 variables representing the classical HLA alleles (14 alleles for both HLA-A and HLA-B and 20 alleles from HLA-C).
Our primary analysis of the discovery sample was a stepwise conditional regression on the full data set consisting of typed SNPs, imputed SNPs and classical alleles. We detected five loci with P-values for association of <1 × 10−6. LD between each was low (r2 < 0.01 for all pairwise comparisons in controls), supporting the hypothesis that they represented independent loci (Table 1 and Fig. 1). Only one of these signals represents a classical allele. When all SNPs are included in the models, the P-values for each SNP are still ≤1 × 10−5, also supporting the hypothesis that they are independent loci. We next repeated the stepwise conditional analysis without the imputed classical alleles in our data set and four of the five loci gave concordant results, that is at each locus a SNP or a proxy (r2 > 0.98) had a similar P-value (Supplementary Material, Table S1). The SNP rs142291993 has a low minor allele frequency, raising concerns for the reliability of the imputation and was not taken forward for replication. In addition to the confirmation of four loci, another SNP, rs380924, had a P-value of <1 × 10−6; as the P-value for this SNP from the primary analysis (P = 6.24 × 10−6) was close to the cut-off for significance, we took this SNP forward for replication. We next repeated the stepwise conditional analysis with only the imputed classical alleles, and found five independent hits (including HLA-C*0602) at P < 1 × 10−6 (Table 2) . Finally, we conditioned on these five classical alleles and looked for further independent signals in SNP data. We found two SNPs with independent signals (Table 2), indicating that the imputed classical alleles alone cannot account for all the association signals in the data. One of these SNPs appears to represent the same signal as a SNP identified our primary analysis. rs2517670 lies within 1.2 kb of rs380924 and has an r2 of 0.93. Once the classical alleles and the two additional SNPs are included in the model, nothing else emerges with a conditional P-value <1 × 10−6, although rs2507971 (also from our primary analysis) has a P-value of 4.43 × 10−6.
Table 1.
Independent association signals in the discovery arm from stepwise conditional analysis, using both SNPs and imputed classical alleles
| ID | Position (bp), Build 37 | Total MAF (case–control) | Single marker P-value | Conditional P-value* | Odds ratio (95% CI) | Full-model P-value | Full-model odds ratio (95% CI) |
|---|---|---|---|---|---|---|---|
| HLA-Cw*0602 | 31 236 526–31 239 913 | 0.16 (0.31–0.09) | 2.20 × 10−236 | 2.20 × 10−236 | 4.37 (3.98–4.79) | 3.64 × 10−148 | 4.75 (4.48–5.05) |
| rs2507971 | 31 461 372 | 0.37 (0.31–0.39) | 8.04 × 10−26 | 6.73 × 10−14 | 1.45 (1.35–1.57) | 1.84 × 10−8 | 1.30 (1.24–1.36) |
| rs142291993 | 31 386 333 | 0.02 (0.02–0.01) | 5.00 × 10−8 | 1.15 × 10−13 | 1.74 (1.35–2.25) | 2.57 × 10−12 | 3.92 (3.23–4.77) |
| rs9260313 | 29 916 885 | 0.26 (0.22–0.28) | 3.60 × 10−14 | 7.93 × 10−09 | 0.71 (0.66–0.78) | 1.18 × 10−5 | 0.78 (0.73–0.82) |
| rs66609536 | 31 362 120 | 0.26 (0.34–0.22) | 2.21 × 10−58 | 3.54 × 10−07 | 1.85 (1.71–2.00) | 1.23 × 10−7 | 1.32 (1.26–1.40) |
| rs380924a | 29 939 885 | 0.37 (0.30–0.40) | 8.75 × 10−29 | 6.24 × 10−06 | 1.55 (1.43–1.67) | 3.88 × 10−6 | 1.25 (1.19–1.31) |
MAF, minor allele frequency.
aSNP rs380924 did not reach the threshold of significance required in this analysis but it did in the analysis of the data without the classical alleles and was therefore taken forward for replication. Boldfaced SNPs were taken forward for replication.
*P-value from stepwise regression conditional on all SNPs in rows above.
Figure 1.
Large-scale conditional regional association plots of independent MHC signals. The first panel shows the association signals with no conditioning. Subsequent panels show association signals conditioned on the most significant SNPs from each previous panel (HLAC, HLA-Cw*0602; rs2, rs2507971; rs1, rs142291993; rs9, rs9260313 and rs6, rs66609536. For all plots, the y-axis is the −log 10 of the P-value and the x-axis the base pair position of the SNP. CI, CII and CIII, classes I, II and III; Extended C1, the extended class I; and X2, the extended class II region.
Table 2.
Independent association signals from analysis prioritizing classical alleles
| Classical allele | MAF | Conditional P-value | Odds ratio and 95% CI |
|---|---|---|---|
| HLAC_0602 | 0.15 | 2.20 × 10−236 | 4.37 (3.98–4.79) |
| HLAB_3901 | 0.01 | 1.20 × 10−9 | 2.00 (1.51–2.62) |
| HLAB_1518 | 0.004 | 1.50 × 10−7 | 2.13 (1.3–3.49) |
| HLAB_5701 | 0.08 | 4.09 × 10−7 | 4.64 (4.11–5.25) |
| HLAB_3801 | 0.01 | 6.78 × 10−7 | 1.73 (1.25–2.40) |
| rs2517670 | 0.34 | 7.67 × 10−12 | 1.51 (1.40–1.64) |
| 6-31367052 | 0.07 | 2.86 × 10−8 | 1.11 (0.97–1.27) |
MAF, minor allele frequency.
Top section: independent association signals from stepwise conditional analysis of imputed classical alleles only. Bottom section (italics): further independent association signals from stepwise conditional analysis using genotypes and imputed SNPs and conditioning on all classical alleles nominated in the top section of the table.
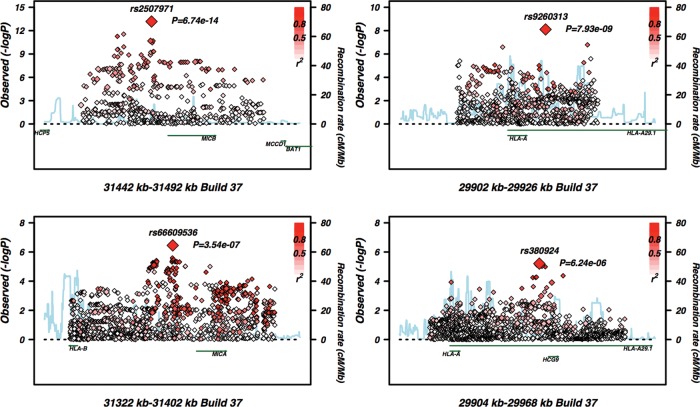
Our replication cohort consisted of 12 107 samples composed of 2913 cases and 9194 controls. All five independent signals were replicated by proxy SNPs contained within the Immunochip data set at values of P = 0.01 or below (Table 3). When all P-values were included in the model, the P-value for SNP rs3130922 became non-significant. However, this is the least effective proxy, so the combination of signal reduction and additional degrees of freedom reduces the signal strength. As expected, the strongest evidence for association appeared with the imputed HLA-Cw*0602 allele (Fig. 2). The other SNPs are located near MICB (rs2507971), HLA-A (rs9260313) and HCG9 (rs380924) (Fig. 3). The rs66609536/rs13437088 SNP near MICA has been reported in a study by Feng et al. (17). We looked for interaction between each of the five independent association signals and SNPs from the GWAS. None was significant at the genome-wide level. The previously reported and replicated interaction between ERAP and HLA-C had a P-value of 10−5.
Table 3.
Independent association signals in the replication arm from conditional regression
| Discovery arm SNP | I-chip proxy | MAF | LD# | Conditional P-value | Odds ratio (95% CI) | Full-model P-value | Full-model odds ratio (95% CI) |
|---|---|---|---|---|---|---|---|
| HLA-C_0602 | rs12199223 | 0.13 | 0.99 (0.99) | 4.72 × 10−213 | 4.3 (3.92–4.71) | 1.56 × 10−234 | 1.29 (1.28–1.30) |
| rs2507971 | rs3130922 | 0.31 | 0.77 (0.76) | 1.08 × 10−2 | 1.10 (1.19–1.02) | 2.83 × 10−1 | 1.01 (1.00–1.01) |
| rs9260313 | rs7745413 | 0.18 | 0.58 (0.59) | 8.02 × 10−4 | 0.85 (0.78–0.95) | 3.42 × 10−5 | 0.97 (0.97–0.98) |
| rs66609536 | rs13437088 | 0.27 | 0.98 (0.98) | 1.70 × 10−13 | 1.32 (1.23–1.44) | 1.33 × 10−14 | 1.04 (1.04–1.05) |
| rs380924 | rs2735079 | 0.38 | 0.98 (0.98) | 5.27 × 10−4 | 1.14 (1.22–1.05) | 4.42 × 10−4 | 1.02 (1.01–1.02) |
MAF, minor allele frequency; LD#, linkage disequilibrium r2 values, calculated from the controls in the discovery arm.
SNPs were added to the model in the order they were selected in the discovery arm.
Figure 2.
Fine-scale conditional regional association plots of the HLA-C signal generated using the SNAP tool (33). Imputed SNPs are shown as diamonds and genotyped SNPs as squares. The base pair position is on the x-axis, −log 10 of the P-value on the y-axis and the recombination fraction on the z-axis.
Figure 3.
Fine-scale conditional regional association plots of the other four independent signals generated using the SNAP tool (33). Imputed SNPs are shown as diamonds and genotyped SNPs as squares. The base pair position is on the x-axis, −log 10 of the P-value on the y-axis and the recombination fraction on the z-axis.
Finally, we mined publicly available transcriptome data (12) to assess whether any of the genes lying in proximity of association signals showed differential expression in psoriatic skin. The only gene that appeared to be up-regulated was MICB. Although the change in gene expression was modest (1.68-fold increase on average), it was consistent and statistically very significant (P = 8.8 × 10−9, using a paired t-test). An up-regulation of MICB in lesional skin is also apparent in other psoriasis transcriptome datasets, suggesting that the trend we observed is likely to be genuine (19).
None of the novel disease-associated SNPs uncovered here or their proxies appears to be eQTLs according to a list defined in psoriatic skin, uninvolved skin and normal skin (20).
DISCUSSION
Linkage and association studies have long demonstrated that the MHC region harbours the major genetic determinant for psoriasis susceptibility. Although the association with HLA-Cw*0602 is well substantiated, the existence of other MHC loci has been investigated in less detail.
Here, we exploited the statistical power of extended case–control data sets to robustly identify further MHC susceptibility determinants. We identify five independent hits in the class I region. rs9260313 (29 917 kb distal to HLA-A) is the most telomeric but is within 25 kb of rs380924 (29 940 kb proximal to HCG9)—both lie within the HLA-G transcript. Over 1 Mb away at intervals of ∼120 and 100 kb, respectively, lie HLA-C (31 237–31 240 kb), rs66609536 (31 362 kb proximal to MICA) and rs2507971 (31 461 kb proximal to MICB). HLA-Cw*0602 is the well-known risk allele for psoriasis and the signal at rs66609536 was previously described by Feng et al. (17).
Our study was not able to replicate a signal detected within the C6orf10 transcript (16). However, and of note, the C6orf10 association was not formally validated in the Feng et al. study, as the SNPs showing association in the discovery and replication data sets were not genetically correlated, suggesting they may not be representing the same causal variant. The three novel association signals emerging from our study lie in close proximity to MICB (rs2507971), HLA-A (rs9260313) and the HCG9 non-coding RNA gene (rs380924). None of the replicated SNPs are in high LD with common class I classical MHC alleles (r2 < 0.27 in controls and r2 < 0.33 in the whole sample).
Recently, there has been renewed interest in the exclusive use of imputed classical alleles in association studies, both in psoriasis and in other diseases. Results from our classical allele-only conditional analyses of the MHC are broadly consistent with others (21). The implicit assumption with these analyses is that classical alleles are a priori more likely to be truly causal than non-coding SNPs. It is also possible that a SNP in complete LD with a causal classical allele could, by chance, end up with a higher association signal than the classical allele itself, particularly when using data where the number of SNPs greatly outnumber the imputed classical alleles. Although we agree these arguments hold some weight, we note that even when we conditioned on the set of all imputed classical alleles with independent signals in our data, we still found additional SNP signals requiring explanation. Thus, we consider the question of the relative importance of classical alleles versus other genetic mechanisms (such as the regulation of gene expression) in the MHC-related pathobiology of psoriasis to be an unresolved issue. Fine mapping of the MHC locus has also been carried out in ulcerative colitis and rheumatoid arthritis but neither disease shows association with the loci we highlight here (22,23).
The identification of multiple psoriasis risk signals within the class I MHC region is noteworthy, especially as the critical SNPs appear to cluster in proximity to functionally related genes. HLA-A, HLA-C and HLA-G all encode cell-surface proteins that play a fundamental role in the presentation of intracellular antigens to cytotoxic T-lymphocytes. Conversely, MICA and MICB encode ligands for the natural killer cell NKG2D receptor, which is essential to the immune surveillance of epithelial tissues (24).
The large number of genetic markers and individuals in this study provide excellent tools for the fine mapping of independent signals in the region. Thus, further refinement and functional characterization of these association signals hold the promise to generate significant mechanistic insights into the dysregulation of immune responses in psoriasis.
MATERIALS AND METHODS
Discovery samples and genotypes
The 2178 cases were typed on the Illumina Human660W-Quad GWAS platform and the 5175 controls on the Illumina custom Human1.2M-Duo. Further details of the sample, genotyping and quality control are described in the original paper. Analysis was performed on all SNPs passing quality control within the extended MHC region.
Replication samples and genotypes
For replication, we used the Genetic Analysis of Psoriasis Consortium (GAPC) data set, with additional controls from other collaborators. Subjects were of self-declared European Caucasian ancestry. Informed consent was obtained in adherence to the Declaration of Helsinki principles. A breakdown of sample origins is provided in Supplementary Material, Table S2. All cases were diagnosed by an experienced dermatologist and documented to have clinical features of chronic psoriasis. DNA was isolated from blood or lymphoblastoid cell lines established from B-cells, using standard methods. Samples were genotyped at the Sanger Institute through the Wellcome Trust Case Control Consortium 2, using the Immunochip, which is a custom Illumina Infinium high-density array consisting of 196 524 variants compiled largely from associated regions identified in previous GWAS on 12 different immune-mediated inflammatory diseases, including psoriasis (25).
SNPs were excluded on the basis of a call rate of <0.95. Samples with call rates <0.98 were excluded as were those that appeared to have outlying ethnicity from a principal component analysis, leaving 12 107 samples (2913 cases and 9194 controls).
Imputation of SNPs and classical alleles
IMPUTE2 (26) was used to impute additional SNPs in the discovery sample, using the European 1000 Genomes data set (December 2010 data update, from the 20100804 sequence and alignment release) as a reference. Imputed SNPs with an info score <0.5, minor allele frequency <1% and extreme departures from Hardy–Weinberg equilibrium (P < 10−6) were excluded. This method produces highly accurate HLA imputations at class I and class II loci with call rates of 95–99% and accuracy between 92 and 98% at the four-digit level. Classical alleles for HLA-C, HLA-B and HLA-A were imputed using HLA*IMP (27,28). We analysed alleles with >1% frequency. Each allele was recoded as if it were a binary locus, so individuals would have no, one or two copies of the allele in question.
Identification of independent signals
Analysis was carried out on a data set that included genotyped SNPs, imputed SNPs and imputed classical alleles. Investigation of independent signals was undertaken using stepwise conditional analysis, originally suggested for the analysis of the HLA region and genetic data by Cordell and Clayton (29). Analysis was performed using SNPTEST, a program that performs association tests while accounting for the uncertainty in imputed genotypes (30). First, unconditional association analyses, using additive genetic models, were performed for all SNPs plus imputed classical alleles in the region. After identifying the most significant polymorphism from this scan, we re-ran the analysis, using an additive model for the remaining SNPs but conditioning on both the additive and dominant effects of the previously identified SNP to ensure that false downstream signals would not be generated by departures from additivity in the signals identified so far. We repeated this procedure conditioning on all previously identified SNPs in each step until no SNP had a conditional P-value of <1 × 10−6. Determination of an appropriate P-value is problematic; Bonferroni correction would suggest a P-value threshold of 9 × 10−7 but would be too extreme given the large number of correlated SNPs. We therefore chose a slightly less stringent P-value of 1 × 10−6. Having identified five polymorphisms that appeared to have independent signals in the combined analysis of SNPs plus classical alleles, we performed a logistic regression using the R statistical package to fit a model that included all polymorphisms as additive effects and we examined all regression coefficients to ensure they remained significant. All analyses included the first principal component from the genome-wide genetic covariance matrix as a covariate to correct for English/Irish population structure, as described in Strange et al. (13).
To further investigate the role of the classical alleles, we re-performed the stepwise procedure using only classical alleles until none remained in the model with a P-value of <1 × 10−6. At this point, we re-introduced the genotyped and imputed SNPs to see whether residual signals remained that could not be explained by the classical alleles already in the model.
We also performed stepwise regression on a data set that excluded the imputed classical alleles.
Replication analysis
We examined conditional association signals by proxy in the Immunochip data set. Tagging r2 values (calculated from controls) are given in Table 3. SNP rs7745413 had a Hardy–Weinberg equilibrium P-value of 3 × 10−8 in the controls but was included as such deviations are often seen in the MHC region. We performed conditional analysis to determine both whether the same hits were present and whether they were independent. We entered the SNPs into the model in the order of selection in the discovery data set. We used principal components from the genetic covariance matrix (across all Immunochip SNPs) as covariates to control for the multiple European origins of the samples. The first 10 principal components were used as covariates, as this was deemed to adequately control the inflation in association statistics (lambda = 1.13).
Hyperlasso
Stepwise conditional analysis is a popular method for model selection because it reflects a straightforward parsimony approach to the selection of new hits. At each step, a specific test is made of the evidence for or against expanding the current set of independent signals. However, since the procedure depends on local optimization at each step, it is possible that the final set of hits arrived at may not be globally optimal. An alternative approach that has been applied to searching for association signals in the MHC region is the Hyperlasso (31,32), a Bayesian regression method that starts with a normal exponential gamma prior for the association coefficient (log odds ratio) assigned to each SNP. We applied the Hyperlasso to our data, but found that the method tended to pick two SNPs in LD in place of a single SNP to represent an association signal. It is possible that further tuning of the parameters would have improved its performance but as the results from the stepwise regression replicated we did not pursue this method any further.
Interaction
We checked for interaction between each independent locus and SNPs throughout the rest of the genome using logistic regression, with the phenotype as the dependent variable. For each SNP in the GWAS, we investigated five models, each model included the main effect of the GWAS SNP, the main effects of the five independent loci, the principal component to control for ancestry and an interaction term between the GWAS SNP and one of the five independent loci. We coded the HLA-Cw*0602 as dominant according to its suggested effect. We followed up results with P-values <1 × 10−8, but none replicated in the Immunochip data.
SUPPLEMENTARY MATERIAL
FUNDING
This work was also supported by an MRC Programme grant to R.C.T. J.N.B., A.H. and F.N. (G0601387). Funding for the Wellcome Trust Case Control Consortium 2 project was provided by the Wellcome Trust (085475/B/08/Z and 085475/Z/08/Z). We acknowledge the funding support of the UK Department of Health via the National Institute for Health Research (NIHR) Comprehensive Biomedical Research Centre awards to Guy's and St Thomas' NHS Foundation Trust in partnership with King's College London.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are very grateful to all the participants in both the discovery and replication arms of our study.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Nestle F.O., Kaplan D.H., Barker J. Psoriasis. New Engl. J. Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 2.Capon F., Burden A.D., Trembath R.C., Barker J.N. Psoriasis and other complex trait dermatoses: from loci to functional pathways. J. Invest. Dermatol. 2012;132:915–922. doi: 10.1038/jid.2011.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trembath R.C., Clough R.L., Rosbotham J.L., Jones A.B., Camp R.D., Frodsham A., Browne J., Barber R., Terwilliger J., Lathrop G.M., et al. Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by a two stage genome-wide search in psoriasis. Hum. Mol. Genet. 1997;6:813–820. doi: 10.1093/hmg/6.5.813. [DOI] [PubMed] [Google Scholar]
- 4.Fan X., Yang S., Huang W., Wang Z.M., Sun L.D., Liang Y.H., Gao M., Ren Y.Q., Zhang K.Y., Du W.H., et al. Fine mapping of the psoriasis susceptibility locus PSORS1 supports HLA-C as the susceptibility gene in the Han Chinese population. PLoS Genet. 2008;4:e1000038. doi: 10.1371/journal.pgen.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms C., Saccone N.L., Cao L., Daw J.A., Cao K., Hsu T.M., Taillon-Miller P., Duan S., Gordon D., Pierce B., et al. Localization of PSORS1 to a haplotype block harboring HLA-C and distinct from corneodesmosin and HCR. Hum. Genet. 2005;118:466–476. doi: 10.1007/s00439-005-0048-2. [DOI] [PubMed] [Google Scholar]
- 6.Nair R.P., Stuart P.E., Nistor I., Hiremagalore R., Chia N.V., Jenisch S., Weichenthal M., Abecasis G.R., Lim H.W., Christophers E., et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am. J. Hum. Genet. 2006;78:827–851. doi: 10.1086/503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capon F., Bijlmakers M.J., Wolf N., Quaranta M., Huffmeier U., Allen M., Timms K., Abkevich V., Gutin A., Smith R., et al. Identification of ZNF313/RNF114 as a novel psoriasis susceptibility gene. Hum. Mol. Genet. 2008;17:1938–1945. doi: 10.1093/hmg/ddn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cargill M., Schrodi S.J., Chang M., Garcia V.E., Brandon R., Callis K.P., Matsunami N., Ardlie K.G., Civello D., Catanese J.J., et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am. J. Hum. Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellinghaus E., Ellinghaus D., Stuart P.E., Nair R.P., Debrus S., Raelson J.V., Belouchi M., Fournier H., Reinhard C., Ding J., et al. Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nat. Genet. 2010;42:991–995. doi: 10.1038/ng.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huffmeier U., Uebe S., Ekici A.B., Bowes J., Giardina E., Korendowych E., Juneblad K., Apel M., McManus R., Ho P., et al. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat. Genet. 2010;42:996–999. doi: 10.1038/ng.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y., Helms C., Liao W., Zaba L.C., Duan S., Gardner J., Wise C., Miner A., Malloy M.J., Pullinger C.R., et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 2008;4:e1000041. doi: 10.1371/journal.pgen.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair R.P., Duffin K.C., Helms C., Ding J., Stuart P.E., Goldgar D., Gudjonsson J.E., Li Y., Tejasvi T., Feng B.J., et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat. Genet. 2009;41:199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strange A., Capon F., Spencer C.C., Knight J., Weale M.E., Allen M.H., Barton A., Band G., Bellenguez C., Bergboer J.G., et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat. Genet. 2010;42:985–990. doi: 10.1038/ng.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuart P.E., Nair R.P., Ellinghaus E., Ding J., Tejasvi T., Gudjonsson J.E., Li Y., Weidinger S., Eberlein B., Gieger C., et al. Genome-wide association analysis identifies three psoriasis susceptibility loci. Nat. Genet. 2010;42:1000–1004. doi: 10.1038/ng.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun L.D., Cheng H., Wang Z.X., Zhang A.P., Wang P.G., Xu J.H., Zhu Q.X., Zhou H.S., Ellinghaus E., Zhang F.R., et al. Association analyses identify six new psoriasis susceptibility loci in the Chinese population. Nat. Genet. 2010;42:1005–1009. doi: 10.1038/ng.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X.J., Huang W., Yang S., Sun L.D., Zhang F.Y., Zhu Q.X., Zhang F.R., Zhang C., Du W.H., Pu X.M., et al. Psoriasis genome-wide association study identifies susceptibility variants within LCE gene cluster at 1q21. Nat. Genet. 2009;41:205–210. doi: 10.1038/ng.310. [DOI] [PubMed] [Google Scholar]
- 17.Feng B.J., Sun L.D., Soltani-Arabshahi R., Bowcock A.M., Nair R.P., Stuart P., Elder J.T., Schrodi S.J., Begovich A.B., Abecasis G.R., et al. Multiple Loci within the major histocompatibility complex confer risk of psoriasis. PLoS Genet. 2009;5:e1000606. doi: 10.1371/journal.pgen.1000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horton R., Wilming L., Rand V., Lovering R.C., Bruford E.A., Khodiyar V.K., Lush M.J., Povey S., Talbot C.C., Jr, Wright M.W., et al. Gene map of the extended human MHC. Nat. Rev. Genet. 2004;5:889–899. doi: 10.1038/nrg1489. [DOI] [PubMed] [Google Scholar]
- 19.Yao Y., Richman L., Morehouse C., de los Reyes M., Higgs B.W., Boutrin A., White B., Coyle A., Krueger J., Kiener P.A., et al. Type I interferon: potential therapeutic target for psoriasis? PLoS ONE. 2008;3:e2737. doi: 10.1371/journal.pone.0002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding J., Gudjonsson J.E., Liang L., Stuart P.E., Li Y., Chen W., Weichenthal M., Ellinghaus E., Franke A., Cookson W., et al. Gene expression in skin and lymphoblastoid cells: refined statistical method reveals extensive overlap in cis-eQTL signals. Am. J. Hum. Genet. 2010;87:779–789. doi: 10.1016/j.ajhg.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H., Hayashi G., Lai O.Y., Dilthey A., Kuebler P.J., Wong T.V., Martin M.P., Fernandez Vina M.A., McVean G., Wabl M., et al. Psoriasis patients are enriched for genetic variants that protect against HIV-1 disease. PLoS Genet. 2012;8:e1002514. doi: 10.1371/journal.pgen.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Achkar J.P., Klei L., de Bakker P.I., Bellone G., Rebert N., Scott R., Lu Y., Regueiro M., Brzezinski A., Kamboh M.I., et al. Amino acid position 11 of HLA-DRbeta1 is a major determinant of chromosome 6p association with ulcerative colitis. Genes. Immun. 2012;13:245–252. doi: 10.1038/gene.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raychaudhuri S., Sandor C., Stahl E.A., Freudenberg J., Lee H.S., Jia X., Alfredsson L., Padyukov L., Klareskog L., Worthington J., et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat. Genet. 2012;44:291–296. doi: 10.1038/ng.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayday A.C. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Cortes A., Brown M.A. Promise and pitfalls of the Immunochip. Arthritis Res. Ther. 2011;13:101. doi: 10.1186/ar3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howie B.N., Donnelly P., Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dilthey A.T., Moutsianas L., Leslie S., McVean G. HLA*IMP—an integrated framework for imputing classical HLA alleles from SNP genotypes. Bioinformatics. 2011;27:968–972. doi: 10.1093/bioinformatics/btr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leslie S., Donnelly P., McVean G. A statistical method for predicting classical HLA alleles from SNP data. Am. J. Hum. Genet. 2008;82:48–56. doi: 10.1016/j.ajhg.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cordell H.J., Clayton D.G. A unified stepwise regression procedure for evaluating the relative effects of polymorphisms within a gene using case/control or family data: application to HLA in type 1 diabetes. Am. J. Hum. Genet. 2002;70:124–141. doi: 10.1086/338007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchini J., Howie B., Myers S., McVean G., Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat. Genet. 2007;39:906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 31.Hoggart C.J., Whittaker J.C., De Iorio M., Balding D.J. Simultaneous analysis of all SNPs in genome-wide and re-sequencing association studies. PLoS Genet. 2008;4:e1000130. doi: 10.1371/journal.pgen.1000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vignal C.M., Bansal A.T., Balding D.J. Using penalised logistic regression to fine map HLA variants for rheumatoid arthritis. Ann. Hum. Genet. 2011;75:655–664. doi: 10.1111/j.1469-1809.2011.00670.x. [DOI] [PubMed] [Google Scholar]
- 33.Johnson A.D., Handsaker R.E., Pulit S.L., Nizzari M.M., O'Donnell C.J., de Bakker P.I. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.