Abstract
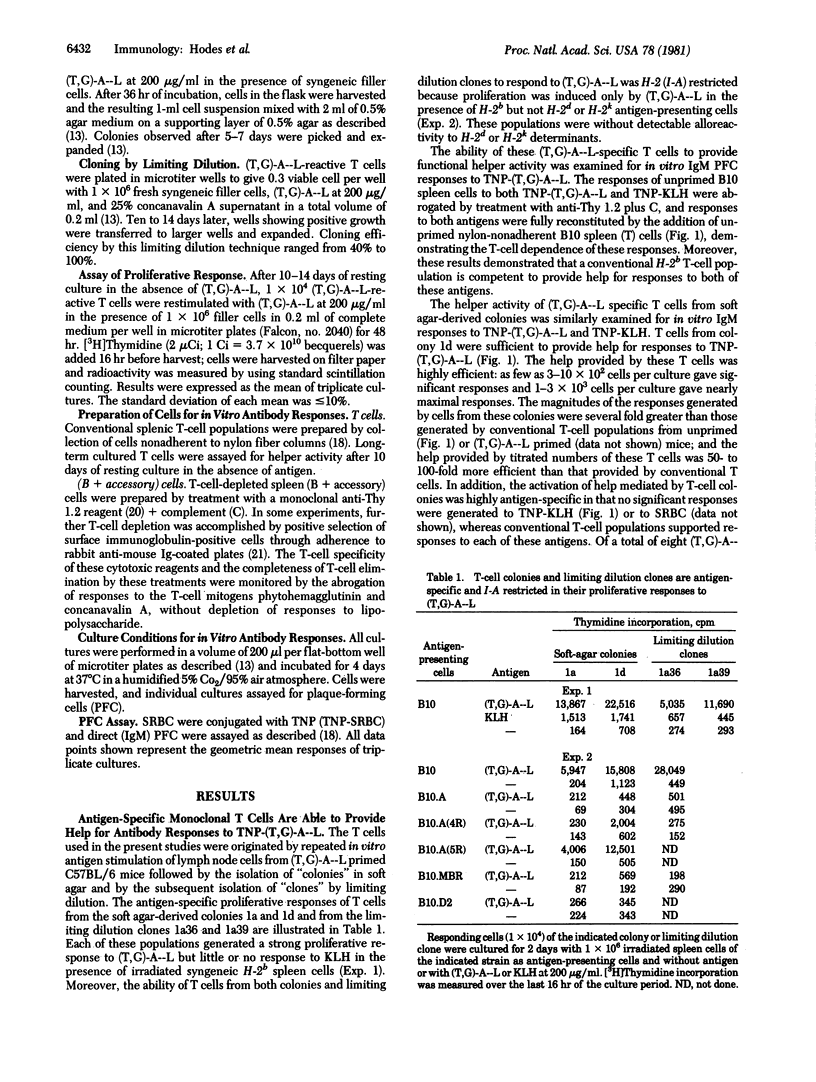
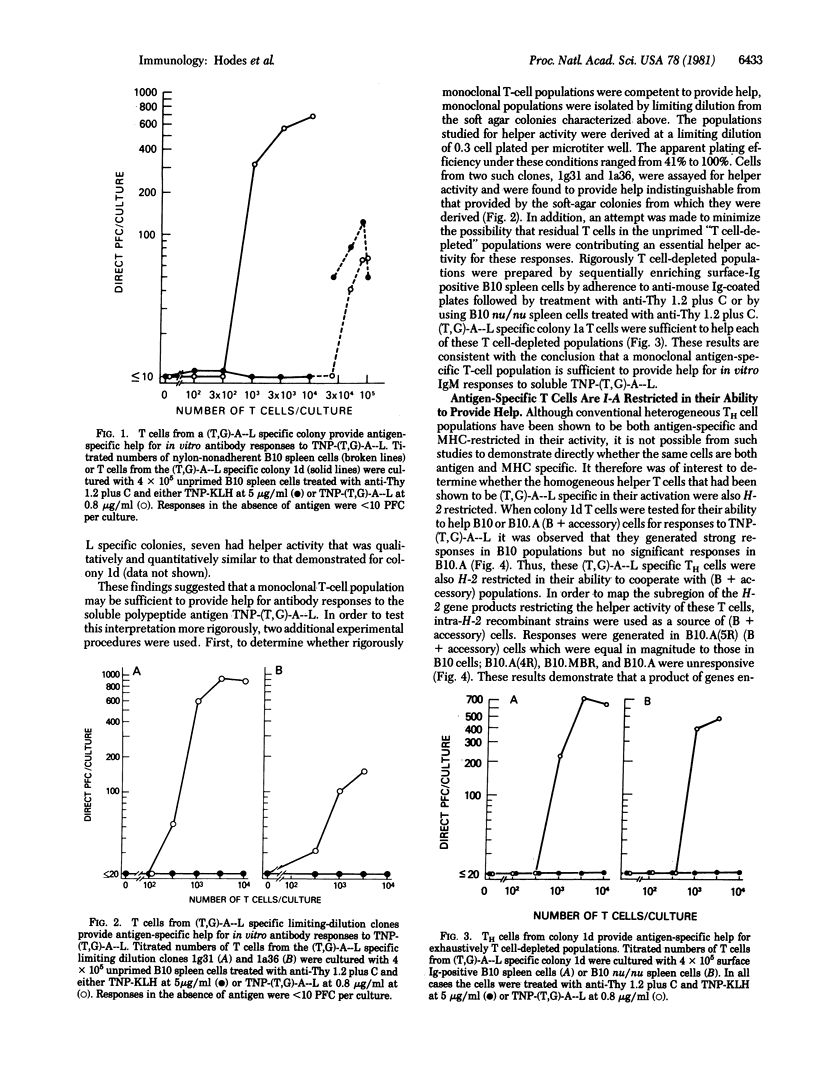
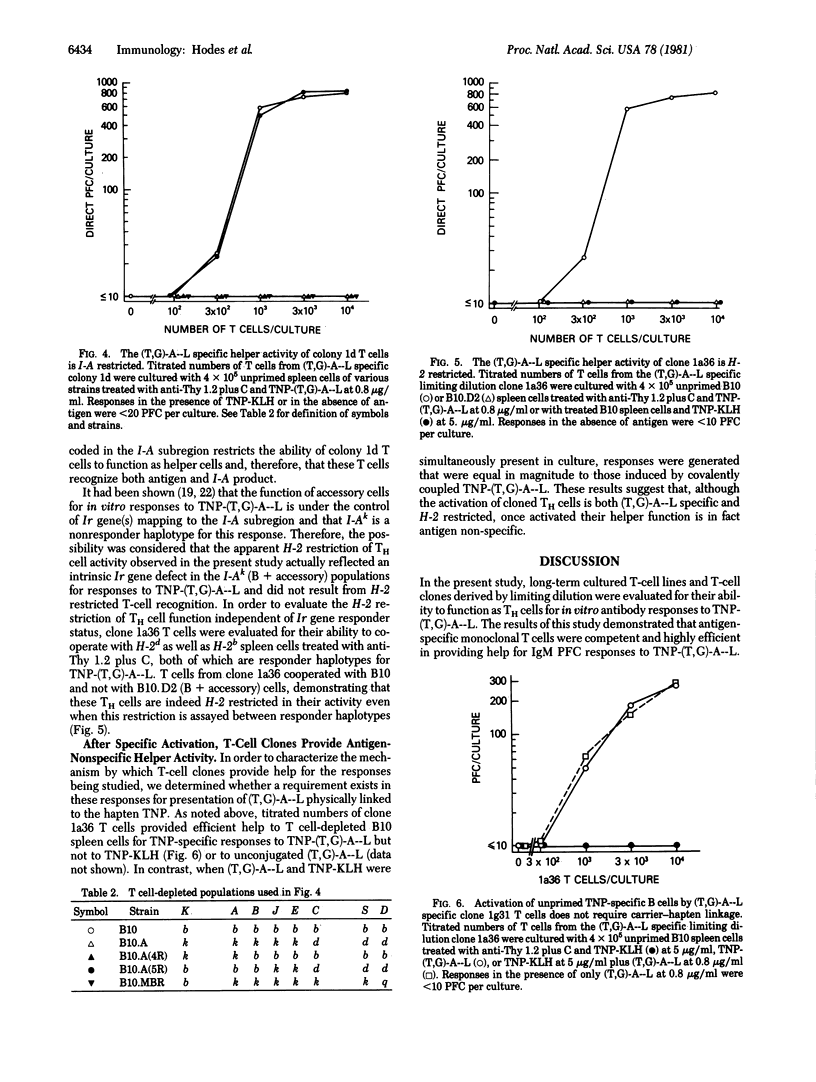
The ability of long-term cultured and monoclonal T cell populations to provide antigen-specific help was assessed in a system of Ir gene-controlled in vitro antibody responses to soluble antigens. T-cell colonies and monoclonal T-cell lines were generated which proliferated specifically in response to poly(LTyr,Glu)-poly(DLAla)--poly(LLys) [(T,G)-A--L] and were I-A restricted in these proliferative responses. These (T,G)-A--L-specific T-cell populations were evaluated for their ability to help unprimed and T-cell depleted spleen cell populations in the generation of antibody responses to trinitrophenyl (TNP)-(T,G)-A--L in vitro. It was found that long-term T-cell lines, including monoclonal T-cell populations derived by limiting dilution, were highly efficient helper cells for IgM responses to TNP-(T,G)-A--L. These helper T cells were both antigen-specific and I-A restricted in their ability to be activated and to cooperate with T-cell depleted spleen cell populations. Once specifically activated, however, these clones provided help that was antigen nonspecific. These studies have thus demonstrated the ability of antigen-specific and H-2-restricted monoclonal T-cell populations to provide help for responses to soluble antigens in vitro.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binz H., Lindenmann J., Wigzell H. Cell-bound receptors for alloantigens on normal lymphocytes. II. Antialloantibody serum contains specific factors reacting with relevant immunocompetent T lymphocytes. J Exp Med. 1974 Sep 1;140(3):731–741. doi: 10.1084/jem.140.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomly K., Maurer P. H. Antigen-specific helper T cells required for dominant production of an idiotype (THId) are not under immune response (Ir) gene control. J Exp Med. 1980 Dec 1;152(6):1571–1582. doi: 10.1084/jem.152.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann K. Idiotype suppression. II. Amplification of a suppressor T cell with anti-idiotypic activity. Eur J Immunol. 1975 Aug;5(8):511–517. doi: 10.1002/eji.1830050802. [DOI] [PubMed] [Google Scholar]
- Erb P., Feldmann M. The role of macrophages in the generation of T-helper cells. II. The genetic control of the macrophage-T-cell interaction for helper cell induction with soluble antigens. J Exp Med. 1975 Aug 1;142(2):460–472. doi: 10.1084/jem.142.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathman C. G., Hengartner H. Clones of alloreactive T cells. Nature. 1978 Apr 13;272(5654):617–618. doi: 10.1038/272617a0. [DOI] [PubMed] [Google Scholar]
- Fathman C. G., Kimoto M. Studies Utilizing murine T cell clones: Ir genes, Ia antigens and MLR stimulating determinants. Immunol Rev. 1981;54:57–79. doi: 10.1111/j.1600-065x.1981.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Hodes R. J., Singer A. Cellular and genetic control of antibody responses in vitro. I. Cellular requirements for the generation of genetically controlled primary IgM responses to soluble antigens. Eur J Immunol. 1977 Dec;7(12):892–897. doi: 10.1002/eji.1830071214. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Bert D. L., Shen F. W. Cell cooperation during in vivo anti-hapten antibody responses. V. Two synergistic Ly-1+23- helper T cells with distinctive specificities. Eur J Immunol. 1980 Apr;10(4):231–236. doi: 10.1002/eji.1830100402. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Hamaoka T., Benacerraf B. Cell interactions between histoincompatible T and B lymphocytes. II. Failure of physiologic cooperative interactions between T and B lymphocytes from allogeneic donor strains in humoral response to hapten-protein conjugates. J Exp Med. 1973 Jun 1;137(6):1405–1418. doi: 10.1084/jem.137.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto M., Fathman C. G. Antigen-reactive T cell clones. II. Unique homozygous and (high responder x low responder)F1 hybrid antigen-presenting determinants detected using poly(Tyr, Glu)-poly D, L-Ala--poly Lys-reactive T cell clones. J Exp Med. 1981 Feb 1;153(2):375–385. doi: 10.1084/jem.153.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake P., Clark E. A., Khorshidi M., Sunshine G. H. Production and characterization of cytotoxic Thy-1 antibody-secreting hybrid cell lines. Detection of T cell subsets. Eur J Immunol. 1979 Nov;9(11):875–886. doi: 10.1002/eji.1830091109. [DOI] [PubMed] [Google Scholar]
- Mage M. G., McHugh L. L., Rothstein T. L. Mouse lymphocytes with and without surface immunoglobulin: preparative scale separation in polystyrene tissue culture dishes coated with specifically purified anti-immunoglobulin. J Immunol Methods. 1977;15(1):47–56. doi: 10.1016/0022-1759(77)90016-3. [DOI] [PubMed] [Google Scholar]
- Melchers F., Andersson J., Lernhardt W., Schreier M. H. H-2-unrestricted polyclonal maturation without replication of small B cells induced by antigen-activated T cell help factors. Eur J Immunol. 1980 Sep;10(9):679–685. doi: 10.1002/eji.1830100905. [DOI] [PubMed] [Google Scholar]
- Mozes E., Haimovich J. Antigen specific T-cell helper factor cross reacts idiotypically with antibodies of the same specificity. Nature. 1979 Mar 1;278(5699):56–57. doi: 10.1038/278056a0. [DOI] [PubMed] [Google Scholar]
- Nabholz M., Engers H. D., Collavo D., North M. Cloned T-cell lines with specific cytolytic activity. Curr Top Microbiol Immunol. 1978;81:176–187. doi: 10.1007/978-3-642-67448-8_29. [DOI] [PubMed] [Google Scholar]
- Singer A., Cowing C., Hathcock K. S., Dickler H. B., Hodes R. J. Cellular and genetic control of antibody responses in vitro. III. Immune response gene regulation of accessory cell function. J Exp Med. 1978 Jun 1;147(6):1611–1620. doi: 10.1084/jem.147.6.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A., Dickler H. B., Hodes R. J. Cellular and genetic control of antibody responses in vitro. II. Ir gene control of primary IgM responses to trinitrophenyl conjugates of poly-L-(Tyr,Glu)-poly-D,L-Ala--poly-L-Lys and poly-L-(His,Glu)-poly-D,L-Ala--poly-L-Lys. J Exp Med. 1977 Oct 1;146(4):1096–1107. doi: 10.1084/jem.146.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A., Hathcock K. S., Hodes R. J. Cellular and genetic control of antibody responses. V. Helper T-cell recognition of H-2 determinants on accessory cells but not B cells. J Exp Med. 1979 May 1;149(5):1208–1226. doi: 10.1084/jem.149.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A., Morrissey P. J., Hathcock K. S., Ahmed A., Scher I., Hodes R. J. Role of the major histocompatibility complex in T cell activation of B cell subpopulations Lyb-5+ and Lyb-5- B cell subpopulations differ in their requirement for major histocompatibility complex-restricted T cell recognition. J Exp Med. 1981 Aug 1;154(2):501–516. doi: 10.1084/jem.154.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J. Restricted helper function of F1 hybrid T cells positively selected to heterologous erythrocytes in irradiated parental strain mice. II. Evidence for restrictions affecting helper cell induction and T-B collaboration, both mapping to the K-end of the H-2 complex. J Exp Med. 1978 Apr 1;147(4):1159–1174. doi: 10.1084/jem.147.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sredni B., Tse H. Y., Schwartz R. H. Direct cloning and extended culture of antigen-specific MHC-restricted, proliferating T lymphocytes. Nature. 1980 Feb 7;283(5747):581–583. doi: 10.1038/283581a0. [DOI] [PubMed] [Google Scholar]
- Swierkosz J. E., Rock K., Marrack P., Kappler J. W. The role of H-2 linked genes in helper T-cell function. II. Isolation on antigen-pulsed macrophages of two separate populations of F1 helper T cells each specific for antigen and one set of parental H-2 products. J Exp Med. 1978 Feb 1;147(2):554–570. doi: 10.1084/jem.147.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Takemori T., Okumura K., Nonaka M., Tokuhisa T. Two distinct types of helper T cells involved in the secondary antibody response: independent and synergistic effects of Ia- and Ia+ helper T cells. J Exp Med. 1978 Feb 1;147(2):446–458. doi: 10.1084/jem.147.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland R., Cantor H. Idiotype-specific T helper cells are required to induce idiotype-positive B memory cells to secrete antibody. Eur J Immunol. 1978 Aug;8(8):600–606. doi: 10.1002/eji.1830080812. [DOI] [PubMed] [Google Scholar]



