Abstract
A decade after the sequencing of the human genome, the National Human Genome Research Institute announced a strategic plan for genomic medicine. It calls for evaluating the structure and biology of genomes, understanding the biology of disease, advancing the science of medicine, and improving the effectiveness of health care.
Fulfilling the promise of genomics urgently requires a population perspective to complement the bench-to-bedside model of translation.
A population approach should assess the contribution of genomics to health in the context of social and environmental determinants of disease; evaluate genomic applications that may improve health care; design strategies for integrating genomics into practice; address ethical, legal, and social issues; and measure the population health impact of new technologies.
It has been more than 20 years since the National Human Genome Research Institute and the Department of Energy launched the Human Genome Project and 10 years since the completion of the initial draft of the human genome sequence. On February 10, 2011, the institute announced its ambitious plan “charting a course for genomic medicine from base pairs to bedside.”1(p24) The plan is organized around several domains, extending from basic research to bedside applications: (1) understanding the structure and biology of genomes, (2) understanding the biology of disease, (3) advancing the science of medicine, and (4) improving the effectiveness of health care. The plan reflects the view that the most effective way to improve human health is to understand normal biology (including genome biology) and its perturbations as the basis for developing diagnostics and therapeutics to improve health care.1
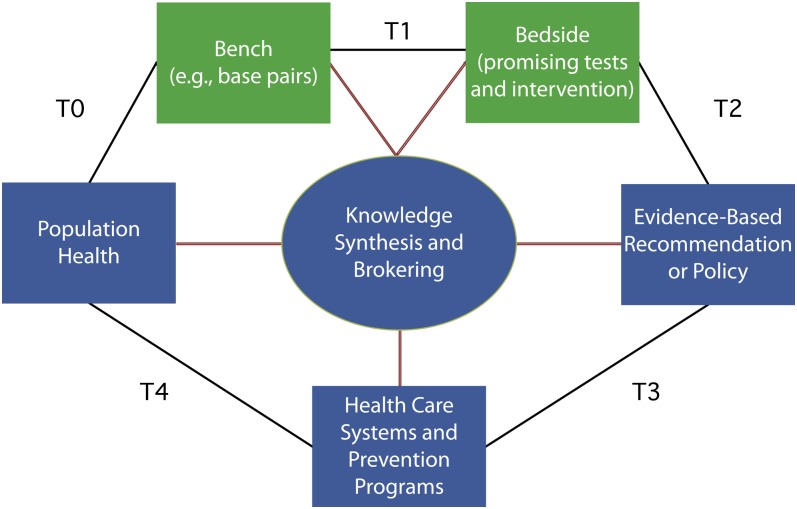
Fulfilling the promise of genomics in improving health requires a population research agenda to complement the “push” model of clinical translation, often described as the first phase of translation (bench to bedside; Figure 1).3 An expanded multidisciplinary research agenda is needed to understand and address the “pull” forces on genomics to improve health at the population level (beyond bench to bedside; Figure 1). A population approach to genomic medicine is needed to assess the contribution of genomics to health in the larger context of social and environmental determinants of disease,4 evaluate promising genomic applications for their potential to improve health and health care, design appropriate implementation strategies for integrating genomics into clinical and public health practice, and measure the population health impact of new technologies. A population approach to genomics should be informed by the public health code of ethics, which addresses fundamental tensions between the rights of individuals and the good of the community.5,6 An expanded research agenda should focus not only on the bench-to-bedside translation phase but also on what is often called the second phase of translation (steps T2–T4 in Figure 1).2 Although we and others have made some of these points before,7–10 we see an ongoing need to emphasize the public health perspective in the context of the new strategic plan.
FIGURE 1—
A framework for multidisciplinary research in genomics and health beyond bench to bedside, with green representing the first phase of translation (T1) and blue representing the second phase of translation (T2–T4), with a feedback loop to basic science discoveries (T0).
Source. Adapted from Khoury et al.2
A population approach is needed to explore how genomics can contribute to our understanding of health and disease. Characterizing health-related metrics at the individual level of what is typical biology (including the genome) can only be obtained from well-designed, large epidemiological studies with representative population samples, including minority populations, which have been difficult to involve in clinical and public health research. For example, in the United States, reference populations can be obtained from public health data systems such as the National Health and Nutrition Examination Survey, a large multiethnic representative sample of the population with phenotypic, genotypic, and disease risk factor data on thousands of individuals.11
A population perspective is also needed to assess how genomics fits in an overall ecological model of health and disease that considers the interaction of individuals' genes and other clinical and behavioral characteristics with familial, social, environmental, and health system determinants.12,13 The study of genetic variation in relation to the effects of infections and environmental, behavioral, social, and other modifiable factors across the life span can contribute to a better understanding of complex disease processes. In addition, emerging scientific areas, such as epigenetics,14 can contribute more directly to our understanding of how environmental factors can lead to heritable changes in gene expression and their impact on disease processes. Most common chronic diseases with global health impact have known environmental, social, and behavioral determinants (e.g., tobacco use; physical activity; diet; racial, ethnic, and economic factors; and differential access to health care). Therefore, a comprehensive research agenda for genomics should address the interactions of genes with other factors as they contribute to the antecedents of disease determinants (e.g., antecedents of tobacco use and its endophenotypes15) as well as the determinants of disease outcomes (e.g., survival and quality of life after the occurrence of cancer16).
By using advances in genomics to address global public health problems with complex environmental causation,17,18 we can assess whether existing strategies (e.g., medications; social, behavioral, and policy interventions) will work across all segments of the population or will need to be modified on the basis of individual susceptibility and disease molecular subtypes. The outcomes of such assessment can reflect the added value of genomics in ways that may not necessarily lead to new therapies or genomic testing for individuals or population groups. For example, Mendelian randomization19 could identify environmental factors amenable to intervention at the policy level, for example, by helping to determine maximum allowed exposure levels among the most susceptible population subgroups.20 More intensive environmental or behavioral interventions could be targeted to susceptible groups. Clearly, discovering the genetic components of disease and other health outcomes is not sufficient. A population-driven, multidisciplinary research agenda is needed to establish whether these discoveries merit a place in the armamentarium for disease treatment and prevention.
A multidisciplinary research agenda is needed to fill in knowledge gaps beyond bench to bedside, that is, from the bedside to improved population health. Public health and health services researchers tend to view translational research as a process for developing evidence-based interventions and implementing them in practice, thus ensuring that new interventions actually reach the populations for whom they are intended and are implemented correctly. The endpoint for bench-to-bedside research, typically the production of a new drug or intervention or a new genome-based test, is the starting point for the second phase of translation.
The Institute of Medicine's Clinical Research Roundtable defined two broad phases of translation in relation to two broad research gaps: (1) “the transfer of new understandings of disease mechanisms gained in the laboratory into the development of new methods for diagnosis, therapy, and prevention and their first testing in humans” and (2) “the translation of results from clinical studies into everyday clinical practice and health decision making.”3(p1278) For genomic medicine, spanning the second translation gap involves scientific evaluation of promising applications, such as diagnostics, drugs, behavioral and policy interventions (T2), implementation science (T3), and comparative effectiveness research and public health surveillance (T4).2,21
This second phase of translational research, which currently composes less than 2% of total research in genomics,2,21 is a crucial link between genome-based discoveries and population health impact. Multiple factors could underlie the imbalance between the first and second phases of translation, including the preferential emphasis of federal funding on discovery research.21 Even the recently established Center for the Advancement of Translational Sciences at the National Institutes of Health seems to be focused on translational research in therapeutics for clinical applications rather than on a broader translational research agenda to serve public health goals.22 Some public health researchers and practitioners are also concerned that undue attention to genetic causes of disease could detract from efforts to address environmental or social determinants of health and health disparities.23 Fortunately, an increased emphasis on gene–environment interaction (such as through epigenetics) should begin to bridge this gap in translational research.
Translation of promising genomic applications into day-to-day health practice is slow and uncertain, with major issues in implementation, access, and disparities across segments of our population. For example, although testing for BRCA1, a gene discovered in 1994, is well established, evaluation of the implementation, effectiveness, and impact of testing among high-risk women has been slow to accrue. Evidence-based recommendations for BRCA1 testing were published in 200524; however, major racial disparities persist in testing utilization,25 and provider knowledge and practices are far from optimal.26 Another well-established genomic application is somatic HER2 tumor testing to target trastuzumab treatment of patients with breast cancer.27 Although this testing is in widespread use, the high cost of trastuzumab therapy is accompanied by persistent gaps in knowledge about how to most efficiently target such therapy.27
More than 2000 genetic tests are already available,28 and more than 270 genome-based applications appeared on the horizon since 2010.29 Clearly, an evidence gap is growing between emerging applications and the data that show their effectiveness in practice and the best approaches for implementation and dissemination. With the expected growth of new genomic applications, including the potential deployment of clinical whole-genome sequencing in the next few years,30 we urgently need to evaluate the balance of benefits and harms of these new tools for individuals, families, and communities—an essential component of the evidence base for their use in practice. These developments also raise concerns about privacy and confidentiality of genetic information and create a need for enhanced provider and consumer education.
The emergence of the direct-to-consumer movement in personal genomics31 raises the prospect of genomic testing delivered not only within health care settings, but also in community settings and for personal use. This trend has potential implications for public health roles in policymaking as well as for educating health care providers and the public. Although increasing availability and consumer demand are likely to fuel the integration of new genomic tools in health care and disease prevention, we still need to understand their potential benefits, harms, and costs from the perspectives of individuals, populations, and health care systems.32 Knowledge of biological pathways and gene–disease associations is not enough to demonstrate the value of genomic information in improving health.32 Although many genomic applications—such as diagnostic tests, new therapies, and pharmacogenomics—will be used in health care settings, others may become part of public health practice, either through existing public health programs (e.g., newborn screening33) or by informing public health efforts to combat infectious, environmental, and chronic diseases.
Finally, a population perspective is needed to understand the many push and pull stakeholder forces that can accelerate or slow down the translation process. These include, for example, public and private investments, policy and legal frameworks, oversight and regulation, product marketing, insurance coverage and reimbursement, consumer education, provider awareness, and differential access to services.34 In addition, conflicts may arise among stakeholders in determining when specific genomic applications are ready for clinical implementation.35 For example, payers tend to require a higher level of evidence of clinical utility than do genomic researchers or test developers. Understanding these multiple forces and how to act on them is a research agenda in itself.
To achieve a shared vision of genomic medicine, a population “honest broker” perspective is needed to address stakeholders’ issues and differences and to harmonize their demands regarding the nature, quantity, and quality of evidence called for to drive decision-making by individuals and health systems.36 Such stakeholder collaboration links researchers and decision-makers, facilitates their interaction so that they can better understand each other's goals and professional culture, forges new partnerships, and applies evidence from translational research. A foundational basis, consisting of at least some level of evidence derived from a broad spectrum of translational research (steps T2–T4) is required to allow productive knowledge brokering between stakeholder groups, a process that is ultimately about developing and using evidence-based decision-making to deliver health services and public health interventions.35 Because of the rapid advances in genomic science and technology, knowledge brokering in this field will require full stakeholder engagement in the formulation and application of novel evidentiary processes that result in ongoing knowledge synthesis and evidence-based recommendations. These processes are critical in appropriately deploying promising genomic tests and therapeutics that can improve health, while minimizing premature or inappropriate use of technologies that lead to unnecessary health care expenditures or harm individuals and populations.36
Only a population perspective can fulfill the promise of genomic medicine. The scientific landscape for genomics is exciting, and the promise for improving health is great. Applying genomic tools in clinical and public health practice will require a multidisciplinary research collaboration of basic sciences with clinical and population sciences (e.g., epidemiologists; behavioral, social, and communication scientists; health services researchers; and public health practitioners).37,38 A comprehensive research agenda will allow us to maximize the value of genomic discoveries in improving health by deploying them effectively and responsibly for the benefit of all.
Acknowledgments
We thank Katie Kolor for her comments.
References
- 1.Green ED, Guyer MS, National Human Genome Research Institute Charting a course for genomic medicine from base pairs to bedside. Nature. 2011;470(7333):204–213 [DOI] [PubMed] [Google Scholar]
- 2.Khoury MJ, Gwinn M, Yoon PW, Dowling N, Moore CA, Bradley L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet Med. 2007;9(10):665–674 [DOI] [PubMed] [Google Scholar]
- 3.Sung NS, Crowley WF, Jr, Genel Met al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289(10):1278–1287 [DOI] [PubMed] [Google Scholar]
- 4.Leischow SJ, Best A, Trochim WMet al. Systems thinking to improve the public's health. Am J Prev Med. 2008;35(2)(suppl):S196–S203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas JC, Sage M, Dillenberg J, Gillory VJ. A code of ethics for public health. Am J Public Health. 2002;92(7):1057–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas JC, Irwin DE, Zuiker ES, Millikan RC. Genomics and the public health code of ethics. Am J Public Health. 2005;95(12):2139–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury MJ, Bowen MS, Burke Wet al. Current priorities in public health practice in addressing the role of human genomics in improving population health. Am J Prev Med. 2011;40(4):486–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall WD, Matthews R, Morley KI. Being more realistic about the public health impact of genomic medicine. PLoS Med. 2010;7(10):e1000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke W, Burton H, Hall AEet al. Extending the reach of public health genomics: what should be the agenda for public health in an era of genome-based and personalized medicine? Genet Med. 2010;12(12):785–791 [DOI] [PubMed] [Google Scholar]
- 10.O'Leary P, Zimmern RL. Genomics and public health: translating research into public benefit. Public Health Genomics. 2010;13(4):193–196 [DOI] [PubMed] [Google Scholar]
- 11.Chang MH, Lindegren ML, Butler MAet al. Prevalence in the United States of selected candidate gene variants: Third National Health and Nutrition Examination Survey, 1991–1994. Am J Epidemiol. 2009;169(1):54–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kindig D, Stoddart G. What is population health? Am J Public Health. 2003;93(3):380–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Institute of Medicine Genes, Behavior and the Social Environment: Moving Beyond the Nature/Nurture Debate. Washington, DC: National Academies Press; 2006 [PubMed] [Google Scholar]
- 14.Mathers JC, McKay JA. Epigenetics—potential contributions to fetal programming. Adv Exp Med Biol. 2009;646:119–123 [DOI] [PubMed] [Google Scholar]
- 15.Phenotypes and Endophenotypes: Foundations for Genetic Studies of Nicotine Use and Dependence. Bethesda, MD: National Cancer Institute; 2009 [Google Scholar]
- 16.Travis LB, Beard C, Allan JMet al. Testicular cancer survivorship: research strategies and recommendations. J Natl Cancer Inst. 2010;102(15):1114–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoury MJ, Davis RL, Gwinn M, Lindegren ML, Yoon P. Do we need genomic research for the prevention of common diseases with environmental causes? Am J Epidemiol. 2005;161(9):799–805 [DOI] [PubMed] [Google Scholar]
- 18.Galea S, Riddle M, Kaplan GA. Causal thinking and complex system approaches in epidemiology. Int J Epidemiol. 2010;39(1):97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davey Smith G. Mendelian randomization for strengthening causal inference in observational studies: applications to gene × environment interactions. Perspect Psychol Sci. 2010;5(5):527–545 [DOI] [PubMed] [Google Scholar]
- 20.Christiani DC, Mehta AJ, Yu CL. Genetic susceptibility to occupational exposures. Occup Environ Med. 2008;65(6):430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schully SD, Benedicto CB, Gillanders EM, Wang SS, Khoury MJ. Translational research in cancer genetics: the road less traveled. Public Health Genomics. 2011;14(1):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Usdin S. Translation under one roof. Sci Bus Exch. 2010;3(48):doi:10.1038/scibx.2010.1430 [Google Scholar]
- 23.Buchanan AV, Weiss KM, Fullerton SM. Dissecting complex disease: the quest for the philosopher's stone? Int J Epidemiol. 2006;35(3):562–571 [DOI] [PubMed] [Google Scholar]
- 24.Nelson HD, Huffman LH, Fu R, Harris EL, U.S. Preventive Services Task Force Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;143(5):362–379 [DOI] [PubMed] [Google Scholar]
- 25.Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293(14):1729–1736 [DOI] [PubMed] [Google Scholar]
- 26.Bellcross CA, Kolor KK, Goddard KA, Coates RJ, Reyes M, Khoury MJ. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40(1):61–66 [DOI] [PubMed] [Google Scholar]
- 27.Phillips KA. Closing the evidence gap in the use of emerging testing technologies in clinical practice. JAMA. 2008;300(21):2542–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Center for Biotechnology Information GeneTests. Available at: http://www.ncbi.nlm.nih.gov/sites/GeneTests/?db=GeneTests. Accessed February 1, 2011
- 29.Gwinn M, Grossnicklaus DA, Yu Wet al. Horizon scanning for new genomic tests. Genet Med. 2011;13(2):161–165 [DOI] [PubMed] [Google Scholar]
- 30.Ashley EA, Butte AJ, Wheeler MTet al. Clinical assessment incorporating a personal genome. Lancet. 2010;375(9725):1525–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med. 2011;364(6):524–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khoury MJ, McBride CM, Schully SDet al. The scientific foundation for personal genomics: recommendations from a National Institutes of Health–Centers for Disease Control and Prevention multidisciplinary workshop. Genet Med. 2009;11(8):559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lloyd-Puryear MA, Brower A. Long-term follow up after newborn screening: a systems approach for improving health outcomes. Genet Med. 2010;12(12)(suppl):S256–S260 [DOI] [PubMed] [Google Scholar]
- 34.Khoury MJ, Berg A, Coates RC, Evans J, Teutsch SM, Bradley LA. The evidence dilemma in genomic medicine. Health Aff (Millwood). 2008;27(6):1600–1611 [DOI] [PubMed] [Google Scholar]
- 35.Khoury MJ. Dealing with the evidence dilemma in genomics and personalized medicine. Clin Pharmacol Ther. 2010;87(6):635–638 [DOI] [PubMed] [Google Scholar]
- 36.Veenstra DL, Roth JA, Garrison LP, Jr, Ramsey SD, Burke W. A formal risk–benefit framework for genomic tests: facilitating the appropriate translation of genomics into clinical practice. Genet Med. 2010;12(11):686–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garber AM, Tunis SR. Does comparative effectiveness research threaten personalized medicine? N Engl J Med. 2009;360(19):1925–1927 [DOI] [PubMed] [Google Scholar]
- 38.McBride CM, Bowen D, Brody LCet al. Future health applications of genomics: priorities for communication, behavioral, and social sciences research. Am J Prev Med. 2010;38(5):556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]