Abstract
Purpose.
To determine the role of Notch signaling in corneal epithelial migration and wound healing.
Methods.
Immunolocalization of Notch1 was performed during epithelial wound healing in vivo in mouse corneal epithelial debridement wounds and in vitro in primary human corneal epithelial cells following a linear scratch wound. The effects of Notch inhibition, using the γ-secretase inhibitor N-(N-[3,5-difluorophenacetyl]-l-alanyl)-S-phenylglycine t-butyl ester (DAPT) or following stable transfection with Notch1-short hairpin RNA (shRNA), was evaluated in a scratch assay and transwell migration assay. Likewise, in vitro adhesion, proliferation and the actin cytoskeleton was examined. The DAPT effect was also evaluated in vivo in a mouse model of corneal epithelial wound healing.
Results.
The expression of Notch1 was reduced at the leading edge of a healing corneal epithelium both in vivo and in vitro. Notch inhibition using DAPT and using Notch1-shRNA both enhanced in vitro migration in scratch and transwell migration assays. Consistent with this increased migratory behavior, Notch inhibited cells demonstrated decreased cell-matrix adhesion and enhanced lamellipodia formation. Notch inhibition by DAPT was also found to accelerate corneal epithelial wound closure in an in vivo murine model without affecting proliferation.
Conclusions.
The results highlight the role of Notch in regulating corneal epithelial migration and wound healing. In particular, Notch signaling appears to decrease in the early stages of wound healing which contributes to cytoskeletal changes with subsequent augmentation of migratory behavior.
In this study, we examined the involvement of Notch signaling in corneal epithelial wound healing and migration. Notch signaling appears to decrease in the early stages of wound healing, which contributes to cytoskeletal changes with subsequent augmentation of migratory behavior.
Introduction
The corneal epithelium protects the cornea against pathogen invasion and is essential for maintaining the integrity and clarity of the cornea. It is constantly regenerated by a reservoir of stem and progenitor cells located primarily in the limbal region. Following an injury that leads to the loss of the epithelium, the remaining epithelial cells undergo a programmed repair mechanism to immediately close the defect.1 This highly coordinated process involves a number of cellular functions including migration, proliferation, and differentiation, which in many ways recapitulate the same pathways involved during development. While many of the regulatory mechanisms governing corneal epithelial wound healing have been studied before,2 the role of Notch signaling, a critical pathway during development, has not been completely defined.
The Notch signaling pathway is a highly conserved network that orchestrates cell fate decisions in many tissues and organisms.3,4 Notch proteins are membrane bound receptors with corresponding membrane bound ligands, Delta and Jagged. Upon binding of the ligand, the Notch receptor is externally cleaved by a disintegrin and metalloprotease (ADAM) and then internally by the γ-secretase complex.5,6 This releases the Notch intracellular (NotchIC) fragment, which in the canonical signaling pathway translocates into the nucleus and associates most commonly with CBF1/RBPJκ to transactivate target genes such as Hairy/Enhancer of Split (Hes).7,8
The importance of Notch signaling in the corneal epithelium has been highlighted by several studies.9–14 Previously, we reported down-regulation of Notch1 during the initial stages of wound healing in the corneal epithelium.11 At the time, we correlated this decrease in Notch signaling to the increased proliferative status of the corneal epithelium and proposed a negative correlation between Notch activation and proliferation. However, as shown in the present study, the decrease in Notch1 at the immediate phase of wound healing may in fact be more closely correlated with the increased migratory capacity of corneal epithelial cells. We demonstrated that Notch1 was specifically reduced at the leading edge of a healing corneal epithelium, and that exogenously inhibiting Notch enhanced the migration of corneal epithelial cells. We further showed that inhibition of Notch induced changes in the actin cytoskeleton that are consistent with the increased migratory phenotype.
Methods
Corneal Epithelial Cell Culture
Human corneal epithelial cell cultures were initiated from cadaver corneas and kindly provided by the Illinois Eye Bank. The limbal rings were treated with Dispase (2 mg/mL; Gibco, Grand Island, NY) at 37°C for 2 hours to separate the epithelial sheets, then digested in 0.25% trypsin-EDTA for 5 to 10 minutes. Cells were washed and resuspended in keratinocyte serum free medium (KSFM; Invitrogen, Grand Island, NY) and plated in collagen coated tissue culture plates. In addition to primary corneal epithelial cells, an SV40 transduced human corneal epithelial cell line (HCE-T) was used for some of the experiments.15 HCE-T cells were grown in Dulbelcco's modified Eagle's medium (DMEM)/F12 (1:1) with 5% fetal bovine serum (FBS).
Immunofluorescence Staining and Microscopy
Mouse corneal sections as well as corneal epithelial cells grown on chamber slides were stained according to previously described protocols.11 The following primary antibodies were used: rabbit polyoclonal anti-Ki67 (dilution 1:1000; Abcam, Cambridge, MA) and anti-Notch1 (dilution 1:100; Santa Cruz Biotechnology, Santa Cruz, CA) antibodies. For negative control, the sections were incubated with an irrelevant (no epithelial expression of the antigen) rabbit antibody. The sections were examined using a spinning disc confocal microscope (Z1; Carl Zeiss, Jena, Germany), and photographed with an AxioCam camera (Carl Zeiss).
Notch1 Knockdown in HCE-T Cells
HCE-T cells were transduced with GIPZ Lentiviral shRNA viral particles (Open Biosystems, Lafayette, CO) at multiplicity of infection (MOI) of five. Two human Notch1 small hairpin RNA (shRNA) constructs (target sequences ACGGACTGCGTGGACAGCT, AGGTGCAGCCACAAAACTT) were used with titers of 1.20 × 108 and 1.04 × 108 transducing units (TU)/mL, respectively. Nonsilencing (NS) shRNA was used as negative control and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) shRNA was used as positive control (Open Biosystems). The transduced cells were identified based on green fluorescent protein (GFP) expression given the GFP component in the viral vector (CMV-tGFP-IRES-puromycin-shRNA). Two stable transfections of Notch1-shRNA (corresponding to each target sequence) and a stable transfection of NS shRNA was established using puromycin (4 μg/mL) treatment for 2 to 3 weeks (puromycin resistance factor also included in the backbone). Notch1 knockdown was evaluated by RT-PCR and Western blotting.
In Vitro Scratch Assay
Primary human corneal epithelial cells or HCE-T were grown to confluence on collagen coated plates. Primary cells were maintained in KSFM, but the calcium was increased to 0.5 mM for 24 hours while HCE-T cells were switched to media with no serum for 24 hours. The cells were exposed to the γ-secretase inhibitor N-(N-[3,5-difluorophenacetyl]-l-alanyl)-S-phenylglycine t-butyl ester (DAPT) (Selleckchem, Houston, TX), 100 nM to 50 μM, or vehicle (dimethyl sulfoxide [DMSO], maximum concentration 0.02%) for 12 to 24 hours before a linear scratch was made using a sterile 200-μL pipette tip.16 Immediately after the scratch and at 12 and 24 hours images of the scratch area were captured using the Zeiss AxioCam MR digital camera on a Zeiss Axioskop2 light microscope (Carl Zeiss). The remaining wound area was measured using ImageJ software (nih.gov, Bethesda, MD) and normalized to time 0 wounds. To minimize the effect of proliferation, experiments were also repeated in primary cells they had been previously treated with mitomycin-C (Bedford Laboratories, Bedford, OH) 5 to 10 μg/mL for 3 hours. A scratch assay was also similarly performed in HCE-T stably transfected with Notch1 and NS shRNA.
Transwell Migration Assay
HCE-T cells (shRNA transduced or previously treated with DAPT, Jagged1, or DMSO) were plated in 8.0-μM pore size transwell inserts (Beckton Dickinson, Downers Grove, IL) in serum free DMEM. The lower compartment was filled with DMEM plus 10% FBS as chemoattractant. After 12 hours, the cells on the upper side of the insert were removed by scraping and the cells that had migrated through were fixed on the lower side of membrane with 10% formalin then stained with crystal violet and quantified by counting the number of cells in 20 separate fields.
Proliferation Assay
The effect of DAPT on cell proliferation was assessed using a thiazolyl blue tetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, MO) assay. Primary human corneal epithelial cells were plated in a 48-well plate at a density of 75,000 per well and grown to 60% confluence. The growth factors were eliminated from the media overnight and the cells were treated with DMSO (0.02%) or DAPT (5 μM) for 24 hours. After complete removal of the media, 20 μL of MTT solution was replaced in each well and incubated at 37°C for 3 hours; the solution was then removed and 500 μL of DMSO was replaced in each well. The concentration was determined by optical densitometry at the 560-nm wavelength.
The in vitro proliferation rate was also measured by immunofluorescence staining for the proliferation marker, Ki67. Primary human cells were plated in equal numbers in a 4-well chamber slides and grown to 40% confluence then treated in the same fashion with DAPT or DMSO. The cells were fixed 24 hours later and stained for Ki67 as described above.
Cell Adhesion Assay
HCE-T transduced with shRNA and primary human corneal epithelial cells treated with DAPT, Jagged1, or DMSO for 24 hours, were trypsinized and plated equally in a 96-well flat bottom plate coated with fibronectin-collagen plus 3% BSA (Sigma-Aldrich). After 30 minute incubation at 37°C, the cell culture media was removed and the plate was washed multiple times with PBS. The adherent cells were fixed with 100% ethanol then stained with crystal violet after which the absorbance in each well was measured at 590-nm wavelength.
Phalloidin Staining
Primary human corneal epithelial cells or shRNA transduced HCE-T cells were cultured to confluence on chamber slides. After serum starvation overnight, a linear scratch was made on the slides. The slides were stained 6 hours after the scratch by the following protocol: after fixation with periodate-lysine-paraformaldehyde (PLP) for 20 minutes, the cells were permeabilized using Tris buffered saline (TBS) and 0.3% Triton X-100 for 5 minutes, washed in PBS three times, and then incubated in rhodamine-phalloidin (1:40 dilution in PBS containing 1% BSA; Cytoskeleton, Inc., Denver, CO) for 20 minutes. After three PBS washes, the slides were mounted using DAPI fluoromount-G medium.
Reverse-Transcriptase (RT) Polymerase-Chain Reaction (PCR)
RNA isolation from epithelial cell cultures and form mouse corneal epithelium was performed as previously described.11 RT reaction was likewise completed as before, followed by PCR for human Notch1, human GAPDH, human Hes1, human β-actin, mouse Hes1, and mouse β-actin (sequences published before11 otherwise available upon request).
Western Blots
Western blots were performed as previously described.11 The following antibodies were used: rabbit anti-cleaved-Notch1 Val1744 (dilution 1:500), rabbit anti-GAPDH (1:5000) both from Cell Signaling (Danvers, MA). Detection was performed by ImageQuant LAS 1040 detection system and quantified using ImageQuant software (both from GE Healthcare, Piscataway, NJ).
Mouse Model of Corneal Epithelial Wound Healing
Corneal wounding experiments in mice were conducted in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Six-month-old C57BL/6J mice were anesthetized with intraperitoneal injection of ketamine (100 mg/kg) and xylazine (5 mg/kg). After applying two drops of topical 0.5% proparacaine, a 2.0-mm area of the central epithelium was demarcated and removed by gentle scraping using a blunt corneal scraper (for Notch1 localization studies a 1.5-mm wound was also used). In the treatment (N = 7) or the control (N = 7) group, DAPT (20 μM) or vehicle (DMSO 0.02%) (20–30 μL) was applied to the cornea every 15 minutes for 2 hours. At 24 hours, the corneas were stained with fluorescein and photographed using a Nikon FS-2 photo-slit lamp with a Nikon D200 camera (Melville, NY). Wound sizes were compared with the baseline for each mouse and the percentages of wound closure was measured using ImageJ software.
Results
Notch1 Is Reduced at the Leading Edge of Migrating Corneal Epithelium
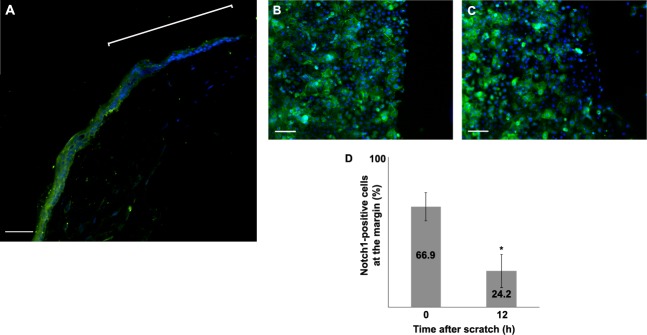
Using immunofluorescence staining, the expression of Notch1 in the mouse corneal epithelium 6 hours after 1.5-mm and 2.0-mm debridement wound was examined. As shown in Figure 1A, Notch1 expression was reduced in the epithelium near the leading edge (images not shown for 2.0 mm). Similar results were found at 2 hours after wounding (data not shown). We further examined the expression of Notch1 in a two-dimensional in vitro wound healing model. Primary human corneal epithelial cells grown to confluence were subjected to a scratch wound. The percentage of cells expressing Notch1 in the leading edge at 12 hours after the scratch was 42% lower compared with baseline (P < 0.001) (Figs. 1B–D).
Figure 1. .
The expression of Notch1 was evaluated in the mouse corneal epithelium at 6 hours after a debridement wound. Notch1 staining was distinctly reduced in the cells near the leading edge (the bracket) (A). The expression of Notch1 was evaluated in primary human corneal epithelial cells subjected to a scratch assay at baseline (B) and after 12 hours (C). The percentage of cells that stained for Notch1 in the leading edge was 42% lower at 12 hours compared with immediately (0 hour) after the scratch (D) (P < 0.001). (Green: FITC, blue: DAPI); scale bar is 40 μm for (A) and 100 μm for (B) and (C). Asterisk represents statistically significant data.
Notch Inhibition Accelerates Human Corneal Epithelial Migration and Wound Closure
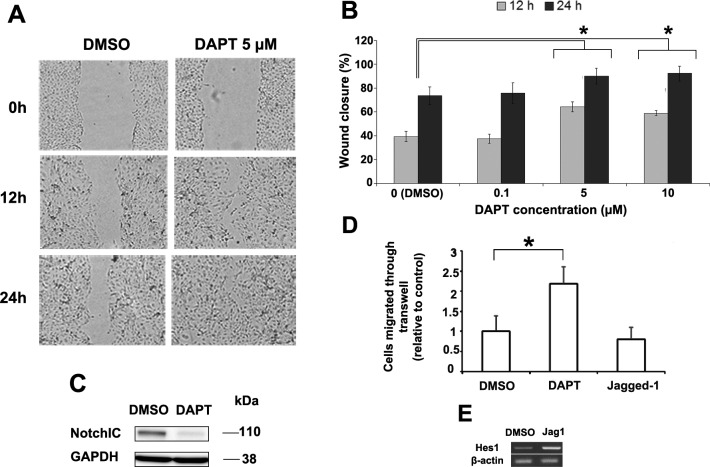
A scratch assay was used to examine the effect of Notch inhibition in human corneal epithelial cells (primary cells and HCE-T). To inhibit Notch signaling broadly, we used the γ-secretase inhibitor DAPT (100 nM–10 μM). The results demonstrated that DAPT 1 to 10 μM consistently accelerated wound closure compared with control (0.02% DMSO) (Figs. 2A, 2B). Concentrations less than 1 μM had no effect while those above 10 μM demonstrated an inhibitory effect on migration (data not shown for concentration above 10 μM since toxicity could not be excluded at these higher doses) (Figs. 2A, 2B). Notch inhibition with 10 μM DAPT was measured by Western blotting for Notch1IC confirming that Notch had been effectively inhibited (Fig. 2C). To examine migration more specifically, HCE-T cells were also subjected to a transwell migration assay. The results demonstrated significantly enhanced migration in cells pretreated with DAPT (218% ± 42%) compared with control (arbitrarily set to 100%) (P < 0.0001). In contrast, Notch activation with Jagged1 appeared to delay migration (80% ± 30%) compared with control but this did not reach statistical significance (P = 0.077) (Figs. 2D, 2E).
Figure 2. .
Human corneal epithelial cells were subjected to a scratch assay and then treated with DAPT or DMSO (control) (A). The effect of DAPT concentration on scratch assay wound closure rate was measured (B). * Represents a statistically significant difference compared to control (P < 0.001). Western blot for Notch1IC confirmed that 10-μM DAPT can effectively inhibit Notch activation (C). HCE-T cells pretreated with DAPT migrated 2.2 times faster than control in transwell migration assay (P < 0.0001) while Jagged1 treated cells migrated 20% slower but did not reach statistical significance (P = 0.077) (D). Jagged1 treatment was found to activate Notch by inducing the expression of its downstream Hes1 (E). Asterisk represents statistically significant data.
Since γ-secretase has additional substrates besides Notch that may potentially affect migration, we used Notch1-shRNA to knockdown Notch1 expression more specifically. HCE-T cells were stably transfected with Notch1-shRNA resulting in 66% knockdown of Notch1 by RT-PCR and reduced Notch1IC by Western blot (Figs. 3A, 3B). Notch1-shRNA transduced cells demonstrated significantly enhanced scratch wound closure and transwell migration compared with NS shRNA transduced cells (Figs. 3C–F). These results were confirmed in a second Notch1-shRNA (different target sequence) transduced cell line which knocked down Notch1 expression by 56% and similarly accelerated migration (data not shown).
Figure 3. .
HCE-T cells stably transfected with Notch1-shRNA demonstrating that Notch1 was knocked down by 66% by RT-PCR compared with NS shRNA (A) with a corresponding decrease in Notch1IC protein level by Western blot (B). Notch1-shRNA transfected HCE-T cells closed scratch wounds faster (P < 0.004) (C, D) and migrated 4 times faster in transwell migration assay (P < 0.0001) (E, F) compared with NS shRNA transduced control cells. Asterisk represents statistically significant data.
Notch Inhibition Does Not Affect Cell Proliferation
Additional studies were done to determine whether the enhanced wound healing observed with γ-secretase inhibition was due to increased proliferation. First, the scratch assay was repeated in primary cells that had been mitotically inactivated with mitomycin C. The results indicated that DAPT enhanced wound closure with or without mitomycin treatment (data not shown) suggesting that cell proliferation was not a factor for the enhanced wound healing. The effect of DAPT on cell proliferation was further assessed in primary human corneal epithelial cells using both Ki67 staining and MTT assays. As shown in Figure 4, the proliferation rate was similar in cells with Notch inhibition compared with control. Although there was no difference in the rate of proliferation based on the MTT assay and Ki67 staining, since this was not done in a scratch assay we cannot completely exclude the possibility that Notch inhibition may have also enhanced scratch wound closure by also affecting proliferation. Nonetheless, based on the results of the transwell migration, which is a more specific assay for migration, it appears that Notch inhibition does have an effect on cell migration.
Figure 4. .
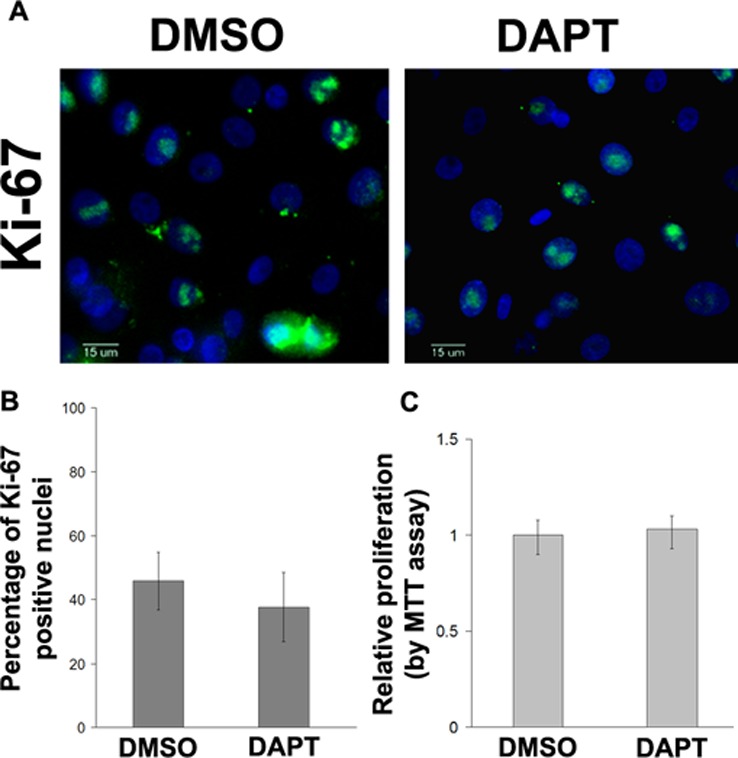
Primary human corneal epithelial cells were treated with DMSO or DAPT (5 μM) and subjected to Ki67 staining (A). The percentage of Ki67-positive cell nuclei was quantified. No difference was found between DAPT-treated and DMSO vehicle control cells (P = 0.254) (B). (Green: FITC; blue: DAPI), scale bar: 15 μm; Cell proliferation was also compared using MTT assay (P = 0.302) (C).
Notch Inhibition Reduces and Notch Activation Increases Cell-Matrix Adhesions
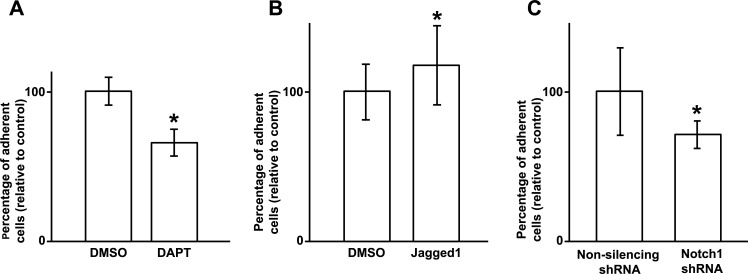
Typically during cell locomotion, cell-matrix adhesions are reduced to allow the cells to migrate.17 Therefore, we sought to determine the effect of Notch inhibition on corneal epithelial cell-matrix adhesions. Primary human corneal epithelial cells exposed to DAPT, Jagged1, or DMSO were evaluated for their ability to adhere to fibronectin-collagen coated plates. The results revealed that after Notch inhibition, the cells adhered 34.5% less efficiently to the matrix compared with DMSO treated controls (Fig. 5A). In contrast, Notch activation with Jagged1 caused the cells to become more adherent to the matrix (Fig. 5B). Notch1-shRNA transduced cells similarly demonstrated reduced adhesion to the matrix compared with NS shRNA transduced cells (Fig. 5C). Such reduction is consistent with the increased migratory capacity of the cells after Notch inhibition.
Figure 5. .
Notch inhibition with DAPT (5 μM) reduces corneal epithelial cell attachment to fibronectin-collagen coated plates by 34.5% compared with DMSO (control). (P < 0.001) (A). In contrast, Jagged1 treated cells were 18% more adherent to the matrix compared with control (P < 0.01) (B). HCE-T transduced with Notch1 (N1) shRNA likewise demonstrated 29% lower matrix attachment compared with NS shRNA transduced HCE-T cells (P < 0.001) (C). Asterisk represents statistically significant data.
Notch Inhibition Affects the Actin Cytoskeleton
Given the importance of the cytoskeletal function in cell migration, rhodamine-phalloidin staining was performed to examine the effect of Notch inhibition on the actin structures. As seen in Figures 6A–D, DAPT treatment enhanced the formation of lamellipodia in the cells near the healing edge. Lamellipodia are important cytoskeletal structures that contribute to cell migration.18–20 In parallel with the more prominent membrane protrusions, the cells also displayed a loss of marginal actin bundle (Figs. 6C, 6D, asterisks). The correlation between the loss of marginal actin bundle and enhanced membrane protrusion (e.g., lamellipodia) has been reported before.20,21 Examination of the Notch1-shRNA transduced cells similarly demonstrated enhanced lamellipodia near the leading edge compared with NS shRNA (Figs. 6E, 6F).
Figure 6. .
F-Actin (rhodamine-phalloidin) staining of human corneal epithelial cells 6 hours after scratch demonstrating enhanced lamellipodia (arrows) formation in DAPT compared with control (A–D). Cells treated with DAPT (10 μM) also demonstrate loss of marginal actin bundle (D, *) compared with control (C, *). HCE-T cells stably transfected with Notch1-shRNA similarly demonstrate enhanced lamellipodia at the wound age at 6 hours compared with NS shRNA transduced cell (E, F). Stable expression of the shRNA vector is evident based on GFP (green) expression, which often overlaps DAPI (blue) nuclear stain. Red: rhodamine-F-actin; scale bar: 100 μm for (A, B) and 50 μm for (E, F).
Inhibiting Notch Accelerates Corneal Epithelial Wound Closure in a Murine Model
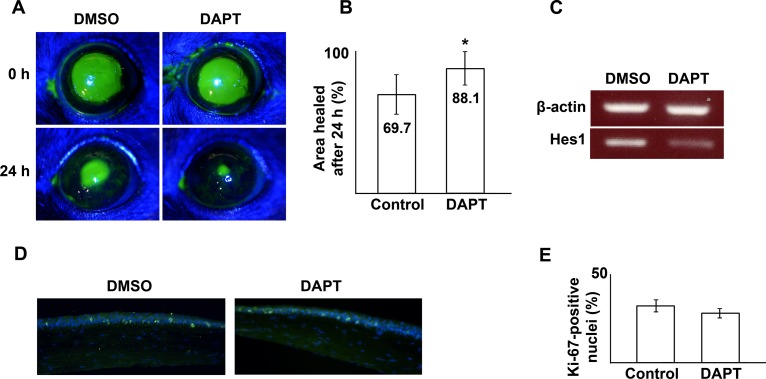
We finally examined the effect of Notch inhibition in an in vivo model of corneal epithelial wound healing. A 2.0-mm corneal epithelial debridement wound was created in mice followed by application of DAPT or vehicle to the cornea for 2 hours. At 24 hours, the corneas treated with DAPT demonstrated a higher percentage area of wound closure compared with DMSO (Figs. 7A–C). Histologic examination of the proliferation marker Ki67 at 24 hours did not demonstrate any difference between Notch inhibited and DMSO control groups, suggesting that migration was the predominant process affected (Figs. 7D, 7E).
Figure 7. .
Treatment with DAPT (20 μM) accelerates wound healing after a 2.0-mm corneal epithelial debridement wound in mice (A, B) (P = 0.025). RT-PCR of the mouse corneal epithelium at 24 hours demonstrates reduced Hes1 in DAPT treated corneas, consistent with suppression of Notch signaling (C). Immunofluorescence examination of the proliferation marker, Ki67 (green: FITC), in the limbal area did not show a significant difference between DAPT-treated and control (DMSO) specimens at 24 hours post wounding (P = 0.102) (blue: DAPI nuclear stain) (D, E). Asterisk represents statistically significant data.
Discussion
Corneal epithelial wound healing is a highly coordinated process that involves numerous cell-cell signaling pathways. In this study, we examined the involvement of Notch signaling in corneal epithelial wound healing and found a specific role in epithelial cell migration. Previous studies have primarily focused on the role of Notch in proliferation and differentiation and its role in migration has not been investigated specifically in the cornea.12–14 In an earlier study, we had reported an inverse correlation between Notch and epithelial proliferation in corneal epithelial wound healing.11 However, based on the results of the present study, it appears that the inverse correlation may be more closely associated with migration particularly in the cells near the leading edge. We found that in the first 6 hours after wounding, Notch1 is down-regulated in the leading edge, promoting a more migratory phenotype in these cells.
Our results, demonstrating faster migration with Notch inhibition, are consistent with previous findings in skin epithelial cells.22 Dotto and colleagues22 transfected human skin keratinocytes with a retroviral dominant negative construct to inhibit all canonical Notch signaling and found that Notch inhibited cells had significantly increased migratory behavior both by individual cell velocity measurements and by Matrigel invasion assay. They implicated Rho-Rho associated protein kinase (ROCK) signaling as the mechanism of action.
In another study, Ma et al.13 investigated the effects of Notch activation and inhibition on corneal epithelial wound healing in organ cultured rat eyes. They found that enhancing Notch signaling with a soluble ligand (Jagged1) retarded wound closure at 24 hours without affecting proliferation, suggesting that migration may have been inhibited. In contrast to our results, they did not find any enhancement in wound closure rate with γ-secretase inhibition. Besides the differences in the experimental models, the difference between our results and theirs may be due to the varying degrees of Notch inhibition. In this study, we carefully examined the various concentrations of γ-secretase inhibitor and found the effective dose for enhancing migration to be 1 to 10 μM, which is a 10-fold lower equivalent dose compared with the specific gamma secretase inhibitor and concentrations used by Ma et al.13 The lower concentration of the γ-secretase inhibitor used in our study is able to inhibit Notch effectively as shown by the reduced levels of Notch1IC (Fig. 2C). Using lower concentrations of the γ-secretase inhibitor DAPT is not only advantageous to minimize non Notch effects, but perhaps because only partial inhibition of Notch would result in enhanced wound healing. Higher concentrations of DAPT were found in our experiments to inhibit wound healing, although we could not exclude the possibility of toxicity. Previous studies have demonstrated that Notch is a highly dosage-dependent signaling system such that high levels of Notch activation may have completely different effects compared with low levels of Notch activity.23,24
A recent study reported that over-expressing Notch1 in transgenic mice also enhances corneal epithelial wound healing.14 The investigators developed mice that over-express Notch1IC (active Notch1) in the corneal epithelium and found that in the transgenic mice corneal epithelial debridement wounds healed faster than controls. These results may at first seem to contradict ours, which showed enhanced wound healing with Notch inhibition. However, a different mechanism may underlie the Notch1IC transgenic findings, namely, the transgenic epithelium may exhibit a quicker onset of proliferation compared with control mice.14 While more studies are needed to dissect the role of Notch in wound healing, based on findings from Lu et al.14 and ours, one might speculate that Notch1 plays multiple roles involving both migration and proliferation. Further studies are also needed to dissect the role of other Notch receptors (Notch2 and Notch3) in corneal epithelial wound healing.
A novel finding of our study is that Notch inhibition promoted cytoskeletal changes that facilitate cell migration. In particular, Notch inhibited cells demonstrated enhanced lamellipodia along with loss of marginal actin bundle. We hypothesize that these changes are likely mediated through Rho GTPases. As mentioned earlier, a previous study has implicated Rho-ROCK signaling pathway in the enhanced migratory behavior of skin keratinocytes after Notch inhibition.22 Additional investigations are necessary to determine specific mechanisms by which Notch might regulate Rho-ROCK activity in the corneal epithelium.
In summary, we have implicated Notch signaling in the regulation of corneal epithelial cell migration. Based on current results, we speculate that treatment with agents that inhibit Notch signaling may provide a novel approach for enhancing corneal epithelial migration and wound healing clinically. This is particularly exciting since a number of Notch inhibitors are currently in clinical trials as cancer therapies,25,26 making them potentially available for clinical application to the cornea.
Acknowledgments
The authors thank Ruth Zelkha, MS, for her generous technical assistance in imaging, Deepak Shukla, PhD, for providing HCE-T cells, and the Illinois and Michigan Eye Banks for providing human corneal tissue.
Footnotes
Supported by the National Eye Institute of the National Institutes of Health with Career Development Grant K08EY017561-A1 (ARD) and Core Grant EY01792, the Cless Family Foundation, and a Career Developmental Award (ARD) and unrestricted departmental grant from Research to Prevent Blindness.
Disclosure: A. Movahedan, None; M. Majdi, None; N. Afsharkhamseh, None; H.M. Sagha, None; N.S. Saadat, None; K. Shalileh, None; B.Y. Milani, None; H. Ying, None; A.R. Djalilian, None
References
- 1.Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med. 2001;226:653–664 [DOI] [PubMed] [Google Scholar]
- 2.Yu FS, Yin J, Xu K, Huang J. Growth factors and corneal epithelial wound healing. Brain Res Bull. 2010;81:229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenwald I. LIN-12/Notch signaling: lessons from worms and flies. Genes Dev. 1998;12:1751–1762 [DOI] [PubMed] [Google Scholar]
- 4.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776 [DOI] [PubMed] [Google Scholar]
- 5.Struhl G, Greenwald I. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature. 1999;398:522–525 [DOI] [PubMed] [Google Scholar]
- 6.Brou C, Logeat F, Gupta N, et al. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–216 [DOI] [PubMed] [Google Scholar]
- 7.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689 [DOI] [PubMed] [Google Scholar]
- 8.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386 [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Ohtsuka T, Sekiyama E, et al. Hes1 regulates corneal development and the function of corneal epithelial stem/progenitor cells. Stem Cells. 2008;26:1265–1274 [DOI] [PubMed] [Google Scholar]
- 10.Thomas PB, Liu YH, Zhuang FF, et al. Identification of Notch-1 expression in the limbal basal epithelium. Mol Vis. 2007;13:337–344 [PMC free article] [PubMed] [Google Scholar]
- 11.Djalilian AR, Namavari A, Ito A, et al. Down-regulation of Notch signaling during corneal epithelial proliferation. Mol Vis. 2008;14:1041–1049 [PMC free article] [PubMed] [Google Scholar]
- 12.Ma A, Boulton M, Zhao B, Connon C, Cai J, Albon J. A role for notch signaling in human corneal epithelial cell differentiation and proliferation. Invest Ophthalmol Vis Sci. 2007;48:3576–3585 [DOI] [PubMed] [Google Scholar]
- 13.Ma A, Zhao B, Boulton M, Albon J. A role for Notch signaling in corneal wound healing. Wound Repair Regen. 2011;19:98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu H, Lu Q, Zheng Y, Li Q. Notch signaling promotes the corneal epithelium wound healing. Mol Vis. 2012;18:403–411 [PMC free article] [PubMed] [Google Scholar]
- 15.Araki K, Ohashi Y, Sasabe T, et al. Immortalization of rabbit corneal epithelial cells by a recombinant SV40-adenovirus vector. Invest Ophthalmol Vis Sci. 1993;34:2665–2671 [PubMed] [Google Scholar]
- 16.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333 [DOI] [PubMed] [Google Scholar]
- 17.Stepp MA, Liu Y, Pal-Ghosh S, et al. Reduced migration, altered matrix and enhanced TGFbeta1 signaling are signatures of mouse keratinocytes lacking Sdc1. J Cell Sci. 2007;120:2851–2863 [DOI] [PubMed] [Google Scholar]
- 18.Harms BD, Bassi GM, Horwitz AR, Lauffenburger DA. Directional persistence of EGF-induced cell migration is associated with stabilization of lamellipodial protrusions. Biophys J. 2005;88:1479–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farooqui R, Fenteany G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J Cell Sci. 2005;118:51–63 [DOI] [PubMed] [Google Scholar]
- 21.Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM. Rho-dependent formation of epithelial “leader” cells during wound healing. Proc Natl Acad Sci U S A. 2003;100:10788–10793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefort K, Mandinova A, Ostano P, et al. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKalpha kinases. Genes Dev. 2007;21:562–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzone M, Selfors LM, Albeck J, et al. Dose-dependent induction of distinct phenotypic responses to Notch pathway activation in mammary epithelial cells. Proc Natl Acad Sci U S A. 2010;107:5012–5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guentchev M, McKay RD. Notch controls proliferation and differentiation of stem cells in a dose-dependent manner. Eur J Neurosci. 2006;23:2289–2296 [DOI] [PubMed] [Google Scholar]
- 25.Nickoloff BJ, Osborne BA, Miele L. Notch signaling as a therapeutic target in cancer: a new approach to the development of cell fate modifying agents. Oncogene. 2003;22:6598–6608 [DOI] [PubMed] [Google Scholar]
- 26.Pannuti A, Foreman K, Rizzo P, et al. Targeting Notch to target cancer stem cells. Clin Cancer Res. 2010;16:3141–3152 [DOI] [PMC free article] [PubMed] [Google Scholar]