Abstract
The concept of system 1 and system 2 ethylene biosynthesis during climacteric fruit ripening was initially described four decades ago. Although much is known about fruit development and climacteric ripening, little information is available about how ethylene biosynthesis is regulated during the postclimacteric phase. A targeted systems biology approach revealed a novel regulatory mechanism of ethylene biosynthesis of tomato (Solanum lycopersicum) when fruit have reached their maximal ethylene production level and which is characterized by a decline in ethylene biosynthesis. Ethylene production is shut down at the level of 1-aminocyclopropane-1-carboxylic acid oxidase. At the same time, 1-aminocyclopropane-1-carboxylic acid synthase activity increases. Analysis of the Yang cycle showed that the Yang cycle genes are regulated in a coordinated way and are highly expressed during postclimacteric ripening. Postclimacteric red tomatoes on the plant showed only a moderate regulation of 1-aminocyclopropane-1-carboxylic acid synthase and Yang cycle genes compared with the regulation in detached fruit. Treatment of red fruit with 1-methylcyclopropane and ethephon revealed that the shut-down mechanism in ethylene biosynthesis is developmentally programmed and only moderately ethylene sensitive. We propose that the termination of autocatalytic ethylene biosynthesis of system 2 in ripe fruit delays senescence and preserves the fruit until seed dispersal.
In 1972, McMurchie et al. introduced system 1 and system 2 ethylene biosynthesis during fruit development and ripening based upon differences in ethylene production and respiration between climacteric and nonclimacteric fruits (McMurchie et al., 1972). Since its inception, the concept of two systems of ethylene biosynthesis has been broadly adopted and refined. System 1 is characterized by a basal level of ethylene production, is autoinhibitory, and is applicable to all vegetative tissues including developing fruits (Alexander and Grierson, 2002). At a certain moment, coordinated by a yet unknown developmental switch, climacteric fruit start to ripen and ethylene production rises autocatalytically. Ethylene production during system 2 was later characterized to be partially autocatalytically and partially developmentally regulated (Yokotani et al., 2009). The drop in ethylene production after the autocatalytic rise is less investigated. Nonclimacteric fruit ripen without an autocatalytic burst in ethylene production.
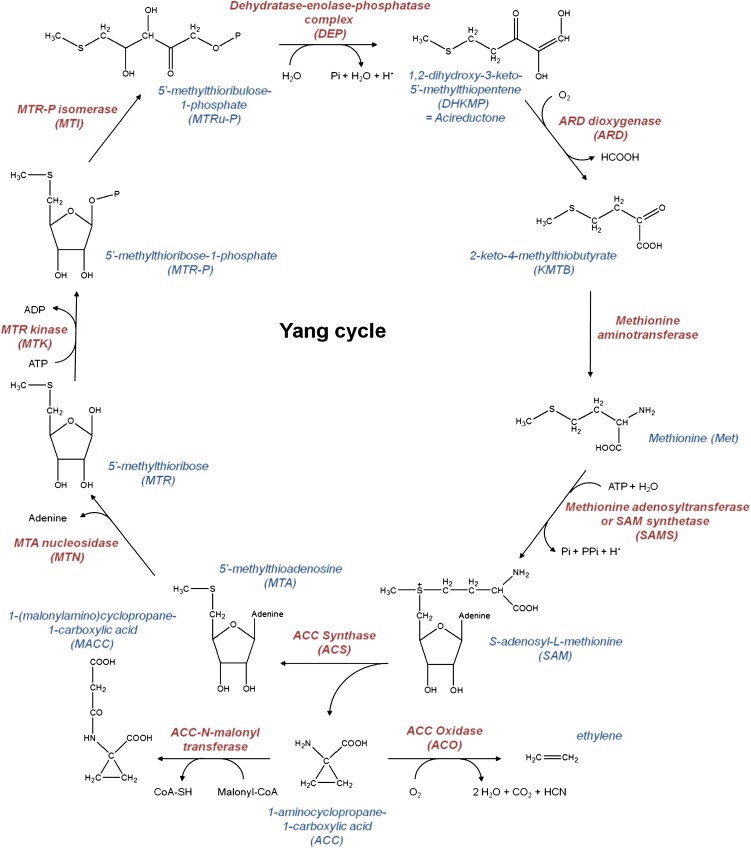
The ethylene biosynthesis pathway was unraveled mainly during the late 1970s and early 1980s (Fig. 1). It was shown that 1-aminocyclopropane-1-carboxylic-acid (ACC) is the precursor of ethylene and originates from the amino acid Met (Lieberman and Kunishi, 1966; Adams and Yang, 1979). ACC is formed by ACC synthase (ACS) using S-adenosyl-l-Met (SAM) as a substrate (Murr and Yang, 1975; Boller et al., 1979). SAM itself is produced from the amino acid Met by SAM synthetase requiring ATP. For each ACC molecule synthesized by ACS, one 5′-methylthioadenosine (MTA) residue is released (Adams and Yang, 1977). MTA is recycled back to Met by the Met or Yang cycle for another round of ethylene biosynthesis. During the first step of Met recycling, MTA is converted to 5′-methylthioribose by 5′-methylthioadenosine nucleosidase (MTN; Adams and Yang, 1977; Wang et al., 1982; Rzewuski et al., 2007). 5′-methylthioribose is phosphorylated to 5′-methylthioribose-1-phosphate (MTR-P) by 5′-methylthioribose kinase (MTK; Kushad et al., 1982; Sauter et al., 2004). Just recently, Pommerrenig et al. (2011) characterized two more enzymes of the Yang cycle in plants. The first is MTR-P isomerase (MTI), which catalyzes the isomerization of MTR-P into 5′-methylthioribulose-1-P. In a second step, 5′-methylthioribulose-1-P is converted to 1,2-dihydroxy-3-keto-5-methylthiopentene by a dehydratase-enolase-phosphatase (DEP). 1,2-dihydroxy-3-keto-5-methylthiopentene (also termed acireductone) is converted into 2-keto-4-methylthiobutyrate by acireductone dioxygenase (ARD) in the presence of oxygen (Sauter et al., 2005; Bürstenbinder et al., 2007). Finally, 2-keto-4-methylthiobutyrate is further metabolized to Met by a yet unknown transaminase (Kushad et al., 1983; Pommerrenig et al., 2011).
Figure 1.
Structural representation of the ethylene biosynthesis and the Met or Yang cycle with corresponding enzymes in its most present form. [See online article for color version of this figure.]
The ethylene precursor ACC is converted into ethylene by ACC oxidase (ACO, previously called the ethylene-forming enzyme) in the presence of oxygen (Hamilton et al., 1991; Dong et al., 1992). ACC can also be conjugated to the biologically inactive metabolite 1-(malonylamino)cyclopropane-1-carboxylic acid (MACC) by ACC-N-malonyltransferase (Hoffman et al., 1982; Liu et al., 1983). MACC was thought to be an end metabolite, but Jiao et al. (1986) and Hanley et al. (1989) showed that MACC can be reconverted into ACC. Later it was found that a minor amount of ACC can also be converted into the low abundant conjugate 1-(γ-glutamylamino)cyclopropane-1-carboxylic acid (Martin et al., 1995). More recently, Staswick and Tiryaki (2004) showed the presence of a low abundant ACC derivative of jasmonic acid in Arabidopsis (Arabidopsis thaliana).
The enzymes solely involved in ethylene biosynthesis are encoded by multigene families. So far, five different isoforms have been identified for ACO in tomato (Solanum lycopersicum), and they are differentially expressed during fruit development and ripening. During system 1, ACO1 and ACO4 are expressed moderately, whereas ACO3 is transiently expressed during the transition phase. During system 2, ACO1 and ACO4 expression increases severalfold, peaking at the orange stage (Barry et al., 1996; Nakatsuka et al., 1998). A fifth member of the ACO family (ACO5) shows an anaerobically induced expression in both fruit and leaves, but has not yet been profiled during fruit development and ripening (Sell and Hehl, 2005).
For ACS, nine genes have been identified in tomato so far (ACS1A, ACS1B, ACS2–ACS8) of which five are expressed during fruit ripening. ACS1A and ACS3 are constitutively expressed at a low level during system 1, whereas ACS6 shows a higher expression (Nakatsuka et al., 1998; Barry et al., 2000). During system 2, ACS2 and ACS4 are highly expressed (Yip et al., 1992; Nakatsuka et al., 1998; Barry et al., 2000).
The expression of both ACS and ACO genes is regulated by recently identified transcription factors in tomato. HB-1 is a homeobox Leu zipper protein that binds to the promoter of ACO1, altering its expression (Lin et al., 2008), whereas ETHYLENE RESPONSE FACTOR2 (ERF2) was shown to interact with the GCC box of the ACO3 promoter, stimulating ACO3 expression (Zhang et al., 2009). The RIPENING INHIBITOR (RIN) transcription factor was also shown to interact with the promoter region of ACS-encoding genes ACS2 and ACS4 (Ito et al., 2008; Fujisawa et al., 2011). Martel et al. (2011) showed a clear correlation between RIN expression, RIN abundance, and ACS2 expression.
Early biochemical studies led to the structural and functional characterization of the two enzymes specific for ethylene biosynthesis. The ACO protein belongs to the superfamily of oxygenases and oxidases with a requirement for iron as cofactor and bicarbonate as activator (Dong et al., 1992; Zhang et al., 2004). Ascorbic acid is an essential additive during in vitro activity measurements and most probably also for in vivo functionality, although the exact role of this effector remains uncertain (Rocklin et al., 2004). ACO was localized in the cytosol by immunocytolocalization (Reinhardt et al., 1994; Chung et al., 2002; Hudgins et al., 2006), but some authors reported ACO localization at the plasma membrane (Rombaldi et al., 1994; Ramassamy et al., 1998). Its three-dimensional structure was determined by x-ray crystallography (Zhang et al., 2004). In vitro analysis of ectopically expressed ACO proteins revealed that each isoform has a specific activity with ACO1 being the most active member (Bidonde et al., 1998).
The ACS protein belongs to the pyridoxal-5′-P (vitamin B6)-dependent enzymes, requiring pyridoxal-5′-P as an essential cofactor and is located in the cytosol (Boller et al., 1979). ACS can be posttranslationally stabilized by phosphorylation to prevent degradation by the ubiquitin/proteasome pathway (Tatsuki and Mori, 2001; Joo et al., 2008; Kamiyoshihara et al., 2010). The three-dimensional structure of apple (Malus domestica) ACS homodimer was obtained by Capitani et al. (1999). It was shown that ACS proteins can form heterodimers that increase both the structural and functional complexity (Tsuchisaka and Theologis, 2004).
Climacteric fruit ripening was most extensively studied in tomato (Alexander and Grierson, 2002). ACC content has been profiled in the past during system 1 and system 2 and shows a close correlation with the level of fruit ethylene production (Hoffman and Yang, 1980; Su et al., 1984). ACC levels decline after the climacteric peak, but rise again at later stages (Hoffman and Yang, 1980). MACC follows a similar pattern, although this metabolite has not yet been profiled during postharvest stages to our knowledge. Met, on the other hand, was rarely investigated in relation to ethylene biosynthesis. Metabolome analysis showed that Met levels increase during tomato fruit ripening (Carrari and Fernie, 2006; Oms-Oliu et al., 2011). To our knowledge, no information is available on SAM and MTA profiles in ripening fruit.
In this study, we aimed to investigate fruit development, climacteric ripening, and postclimacteric development during postharvest storage of tomato by means of a targeted systems biology approach. Our intent was to integrate metabolic, enzymatic, and transcriptomic profiles to characterize the regulation of the ethylene biosynthesis pathway including the Yang cycle. The current understanding of preclimacteric and climacteric fruit ripening (mainly confirming literature) is expanded and linked to new findings on the poorly investigated postclimacteric phase of ethylene biosynthesis. This approach revealed a novel regulatory mechanism for ethylene biosynthesis during the postclimacteric ripening of tomato fruit.
RESULTS
Fruit Physiology and Quality
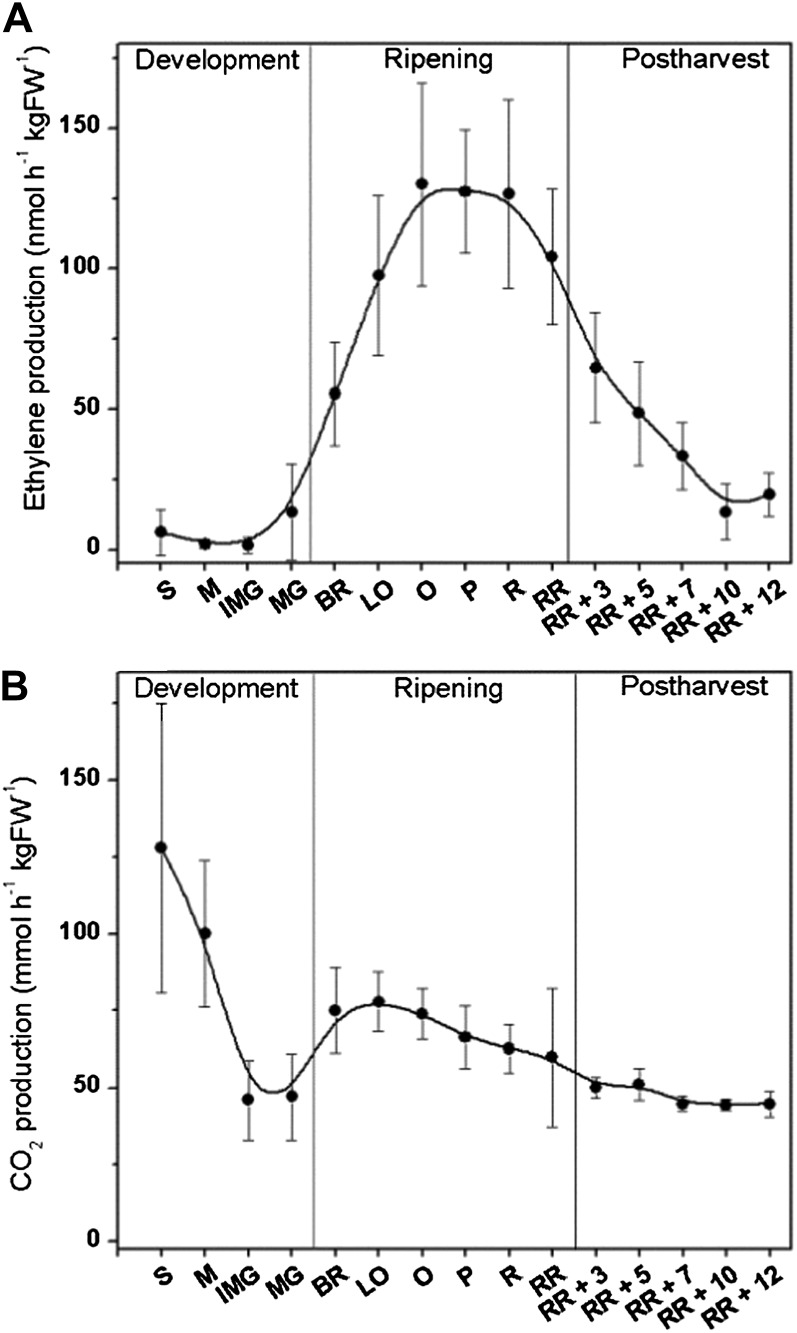
Various physiological parameters were measured to obtain a representative data set describing fruit development, climacteric ripening, and postharvest storage. Straight after sampling, fruit ethylene production and respiration rates were measured together with color and firmness, two important fruit quality parameters. Overall, fruit ethylene production showed a typical climacteric behavior with low ethylene production during development (system 1) and a climacteric rise during ripening (system 2; Fig. 2A). During postclimacteric development ethylene production decreased close to, without quite reaching, the basal level. A typical high respiration rate was observed for very small developing fruit that declined to a basal level at the immature green stage. Fruit respiration rate subsequently showed a distinct increase at the onset of fruit ripening and declined again during further ripening and storage (Fig. 2B). Fruit color showed a logistic shift from green to red during ripening (Supplemental Fig. S1A). Fruit firmness measurements showed that softening began at the breaker stage and continued until the red stage was reached (Supplemental Fig. S1B).
Figure 2.
Ethylene production and respiration rate during different tomato fruit development stages. A, In vivo ethylene production (nmol h−1 kg FW−1) and (B) in vivo CO2 production (mmol h−1 kg FW−1) during tomato fruit development, ripening, and postharvest storage. Error bars represent the sd of the mean of 10 biological replicates. S, Small-sized fruit; M, medium-sized fruit; IMG, immature green fruit; MG, mature green fruit; BR, breaker fruit; LO, light orange fruit; O, orange fruit; P, pink fruit; R, red fruit; RR, red ripe fruit; RR + 3, 3 d post harvest; RR + 5, 5 d post harvest; RR + 7, 7 d post harvest; RR + 10, 10 d post harvest; RR + 12, 12 d post harvest; FW, fresh weight.
Metabolic Profiling Reveals that ACC Is Not Limiting for Postclimacteric Ethylene Production
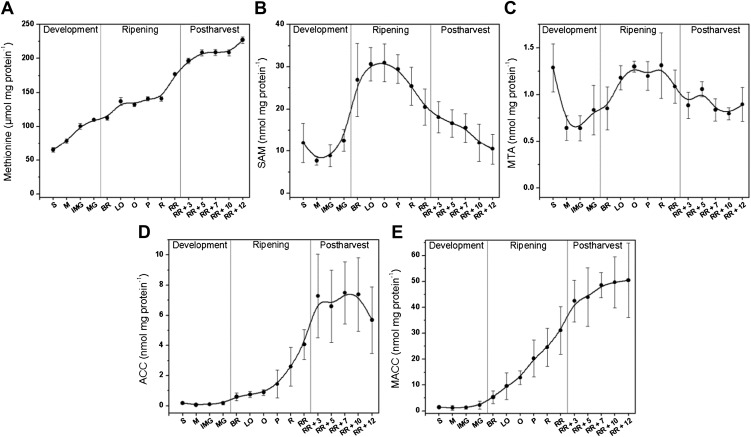
The relation between ethylene production and intermediate metabolites was further investigated by metabolic profiling. Free Met (Fig. 3A) continuously accumulated throughout fruit development and postharvest storage. Met is a general metabolite that appeared not to be limiting because it was present at a micromolar concentration compared with the nanomolar concentration of other metabolites. SAM and MTA profiles have not yet been previously published for tomato. The SAM profile (Fig. 3B) showed a clear correlation with ethylene production: low during fruit development, followed by a drastic increase during ripening and a steady decline during the postclimacteric stages. SAM levels began to increase in parallel with ethylene production but reached maximal levels at the breaker stage prior to the ethylene maximum, which was reached at the orange stage. MTA followed a similar profile like SAM, albeit less pronounced and around 20 times lower in concentration (Fig. 3C).
Figure 3.
Metabolic profiles during different tomato fruit development stages. A, Met (µg mg protein−1); B, SAM (nmol mg protein−1); C, MTA (nmol mg protein−1); D, ACC (nmol mg protein−1), and E, MACC (nmol mg protein−1) content during tomato fruit development, ripening, and postharvest storage. Error bars represent the sd of the mean of 10 biological replicates. See Figure 2 for maturity stage annotations.
ACC and MACC metabolic profiles were similar to previously published results (Fig. 3, D and E), with low levels during preclimacteric development and a strong increase during climacteric ripening (Hoffman and Yang, 1980; Su et al., 1984). During postharvest storage, ACC content leveled off, whereas MACC content slowly continued to increase. MACC content was about 60 times higher than ACC content and steadily increased, indicating that MACC is most probably an end product. In conclusion, the high ACC content during the postclimacteric stages suggests that ACC content was not limiting for ethylene biosynthesis during these stages.
Enzymatic Profiling Reveals that ACO Catalyzes the Rate-Limiting Step during Postclimacteric Ethylene Biosynthesis
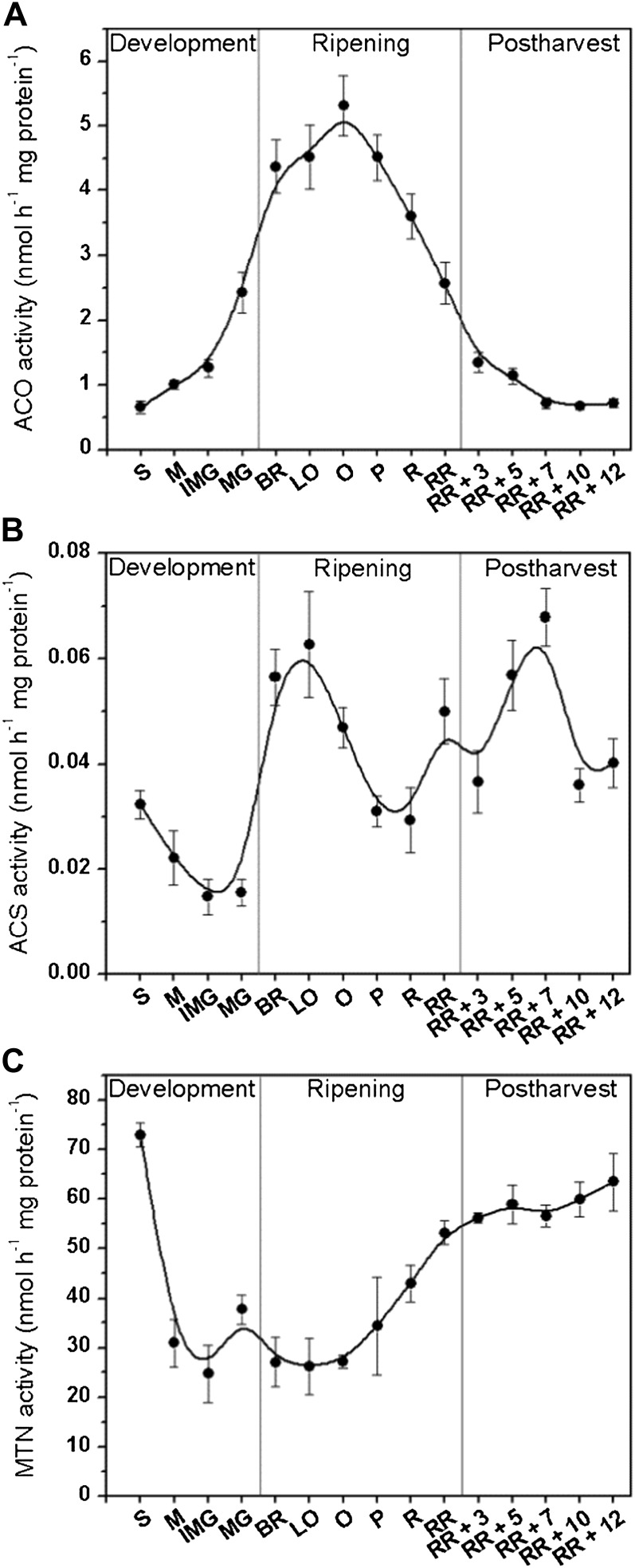
To relate metabolic changes to key enzymes of ethylene biosynthesis, we determined the in vitro enzyme activities of ACO, ACS, and MTN during fruit development, ripening, and postharvest storage (Fig. 4). Large differences in absolute activity values were observed between ACS and the other enzymes analyzed. ACS is known to have a low abundance and stability. Together with the tedious extraction procedure, this might have caused the low in vitro activity. During preclimacteric development, ACO activity increased whereas ACS activity decreased, supporting the general observation that ACS is the rate-limiting enzyme for ethylene biosynthesis. Once the fruit started to ripen, ACO and ACS activities increased 2- to 3-fold. At the light orange to orange stage, when ethylene production had reached its maximum, ACO and ACS activities both declined. Surprisingly, ACS activity rose again at the end of ripening and during subsequent postharvest storage, whereas ACO activity dropped during storage, almost to the low activity observed in preclimacteric fruit. Reduced ACO activity and a sustained activity of ACS resulted in the accumulation of ACC (Fig. 3D). This strongly suggests that ACO is rate limiting for ethylene biosynthesis during the postclimacteric stages.
Figure 4.
Enzyme activities of ACO, ACS, and MTN during different tomato fruit development stages. A, In vitro ACO enzyme activity (nmol h−1 mg protein−1); B, in vitro ACS enzyme activity (nmol h−1 mg protein−1); and C, in vitro MTN enzyme activity (nmol h−1 mg protein−1) during tomato fruit development, ripening, and postharvest storage. Error bars represent the sd of the mean of 10 biological replicates. See Figure 2 for maturity stage annotations.
MTN activity was high during early fruit development but rapidly dropped to an intermediate level during preclimacteric ethylene production and remained low during climacteric ripening (Fig. 4C). The activity rose again at the pink to red ripe stage, just prior to the decrease in MTA levels (Fig. 3C). This delay may indicate a feed-forward activation of MTN by MTA. During postharvest storage, MTN activity reached a maximum similar to ACC and MACC. All in all, MTN activity shows no direct correlation with climacteric ethylene production but correlated well with ACC/MACC synthesis during the postclimacteric stages.
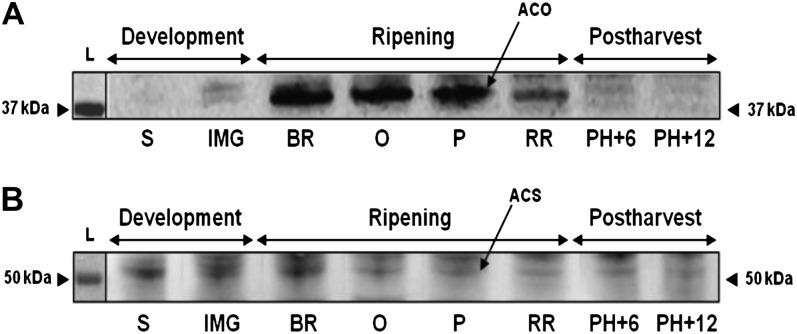
Western-Blot Analysis Confirms Both ACO and ACS Activity
To study the regulation of ethylene biosynthesis at the protein level, ACO and ACS protein content was visualized by western blotting (Fig. 5). The antibodies used were designed to recognize a consensus peptide epitope of four tomato ACO isoforms (ACO1 to -4) and of four tomato ACS isoforms (ACS1, ACS2, ACS4, and ACS6). The ACO western blot showed a clear band just above the 37-kD marker corresponding to the predicted masses of all tomato ACO isoforms. During early fruit development, hardly any ACO protein was detected, although a very faint band was visible prior to ripening. At the onset of ripening, ACO content increased toward a maximum around the breaker to pink stage. From the red ripe stage onward, ACO protein levels decreased again. Abundance of the 37-kD ACO protein thus correlated well with high ACO activity observed during fruit ripening (Fig. 4A).
Figure 5.
Western-blot analysis of (A) ACO and (B) ACS protein content during tomato fruit development, ripening, and postharvest storage. The 37- and 50-kD markers are visualized by the arrow heads next to the ladder (L). See Figure 2 for maturity stage annotations. kDa, kD.
Western-blot analysis of ACS showed a faint band just above 50 kD, corresponding to the mass of ACS (Fig. 5B). The weak signal is probably caused by the low ACS abundance in tomato pericarp and the weak affinity of the anti-ACS antibodies. An unspecific band, just slightly higher than the ACS band, blurs the signal. Nevertheless, ACS appeared to be present during early fruit development with the highest signal intensity observed at the breaker stage corresponding to the high ACS activity measured at this stage (Fig. 4B). During further ripening, the ACS protein level remained constant and was still clearly detectable during the postclimacteric stages when high ACS activity was observed. Overall changes in ACS protein abundance were similar to the changes observed for ACS activity.
Transcriptional Profiling Reveals Similarities between ACS and Yang Cycle Gene Expression
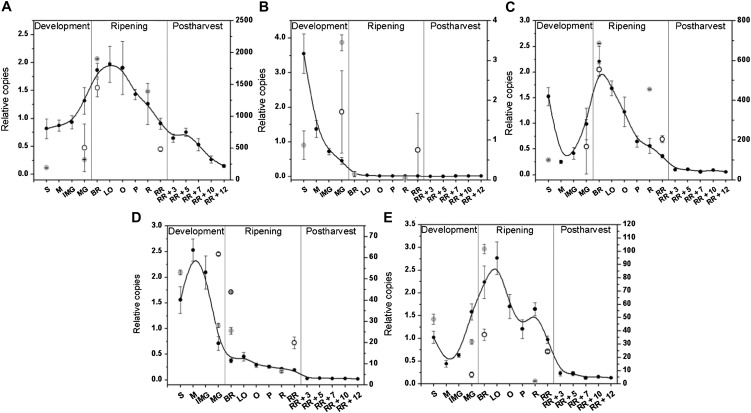
The burst of ethylene synthesis is a hallmark of climacteric tomato fruit ripening. Surprisingly, postclimacteric development with low ethylene production was accompanied by high ACS activity and abundance and an accumulation of ACC. To better understand the regulation of ethylene biosynthesis and Met recycling at the transcriptional level, expression profiles of ACO, ACS, SAMS, and Yang cycle genes were studied throughout tomato fruit development, ripening, and postharvest storage by real-time quantitative PCR (qPCR). These expression profiles were compared with the recently obtained RNA sequencing data of the Tomato Genome Consortium for tomato fruit of the same maturity stages for both the greenhouse cultivar Heinz 1706 and the old species Solanum pimpinellifolium (Tomato Genome Consortium, 2012). Figure 6 shows the expression profiles of all known ACO isoforms in tomato. The results confirm previous reports on ACO expression patterns visualized by northern blotting but also revealed some differences (Barry et al., 1996; Nakatsuka et al., 1998). ACO2 and ACO4 transcript levels are highest during system 1, whereas in a previous report ACO2 transcripts were not detected (Barry et al., 1996). ACO1 was moderately expressed during preclimacteric development. During the transition stage ACO1 and ACO3 transcript levels increased, whereas the expression levels of ACO2 and ACO4 dropped. ACO1 was the only ACO gene still expressed at elevated levels in the postclimacteric stage, likely contributing to the low rate of ethylene synthesis observed in these fruit. In parallel with ACO1 expression, ACO abundance (Fig. 5A), ACO activity (Fig. 4A), and ethylene production (Fig. 2A) continuously declined during fruit storage. ACO3 expression dropped from the light orange stage on to a very low level that was maintained during postharvest storage. The expression level of ACO4 was very low during ripening, contrary to the report by Nakatsuka et al. (1998). The yet uncharacterized expression profile of ACO5 showed a similar expression pattern as ACO3 with elevated levels during ripening. In general, the RNA sequencing data matched the expressions profiles for all ACO isoforms.
Figure 6.
Gene expression (relative copies) of ACO1 (A), ACO2 (B), ACO3 (C), ACO4 (D), and ACO5 (E) during fruit development, ripening, and postharvest storage. Error bars represent the sd of the mean of six biological replicates. See Figure 2 for maturity stage annotations. For comparison, normalized RNA sequencing data (secondary y axis) for defined maturity stages from cv Heinz 1706 (light gray circles) and S. pimpinellifolium (open circles) were included.
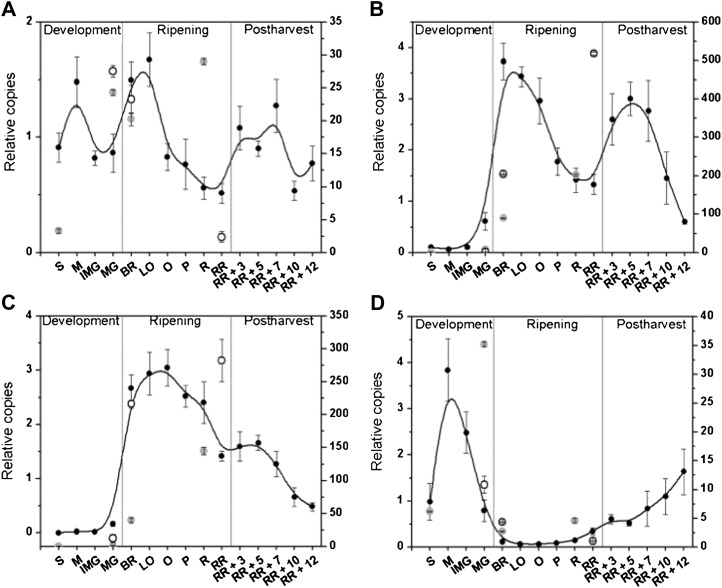
Figure 7 shows the expression profiles of the ACS gene family. The expression profile of ACS1 (A and B together) fluctuated throughout fruit development. During preclimacteric development, ripening, and again during postharvest storage, ACS1 was transiently up-regulated. Transcripts of ACS2 and ACS4 were not detected during initial development, but expression strongly increased during the autocatalytic ethylene production phase of system 2. Expression of both genes peaked during the breaker stage along with the peak in ACS abundance (Fig. 5B) and activity at this stage (Fig. 4B). ACS2 and ACS4 transcript levels declined during further ripening and peaked again during the postclimacteric stages matching ACS activity. ACS6 was expressed during early fruit development, but mRNA levels quickly decreased. During ripening, ACS6 was not expressed, but transcript levels gradually increased during postharvest storage.
Figure 7.
Gene expression (relative copies) of ACS1 (A), ACS2 (B), ACS4 (C), and ACS6 (D) during fruit development, ripening, and postharvest storage. Error bars represent the sd of the mean of six biological replicates. See Figure 2 for maturity stage annotations. For comparison, normalized RNA sequencing data (secondary y axis) for defined maturity stages from cv Heinz 1706 (light gray circles) and S. pimpinellifolium (open circles) were included.
Transcripts of the other four ACS members (ACS3, ACS5, ACS7, and ACS8) were not detected at any stage. A phylogenetic relationship analysis of all ACS genes in tomato revealed that the fruit ripening-related ACS genes group together, whereas the remaining four nonfruit-ripening specific members that were not expressed also grouped (Supplemental Fig. S2). The RNA sequencing data matched the qPCR expression profiles for all ACS genes and confirmed high expression of ACS2 and ACS4 at the red stage. No RNA reads were found for ACS3, ACS5, ACS7, and ACS8.
It has been proposed that high rates of ethylene biosynthesis in climacteric fruit are supported by recycling of the ethylene precursor Met via the Yang cycle (Baur and Yang, 1972). To test this hypothesis, we analyzed transcript abundance of the known Yang cycle genes MTN, MTK, ARD1, ARD2, MTI, and DEP in relation to ethylene biosynthesis in tomato fruit (Fig. 8). MTN, MTK, ARD1, and ARD2 showed a coordinated expression pattern. During fruit development, expression levels were low and increased just prior to ripening at the mature green stage. Around the orange stage, transcript levels dropped slightly before increasing to even higher levels during postharvest storage. These expression profiles showed a striking similarity to the expression profiles of ACS1, ACS2, and ACS4 and to ACS activity. In contrast, the recently identified phloem-specific Yang cycle genes DEP and MTI (Pommerrenig et al., 2011) did not show a comparable pattern. Both genes were mainly expressed in young developing fruit and showed a moderate constitutive expression during ripening and postharvest storage. Again the RNA sequencing data confirmed the expression profiles for all Yang cycle genes except for MTN, for which no RNA reads were found, although the qPCR results provided in this study show a strong up-regulation.
Figure 8.
Gene expression (relative copies) of Yang cycle genes MTN (A), MTK (B), ARD1 (C), ARD2 (D), DEP (E), and MTI (F) during fruit development, ripening, and postharvest storage. Error bars represent the sd of the mean of six biological replicates. See Figure 2 for maturity stage annotations. For comparison, normalized RNA sequencing data (secondary y axis) for defined maturity stages from cv Heinz 1706 (light gray circles) and S. pimpinellifolium (open circles) were included.
Although SAMS is not exclusively used for ethylene synthesis, the production of SAM from Met is an important requirement in the ethylene pathway. Four SAMS genes (SAMS1–SAMS4) were identified in tomato, for which the expression profiles during fruit development are shown in Supplemental Figure S3. SAMS1 and SAMS4 showed a similar profile with only a high expression during fruit development. SAMS2 and SAMS3 were also highly expressed during fruit development, but SAMS2 was also up-regulated during postharvest storage. It is worth mentioning that none of the SAMS genes were expressed at high levels during climacteric ripening, although SAM levels peaked during these stages. The RNA sequencing data also matched the SAMS expression profiles in general. Despite technical and cultivar differences, the RNA sequencing data of the Tomato Genome Consortium (2012) largely confirmed all measured expression profiles. This indicates that the observed profiles are representative for modern greenhouse cultivars (cv Bonaparte and Heinz 1706) as well as for the old species S. pimpinellifolium, and likely for tomato fruit in general.
Effect of Harvest on Ethylene Biosynthesis
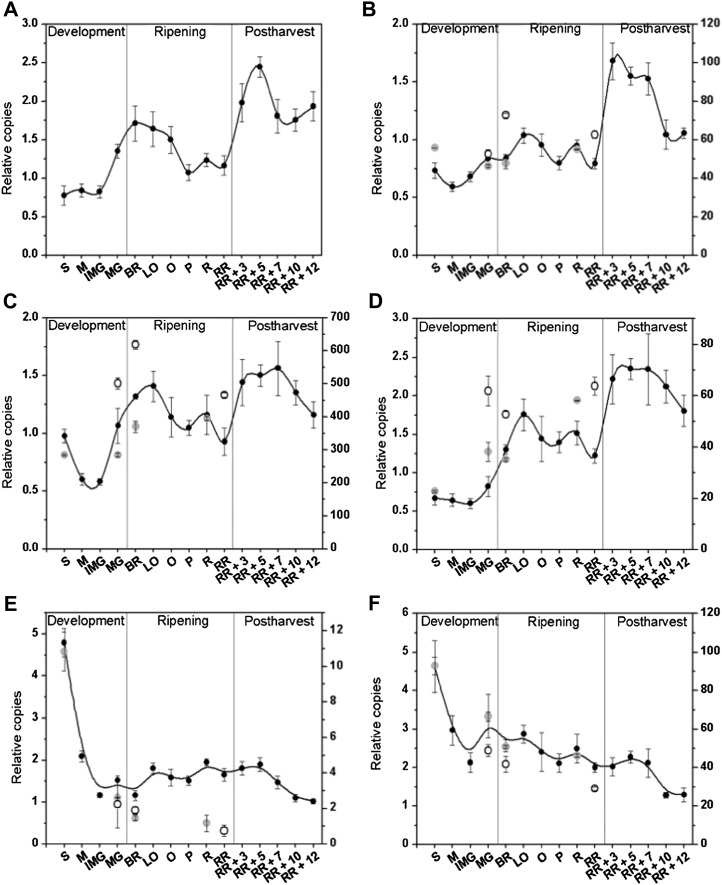
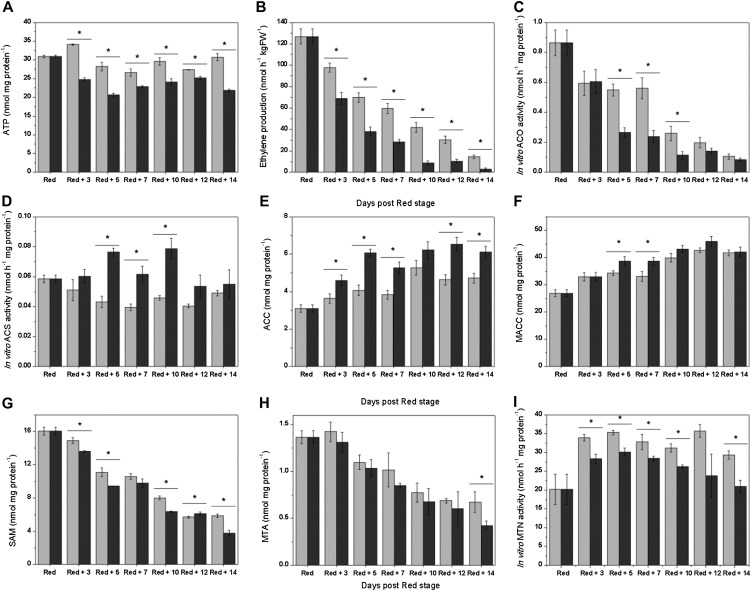
When fruit have reached their red ripe stage and ethylene biosynthesis declines, ACS activity and expression, together with the expression of Yang cycle genes, increased. As these changes coincided with harvest, we decided to investigate whether they occurred as a result of fruit detachment. Figure 9 shows the effect of harvesting red tomatoes on fruit ethylene production, related enzyme activities, and metabolites. Detached red fruit showed a reduced level of free ATP (Fig. 9A), indicating that energy supply was more limiting in detached fruit. Ethylene profiles for both attached and detached fruit showed the characteristic decline in ethylene production for postclimacteric fruit (Fig. 9B). However, fruit left on the plant had a significantly higher ethylene production rate than fruit removed from the vine. This was also reflected by a higher ACO activity (Fig. 9C). It is interesting to note that red fruit kept on the plant did not show an increase in ACS activity, as was observed in detached fruit (Fig. 9D). The increase in ACS activity, together with a lower consumption by ACO, resulted in an ACC content that was significantly higher in detached fruit as compared with attached fruit (Fig. 9E). Differences in MACC levels were also observed, albeit less pronounced (Fig. 9F). As a consequence of the increased ACS activity in detached fruit, SAM levels were slightly lower (Fig. 9G). MTA levels, on the other hand, did not differ significantly (Fig. 9H). Although one would expect a higher MTN activity to cope with the additional MTA coming from the high ACS activity, MTN activity was significantly lower in detached fruit (Fig. 9I). Perhaps other pathways (e.g. polyamine biosynthesis) might have reduced their MTA input because of the detachment. Nonetheless, the findings indicated that MTA levels are tightly regulated, independent of harvest. Taken together, these results showed that harvesting red tomatoes triggers ACS activity and, at the same time, inhibits ACO activity with a concomitant effect on ethylene biosynthesis metabolites.
Figure 9.
Metabolic and enzymatic profiles of red tomatoes harvested and stored off the plant (dark gray) and red tomatoes kept on the plant (light gray) for 14 d. A, ATP content (nmol mg protein−1). B, Ethylene production (nmol h−1 kg FW−1). FW, Fresh weight. C, ACO in vitro activity (nmol h−1 mg protein−1). D, ACS in vitro activity (nmol h−1 mg protein−1). E, ACC content (nmol mg protein−1). F, MACC content (nmol mg protein−1). G, SAM content (nmol mg protein−1). H, MTA content (nmol mg protein−1). I, MTN in vitro activity (nmol h−1 mg protein−1). Error bars represent the sd of the mean of 10 biological replicates. Statistically significant differences (P < 0.05) between attached and detached fruit are indicated with an asterisk.
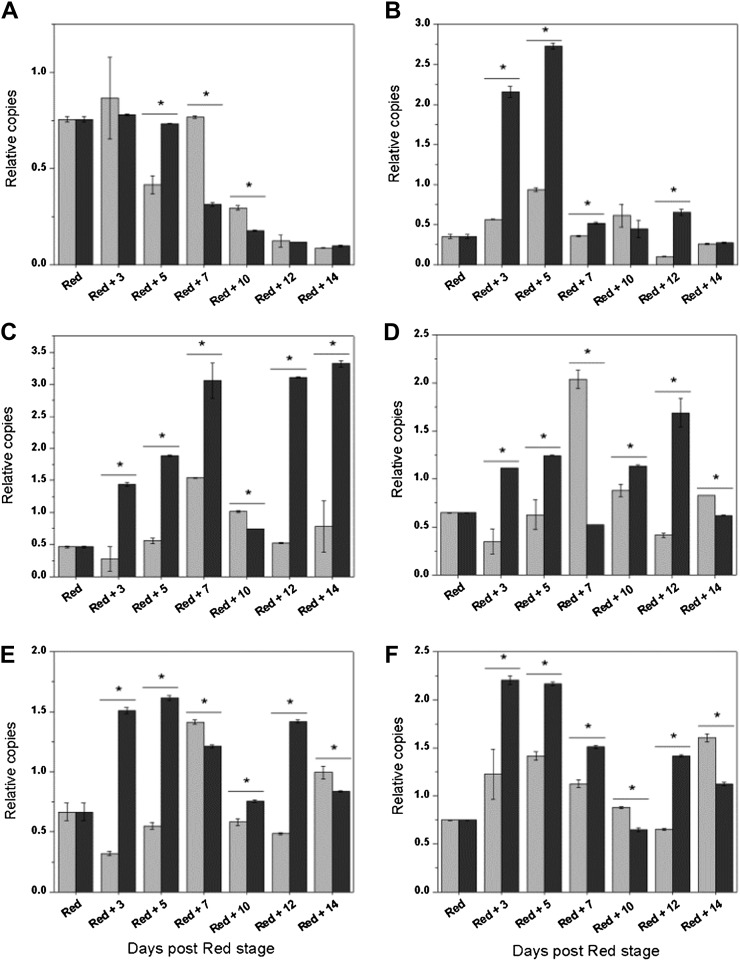
Figure 10 shows expression profiles of some ACO, ACS, and Yang cycle genes. ACO1 expression was slightly higher in attached fruit concomitant with elevated ethylene production of fruit on the vine (Fig. 10A). Both attached and detached fruit showed up-regulation of ACS2 within 5 d after harvest, but this was more pronounced in detached fruit (Fig. 10B). Expression of MTN, MTK, ARD1, and ARD2 was also up-regulated in postclimacteric fruit (Fig. 10, C–F). Again, tomatoes that were stored off the plant showed a more pronounced increase of transcripts. Note that the higher MTN expression in detached fruit did not result in an increased MTN activity (Fig. 9D). Overall, these profiles suggest that harvesting red fruit promotes ACS activity through an elevated expression of ACS2 and inhibits ACO activity and ethylene production. At the same time, the expression of Yang cycle genes is elevated, activating the recycling part of the ethylene pathway likely to metabolize the ACS side-product, MTA.
Figure 10.
Gene expression (relative copies) profiles of ACO1 (A), ACS2 (B), MTN (C), MTK (D), ARD1 (E), and ARD2 (F) for red tomatoes harvested and stored off the plant (dark gray) and red tomatoes kept on the plant (light gray) for 14 d. Error bars represent the sd of the mean of six biological replicates. Statistical significant differences (P < 0.05) between attached and detached fruit are indicated with an asterisk.
Ethylene Sensitivity Is Lost during Postclimacteric Fruit Development
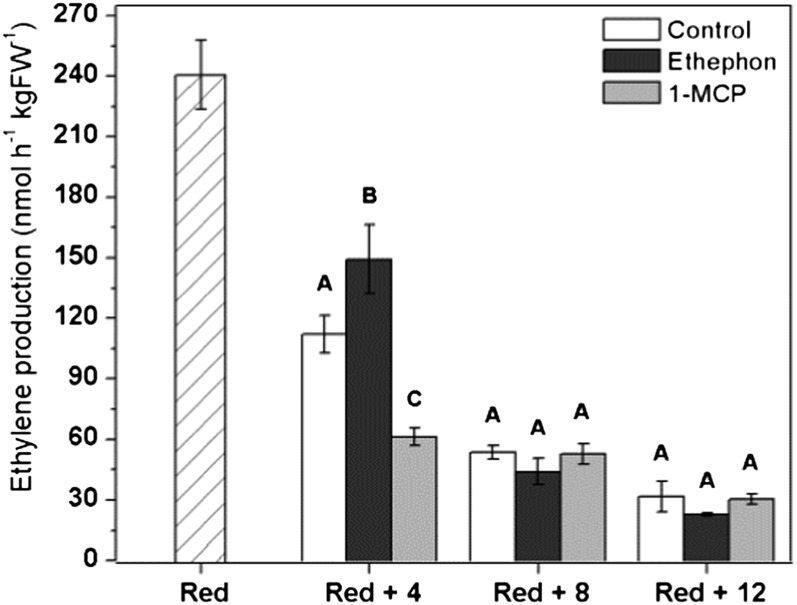
System 1 and system 2 have previously been characterized as autoinhibitory and autocatalytic mechanisms to regulate ethylene biosynthesis. To test whether postclimacteric tomatoes with a declining ethylene production are susceptible to ethylene-dependent feedback regulation, experiments were carried out with exogenously applied ethylene and with 1-methylcyclopropane (1-MCP), an inhibitor of ethylene perception. Application of the ethylene-releasing compound ethephon to red fruit transiently increased ethylene production within 4 d whereas 1-MCP transiently reduced ethylene biosynthesis compared with control fruit (Fig. 11). At 8 d and later, control fruit had an equal ethylene production compared with treated fruit, suggesting only a temporal effect. The overall decline in ethylene production observed in the postclimacteric stage could not be overcome by any of the treatments, indicating that down-regulation of ethylene biosynthesis after fruit maturation was developmentally programmed and only moderately ethylene sensitive.
Figure 11.
Ethylene production (nmol h−1 kg FW−1) of red ripe harvested fruit was assessed for 12 d after 1-MCP and ethephon treatments for 24 h at 18°C. Error bars represent the sd of the mean of 10 biological replicates. Statistically significant differences (P < 0.05) between treatments are indicated with A, B, and C. FW, Fresh weight.
PCA Reveals Profile Similarities
To further analyze this large and complex data set, principal component analysis (PCA) was performed on all parameters measured. The correlation loadings plot of the first two PCs is shown in Supplemental Figure S4. PC1 explained 33%, and PC2 explained 25% of the total variance. Some variables were closely clustered, indicating that those metabolites/proteins/genes showed a similar profile. ACC and MACC grouped and were associated with postharvest storage. Fruit firmness and respiration (CO2) were associated more with small developing fruit. The PCA also confirmed that the Yang cycle genes (MTN, MTK, ARD1, and ARD2) correlated well with ACS activity. ACO activity correlated with the expression of ACO1, ACO3, and ACO5 whereas ethylene production correlated strongly with SAM and MTA content and with ACS2 and ACS4 expression, and was associated mainly with breaker to orange fruit.
DISCUSSION
Regulation of Postclimacteric Ethylene Biosynthesis Is Distinct from System 1 and System 2 Ethylene Production
A targeted systems biology approach allowed us to characterize the ethylene biosynthesis pathway and the Yang cycle of developing tomato fruit in depth. Based upon the results described above and previous findings from literature, the regulation of postclimacteric ethylene biosynthesis was found to be different from the autocatalytic phase (system 2) and different from the autoinhibitory phase (system 1). The postclimacteric ethylene production is characterized by a gradual decline in ethylene biosynthesis when pink-red fruit have reached their maximum in autocatalytic ethylene production. It is essential that tomato fruit stop the autocatalytic rise when the ripening process is nearly accomplished. Additional ethylene from this point on will enhance senescence and fruit decay. It may be advantageous that red fruit delay their senescence phase to maximize the time available for seed dispersal. The exact mechanism by which autocatalytic ethylene production is shut down is only poorly understood. We found that the regulatory mechanisms by which ethylene biosynthesis is shut down operate at the level of ACO1 transcript and ACO protein abundance. On the other hand, ACS gene expression and activity increase again during postharvest storage of tomato. Enhanced ACS activity, together with reduced ACO activity, results in an accumulation of ACC indicating that ACO is the rate-limiting step of postclimacteric ethylene biosynthesis.
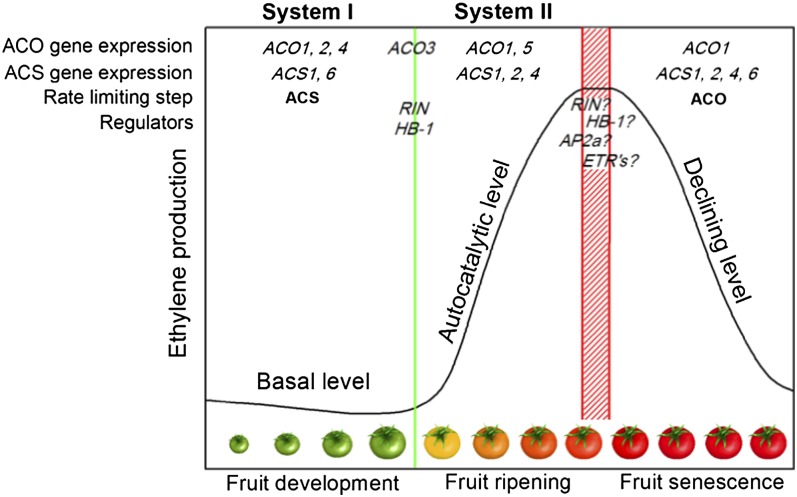
How ACO and ACS expression is controlled during the postclimacteric phase remains unknown. Elucidation of the initiation of fruit ripening and the associated climacteric ethylene production can provide some clues. A few key transcriptional regulators were identified as essential activators of the ripening process. RIN-MADS is a MADS-box transcription factor that binds to the promoter of ACS2, ACS4, and other ripening-associated genes. Therefore, RIN is a crucial and early regulator of fruit ripening (Vrebalov et al., 2002; Fujisawa et al., 2011). Perhaps ACS2 expression during postclimacteric ripening could be induced by a sustained activity of RIN-MADS. HB-1 encodes a homeodomain-zip homeobox transcription factor that directly interacts with the promoter of ACO1 and is responsible for the activation of ACO1 expression during ripening (Lin et al., 2008). Loss of HB-1 activity might terminate autocatalytic ethylene production by reducing ACO1 expression. If and how these regulators shut down ethylene biosynthesis remains to be investigated. It is also plausible that ethylene response factors (ERFs) are responsible for the direct (interaction with ACO and/or ACS promoter regions, like ERF2) or indirect (through deactivating the expression of the regulators mentioned above) shut down of ethylene biosynthesis. The ERF gene AP2a was identified as a negative regulator of ethylene biosynthesis and was also found to be ethylene induced (Karlova et al., 2011). Repression of AP2a expression resulted in an overproduction of ethylene (Chung et al., 2010; Karlova et al., 2011). Down-regulation of ethylene biosynthesis by ethylene-induced ERFs would mean that ethylene turns down its own production during postclimacteric ripening, similar to system 1 and system 2 ethylene biosynthesis. An overview summarizing the main ethylene-related features of system 1 and system 2 and of the regulatory mode of postclimacteric ripening described here is given in Figure 12 and Supplemental Table S1.
Figure 12.
Illustration of the main characteristics of tomato fruit development, climacteric ripening, and postclimacteric ripening (postharvest storage) with respect to ethylene biosynthesis.
The Termination of Climacteric Ethylene Production Is Developmentally Programmed
Mature green tomatoes treated with 1-MCP show a reduced climacteric ethylene production and do not ripen like untreated fruit (Hoeberichts et al., 2002; Tassoni et al., 2006). When treated with ethylene, fruit show an increased ethylene production and ripen faster (Sims, 1969). With either treatment, climacteric ethylene biosynthesis was severely altered. By contrast, postclimacteric red fruit that were treated with ethephon or 1-MCP did not show such a pronounced alteration in ethylene production. The decline in ethylene production was not overcome by ethephon nor inhibited by 1-MCP, except for a temporal and minor effect 4 d after treatment. This temporal effect of 1-MCP-treated red ripe tomatoes was also observed by Hoeberichts et al. (2002). How the change in ethylene sensitivity is achieved in postclimacteric tomatoes remains to be elucidated. Ethylene sensitivity is inversely proportional to the amount of ethylene receptors (Hua and Meyerowitz, 1998; Tieman et al., 2000). This means that a high receptor abundance lowers ethylene sensitivity and vice versa. During climacteric ripening of tomato, the expression of ethylene receptors NR (ETR3), ETR4, and ETR6 is induced (Lashbrook et al., 1998, Kevany et al., 2007). Receptor abundance may also be regulated at the level of protein stability (Kevany et al., 2007). However, it remains to be investigated if receptor abundance is key to the reduced ethylene sensitivity observed during postclimacteric development. Regulation at other ethylene signaling components is also conceivable. In any case, the decline in ethylene biosynthesis observed during postclimacteric ripening appears to be developmentally programmed and only moderately influenced by ethylene signaling.
The Yang Cycle Genes Are Coordinately Expressed and Show a Distinct Up-Regulation during Postclimacteric Ripening
The Yang cycle genes MTN, MTK, ARD1, and ARD2 showed very similar expression profiles throughout tomato fruit development, ripening, and postharvest storage. By contrast, MTI and DEP were mainly expressed in a constitutive manner. Arabidopsis AtMTI and AtDEP were described as being specifically expressed in the phloem (Pommerrenig et al., 2011). It was shown that the vascular bundle tissue of tomato fruit pericarp showed a more complex transcriptome profile compared with overall pericarp tissue, as used in this study (Matas et al., 2011). Possibly tomato fruit pericarp possesses other enzymes that catalyze these steps of the Yang cycle than the ones analyzed, although a database search did not reveal additional homologous genes. Perhaps certain Yang cycle metabolites are being translocated between the pericarp tissue and vascular cells for further processing. Recently, it was shown in the Arabidopsis double mtn/mtk mutant that efficient recycling of MTA by the Yang cycle is essential for vascular tissue development (Waduwara-Jayabahu et al., 2012).
The gene expression profiles of MTN, MTK, and ARD show a basal expression during preclimacteric fruit development and an up-regulation with the onset of ethylene production at the mature green stage. This is in accord with the long-proposed function of the Yang cycle to preserve the reduced sulfur of MTA, the by-product of ACS during high rates of ethylene biosynthesis (Baur and Yang, 1972; Bürstenbinder et al., 2007). Nevertheless, these Yang cycle genes show their highest expression during the postclimacteric phase when ethylene production continuously declined. High Yang cycle activity coincides with the up-regulation of ACS genes and ACS enzyme activity. As only little ethylene is produced postclimacteric, the signal that controls the Yang cycle may come from the ACS products ACC or MTA. It was previously described that MTN1 protein abundance and MTN enzyme activity in Arabidopsis were induced by MTA (Bürstenbinder et al., 2010). In rice (Oryza sativa), it was shown that OsMTN expression and MTN activity increased in the youngest internode upon submergence, also linking it to ethylene and ACS (Rzewuski et al., 2007). However, early experiments showed that breaker tomato pericarp discs infiltrated with the ACS activity inhibitor aminoethoxyvinyl-Gly did not alter MTN activity (Wang et al., 1982). Independent of ethylene synthesis or ACS activity, it appears to be crucial to maintain MTA levels within a narrow concentration range. It was shown that loss of MTA metabolism in Arabidopsis mtn knockout plants causes morphological disorders in Arabidopsis (Waduwara-Jayabahu et al., 2012). This evidence points toward a crucial role for MTA in regulating the initial step of the Yang cycle. However, it still remains to be elucidated how this is achieved and how MTK and ARD genes are coordinately regulated. One possibility would be that their respective substrates accumulate with higher MTN activity resulting in an independent, yet coordinated, regulation of the Yang cycle. Alternatively, a common transcription factor could be activated that coordinately regulate MTN, MTK, ARD1, and ARD2 genes in tomato fruit. The data presented here clearly indicate that ethylene is not the crucial signal to regulate the Yang cycle as originally stated by Baur and Yang (1972). Rather, the regulation of the Yang cycle in tomato pericarp is linked to ACS activity and/or MTA levels.
Fruit Detachment Induces ACS and Inhibits ACO
Detachment of fruit from the mother plant is a serious intervention. Fruit are cut off from nutrition and water, causing stress. Resources and energy need to be preserved as the fruit suddenly has to switch from a heterotrophic metabolism to an autotrophic metabolism. This will ultimately result in a lower content of free ATP, as observed in detached tomato fruit. Previous studies demonstrated that detached preclimacteric tomato fruit have a normal climacteric ethylene production rate (Nakatsuka et al., 1997). Preclimacteric harvested apples show a higher ethylene production rate compared with attached fruit (Sfakiota and Dilley, 1973; Lin and Walsh, 2008) whereas harvested melon (Cucumis melo) fruit did the opposite (Bower et al., 2002). Little information is available about ethylene production in fruit detached post ripening. Our results show that the decline in ethylene production during postclimacteric ripening is enhanced when the fruit is harvested. This reduction is caused by an inhibition of both ACO expression and activity. Despite of a reduced ACO activity, ACS expression and activity increase following detachment. It is known that local wounding of the calyx and fruit water stress by dehydration can induce ACS expression in detached persimmon fruit (Nakano et al., 2003). Harvested tomato fruit are also confronted with water loss during storage (Hertog et al., 2004), which might in turn induce ACS expression and activity. Or perhaps another “tree factor” is involved (Sfakiota and Dilley, 1973). Along with ACS activity, expression of Yang cycle genes also increased in detached fruit. This is in accord with the view that regulation of the Yang cycle is closely linked to ACC/MTA production.
CONCLUSION
A targeted systems biology approach revealed a novel mode of regulation of ethylene biosynthesis, which sets in when tomato fruit have reached their climacteric maximum around the pink-red stage. It is characterized by a decline in ethylene production that appears to be developmentally programmed and that is regulated at the level of ACO. Harvest of red tomatoes hastens the decline in ethylene production and at the same time promotes the expression and activity of ACS. Increased ACS activity causes an influx of MTA into the Yang cycle, which is highly active during postclimacteric ripening, revealing a close link between ACC synthesis and Yang cycle regulation. Finally, we observed that Met recycling from MTA is ensured by coordinated regulation of Yang cycle genes.
MATERIALS AND METHODS
Plant Material
Tomato plants (Solanum lycopersicum ‘Bonaparte’) were grown in a greenhouse at the Research Station for Vegetable Production of Hoogstraten (Belgium). Plants were cultivated hydroponically on rock wool substrate under natural daylight with controlled humidity (70%) and temperature (23°C/21°C d/night). Fruit of different developmental and ripening stages were harvested (August 2009), transported to the lab, analyzed for ethylene production and fruit quality attributes (color and firmness), and further processed the same day (Van de Poel et al., 2012). Ten independent fruits of each developmental stage were selected, and pericarp tissue of individual fruit was flash frozen in liquid nitrogen, crushed with a Grindomixer (Retsch) and stored at −80°C. Subsequently, a postharvest experiment was conducted by harvesting red ripe tomato fruit and storing them for 12 d at shelf life conditions (18°C and 80% relative humidity) with regular sampling (10 fruit each time) as described above.
The Effect of Harvest on Ethylene Biosynthesis
In a separate experiment, the effect of harvest on ethylene biosynthesis was investigated by comparing red ripe tomato fruit attached and detached from the plant in time (June 2010). For the detached fruit, red ripe tomatoes were harvested from the greenhouse (as described above) and stored for 14 d at shelf life conditions (18°C and 80% relative humidity). At the same time, red ripe fruit that remained attached to the vine were marked and kept on the plant for the same 14 d. Both attached and detached fruit were sampled simultaneously several times during the same storage period (10 independent fruit for each sampling time/treatment). Ethylene production was assessed the same day of sampling and pericarp tissue of individual fruit was subsequently flash frozen in liquid nitrogen, crushed, and stored at −80°C.
1-MCP and Ethephon Treatment of Red Harvested Tomatoes
Red ripe tomatoes were harvested and treated for 24 h at 18°C in airtight containers with 5 µL L−1 1-MCP (SmartFresh, AgroFresh) or 300 µL of ethephon Classic (a kind gift from Bayer CropScience N.V.). The ethephon solution was adjusted to pH 6.0 to facilitate rapid ethylene release. Control fruit were also kept at 18°C in similar sealed containers during the experimental period. Subsequent sampling was done on 10 fruits per time point per treatment. Ethylene production was measured, and pericarp tissue was flash frozen in liquid nitrogen, crushed, and stored at −80°C.
Ethylene and Metabolite Quantification
Fruit ethylene production was quantified after 1 h incubation according to the procedure described by Bulens et al. (2011). Fruit respiration rate was quantified by measuring CO2 production simultaneously with ethylene production by gas chromatography (CompactGC, Interscience). ACC was extracted and quantified by the Lizada and Yang (1979) method recently optimized by Bulens et al. (2011). MACC was obtained after acidic hydrolysis according to Hoffman et al. (1982) and also recently updated by Bulens et al. (2011). SAM was extracted and quantified by capillary electrophoresis (P/ACE MDQ, Beckman Coulter) with UV detection according to Van de Poel et al. (2010). Met content was measured by gas chromatography-mass spectrometry (Agilent) as described by Oms-Oliu et al. (2011). MTA and ATP were quantified by HPLC after chloroacetaldehyde derivatization according to Bürstenbinder et al. (2007).
Protein Extraction and in Vitro Activity
ACO was extracted and in vitro activity determined according to Ververidis and John (1991) updated by Bulens et al. (2011). The extraction buffer consisted of a 100-µM Tris buffer, pH 8.0, instead of the described 400-µM 3-(N-morpholino)propanesulfonic acid buffer, pH 7.2. The incubation time was optimized to 15 min. ACS was extracted and in vitro activity determined exactly as described by Bulens et al. (2011). MTN was extracted with a cold 50 mm of potassium phosphate, pH 7.2, from 200 mg of crushed frozen tissue. The mixture was vortexed vigorously and incubated on ice for 10 min. Subsequently, it was centrifugated at 18.320g for 30 min at 4°C. The supernatant was collected for the in vitro activity assay. The enzymatic MTN activity was measured spectrophotometrically according to Dunn et al. (1994), updated by Bürstenbinder et al. (2010). Briefly, 15 µg of total protein yield was used together with 50 mm of potassium phosphate buffer, pH 7.2, 1 mm MTA, and 2.5 mm of iodonitrotetrazolium chloride (prepared fresh). This mixture was incubated for 2 h with 0.2 units of xanthine oxidase (Grade III), and absorption was measured periodically at 470 nm with a spectrophotometer (SpectraMax M2, Molecular Devices).
Western Blotting
Polyclonal antibodies were developed (GenScript, GE Healthcare) against a consensus epitope for four ACO isoforms (ACO1, ACO2, ACO3, and ACO4: CQDDKVSGLQLLKDE) and a consensus epitope for four ACS isoforms (ACS1, ACS2, ACS4, and ACS6: LADPGDAFLVP). Total protein content of ACO and ACS extracts was determined by the Bradford assay (Bradford, 1976). For ACO and ACS, 15 and 25 µg of total protein content, respectively, was mixed with 10× SDS-sample buffer (Laemmli buffer), denatured for 5 min at 95°C, and loaded on a 12-well Criterion XT 6% to 12% Bis-Tris precast gel (Bio-Rad). SDS-PAGE was performed for 1 h at 180 V with an XT-MES buffer (Bio-Rad). Subsequent electroblotting was carried out for 1 h and 20 min at 100 V on a Protran nitrocellulose membrane (Whatman, Maidstone) or a polyvinylidene difluoride membrane (GE Healthcare) in the presence of transfer buffer (25 mm Tris, 140 mm Gly, 20% [v/v] methanol). The membrane was blocked for 1 h in TBS-T (Tris-buffered saline plus Tween 20; 25 mm Tris, 125 mm NaCl, and 0.1% [v/v] Tween 20) containing 5% milk powder for ACO and 5% bovine serum albinum for ACS. After blocking, the membrane was incubated overnight at 4°C with primary antibody solution (1/1000 anti-ACO AB in TBS-T with 5% milk powder or 1/100 anti-ACS AB in TBS-T with 5% bovine serum albinum). Subsequently, the membrane was washed 5 times for 5 min in TBS-T. Secondary antibody (1/2000 anti-rabbit-horseradish peroxidase-linked AB; Cell Signaling Technologies, Inc.) incubation was carried out for 2 h at 4°C in the presence of TBS-T with 5% milk powder or 5% bovine serum albinum. Again, the membrane was washed. Enhanced chemoluminescence was performed with Pierce ECL western-blotting substrate (Thermo Fischer Scientific), and band intensity was detected with the ImageQuant LAS4000 system (GE Healthcare).
RNA Extraction and Reverse Transcription-qPCR
All procedures described below are in accordance with the Minimum Information for Publication of Quantitative Real-Time PCR Experiments guidelines where applicable (Bustin et al., 2009). Total RNA was extracted with hot (65°C) RNA extraction buffer (55 mm cetyl trimethyl ammonium bromide, 1.34 m NaCl, 25 mm EDTA, 2% [w/v] polyvinylpyrrolidon, 2% [v/v] β-mercaptoethanol, 0.05% [v/v] spermidine) from 4 g of frozen crushed tissue. The mixture was heated for 2 min at 65°C and subsequently homogenized for 2 min and further incubated at 65°C for 15 min. An equal volume of chloroform:isoamyl alcohol (24:1, v/v) was added, shaken vigorously, and subsequently centrifuged at 3,090g for 5 min at 4°C. The upper phase was collected and mixed with another volume of chloroform:isoamyl alcohol followed by a second round of centrifugation. The upper phase was collected and aliquoted (1.5 mL), and RNA was precipitated with 0.5 mL of 7.5 m LiCl overnight at 4°C. The mixture was centrifugated at 14,000g for 30 min at 4°C, and the supernatant was discarded. The RNA pellet was washed with 300 µL of 70% cold ethanol and centrifugated again at 14,000g for 10 min at 4°C. The ethanol supernatant was poured away and ethanol leftovers were vaporized under vacuum for 15 min at room temperature. The pellet was dissolved in 75 µL RLT buffer and subsequent purification, and DNA removal was done with the Qiagen RNeasy Plus Mini Kit according to the manufacturer’s protocol (Qiagen GmbH). RNA integrity was checked on a 1% agarose gel stained with ethidium bromide and RNA content was quantified spectroscopically in a 384-well microtiter plate (UV-star, Greiner Bio-One GmbH) by measuring absorption at 260 nm. One microgram of the purified RNA was reverse transcribed into complementary DNA (cDNA) by the QuantiTect Rev. Transcription Kit (Qiagen) according to the manufacturer’s protocol and subsequently stored at −80°C.
The reverse transcription-qPCR reaction consisted of forward and reverse primer (3.75 µM), reverse transcription-template, water, and Absolute QPCR SYBR Green mix (ABgene, Ltd.). Primers and their properties are listed in Supplemental Table S2. qPCR was performed with the Rotor-Gene Q (Qiagen) for 35 to 45 cycles (depending on primers used). At the end of each PCR run, high-resolution melting curve analysis was performed to check product specificity. For each gene, six biological replicates were used and normalized against the average expression of five reference genes (ACT, GAPDH, EF1a, PP2Ac, and RPL2; Løvdal and Lillo, 2009). Relative quantification was calculated by including a calibration curve in duplex (a dilution series of three orders of log-linear dynamic range) in each run, which also allowed us to determine individual PCR efficiency.
Phylogenetic Analysis
Phylogenetic analyses were executed with Mr. Bayes 3.2 freeware (Ronquist and Huelsenbeck, 2003) using cDNA sequences of all nine ACS isoforms (for annotation see Supplemental Table S1). Default settings of the 4by4 Nucmodel were used and ran for 1 million generations.
Statistical Analysis, PCA, and Trend Visualization
Statistical differences were analyzed with the one-way ANOVA procedure using the Statistical Analysis Software (SAS Enterprise Guide 4.2; SAS Institute, Inc.). Confidence intervals were set at 95%. PCA was preformed with The Unscrambler X 10.1 (CAMO software AS). Graphs were made with Origin8 (OriginLab Corporation), and the trend was visualized using an interpolating smoothing spline.
Sequence data from this article can be found in the GenBank/EMBL data libraries and are listed in Supplemental Table S2.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Quality attributes of tomato during ripening.
Supplemental Figure S2. Phylogenetic tree showing the nine different tomato ACS cDNAs.
Supplemental Figure S3. Gene expression profiles of SAMS.
Supplemental Figure S4. PCA correlation loadings of all variables measured.
Supplemental Table S1. Summary of the most important biochemical features of the ethylene biosynthesis during ripening of tomato.
Supplemental Table S2. List of primers used and their properties.
Supplementary Material
Acknowledgments
The Research Station for Vegetable Production of Hoogstraten (Belgium) and G. Pittoors from Pitoma BVBA are acknowledged for supplying plant material. J. Shukhtina (KU Leuven) is acknowledged for her help with certain analyses.
B.V.d.P. and A.M. performed the research. B.V.d.P., I.B., M.L.A.T.M.H., J.K., E.W., M.P.D.P., M.S., B.M.N., and A.H.G. designed the research. I.B., R.D., M.W., S.V., Y.O., and R.H. helped with specific analytical, biochemical and molecular measurements. B.V.d.P., I.B., M.L.A.T.M.H., S.V., M.S., and A.H.G. analyzed the data. The manuscript was written by B.V.d.P. and approved by all other authors.
Glossary
- qPCR
real-time quantitative PCR
- cDNA
complementary DNA
- PCA
principal component analysis
- ACC
1-aminocyclopropane-1-carboxylic-acid
- ACS
ACC synthase
- SAM
S-adenosyl-l-Met
- MTA
5′-methylthioadenosine
- MTN
5′-methylthioadenosine nucleosidase
- MTK
5′-methylthioribose kinase
- MTI
5′-methylthioribose-phosphate isomerase
- DEP
dehydratase-enolase-phosphatase
- ARD
acireductone dioxygenase
- ACO
ACC oxidase
- MACC
1-(malonylamino)cyclopropane-1-carboxylic acid
- 1-MCP
1-methylcyclopropane
- ERF
ethylene response factor
- P
phosphate
- TBS-T
Tris-buffered saline plus Tween 20
References
- Adams DO, Yang SF. (1977) Methionine metabolism in apple tissue: implication of S-adenosylmethionine as an intermediate in the conversion of methionine to ethylene. Plant Physiol 60: 892–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DO, Yang SF. (1979) Ethylene biosynthesis: identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci USA 76: 170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander L, Grierson D. (2002) Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot 53: 2039–2055 [DOI] [PubMed] [Google Scholar]
- Barry CS, Blume B, Bouzayen M, Cooper W, Hamilton AJ, Grierson D. (1996) Differential expression of the 1-aminocyclopropane-1-carboxylate oxidase gene family of tomato. Plant J 9: 525–535 [DOI] [PubMed] [Google Scholar]
- Barry CS, Llop-Tous MI, Grierson D. (2000) The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol 123: 979–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur AH, Yang SF. (1972) Methionine metabolism in apple tissue in relation to ethylene biosynthesis. Phytochemistry 11: 3207–3214 [Google Scholar]
- Bidonde S, Ferrer MA, Zegzouti H, Ramassamy S, Latché A, Pech JC, Hamilton AJ, Grierson D, Bouzayen M. (1998) Expression and characterization of three tomato 1-aminocyclopropane-1-carboxylate oxidase cDNAs in yeast. Eur J Biochem 253: 20–26 [DOI] [PubMed] [Google Scholar]
- Boller T, Herner RC, Kende H. (1979) Assay for and enzymatic formation of an ethylene precursor, 1-aminocyclopropane-1-carboxylic acid. Planta 145: 293–303 [DOI] [PubMed] [Google Scholar]
- Bower J, Holford P, Latché A, Pech JC. (2002) Culture conditions and detachment of the fruit influence the effect of ethylene on the climacteric respiration of melon. Postharvest Biol Technol 26: 135–146 [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Bulens I, Van de Poel B, Hertog MLAT, De Proft MP, Geeraerd AH, Nicolaï BM. (2011) Protocol: an updated integrated methodology for analysis of metabolites and enzyme activities of ethylene biosynthesis. Plant Methods 7: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürstenbinder K, Rzewuski G, Wirtz M, Hell R, Sauter M. (2007) The role of methionine recycling for ethylene synthesis in Arabidopsis. Plant J 49: 238–249 [DOI] [PubMed] [Google Scholar]
- Bürstenbinder K, Waduwara I, Schoor S, Moffatt BA, Wirtz M, Minocha SC, Oppermann Y, Bouchereau A, Hell R, Sauter M. (2010) Inhibition of 5′-methylthioadenosine metabolism in the Yang cycle alters polyamine levels, and impairs seedling growth and reproduction in Arabidopsis. Plant J 62: 977–988 [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, et al. (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622 [DOI] [PubMed] [Google Scholar]
- Capitani G, Hohenester E, Feng L, Storici P, Kirsch JF, Jansonius JN. (1999) Structure of 1-aminocyclopropane-1-carboxylate synthase, a key enzyme in the biosynthesis of the plant hormone ethylene. J Mol Biol 294: 745–756 [DOI] [PubMed] [Google Scholar]
- Carrari F, Fernie AR. (2006) Metabolic regulation underlying tomato fruit development. J Exp Bot 57: 1883–1897 [DOI] [PubMed] [Google Scholar]
- Chung MC, Chou SJ, Kuang LY, Charng YY, Yang SF. (2002) Subcellular localization of 1-aminocyclopropane-1-carboxylic acid oxidase in apple fruit. Plant Cell Physiol 43: 549–554 [DOI] [PubMed] [Google Scholar]
- Chung M-Y, Vrebalov J, Alba R, Lee J, McQuinn R, Chung J-D, Klein P, Giovannoni J. (2010) A tomato (Solanum lycopersicum) APETALA2/ERF gene, SlAP2a, is a negative regulator of fruit ripening. Plant J 64: 936–947 [DOI] [PubMed] [Google Scholar]
- Dong JG, Fernández-Maculet JC, Yang SF. (1992) Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proc Natl Acad Sci USA 89: 9789–9793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn SM, Bryant JA, Kerr MW. (1994) A simple spectrophotometric assay for plant 5′-deoxy-5′-methylthioadenosine nucleosidase using xanthine oxidase as a coupling enzyme. Phytochem Anal 5: 286–290 [Google Scholar]
- Fujisawa M, Nakano T, Ito Y. (2011) Identification of potential target genes for the tomato fruit-ripening regulator RIN by chromatin immunoprecipitation. BMC Plant Biol 11: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AJ, Bouzayen M, Grierson D. (1991) Identification of a tomato gene for the ethylene-forming enzyme by expression in yeast. Proc Natl Acad Sci USA 88: 7434–7437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley KM, Meir S, Bramlage WJ. (1989) Activity of ageing carnation flower parts and the effects of 1-(malonylamino)cyclopropane-1-carboxylic acid-induced ethylene. Plant Physiol 91: 1126–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertog MLATM, Ben-Arie R, Roth E, Nicolai BM. (2004) Humidity and temperature effects on invasive and non-invasive firmness measures. Postharvest Biol Technol 33: 79–91 [Google Scholar]
- Hoeberichts FA, Van der Plas LHW, Woltering EJ. (2002) Ethylene perception is required for the expression of tomato ripening-related genes and associated physiological changes even at advanced stages of ripening. Postharvest Biol Technol 26: 125–133 [Google Scholar]
- Hoffman NE, Yang SF. (1980) Changes of 1-aminocyclopropane-1-carboxylic acid content in ripening fruits in relation to their ethylene production-rates. J Am Soc Hortic Sci 105: 492–495 [Google Scholar]
- Hoffman NE, Yang SF, McKeon T. (1982) Identification of 1-(malonylamino)cyclopropane-1-carboxylic acid as a major conjugate of 1-aminocyclopropane-1-carboxylic acid, an ethylene precursor in higher plants. Biochem Biophys Res Commun 104: 765–770 [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Hudgins JW, Ralph SG, Franceschi VR, Bohlmann J. (2006) Ethylene in induced conifer defense: cDNA cloning, protein expression, and cellular and subcellular localization of 1-aminocyclopropane-1-carboxylate oxidase in resin duct and phenolic parenchyma cells. Planta 224: 865–877 [DOI] [PubMed] [Google Scholar]
- Ito Y, Kitagawa M, Ihashi N, Yabe K, Kimbara J, Yasuda J, Ito H, Inakuma T, Hiroi S, Kasumi T. (2008) DNA-binding specificity, transcriptional activation potential, and the rin mutation effect for the tomato fruit-ripening regulator RIN. Plant J 55: 212–223 [DOI] [PubMed] [Google Scholar]
- Jiao XZ, Philosoph-Hadas S, Su LY, Yang SF. (1986) The conversion of 1-(malonylamino)cyclopropane-1-carboxylic acid to 1-aminocyclopropane-1-carboxylic acid in plant tissues. Plant Physiol 81: 637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S, Liu Y, Lueth A, Zhang SQ. (2008) MAPK phosphorylation-induced stabilization of ACS6 protein is mediated by the non-catalytic C-terminal domain, which also contains the cis-determinant for rapid degradation by the 26S proteasome pathway. Plant J 54: 129–140 [DOI] [PubMed] [Google Scholar]
- Kamiyoshihara Y, Iwata M, Fukaya T, Tatsuki M, Mori H. (2010) Turnover of LeACS2, a wound-inducible 1-aminocyclopropane-1-carboxylic acid synthase in tomato, is regulated by phosphorylation/dephosphorylation. Plant J 64: 140–150 [DOI] [PubMed] [Google Scholar]
- Karlova R, Rosin FM, Busscher-Lange J, Parapunova V, Do PT, Fernie AR, Fraser PD, Baxter C, Angenent GC, de Maagd RA. (2011) Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. Plant Cell 23: 923–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevany BM, Tieman DM, Taylor MG, Cin VD, Klee HJ. (2007) Ethylene receptor degradation controls the timing of ripening in tomato fruit. Plant J 51: 458–467 [DOI] [PubMed] [Google Scholar]
- Kushad MM, Richardson DG, Ferro AJ. (1982) 5-Methylthioribose kinase activity in plants. Biochem Biophys Res Commun 108: 167–173 [DOI] [PubMed] [Google Scholar]
- Kushad MM, Richardson DG, Ferro AJ. (1983) Intermediates in the recycling of 5-methylthioribose to methionine in fruits. Plant Physiol 73: 257–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashbrook CC, Tieman DM, Klee HJ. (1998) Differential regulation of the tomato ETR gene family throughout plant development. Plant J 15: 243–252 [DOI] [PubMed] [Google Scholar]
- Lieberman M, Kunishi A, Mapson LW, Wardale DA. (1966) Stimulation of ethylene production in apple tissue slices by methionine. Plant Physiol 41: 376–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SF, Walsh CS. (2008) Studies of the “tree factor” and its role in the maturation and ripening of 'Gala' and 'Fuji' apples. Postharvest Biol Technol 48: 99–106 [Google Scholar]
- Lin ZF, Hong YG, Yin MG, Li CY, Zhang K, Grierson D. (2008) A tomato HD-Zip homeobox protein, LeHB-1, plays an important role in floral organogenesis and ripening. Plant J 55: 301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hoffman NE, Yang SF. (1983) Relationship between the malonylation of 1-aminocyclopropane-1-carboxylic acid and D-amino acids in mung-bean hypocotyls. Planta 158: 437–441 [DOI] [PubMed] [Google Scholar]
- Lizada MCC, Yang SF. (1979) A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem 100: 140–145 [DOI] [PubMed] [Google Scholar]
- Løvdal T, Lillo C. (2009) Reference gene selection for quantitative real-time PCR normalization in tomato subjected to nitrogen, cold, and light stress. Anal Biochem 387: 238–242 [DOI] [PubMed] [Google Scholar]
- Martel C, Vrebalov J, Tafelmeyer P, Giovannoni JJ. (2011) The tomato MADS-box transcription factor RIPENING INHIBITOR interacts with promoters involved in numerous ripening processes in a COLORLESS NONRIPENING-dependent manner. Plant Physiol 157: 1568–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MN, Cohen JD, Saftner RA. (1995) A new 1-aminocyclopropane-1-carboxylic acid-conjugating activity in tomato fruit. Plant Physiol 109: 917–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matas AJ, Yeats TH, Buda GJ, Zheng Y, Chatterjee S, Tohge T, Ponnala L, Adato A, Aharoni A, Stark R, et al. (2011) Tissue- and cell-type specific transcriptome profiling of expanding tomato fruit provides insights into metabolic and regulatory specialization and cuticle formation. Plant Cell 23: 3893–3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurchie EJ, McGlasson WB, Eaks IL. (1972) Treatment of fruit with propylene gives information about the biogenesis of ethylene. Nature 237: 235–236 [DOI] [PubMed] [Google Scholar]
- Murr DP, Yang SF. (1975) Conversion of 5′-methylthioadenosine to methionine by apple tissue. Phytochemistry 14: 1291–1292 [Google Scholar]
- Nakano R, Ogura E, Kubo Y, Inaba A. (2003) Ethylene biosynthesis in detached young persimmon fruit is initiated in calyx and modulated by water loss from the fruit. Plant Physiol 131: 276–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A. (1998) Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol 118: 1295–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka A, Shiomi S, Kubo Y, Inaba A. (1997) Expression and internal feedback regulation of ACC synthase and ACC oxidase genes in ripening tomato fruit. Plant Cell Physiol 38: 1103–1110 [DOI] [PubMed] [Google Scholar]
- Oms-Oliu G, Hertog MLAT, Van de Poel B, Mpofo-Asiama J, Geeraerd AH, Nicolai BM. (2011) Metabolic characterization of tomato fruit during preharvest development, ripening, and postharvest shelf-life. Postharvest Biol Technol 62: 7–16 [Google Scholar]
- Pommerrenig B, Feussner K, Zierer W, Rabinovych V, Klebl F, Feussner I, Sauer N. (2011) Phloem-specific expression of Yang cycle genes and identification of novel Yang cycle enzymes in Plantago and Arabidopsis. Plant Cell 23: 1904–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramassamy S, Olmos E, Bouzayen M, Pech J-C, Latché A. (1998) 1-aminocyclopropane-1-carboxylate oxidase of apple fruit is periplasmic. J Exp Bot 49: 1909–1915 [Google Scholar]
- Reinhardt D, Kende H, Boller T. (1994) Subcellular-localization of 1-aminocyclopropane-1-carboxylate oxidase in tomato cells. Planta 195: 142–146 [Google Scholar]
- Rocklin AM, Kato K, Liu HW, Que L, Jr, Lipscomb JD. (2004) Mechanistic studies of 1-aminocyclopropane-1-carboxylic acid oxidase: single turnover reaction. J Biol Inorg Chem 9: 171–182 [DOI] [PubMed] [Google Scholar]
- Rombaldi C, Lelièvre JM, Latché A, Petitprez M, Bouzayen M, Pech JC. (1994) Immunocytolocalization of 1-aminocyclopropane-1-carboxylic acid oxidase in tomato and apple fruit. Planta 192: 453–460 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574 [DOI] [PubMed] [Google Scholar]
- Rzewuski G, Cornell KA, Rooney L, Bürstenbinder K, Wirtz M, Hell R, Sauter M. (2007) OsMTN encodes a 5′-methylthioadenosine nucleosidase that is up-regulated during submergence-induced ethylene synthesis in rice (Oryza sativa L.). J Exp Bot 58: 1505–1514 [DOI] [PubMed] [Google Scholar]
- Sauter M, Cornell KA, Beszteri S, Rzewuski G. (2004) Functional analysis of methylthioribose kinase genes in plants. Plant Physiol 136: 4061–4071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter M, Lorbiecke R, Ouyang B, Pochapsky TC, Rzewuski G. (2005) The immediate-early ethylene response gene OsARD1 encodes an acireductone dioxygenase involved in recycling of the ethylene precursor S-adenosylmethionine. Plant J 44: 718–729 [DOI] [PubMed] [Google Scholar]
- Sell S, Hehl R. (2005) A fifth member of the tomato 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase gene family harbours a leucine zipper and is anaerobically induced. DNA Seq 16: 80–82 [DOI] [PubMed] [Google Scholar]
- Sfakiota EM, Dilley DR. (1973) Internal ethylene concentrations in apple fruits attached to or detached from tree. J Am Soc Hortic Sci 98: 501–503 [Google Scholar]
- Sims WL. (1969) Effects of ethrel on fruit ripening of tomatoes greenhouse, field and postharvest trials. Calif Agric 23: 12–14 [Google Scholar]
- Staswick PE, Tiryaki I. (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16: 2117–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LY, Mckeon T, Grierson D, Cantwell M, Yang SF. (1984) Development of 1-aminocyclopropane-1-carboxylic acid synthase and polygalacturonase activities during the maturation and ripening of tomato fruit. HortScience 19: 576–578 [Google Scholar]
- Tassoni A, Watkins CB, Davies PJ. (2006) Inhibition of the ethylene response by 1-MCP in tomato suggests that polyamines are not involved in delaying ripening, but may moderate the rate of ripening or over-ripening. J Exp Bot 57: 3313–3325 [DOI] [PubMed] [Google Scholar]
- Tatsuki M, Mori H. (2001) Phosphorylation of tomato 1-aminocyclopropane-1-carboxylic acid synthase, LE-ACS2, at the C-terminal region. J Biol Chem 276: 28051–28057 [DOI] [PubMed] [Google Scholar]
- Tieman DM, Taylor MG, Ciardi JA, Klee HJ. (2000) The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc Natl Acad Sci USA 97: 5663–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomato Genome Consortium (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485: 635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchisaka A, Theologis A. (2004) Heterodimeric interactions among the 1-amino-cyclopropane-1-carboxylate synthase polypeptides encoded by the Arabidopsis gene family. Proc Natl Acad Sci USA 101: 2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Poel B, Bulens I, Hertog MLATM, Van Gastel L, De Proft MP, Nicolai BM, Geeraerd AH. (2012) Model-based classification of tomato fruit development and ripening related to physiological maturity. Postharvest Biol Technol 67: 59–67 [Google Scholar]
- Van de Poel B, Bulens I, Lagrain P, Pollet J, Hertog MLAT, Lammertyn J, De Proft MP, Nicolaï BM, Geeraerd AH. (2010) Determination of S-adenosyl-l-methionine in fruits by capillary electrophoresis. Phytochem Anal 21: 602–608 [DOI] [PubMed] [Google Scholar]
- Ververidis P, John P. (1991) Complete recovery in vitro of ethylene-forming enzyme-activity. Phytochemistry 30: 725–727 [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J. (2002) A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 296: 343–346 [DOI] [PubMed] [Google Scholar]
- Waduwara-Jayabahu I, Oppermann Y, Wirtz M, Hull ZT, Schoor S, Plotnikov AN, Hell R, Sauter M, Moffatt BA. (2012) Recycling of methylthioadenosine is essential for normal vascular development and reproduction in Arabidopsis. Plant Physiol 158: 1728–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SY, Adams DO, Lieberman M. (1982) Recycling of 5′-methylthioadenosine-ribose carbon atoms into methionine in tomato tissue in relation to ethylene production. Plant Physiol 70: 117–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip WK, Moore T, Yang SF. (1992) Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions. Proc Natl Acad Sci USA 89: 2475–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokotani N, Nakano R, Imanishi S, Nagata M, Inaba A, Kubo Y. (2009) Ripening-associated ethylene biosynthesis in tomato fruit is autocatalytically and developmentally regulated. J Exp Bot 60: 3433–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Ren JS, Clifton IJ, Schofield CJ. (2004) Crystal structure and mechanistic implications of 1-aminocyclopropane-1-carboxylic acid oxidase—the ethylene-forming enzyme. Chem Biol 11: 1383–1394 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang H, Quan R, Wang X-C, Huang R. (2009) Transcriptional regulation of the ethylene response factor LeERF2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiol 150: 365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.