Abstract
Panicum mosaic virus (PMV) and its satellite virus (SPMV) together infect several small grain crops, biofuel, and forage and turf grasses. Here, we establish the emerging monocot model Brachypodium (Brachypodium distachyon) as an alternate host to study PMV- and SPMV-host interactions and viral synergism. Infection of Brachypodium with PMV+SPMV induced chlorosis and necrosis of leaves, reduced seed set, caused stunting, and lowered biomass, more than PMV alone. Toward gaining a molecular understanding of PMV- and SPMV-affected host processes, we used a custom-designed microarray and analyzed global changes in gene expression of PMV- and PMV+SPMV-infected plants. PMV infection by itself modulated expression of putative genes functioning in carbon metabolism, photosynthesis, metabolite transport, protein modification, cell wall remodeling, and cell death. Many of these genes were additively altered in a coinfection with PMV+SPMV and correlated to the exacerbated symptoms of PMV+SPMV coinfected plants. PMV+SPMV coinfection also uniquely altered expression of certain genes, including transcription and splicing factors. Among the host defenses commonly affected in PMV and PMV+SPMV coinfections, expression of an antiviral RNA silencing component, SILENCING DEFECTIVE3, was suppressed. Several salicylic acid signaling components, such as pathogenesis-related genes and WRKY transcription factors, were up-regulated. By contrast, several genes in jasmonic acid and ethylene responses were down-regulated. Strikingly, numerous protein kinases, including several classes of receptor-like kinases, were misexpressed. Taken together, our results identified distinctly altered immune responses in monocot antiviral defenses and provide insights into monocot viral synergism.
In the past decades, substantial progress has been made in dissecting interactions between plant and virus proteins and defining the role of virus-encoded proteins in plant cells. Model plant species, such as Arabidopsis (Arabidopsis thaliana) and Nicotiana benthamiana, have been crucial in determining the mechanics of antiviral defenses. Genome-wide expression studies in these model plants identified networks of genes affected commonly by several plant viruses, whereby diverse DNA and RNA viruses alter genes in primary and secondary metabolism, detoxification, cell wall remodeling, and defense responses (Whitham et al., 2003, 2006; Ascencio-Ibáñez et al., 2008; García-Marcos et al., 2009; Elena et al., 2011; Hanssen et al., 2011; Postnikova and Nemchinov, 2012). At the cellular level, specific recognition of viral nucleic acids or viral proteins by host resistance gene products inhibits viral replication or accumulation (Kohm et al., 1993; Bendahmane et al., 1999; Ishibashi et al., 2007). Additionally, tissue-level hypersensitive response and systemic acquired resistance, often mediated by defense hormones such as salicylic acid (SA), both delimit movement of the virus from the primary infected cells and induce a broad-spectrum resistance to systemic infection (Huang et al., 2005; Alamillo et al., 2006; Kachroo et al., 2006; Heil and Ton, 2008; Baebler et al., 2011). Recently, RNA silencing has emerged as a broadly conserved defense response against viruses (Dunoyer and Voinnet, 2005; Alvarado and Scholthof, 2009, 2011). Multiple RNA silencing components, such as Dicer, double-stranded RNA-binding (DRB) protein, HUA ENHANCER1, Argonaute (AGO), and RNA-dependent RNA polymerase (RDR), play critical roles in programming virus-specific RNA-induced silencing complexes that subsequently target viral RNA for degradation (Alvarado and Scholthof, 2009). As a coevolutionary strategy, generally referred to as the Red Queen Hypothesis (Van Valen, 1973), to antagonize host antiviral defenses, many viruses encode suppressor proteins that function to interfere and suppress RNA silencing (Dunoyer and Voinnet, 2005; Alvarado and Scholthof, 2011). These and other host:virus outcomes have been recently summarized by Pallas and García (2011).
Interestingly, viral suppressors were discovered by studying a mixed virus infection that induced a synergism on tobacco (Nicotiana tabacum) plants. This coinfection of Potato virus X (PVX; genus Potexvirus) and Potato virus Y (PVY; genus Potyvirus) on potato (Solanum tuberosum) and tobacco plants resulted in significantly increased levels of PVX compared with single virus infections and greatly enhanced symptoms (Rochow and Ross, 1955; Damirdagh and Ross, 1967; Goodman and Ross, 1974; Vance, 1991). PVY encodes a potent silencing suppressor protein, HC-Pro, which enhances accumulation of PVX in tobacco (Pruss et al., 1997, 2004; González-Jara et al., 2004) but not in N. benthamiana, suggesting that viral synergistic interactions can be host specific (García-Marcos et al., 2009).
Much of our knowledge of virus:host interactions has been determined under laboratory conditions with dicotyledonous host plants, especially N. benthamiana and Arabidopsis. Studies of viruses with host ranges limited to monocots (plants in the Poaceae, including forage and turf grasses and small grains) have lagged due to the lack of a tractable model species. Similarly, few studies of heterogeneous virus infections have been reported on monocot or dicot host plants, resulting in a gap in our molecular understanding of how plants respond to mixed (or synergistic) virus infections. For example, little is known about how viral synergism alters host defenses and whether antiviral defenses are conserved among dicot and monocot plants. Here, we focused on addressing the biological response of Brachypodium (Brachypodium distachyon), an emerging model grass species, to the mixed infection of Panicum mosaic virus (PMV) and its satellite virus, SPMV.
PMV, the type member of the genus Panicovirus in the family Tombusviridae, is a single-stranded, positive-sense RNA with a host range limited to the Poaceae family of grasses (Turina et al., 1998, 2000; Scholthof, 1999). PMV was first described in natural infections of switchgrass (Panicum virgatum; Sill and Pickett, 1957) and subsequently as the causal agent of St. Augustine decline disease on St. Augustinegrass (Stenotaphrum secundatum), a popular turf grass in the southern United States (Cabrera and Scholthof, 1999). Experimental hosts of PMV include foxtail millet (Setaria italica), pearl millet (Pennisetum glaucum), and proso millet (Panicum miliaceum; Buzen et al., 1984). From its 4,326-nucleotide genomic RNA, PMV expresses two replicase-associated proteins (P48 and P112), using translation read-through of the 48-kD open reading frame to express the 112-kD protein (Batten et al., 2006b; Supplemental Fig. S1). Four genes are encoded on the subgenomic RNA that facilitate virus movement (P8, P6.6, and P15) and encapsidate the genomic RNA (26-kD capsid protein [CP]) to form 30-nm (T = 3) virions (Turina et al., 2000; Batten et al., 2006b).
PMV also supports the replication and systemic spread of SPMV, an 824-nucleotide, positive-sense, single-stranded RNA (Niblett and Paulsen, 1975; Masuta et al., 1987; Turina et al., 1998; Scholthof, 1999; Supplemental Fig. S1). SPMV genomic RNA encodes a 17-kD CP (SPMV CP) to form 16-nm (T = 1) icosahedral particles (Ban et al., 1995; Ban and McPherson, 1995; Desvoyes and Scholthof, 2000). Previously, we showed that in addition to encapsidation, SPMV CP localizes to the host plasma membrane, forming puncta near the plasmodesmata (Qi et al., 2008), reflecting its movement-protein like functions. SPMV CP also traffics to the nucleus and nucleolus (Qi et al., 2008) forming Cajal body-like structures similar to other nucleolar-localized viral proteins (Kim et al., 2007). Additionally, SPMV CP induces a nonhost hypersensitive response in N. benthamiana when expressed from viral gene vectors (Qiu and Scholthof, 2004), prevents the accumulation of defective interfering SPMV RNA in PMV mixed infections of millets (Qiu and Scholthof, 2001), and stabilizes foreign gene inserts in cis and in trans when expressed from virus gene vectors (Everett et al., 2010). For all its features as a pathogenicity factor, evidence is lacking to suggest SPMV CP is a suppressor of gene silencing (Qiu and Scholthof, 2004).
Satellite viruses are molecular parasites that depend on their helper viruses for replication and movement in host plants (Scholthof et al., 1999; Hu et al., 2009). Our previous genetic and molecular characterization of PMV and SPMV in millets revealed multiple determinants of the synergism (Desvoyes and Scholthof, 2000; Turina et al., 2000; Qiu and Scholthof, 2001, 2004; Omarov et al., 2005; Batten et al., 2006a, 2006b; Everett et al., 2010). In the case of PMV+SPMV mixed infections, SPMV has the unusual feature of enhancing PMV accumulation in host plants, with a simultaneous increase in accumulation of PMV movement protein (P8) and exacerbation of symptoms on systemically infected (noninoculated) leaves compared with infections of PMV alone (Scholthof, 1999; Supplemental Fig. S1). These effects reflect the historical definition of viral synergism: The mixed infection causes more severe symptoms than a single infection and tends to facilitate an increase in the titer of one of the virus pairs during the acute stage of the disease (Rochow and Ross, 1955; Latham and Wilson, 2008; Syller, 2012). However, the PMV+SPMV synergism stands unique because it involves a satellite virus. Despite our past progress, the lack of a genetic model plant species made it challenging to study the mechanism(s) underlying this synergism and effects on host plant processes.
Recently, Brachypodium was developed as a genomic model for studies of grass biology and host-pathogen interactions (Brkljacic et al., 2011). The Brachypodium genome is syntenic to rice (Oryza sativa) and sorghum (Sorghum bicolor) genomes and is related to several forage, small grains, and bioenergy crop plants, including wheat (Triticum aestivum), barley (Hordeum vulgare), and millets, all of which are in the grass subfamily Pooideae (Powell, 1994; International Brachypodium Initiative, 2010). These temperate and cool season grasses belonging to the Poaceae are the primary source of calories throughout the world (Draper et al., 2001; Huo et al., 2006; Vogel et al., 2006; Vogel and Hill, 2008; Bevan et al., 2010; Garvin et al., 2010; International Brachypodium Initiative, 2010; Brkljacic et al., 2011; Cao et al., 2011). In this study, we established Brachypodium as a systemic host for PMV and SPMV infections and show that the disease etiology mimics biological features previously described in millet plants. Furthermore, using microarray techniques, we identified host pathways affected by PMV and PMV+SPMV infections. From this, we sketched a model that shows the contributions of SPMV as a molecular effector of the helper virus, PMV, and provide a more utilitarian definition of viral synergism that is based not only on the visual symptoms but also on the altered host molecular processes that occur in viral synergism.
RESULTS
PMV and SPMV Infection of Brachypodium Results in a Viral Synergism
To study the synergism associated with PMV and SPMV infections, 1-week-old Brachypodium plants with two to three leaves were inoculated with full-length infectious transcripts of PMV alone or PMV+SPMV (Batten et al., 2006b). The PMV infection induced chlorosis and necrosis of leaves with moderate stunting (Fig. 1, A and B), in comparison to mock inoculated plants. By contrast, PMV+SPMV coinfection exacerbated the symptoms with excessive stunting, severe chlorosis and necrosis, and reduced or lack of seed set (Fig. 1, A and B). Moreover, in the PMV+SPMV-infected plants, PMV CP was consistently detected several days earlier than in plants inoculated with PMV alone (Fig. 1, D and E). Each of these features exemplifies those we previously described in PMV+SPMV-infected millet plants (Scholthof, 1999).
Figure 1.
SPMV exacerbates disease symptoms and enhances PMV accumulation in Brachypodium. A and B, Typical disease symptoms and gross effects of PMV alone and PMV+SPMV coinfection at 42 dpi. PMV alone infection causes mild chlorosis, necrosis, and stunting of roots and shoots, while PMV+SPMV coinfection causes severe stunting, chlorosis, and reduced seed set. The inset image in A shows the severe chlorosis associated with coinfection with SPMV. The progression of PMV and PMV+SPMV infection can be classified into the following: stage I with no visible chlorosis on leaves, stage II with mild chlorosis on leaves, and stage III with prominent chlorosis and necrosis on leaves, stunting, and reduced seed set (C). Immunoblot analysis of CP accumulation at infection stages I, II, and III with PMV alone (D) or PMV+SPMV infection (E). The earlier detection of PMV CP in the mixed infections (E) is associated with SPMV enhancement of either PMV replication or movement, which is a component of the working definition of the SPMV-associated synergism (Scholthof, 1999). The asterisks represent lower molecular weight CPs, possibly produced from leaky scanning (Omarov et al., 2005) or posttranslational modifications. The Ponceau S-stained nitrocellulose membranes serve to indicate approximately equal protein loading among the sample lanes.
To analyze the temporal progression of the disease in detail, and gain consistency in comparisons among independent infections performed at different times, we classified PMV and SPMV infections into three different stages based on the degree of visible symptoms and the abundance of the viral proteins in the infected plants. Infected plants at stage I (typically ≤7 d postinfection [dpi]) showed no visible chlorosis symptoms on the inoculated leaves and had no detectable levels of viral CPs compared with mock-inoculated controls (Fig. 1, C–E). Infected plants at stage II (typically 10–14 dpi) displayed a few chlorotic and necrotic lesions on both the inoculated and newly emerging noninoculated leaves and possessed detectable levels of viral CPs. Finally, stage III infected plants (typically ≥21 dpi) were severely chlorotic and necrotic, were stunted, and had higher accumulation of viral CPs (Fig. 1, C–E). With regards to developmental timeline of the infected plants, stage I and II plants are in their early and late vegetative phase, respectively, while, stage III plants have transitioned to reproductive phase.
Chlorotic symptoms on upper noninoculated leaves of PMV+SPMV-infected plants typically appear by 10 dpi (Stage II) and become prominent by 21 dpi (stage III; Fig. 2, A and B). We determined that the chlorotic leaves had reduced levels of chlorophyll a and chlorophyll b in the PMV+SPMV-infected shoots (Fig. 2C) compared with the mock-inoculated plants. Plants infected with PMV+SPMV at maturity (stage III) also had significantly reduced biomass, based on fresh and dry weights (Fig. 2D). Additionally, PMV+SPMV-infected plants at stage III were severely stunted (Fig. 2B) with shorter internodes and smaller leaves (Fig. 2, E and F), although tiller number was not affected (Fig. 2G), when compared with mock-inoculated plants. Seed set was also greatly or completely abolished in PMV+SPMV-infected Brachypodium at stage III (Fig. 1, A and C), which we also described previously for PMV+SPMV infections in millet plants (Scholthof, 1999). Finally, immunoblot analysis of the PMV+SPMV-infected plants at stage II, detected both PMV CP and SPMV CP in upper noninoculated leaves and root tissues, demonstrating systemic movement of both viruses (Fig. 2H). Taken together, these results show Brachypodium is a good model host plant for studying PMV and its SPMV-associated synergistic interactions.
Figure 2.
Brachypodium infections with PMV+SPMV compared with mock-inoculated plants. Typical outcomes of PMV+SPMV infection result in leaf chlorosis and necrosis (A, indicated by arrows), stunting (B), reduced chlorophyll a and chlorophyll b (C, Chl A and Chl B) accumulation by stage III, decreased fresh weight (FW) and dry weight (D, DW), shorter internodes (E), smaller leaves (F), but no significant effect on tiller number (G). Asterisks in the graph represent significant differences compared with mock controls (P value < 0.01, Student’s t test). H, Immunoblot assays of PMV CP and SPMV CP accumulation in PMV+SPMV-infected roots and shoots at stage III. The asterisk represents a lower molecular weight SPMV CP (see legend of Fig. 1 and Omarov et al., 2005). The Coomassie Brilliant Blue R-stained gel reflects approximate equal sample loading among the mock- and virus-inoculated sample lanes. [See online article for color version of this figure.]
Transcriptome Analysis of PMV- and SPMV-Infected Brachypodium
To identify host molecular pathways altered by PMV and SPMV leading to the disease symptoms, we performed genome-wide gene expression analysis using DNA oligonucleotide microarrays. Because our intent was to identify the altered host molecular processes that preceded the overall disease symptoms of the infected plants, we chose to analyze infected plants before prominent visual chlorosis, necrosis, and stunting symptoms appeared. Inoculated and noninoculated leaves and stems of plants infected with PMV alone or coinfected with PMV+SPMV, with mild chlorosis symptoms (stage II), along with mock-inoculated plants, were harvested for RNA isolation and microarray analysis. A custom-designed Affymetrix tiling array, representing approximately 27,000 genes, by multiple probes, was used to assay three independent biological replicates from each of the inoculation treatments. Normalized microarray expression data were further corrected for false discovery rate (FDR) using the Benjamini and Hochberg method (q-value < 0.05; Benjamini and Hochberg, 1995). In PMV-infected plants, approximately 464 and 326 genes were up- and down-regulated by 2-fold or greater, respectively, in comparison with mock-inoculated plants (Fig. 3A). In PMV+SPMV coinfection, 621 and 433 genes were up- and down-regulated by 2-fold or greater, respectively (Fig. 3A). To validate the microarray results, we selected 20 candidate genes, including multiple protein kinases, transcription factors, and metabolic enzymes (Supplemental Table S1) and performed quantitative reverse transcription (qRT)-PCR analysis using the same RNA used for the microarray analysis. The criteria for choosing the 20 genes for qRT-PCR analysis was to represent genes in diverse functional categories and with varying levels of misexpression in the PMV and PMV+SPMV infections (2- to 50-fold). The results of the qRT-PCR analysis strongly correlated to the microarray data (Pearson correlation coefficient, r > 0.9, and coefficient of determination, R2 > 0.8; Supplemental Table S1).
Figure 3.
PMV and SPMV alter genes in diverse functional categories. Triplicate samples of Brachypodium (Bd21) plants infected with PMV or PMV+SPMV or mock inoculated at stage II (mild chlorosis symptoms) were analyzed using microarrays. Significant changes in gene expression relative to mock were determined using the Benjamini and Hochberg method by correcting for FDR (q-value < 0.05). A, The Venn diagrams represent the number of genes up-regulated and down-regulated in response to PMV and PMV+SPMV infection. B, Summary of the biological processes overrepresented in PMV and PMV+SPMV infections as deduced using AgriGO (Du et al., 2010), Pfam, and PANTHER functional annotations (Thomas et al., 2003; Punta et al., 2012). A detailed list of the GO assignments and SEA analysis is in Supplemental Table S2. [See online article for color version of this figure.]
Next, we performed functional annotation of genes that were commonly induced or repressed in PMV and PMV+SPMV infections using gene ontology (GO) terms for biological processes (GO-P), molecular function (GO-F), and cellular component (GO-C) using the AgriGO analysis tool (Du et al., 2010). To maximize functional annotations for more genes, additional protein families (Pfam; Punta et al., 2012) and protein analysis through evolutionary relationship (PANTHER; Thomas et al., 2003) functional annotations were assigned using the Phytozome resource (Goodstein et al., 2012; http://www.phytozome.net/). Furthermore, gene set enrichment was performed using the AgriGO singular enrichment analysis (SEA), with appropriate statistical corrections (FDR < 0.05). SEA identifies significant GO terms that are overrepresented in a query gene list relative to a precalculated frequency in the reference (or background) genome of a species and allows a general inference of the overrepresented biological processes or GO terms. The SEA revealed that both PMV alone and PMV+SPMV infections altered expression of multiple genes in biological processes, such as primary metabolism, metabolite transport, defense, and cell wall remodeling (summarized in Fig. 3B and expanded details in Supplemental Table S2), suggesting that PMV alone and SPMV-synergistic interactions altered the metabolic fate of the infected cells. Furthermore, among the induced genes, several detoxification and stress-responsive transcripts corresponding to glutathione S-transferases, cytochrome P450, chaperone and heat shock proteins, ribosomal proteins, and proteasome components were overrepresented (Fig. 4A; Supplemental Table S2). Interestingly, multiple nuclear-encoded, photosynthesis-related chlorophyll a/b binding and photosystem proteins were predominant among the down-regulated genes (Fig. 4B; Supplemental Table S2). The latter result lends genetic support for our observations of the leaf chlorosis (Figs. 1A and 2A) and decreased chlorophyll levels (Fig. 2C) in the PMV+SPMV-infected shoots.
Figure 4.
Pseudo-colored heat map representation of Brachypodium gene expression in PMV alone and PMV+SPMV infection. The color bars on the top indicate logarithmic expression values (log2) from the microarray: green for lower expression and red for higher expression. A, The up-regulated GST proteins, chaperone/heat shock proteins (HSPs), cytochrome P450 family proteins, and several genes implicated in protein synthesis are indicated. B, The down-regulated photosynthesis-related genes are indicated (see also Fig. 2C for effects on chlorophyll content). Functional classification of the different genes was deduced using AgriGO (Du et al., 2010), Pfam, and PANTHER annotations (Thomas et al., 2003; Punta et al., 2012). Note that genes with multiple alternatively spliced transcripts occur more than once, and certain alternatively spliced transcripts exhibit differential expression kinetics.
Host Defense Responses to PMV and SPMV
Because high-quality genome sequence information is available for Arabidopsis, and because antiviral defenses are well studied in that model (Whitham et al., 2003; Ascencio-Ibáñez et al., 2008; García-Marcos et al., 2009; Elena et al., 2011; Hanssen et al., 2011), for our subsequent analysis, we chose to compare our transcriptome results with Arabidopsis-virus responses. Moreover, such a comparison allows us to compare and contrast antiviral responses in monocot and dicot plants. Among the altered antiviral silencing components, transcript levels of a SDE3-like helicase (Bradi3g52690), which in Arabidopsis is involved in secondary amplification of silencing and short-interfering RNA production (Dalmay et al., 2001), was suppressed in PMV and PMV+SPMV infections by ≥2-fold compared with mock-inoculated plants (Supplemental Table S1). In Arabidopsis, activation of pathogenesis-related (PR) proteins is a classical marker for basal and induced defense responses involving SA, pathogen-triggered immunity, effector-triggered immunity, and systemic acquired resistance (Pieterse et al., 2009; Vlot et al., 2009; Padmanabhan and Dinesh-Kumar, 2010; An and Mou, 2011). Among the PMV and PMV+SPMV-modulated genes in Brachypodium that are functionally annotated as stress or stimulus responsive (Fig. 3B), two Cys-rich secretory proteins (Bradi1g57580 and Bradi1g57590) were induced >20-fold compared with mock controls (Table I). We identified these as PR-related proteins and, in Arabidopsis, were closest to PR-1. Further candidate gene searches revealed increased expression of several PR genes orthologous to Arabidopsis PR-2, PR-3, PR-4, and PR-5 (Table I) in PMV alone and PMV+SPMV coinfection. Interestingly, multiple Brachypodium PR genes that were strongly induced in PMV infection were relatively less induced in the PMV+SPMV coinfection (Table I). For example, expression of a PR-1-like gene was up-regulated by 40-fold in PMV alone infection compared with mock; however, its expression was induced only 20-fold in PMV+SPMV infections. Similar expression patterns were observed for other PR genes (Table I). Because PMV and PMV+SPMV infection induced multiple PR genes, we next determined whether other components of SA signaling were altered. We found up-regulation of ISOCHORISMATE SYNTHASE1 (ICS1)-like, ABERRANT GROWTH AND DEATH2 (AGD2), ALTERNATIVE OXIDASE (AOX), and certain WRKY factors, while expression of a PHYTOALEXIN DEFICIENT4 (PAD4)-like gene was down-regulated (Table I). The closest orthologs of each of these gene products in Arabidopsis are implicated in SA biosynthesis and signaling (Vlot et al., 2009; An and Mou, 2011).
Table I. Differential expression of putative Brachypodium genes involved in SA signaling.
The relative unlogged fold change (≥1.5-fold) in gene expression of PMV and PMV+SPMV infection compared with mock is indicated. Minus sign represents down-regulated fold change. The gene descriptions were deduced from peptide ortholog analysis with Arabidopsis protein sequences using Phytozome (Goodstein et al., 2012). The percentage similarity of Brachypodium proteins with the orthologous Arabidopsis proteins is indicated in parentheses. Note that some Brachypodium genes with low percentage similarity to their respective Arabidopsis orthologs are indicated as “-like.” For additional phylogenetic analyses, see Fig. 5 and Supplemental Figure S2. The statistical significance of gene expression changes was determined using Student’s t test and was corrected for FDR using the Benjamini and Hochberg method (q-value < 0.05).
| Gene ID | PMV | PMV+SPMV | Description |
|---|---|---|---|
| Bradi1g57580 | 42.53 | 21.90 | PR-1-like (52%) |
| Bradi1g57590 | 40.79 | 22.56 | PR-1-like (52%) |
| Bradi2g60490 | 10.23 | 9.47 | BdPR-2 (51%) |
| Bradi2g47210 | 3.76 | 3.32 | BdPR-3 (71%) |
| Bradi3g48230 | 2.39 | 2.07 | PR-3-like (60%) |
| Bradi4g14920 | 9.29 | 6.61 | BdPR-4 (67%) |
| Bradi1g13060 | 19.13 | 12.87 | PR-5-like (54%) |
| Bradi1g13070 | 26.34 | 19.88 | PR-5-like (48%) |
| Bradi1g67240 | 2.58 | 2.33 | ICS1-like (19%) |
| Bradi1g71530 | 3.36 | 1.83 | BdAGD2 (73%) |
| Bradi4g23367 | −1.86 | −2.18 | PAD4-like (38%) |
| Bradi3g32990 | 1.62 | 1.76 | FMO1-like (32%) |
| Bradi5g20547 | 28.99 | 32.88 | BdAOX1A (90%) |
| Bradi5g20540 | 22.16 | 23.93 | BdAOX1A-like (70%) |
| Bradi5g20557 | 6.01 | 6.38 | BdAOX1B (86%) |
| Bradi2g15877 | 20.59 | 23.90 | WRKY53-like (42%) |
| Bradi2g30695 | 4.96 | 5.28 | WRKY70-like (12.4%) |
| Bradi4g30360 | 3.67 | 2.96 | WRKY18-like (43%) |
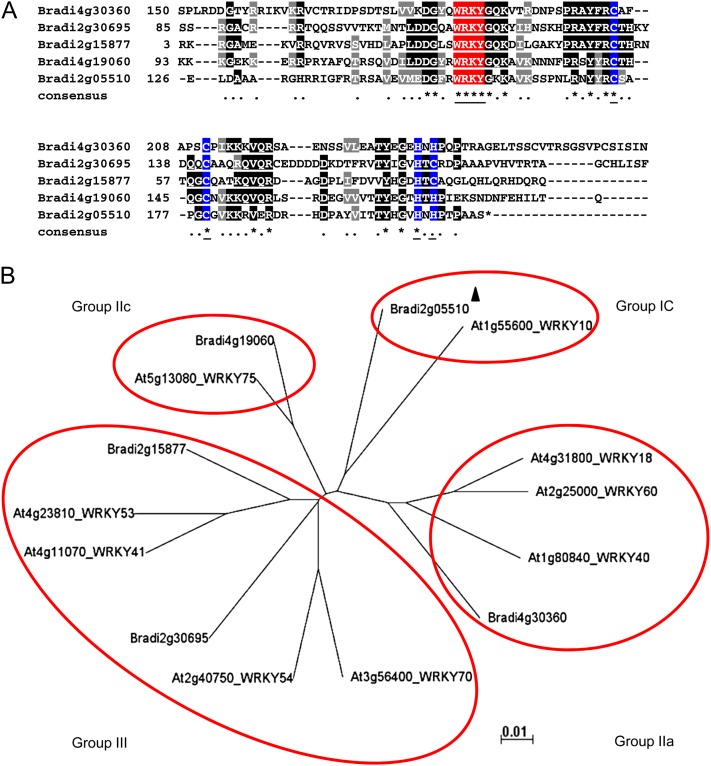
Activation of PR genes by SA or pathogen infection is likely a downstream secondary response, mediated in Arabidopsis by NPR1, TGA, and WRKY family transcriptional regulators (Moore et al., 2011). From our microarray results, transcript levels of NPR1 and TGA family members were not affected by PMV or PMV+SPMV infection (data not shown). However, multiple WRKY genes were up-regulated in PMV and PMV+SPMV infections (Table I; Fig. 5). WRKY factors belong to a superfamily of DNA-binding transcriptional regulators that are crucial for plant growth and development and mediate responses to biotic and abiotic stress and innate immunity (Rushton et al., 2010). To identify PMV- and PMV+SPMV-affected WRKY factors, we performed multiple sequence alignment and phylogenetic comparisons with Arabidopsis WRKY factors. In Arabidopsis, WRKY factors are classified into different groups based on their WRKY and zinc-finger domains (Rushton et al., 2010). As expected, Brachypodium WRKY factors modulated in PMV and PMV+SPMV infection have signature WRKY and zinc-finger motifs (Fig. 5A) and belonged to group IC (Bradi2g05510), group IIa (Bradi4g30360), group IIc (Bradi4g19060), and group III (Bradi2g15877 and Bradi2g30695; Fig. 5B). In Arabidopsis, WRKY18, -53, -54, and -70 have roles in SA-mediated responses to bacterial and fungal diseases (Wang et al., 2006). PMV- and SPMV-modulated WRKY factors were phylogenetically closer to Arabidopsis WRKY10 (group IC); WRKY18, -40, and -60 (group IIa); WRKY75 (group IIc); and WRKY53, -41, -54, and -70 (group III), respectively (Fig. 5B), suggesting their potential roles in mediating SA responses in PMV or PMV+SPMV infection.
Figure 5.
Sequence analysis of PMV- and SPMV-modulated WRKY transcription factors in Brachypodium. A, Multiple sequence alignment of WRKY-domain sequence of WRKY factors misexpressed in PMV and SPMV infection. The consensus symbols are indicated below the alignments with identically conserved residues indicated by black shading and an asterisk. Amino acids with ≥50% identity are shaded gray and marked with a period. The conserved WRKY and zinc-finger motifs are underlined and in red and blue, respectively. B, The phylogenetic relationship of Brachypodium WRKY factors misexpressed in PMV and PMV+SPMV infections with orthologous Arabidopsis WRKY factors. The protein sequences were retrieved from Phytozome (Goodstein et al., 2012), aligned using ClustalX2 (Larkin et al., 2007), and displayed as an unrooted tree using Archeopteryx (Han and Zmasek, 2009). The branch length at bottom right of the phylogram indicates genetic distances. The down-regulated gene is identified by a black triangle. The grouping of WRKY factors into Groups Ic, IIa, IIc, and III was based on the classification described previously (Rushton et al., 2010). [See online article for color version of this figure.]
PMV and SPMV Modulate Expression of Jasmonic Acid and Ethylene Signaling-Related Genes
In addition to SA, two other hormones, jasmonic acid (JA) and ethylene (ET), play important roles in plant defenses against pathogens (Pieterse et al., 2009; Verhage et al., 2010; An and Mou, 2011). Although there are reports of SA and JA synergy (Mur et al., 2006), in general, SA antagonizes JA and ET responses, especially in mediating defense against biotrophic pathogens (Vlot et al., 2009). PMV and PMV+SPMV infections suppressed the expression of putative Brachypodium LIPOXYGENASE2 (LOX2) and ALLENE OXIDE SYNTHASE (AOS) enzymes (Table II), which in Arabidopsis are associated with JA biosynthesis. Although expression of 12-OPDA REDUCTASE3 (OPR3), an enzyme functioning downstream of LOX2 and AOS in JA biosynthesis was up-regulated (Table II), two putative JA-responsive genes, including VEGETATIVE STORAGE PROTEIN1 (VSP1) and FATTY ACID DESATURASE7 (FAD7), were down-regulated (Table II). Few of the PMV- and PMV+SPMV-infected plants transitioned to flowering (Fig. 1): These plants had fewer flowers, reduced or no seed set, and poor viability (Fig. 1; data not shown). Such reproductive defects are also common in several JA-deficient mutants, such as fad3/fad7/fad8, aos, and opr3 in Arabidopsis (Wasternack, 2007), and correlate to the suppressed JA responses in the PMV- and PMV+SPMV-infected plants.
Table II. Differential expression of putative Brachypodium genes involved in JA and ET signaling.
The relative unlogged fold change (≥1.5-fold) in gene expression of PMV and PMV+SPMV infection compared with mock are indicated. Minus sign represents down-regulated fold change. The gene descriptions were deduced from peptide ortholog analysis with Arabidopsis protein sequences using Phytozome (Goodstein et al., 2012). The percentage similarity of Brachypodium proteins with the orthologous Arabidopsis proteins is indicated in parentheses. Note that some Brachypodium genes with low percentage similarity to their respective Arabidopsis orthologs are indicated as “-like.” For additional phylogenetic analyses, see Supplemental Figure S2. The statistical significance of gene expression changes was determined using Student’s t test and was corrected for FDR using the Benjamini and Hochberg method (q-value < 0.05).
| Gene ID | PMV | PMV+SPMV | Description |
|---|---|---|---|
| Bradi1g05870 | 3.60 | 3.07 | BdOPR3 (66%) |
| Bradi2g35907 | 2.43 | 2.51 | OPR3-like (52%) |
| Bradi1g16580 | −2.00 | −3.03 | BdFAD7 (71%) |
| Bradi1g51330 | −1.64 | −1.89 | VSP1-like (44%) |
| Bradi3g01110 | −1.64 | −1.92 | BdAOS (56%) |
| Bradi3g30510 | 4.02 | 4.75 | BdCYP89A6 (65%) |
| Bradi3g07010 | −1.96 | −2.86 | BdLOX2 (66%) |
| Bradi3g07000 | −2.56 | −2.56 | LOX2-like (64%) |
| Bradi3g39980 | 6.30 | 7.68 | LOX-like (62%) |
| Bradi3g11260 | 1.60 | 1.74 | BdMKK3 (64%) |
| Bradi3g50490 | −2.50 | −2.70 | BdERF-1 (42%) |
| Bradi1g72450 | −8.33 | −7.69 | ERF/RAP2.2-like (28%) |
| Bradi5g25570 | −4.76 | −5.26 | ERF3-like (28%) |
| Bradi2g52370 | −2.63 | −2.44 | ERF4-like (31%) |
| Bradi1g75960 | 2.40 | 2.51 | BdACO1 (82%) |
The precursor of ET, 1-aminocyclopropane-1-carboxylic acid (ACC), is synthesized by ACC synthase. Production of ACC is a rate-limiting step in ET biosynthesis. ACC is further converted to ET by ACC oxidase (ACO; Dong et al., 1992). PMV- and PMV+SPMV-infected plants had higher levels of ACO1 transcript compared with mock (Table II). However, expression of several putative ET response factors such as ETHYLENE RESPONSE FACTOR-1 (ERF-1), ERF3-like, ERF4, and ERF/Related to AP2.2 (RAP2.2) were down-regulated (Table II), suggesting suppressed ET signaling in the infected plants.
Together, these results support alteration of JA and ET signaling, as a component of host immune responses to PMV and PMV+SPMV infection, possibly via interactions with SA signaling (Vlot et al., 2009).
Temporal Expression Analysis of Putative SA, JA, and ET Components during the Progression of PMV and SPMV Infection
Because the altered SA, JA, and ET responses in the PMV- and PMV+SPMV-infected plants identified using microarray analysis was only a snapshot of the affected defense responses before the onset of severe disease symptoms (stage II), we analyzed their dynamics during the other stages of infection. For this, we sampled PMV- and PMV+SPMV-infected plants when they displayed no symptoms (stage I), mild chlorosis symptoms (stage II), and severe chlorosis, necrosis, and stunting symptoms (stage III), as described earlier (Fig. 1). Next, we performed qRT-PCR analysis to quantify expression of putative SA (PR-1-, PR-3-, PR-5-, PAD4-like, and AOX1A), JA (LOX2, AOS, and OPR3), and ET (ERF-1 and ERF3-like) components in the infected plants. Based on the microarray data (Tables I and II), the aforementioned genes demonstrated varying levels of misexpression in PMV- and PMV+SPMV-infected plants. The temporal expression analysis revealed activated expression of PR-1, PR-3, and PR-5-like genes in PMV (≥2.8-fold) and in PMV+SPMV-infected plants (≥1.7-fold) when compared with mock-inoculated plants as early as stage I, when visible symptoms were not evident (Fig. 6, A–C). PR gene expression continued to increase in subsequent stages of infection (stages II and III; Fig. 6, A–C). Also consistent with the microarray data, expression of multiple PR genes was significantly less induced in PMV+SPMV-infected plants compared with PMV alone infection (Fig. 6, A–C; Table I). Similarly, expression of AOX1A was induced ≥250-fold in PMV and ≥50-fold in PMV+SPMV-infected plants at stage I and was ≥500-fold at stages II and III (Fig. 6E) in both infections. In contrast with the activated expression of PR and AOX genes that are components downstream of SA signaling in Arabidopsis, expression of a putative PAD4-like gene, an upstream positive regulator of SA responses (Zhou et al., 1998), was significantly down-regulated in the infected plants in stages II and III; however, its expression was slightly induced (approximately 1.3- to 1.4-fold; P value ≤ 0.05) early in the infection (stage I; Fig. 6D). Furthermore, expression of multiple JA and ET components LOX2, AOS, ERF-1, and ERF3-like genes were all uniformly suppressed in PMV- and PMV+SPMV-infected plants, while expression of OPR3 was up-regulated (Fig. 6, F–J), results that are again consistent with the microarray data. Similar to the expression pattern of the PAD4-like gene, expression of LOX2 and ERF3-like genes was significantly higher early in the infection (stage I) but suppressed in stages II and III when compared with the mock-inoculated plants (Fig. 6, D, F, and J). Moreover, multiple SA, JA, and ET components in Brachypodium were developmentally regulated. PR-1, PR-3, and PR-5-like gene expression was highest in plants in their reproductive phase with 2.3-, 2.0-, and 2.3-fold change induction, respectively, when compared with younger plants at stage I (Fig. 6, A–C). Expression of AOS and ERF-1 was also higher early in the vegetative phase (stage I), while their expression was suppressed in late vegetative phase (stage II), as well as in the reproductive phase (stage III; Fig. 6, G and I). LOX2 and ERF3-like genes displayed the opposite pattern, with lowest expression early in the vegetative phase (stage I; Fig. 6, F and J). Interestingly, PMV and PMV+SPMV infection perturbed the normal developmental expression pattern of LOX2 and ERF3-like genes but not AOS and ERF-1 (Fig. 6, F, G, I, and J).
Figure 6.
Temporal expression analysis of SA, JA, and ET components during PMV and SPMV disease progression. Brachypodium plants infected with PMV, PMV+SPMV, or mock inoculated sampled at stages I, II, and III (see Fig. 1C for description of stages) to determine the expression of putative genes in SA responses PR-1-like (A), PR-3-like (B), PR-5-like (C), PAD4-like (D), and AOX1A (E); JA responses LOX2 (F), AOS (G), and OPR3 (H); and ET responses ERF-1 (I) and ERF3-like (J) using qRT-PCR analysis. Transcript level of UBIQUITIN18 was used to normalize the qRT-PCR data. Expression values plotted are average of three biological replicates. Error bars represent sd among the replicates. Significant changes in gene expression were determined using Student’s t test (P value < 0.05). Note that the expression scales for each gene panel are unique and are relative to the mock-inoculated control plants at stage I. Primers used are listed in Supplemental Table S7. [See online article for color version of this figure.]
Together, these analyses not only corroborate the microarray findings, but also reveal the dynamic expression patterns of multiple SA, JA, and ET components during Brachypodium development and their differential perturbation in PMV and PMV+SPMV infections.
PMV and SPMV Infection Modulates Expression of Multiple Receptor-Like Kinase/Pelle Members
PMV and PMV+SPMV infection altered expression of several Ser-Thr kinases (STKs). Expression of 22 STKs was up-regulated by 2-fold or greater (FDR < 0.05) in PMV and PMV+SPMV infection (Table III), with six additional STKs whose expression was affected by 1.5- to 2-fold (data not shown). Based on the predicted protein sequences and the functional domains, the majority of these kinases were identified as being receptor-like kinase/Pelle (RLK) proteins (Shiu et al., 2004). Most plant RLKs are transmembrane proteins or membrane-associated proteins with intracellular kinase domains, although some are localized to the cytosol (Shiu et al., 2004; Padmanabhan and Dinesh-Kumar, 2010). Using multiple sequence alignment analysis and phylogenetic comparisons, we identified PMV- and PMV+SPMV-affected kinases as belonging to RLK subfamilies LRR-VIII-2, LRR-XII, wall-associated kinase (WAK)/LRK10L-1, RLCK-OS2, RLCK-IV, RLCK-IXb, SD-1c, SD-2b, l-LEC, and DUF26, in addition to non-RLK subfamilies, such as MEKK_ste11_MAP3K, KIN1/SNF1/Nim1_like (CAMK_2), and PVPK_like (AGC_8)_kin82y (Fig. 7; Table III). Several of the RLKs possessed additional domains such as Leu-rich repeat (LRR), calcium-binding epidermal growth factor (EGF), legume-lectin (l-LEC), d-Man binding lectin, and U-box domains (Fig. 7), as well as three RLKs possessed domains of unknown function (DUF26). Kinases such as those possessing l-LEC, d-Man binding lectin, and DUF26 also contained putative transmembrane domains (Supplemental Table S3), suggesting that they may function as transmembrane RLKs. The general up-regulation of several host kinases and RLKs suggests that phosphorylation modifications of either host or virus-encoded proteins are crucial signals in PMV and PMV+SPMV infection.
Table III. Differential expression of putative Brachypodium kinases altered in PMV and PMV+SPMV infection.
The relative unlogged fold change (≥2-fold) in gene expression of PMV and PMV+SPMV infection compared with mock are indicated. Minus sign represents down-regulated fold-change. The gene descriptions were deduced from Pfam and PANTHER functional annotations obtained using the Phytozome (Goodstein et al., 2012). The statistical significance of gene expression changes was determined using Student’s t test and was corrected for FDR using the Benjamini and Hochberg method (q-value < 0.05).
| Gene ID | PMV | PMV+SPMV | Description |
|---|---|---|---|
| Bradi4g02020 | 5.15 | 5.36 | LRR protein kinase, RLK plant type, subfamily LRR-VIII-2 |
| Bradi1g32630 | 2.25 | 2.10 | Protein kinase domain, RLK plant type, subfamily LRR-VIII-2 |
| Bradi4g11740 | 23.97 | 21.17 | LRR N-terminal domain, RLK plant type, subfamily LRR-XII |
| Bradi5g21857 | 2.91 | 3.02 | LRR, Di-Glc binding within endoplasmic reticulum, RLK plant type |
| Bradi4g02850 | 5.19 | 6.07 | WAK, RLK plant type, subfamily WAK/LRK10L-1 |
| Bradi1g02210 | 6.88 | 8.33 | Calcium-binding EGF domain, WAK, RLK plant type |
| Bradi3g01157 | 2.66 | 2.55 | Calcium-binding EGF domain, WAK, RLK plant type |
| Bradi4g21990 | 4.05 | 4.53 | Protein kinase domain, RLK plant type, subfamily RLCK-OS2 |
| Bradi1g75140 | 2.40 | 2.31 | Protein kinase domain, RLK plant type, subfamily RLCK-IV |
| Bradi4g38690 | 3.68 | 4.76 | U-box domain, protein kinase domain, RLK plant type, subfamily RLCK-IXb |
| Bradi4g37220 | 5.72 | 5.36 | d-Man binding lectin, S-locus glycoprotein, RLK plant type, subfamily SD-1c |
| Bradi5g02980 | 3.51 | 2.82 | d-Man binding lectin, RLK plant type, subfamily SD-2b |
| Bradi2g20687 | 2.37 | 3.00 | d-Man binding lectin, S-locus glycoprotein, RLK plant type |
| Bradi4g24590 | 3.20 | 4.19 | Protein kinase domain, RLK plant type |
| Bradi2g47510 | −5.26 | −7.14 | MAPKK related, subfamily MEKK_ste11_MAP3K |
| Bradi4g30380 | −3.23 | −3.33 | NAF domain, calcium/calmodulin-dependent protein kinase, subfamily KIN1/SNF1/Nim1_like (CAMK_2) |
| Bradi4g45310 | −2.22 | −2.78 | AGC kinases, PAS fold, subfamily PVPK_like (AGC_8)_kin82y |
| Bradi1g57700 | 6.33 | 6.94 | Protein kinase domain, RLK plant type, subfamily l-LEC |
| Bradi1g23710 | 5.64 | 4.92 | Legume lectin domain, RLK plant type, subfamily l-LEC |
| Bradi1g23730 | 11.32 | 9.39 | Legume lectin domain, RLK plant type, subfamily l-LEC |
| Bradi1g57657 | 5.45 | 7.01 | Legume lectin domain, RLK plant type, subfamily l-LEC |
| Bradi1g57690 | 3.38 | 4.23 | Legume lectin domain, RLK plant type, subfamily l-LEC |
| Bradi5g16760 | 3.37 | 3.17 | Legume lectin domain, RLK plant type, subfamily l-LEC |
| Bradi1g08990 | −2.38 | −2.00 | Protein kinase domain, subfamily l-LEC |
| Bradi1g25647 | 2.32 | 2.56 | Domain of unknown function DUF26, RLK plant type |
| Bradi1g42397 | −2.04 | −2.13 | Domain of unknown function DUF26, RLK plant type |
| Bradi1g25717 | 2.54 | 2.26 | Domain of unknown function DUF26, RLK plant type |
Figure 7.
Phylogenetic classification of Brachypodium Ser-Thr kinases altered in PMV and PMV+SPMV infection. The sequences were retrieved from Phytozome (Goodstein et al., 2012), aligned using ClustalX2 (Larkin et al., 2007), and displayed as an unrooted tree using Archeopteryx (Han and Zmasek, 2009). The functional domains and subfamilies of the kinases were deduced using predicted Pfam and PANTHER annotations (Thomas et al., 2003; Punta et al., 2012) using Phytozome (Goodstein et al., 2012). The kinases were grouped into subfamilies: LRR-VIII-2, LRR-XII, WAK/LRK10L-1, RLCK-OS2, RLCK-IV, RLCK-IXb, SD-1c, SD-2b, MEKK_ste11_MAP3K, KIN1/SNF1/Nim1_like (CAMK_2), PVPK_like_kin82y (AGC_8), l-LEC, and DUF26, following a classification previously described (Shiu et al., 2004). Down-regulated genes are identified by black triangles. Note that among the down-regulated genes, Bradi2g47510, Bradi4g30380, and Bradi4g45310 cluster together, suggesting a transcriptional coregulation of these phylogenetically related kinases.
The SPMV Synergism: Modulation of PMV-Triggered Defense Gene Expression and Alteration of Unique Nuclear Factors
Because SPMV enhanced PMV accumulation and exacerbated disease symptoms (Fig. 1; Scholthof, 1999), we analyzed the effect of SPMV on host molecular processes. Unexpectedly, multiple genes in SA, JA, and ET defenses, particularly PR and AOX genes that were altered in PMV single infections, consistently demonstrated lower fold changes of misexpression in coinfection with SPMV, as determined by microarray and qRT-PCR analysis (Tables I and II; Fig. 6). In contrast with the antagonistic effect on defense gene expression, a large number of genes in diverse functional categories displayed additive or synergistic alteration in their expression patterns during a coinfection of SPMV (Tables IV and V). Intriguingly, few distinctive genes were uniquely altered by SPMV (Table VI), compared with PMV alone or mock-inoculated plants. After verification by qRT-PCR, we identified these unique up-regulated genes as spliceosome-associated factors (Bradi2g09850 and Bradi5g07850), an AP2 family transcription factor (Bradi3g06562), and a protein of unknown function (Bradi4g34700) with a predicted nuclear localization signal (based on PSORT analysis; data not shown).
Table IV. Additively induced Brachypodium genes by SPMV.
The relative unlogged fold change (≥2-fold) in gene expression of PMV and PMV+SPMV infection compared with mock are indicated. Only gene expression changes that are statistically significant (P value < 0.05) between PMV and PMV+SPMV infections are listed. The gene descriptions were deduced from Pfam and PANTHER functional annotations obtained using the Phytozome (Goodstein et al., 2012). An asterisk represents genes that are also present in other tables and figures elsewhere in the article.
| Gene ID | PMV | PMV+SPMV | Description |
|---|---|---|---|
| Bradi3g31720* | 117.20 | 164.83 | Glutathione S-transferase, N-terminal domain |
| Bradi2g49067 | 78.57 | 119.88 | Unknown |
| Bradi1g74970 | 73.33 | 96.13 | Unknown |
| Bradi3g16650 | 52.07 | 65.42 | Unknown |
| Bradi2g48160 | 47.15 | 61.90 | Eukaryotic porin |
| Bradi3g31727 | 40.47 | 53.87 | Unknown |
| Bradi1g06980 | 38.47 | 50.74 | DnaJ domain |
| Bradi2g44820 | 21.24 | 27.73 | UDP-glucoronosyl and UDP-glucosyl transferase |
| Bradi1g21921 | 16.93 | 22.52 | Sulfotransferase4B (ST4B) |
| Bradi2g10857 | 12.29 | 20.97 | Unknown |
| Bradi1g59240 | 9.75 | 18.45 | Unknown |
| Bradi3g21270 | 10.61 | 15.96 | Protein of unknown function (DUF1218) |
| Bradi3g60470 | 6.61 | 11.70 | Metallo-β-lactamase superfamily |
| Bradi3g28120 | 7.78 | 10.83 | Unknown |
| Bradi1g69140 | 7.64 | 10.25 | RST domain of plant C terminus |
| Bradi5g26080 | 8.36 | 10.15 | Dihydroorotate dehydrogenase |
| Bradi3g09120 | 5.93 | 8.87 | Peroxidase |
| Bradi3g53227 | 6.25 | 8.17 | Unknown |
| Bradi2g48540 | 6.55 | 7.94 | Unknown |
| Bradi3g49330* | 4.99 | 7.92 | Ubiquitin family |
| Bradi1g57657 | 5.45 | 7.01 | Unknown |
| Bradi2g18630 | 5.40 | 6.62 | Eukaryotic porin |
| Bradi3g38050 | 4.00 | 6.17 | Carboxylesterase, α/β hydrolase fold |
| Bradi3g48590 | 4.47 | 5.97 | Lipocalin/cytosolic fatty-acid binding protein family |
| Bradi5g08520 | 4.21 | 5.93 | K+ potassium transporter |
| Bradi3g14140 | 3.91 | 5.05 | Transmembrane amino acid transporter protein, Trp/Tyr permease family |
| Bradi2g14610 | 3.38 | 5.03 | Holliday junction DNA helicase ruvB N terminus, ATPase family |
| Bradi1g77680 | 3.39 | 4.83 | Unknown |
| Bradi1g00700 | 3.70 | 4.80 | Sugar (and other) transporter, major facilitator superfamily |
| Bradi4g38690* | 3.68 | 4.76 | Protein kinase domain, universal stress protein family, U-box domain |
| Bradi3g22880 | 3.27 | 4.76 | MatE protein, multidrug resistance pump |
| Bradi4g27850* | 3.40 | 4.67 | AP2 domain |
| Bradi2g55892 | 3.48 | 4.59 | Unknown |
| Bradi2g44150* | 2.74 | 4.30 | Cytochrome P450 |
| Bradi2g50630 | 3.55 | 4.29 | NLI interacting factor-like phosphatase |
| Bradi1g57690* | 3.38 | 4.23 | Legume lectin domain, protein kinase domain |
| Bradi4g24590* | 3.20 | 4.19 | Protein kinase domain |
| Bradi3g21310 | 3.33 | 4.16 | Miro-like protein, RAS family, EF hand associated |
| Bradi1g11780 | 3.24 | 4.15 | ABC1 family |
| Bradi2g47020 | 3.01 | 4.05 | Unknown |
| Bradi2g44200* | 2.48 | 3.96 | Cytochrome P450 |
| Bradi3g16450 | 3.24 | 3.92 | SPFH domain/Band7 family, Stomatin-like integral membrane domain |
| Bradi3g09620 | 2.60 | 3.91 | Unknown |
| Bradi3g52367 | 2.94 | 3.66 | Unknown |
| Bradi1g45470* | 2.50 | 3.66 | AP2 domain |
| Bradi5g12530 | 2.92 | 3.59 | SPFH domain/Band7 family, Stomatin-like integral membrane domain |
| Bradi1g10840 | 2.62 | 3.53 | UDP-glucoronosyl and UDP-glucosyl transferase |
| Bradi1g26850 | 2.44 | 3.35 | C2 domain, Ca2+-dependent phospholipid-binding protein |
| Bradi1g20995 | 2.48 | 3.31 | Unknown |
| Bradi5g07380 | 2.45 | 3.10 | Cleavage site for pathogenic type III effector avirulence factor Avr |
| Bradi1g14350 | 2.49 | 3.09 | MatE protein, multidrug resistance pump |
| Bradi2g20687* | 2.37 | 3.00 | d-Man binding lectin, S-locus glycoprotein, RLK plant type |
| Bradi3g52830 | 2.40 | 2.94 | ATPase family associated with various cellular activities (AAA) |
| Bradi2g51592 | 2.29 | 2.90 | Unknown |
| Bradi5g02980* | 3.51 | 2.82 | d-Man binding lectin, protein kinase domain |
| Bradi3g47580 | 2.14 | 2.62 | Zinc finger, ZZ type, UBA/TS-N domain, ubiquitin-associated |
| Bradi3g48690 | 2.02 | 2.52 | Unknown |
Table V. Additively down-regulated Brachypodium genes by SPMV.
The relative unlogged fold change (≥2-fold) in gene expression of PMV and PMV+SPMV infection compared with mock is indicated. Only gene expression changes that are statistically significant (P value < 0.05) between PMV and PMV+SPMV infections are listed. The gene descriptions were deduced from Pfam and PANTHER functional annotations obtained using the Phytozome (Goodstein et al., 2012). An asterisk represents genes that are also present in other tables and figures elsewhere in the article.
| Gene ID | PMV | PMV+SPMV | Description |
|---|---|---|---|
| Bradi5g05110* | −9.09 | −33.33 | Helix-loop-helix DNA-binding domain |
| Bradi4g42540* | −8.33 | −14.29 | Cytochrome P450 |
| Bradi4g36601 | −3.70 | −12.50 | Aquaporin transporter |
| Bradi2g05510* | −3.45 | −6.67 | WRKY DNA-binding domain |
| Bradi5g17500 | −3.33 | −6.25 | NUDIX domain, phosphohydrolase |
| Bradi2g55050 | −4.00 | −5.88 | Multicopper oxidase |
| Bradi1g12130* | −2.63 | −5.26 | Chlorophyll a/b binding protein |
| Bradi2g05832 | −2.50 | −4.55 | Unknown |
| Bradi2g20380* | −2.78 | −4.55 | Photosystem II reaction center W protein (PsbW) |
| Bradi2g54430 | −2.86 | −4.35 | Unknown |
| Bradi1g42670* | −2.63 | −4.35 | Chlorophyll a/b binding protein |
| Bradi3g32200 | −2.94 | −4.17 | Unknown |
| Bradi2g56030 | −2.56 | −4.00 | Fru-1-6-bisphosphatase |
| Bradi3g37680 | −2.56 | −3.85 | Cupin domain |
| Bradi4g20500 | −2.38 | −3.85 | GUN4-like, magnesium-protoporphyrin IX synthesis |
| Bradi2g62520 | −2.78 | −3.70 | Major intrinsic protein, aquaporin |
| Bradi1g68210 | −2.63 | −3.70 | Senescence-associated protein |
| Bradi3g07190* | −2.56 | −3.70 | Chlorophyll a/b binding protein |
| Bradi5g22290 | −2.17 | −3.57 | Aminomethyltransferase folate-binding domain |
| Bradi3g10770 | −2.56 | −3.33 | Unknown |
| Bradi3g04930 | −2.17 | −3.23 | rRNA adenine dimethylase, ubiE/COQ5 methyltransferase family |
| Bradi2g26280 | −2.50 | −3.23 | Unknown |
| Bradi3g60920 | −2.00 | −3.13 | NAD-dependent epimerase/dehydratase family, male sterility protein |
| Bradi1g55720 | −2.08 | −2.94 | Leu-rich repeat Ser/Thr phosphatase |
| Bradi5g01737 | −2.13 | −2.86 | Unknown |
| Bradi3g13970* | −2.13 | −2.86 | Photosystem II protein Y (PsbY) |
| Bradi4g45310* | −2.22 | −2.78 | Protein kinase domain |
| Bradi1g24870* | −2.00 | −2.63 | Chlorophyll a/b binding protein |
| Bradi5g17480* | −2.04 | −2.63 | AP2 domain |
Table VI. Unique Brachypodium nuclear transcription and splicing factors altered by SPMV.
Differential expression of putative splicing factors and transcription factors uniquely altered in PMV+SPMV infection. The fold changes in gene expression in PMV and PMV+SPMV infections compared with mock are shown. Changes in gene expression in PMV+SPMV infection as determined by microarray and qRT-PCR analysis strongly correlate to each other [Pearson correlation coefficient (r) = 0.95 and coefficient of determination (R2) = 0.92] and are statistically significant compared with mock and PMV infections. Changes in gene expression in PMV infection and mock are insignificant. The statistical significance of qRT-PCR changes in gene expression was determined using Student’s t test (P value < 0.05). For the microarray data, FDR correction using the Benjamini and Hochberg method (q-value < 0.05) was used. Primers used for this study are listed in Supplemental Table S6. The gene descriptions were deduced from Pfam and PANTHER functional annotations obtained using the Phytozome (Goodstein et al., 2012).
| Gene ID | Microarray |
qRT-PCR |
Description | ||
|---|---|---|---|---|---|
| PMV | PMV+SPMV | PMV | PMV+SPMV | ||
| Bradi2g09850 | 1.11 | 1.58 | 0.90 | 1.28 | Pre-mRNA splicing factor PRP21 like protein, splicing factor3A subunit1 |
| Bradi5g07850 | 1.11 | 2.09 | 1.30 | 1.84 | Splicing factor1/branch point binding protein (RRM superfamily), zinc knuckle domain |
| Bradi3g06562 | 1.16 | 1.55 | 1.00 | 1.34 | AP2 domain |
| Bradi4g34700 | 1.02 | 1.63 | 1.10 | 1.50 | Unknown |
Taken together, these results show that SPMV modulates PMV-triggered defense gene expression and uniquely activates expression of putative nuclear-localized proteins involved in transcription and splicing. We hypothesize that the cumulative effect of these processes relates to the exacerbated disease symptoms induced by SPMV in PMV+SPMV coinfections.
DISCUSSION
In contrast with dicot plant virus research, studies with monocot-infecting viruses have lagged due to the lack of a reliable genetic model. Grasses, including rice, maize (Zea mays), switchgrass, wheat, and sorghum have long lifecycles, require an abundance of greenhouse space, and oftentimes lack critical genomic tools (microarrays, annotated genomes, etc.). Recently, Brachypodium has emerged as a promising model for grass biology research (Draper et al., 2001; Huo et al., 2006; Vogel et al., 2006; Vogel and Hill, 2008; Bevan et al., 2010; Garvin et al., 2010; International Brachypodium Initiative, 2010; Brkljacic et al., 2011; Cao et al., 2011). Here, we demonstrated the utility of Brachypodium as a model system for plant pathobiology studies with a focus on the synergism of the mixed infection of PMV and SPMV. PMV- and PMV+SPMV-infected Brachypodium produced the same disease outcome we had previously observed with various millet species (Scholthof, 1999), including severe symptoms of chlorosis and necrosis, stunting, and reduced seed set (Figs. 1 and 2). The earlier detection of PMV CP together with the exacerbated symptoms of PMV+SPMV-infected plants compared with PMV alone infection (Fig. 1) supports a role for SPMV in enhancing PMV accumulation in Brachypodium, a finding consistent with our previous reports of PMV+SPMV synergism in millets (Scholthof, 1999). Because viral synergistic interactions can be host specific (González-Jara et al., 2004), recapitulating the results of these earlier experiments in millet species was a critical first step toward establishing Brachypodium as a robust genetic model species for studying PMV and SPMV interactions.
Our comparative transcriptome analyses of PMV alone and the mixed PMV+SPMV infections in Brachypodium (summarized in Fig. 8) with previously known dicot-infecting plant viruses (Whitham et al., 2003; Ascencio-Ibáñez et al., 2008; García-Marcos et al., 2009; Elena et al., 2011; Hanssen et al., 2011) revealed several distinctive features of virus-host interactions of monocots. PMV and PMV+SPMV infection prominently triggered SA-mediated defense responses, a response that appears conserved with several dicot host:virus interactions (Murphy et al., 1999; Kachroo et al., 2000; Whitham et al., 2003, 2006; Huang et al., 2005; Ascencio-Ibáñez et al., 2008; Hanssen et al., 2011; Pacheco et al., 2012). Multiple genes in the PR protein family were strongly up-regulated in PMV alone and PMV+SPMV infections in Brachypodium (Table I, and summarized in Fig. 8). Brachypodium has two PR-1-like (Bradi1g57580 and Bradi1g57590) and two PR-5-like (Bradi1g13060 and Bradi1g13070) genes that are tandemly arranged on chromosome 1. Expression of both copies of PR-1-like and PR-5-like genes was induced in PMV and PMV+SPMV infections (Table I), suggesting that these genes are transcriptionally coregulated. Similarly, expression of three tandemly arranged subunits of AOX, an enzyme activated by SA and pathogen infection (Lennon et al., 1997), was uniformly up-regulated (Table I, and summarized in Fig. 8). Although Arabidopsis also has tandemly arranged PR and AOX genes, it is not known if they are transcriptionally coregulated in response to pathogen infections. PR gene expression was also activated in healthy Brachypodium plants (mock inoculated) as they matured (Fig. 6). This correlates with their known roles in Arabidopsis developmental leaf senescence (Lim et al., 2007), a process triggered as plants mature. However, PMV and SPMV infection constitutively activated PR-1, PR-3, PR-5-like genes, and AOX1A, although expression of PR3-like gene and AOX1A displayed a trend of down-regulation as the infected plants matured (Fig. 6). Despite the strong activation of PR genes and other SA components, PMV- and PMV+SPMV-infected plants did not display precocious senescence (data not shown), suggesting that the role of PR proteins in defense responses is distinct from their function in developmental senescence.
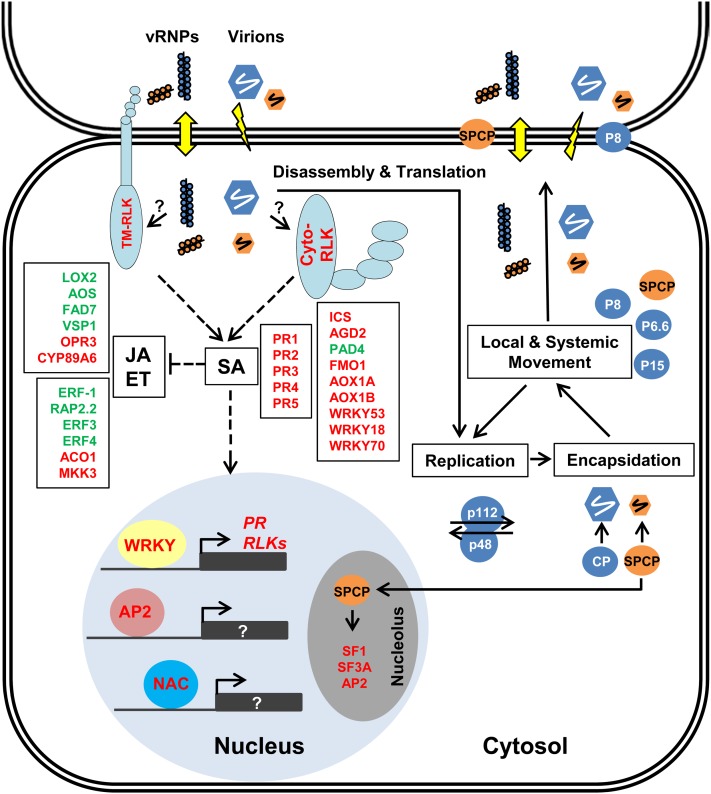
Figure 8.
Proposed model for the host:pathogen interactions induced by PMV alone or the PMV+SPMV synergism in Brachypodium. Briefly, the PMV replicase-associated proteins (P48 and P112), movement-associated proteins (P8, P6.6, and P15), PMV CP, and SPMV capsid protein (SPCP) cooperatively function in translation, replication, encapsidation, and movement of PMV and SPMV in a systemic infection of Brachypodium and other grasses in the Poaceae. PMV and SPMV move cell to cell primarily via plasmodesmata (up-down arrow) as viral ribonucleoprotein complexes, probably with assistance from PMV P8, SPCP (which are also associated with the host cell membrane), P6.6, and P15 (Turina et al., 2000; Qi et al., 2008). Blue and orange beads on a string represent PMV and SPMV vRNPs, respectively. Both PMV virions (blue hexagon) and SPMV virions (orange hexagon) assemble in the cytosol of an infected leaf and are probably introduced into new cells through cell damage (lightning bolt). An as yet unidentified PMV- or SPMV-associated viral component (RNA and/or proteins indicated by “?”) elicits host defense responses activating expression of multiple transmembrane receptor-like kinases (TM-RLK) and cytoplasmic receptor-like kinases (Cyto-RLK). PMV and PMV+SPMV infection also triggers expression of SA, JA, and ET components, possibly as a host defense response (the red and green text indicates up- and down-regulated genes, respectively). SPCP also localizes to the host nucleus and nucleolus (Qi et al., 2008), thereby uniquely modulating expression of putative splicing factors (SF1 and SF3A) and an AP2 factor and may affect alternative splicing of certain host mRNAs. The annotation of Brachypodium genes is based on orthologous Arabidopsis proteins. Further outcomes of the Brachypodium transcriptome changes in response to PMV and PMV+SPMV infections are explained in the “Discussion.” The PMV and SPMV genomes are shown in Supplemental Figure S1.
In support of the antagonistic relationship between SA and JA/ET responses, PMV and PMV+SPMV infection suppressed expression of multiple JA and ET components (Table II; Fig. 6, and summarized in Fig. 8). JA and ET hormones are also implicated in plant development, including senescence (van der Graaff et al., 2006) and flower development (Wasternack, 2007). In Arabidopsis, expression of LOX2 is induced in response to wounding (Bell et al., 1995), methyl-jasmonate treatment (Jensen et al., 2002), and senescence (van der Graaff et al., 2006). Here, we found that the expression of LOX2 was developmentally regulated, with highest expression in mature plants (Fig. 6). Expression of AOS, an enzyme functioning downstream of LOX2 in JA biosynthesis (Wasternack, 2007), was also developmentally regulated; however, its expression appeared antagonistic to LOX2, with lowest expression in the mature plants (Fig. 6).
Although the cross talk between JA and ET signaling in plant-pathogen interactions is widely known, relatively less information is available regarding the transcriptional regulation and feedback between the two pathways. In Arabidopsis, members of AP2/ERF family of transcription factors, such as ERF1, mediate JA-induced transcriptional changes in response to pathogen infections as well as to exogenous methyl-jasmonate or ET (Lorenzo et al., 2003). In periwinkle (Catharanthus roseus), an AP2/ERF-domain transcription factor, ORCA3, binds directly to the jasmonate- and elicitor-responsive element in the promoter of a terpenoid indole alkaloid biosynthetic gene, STRICTOSIDINE SYNTHASE, and activates its expression in response to methyl-jasmonate (van der Fits and Memelink, 2001). PMV and PMV+SPMV infection modulated expression of multiple AP2/ERF-like transcription factors, including ERF-1 and ERF3-like genes (Table II; Supplemental Table S4). Moreover, expression of ERF-1 and ERF3-like genes appeared to be developmentally regulated (Fig. 6) and, interestingly, paralleled the expression patterns of AOS and LOX2, respectively (Fig. 6). Given the role of ERF proteins in transcriptional regulation of JA components (van der Fits and Memelink, 2001; Lorenzo et al., 2003), it is tempting to speculate the Brachypodium ERF-1 and ERF3-like factors we identified here could mediate AOS and LOX2 expression. Nevertheless, the simultaneous suppression of JA and ET components in PMV and PMV+SPMV infection and the overlapping developmental expression patterns of JA biosynthesis genes and ERF transcription factors supports transcriptional coregulation of JA and ET components.
In contrast with the prominent changes in expression of SA, JA, and ET defense components, components of posttranscriptional gene silencing defenses were largely unaffected in PMV and PMV+SPMV infections. Posttranscriptional gene silencing via short-interfering RNA is a conserved mechanism of defense against viruses (Dunoyer and Voinnet, 2005; Alvarado and Scholthof, 2009, 2011). For example, tomato (Solanum lycopersicum) plants infected with Pepino mosaic virus, a Potexvirus, activates multiple Dicer-like (DCL) and AGO genes during the infection (Hanssen et al., 2011). Similarly, Arabidopsis DRB4 expression is induced upon infection of Turnip yellow mosaic virus (Jakubiec et al., 2012), and expression of RDR1 is up-regulated in tobacco plants infected with Tobacco mosaic virus (Xie et al., 2001) and PVY (Rakhshandehroo et al., 2009). Brachypodium has seven DCL, four DRB, two HEN, 14 AGO, six RDR, and three SILENCING DEFECTIVE3 (SDE3)-like genes (data not shown). In contrast with the Pepino mosaic virus, Turnip yellow mosaic virus, and PVY responses (Rakhshandehroo et al., 2009; Hanssen et al., 2011; Jakubiec et al., 2012), expression of DCL, DRB, AGO, HEN, and RDR genes in Brachypodium was not affected by PMV or PMV+SPMV infections (data not shown), although we cannot rule out changes in protein level or activity or changes that occur transiently. However, expression of an SDE3-like helicase was suppressed in both PMV and PMV+SPMV infections (Supplemental Table S1). It is possible that a PMV-encoded protein functions to suppress SDE3 expression. Nevertheless, not activating (or otherwise evading) antiviral RNA silencing surveillance and suppressing SDE3 may be a strategy used by PMV and SPMV to enable productive infection in Brachypodium and, perhaps, in the other temperate grasses that support this mixed infection (Buzen et al., 1984; Scholthof, 1999).
Several transcriptional regulators belonging to TGA, WRKY, and MYB gene families orchestrate SA-mediated transcriptional changes, including activation of PR and other defense-related genes, as well as modulate JA and ET signaling and influence plant defenses (Moore et al., 2011). In Arabidopsis, SA-mediated defense responses are either NPR1 dependent or independent (An and Mou, 2011). Both SA and pathogen infection induce expression of NPR1, in part via the WRKY transcription factors that bind to NPR1 promoter-proximal W-box sequences (TTGACC/T; Yu et al., 2001). Moreover, NPR1 physically interacts with TGA factors within the nucleus, and its localization/activity is posttranslationally regulated by SA (Tada et al., 2008). Although Brachypodium NPR1 (Bradi2g05870) also contains two putative W-box elements within a 50-bp region upstream of the transcriptional start site (−242 to −292 bp), its expression was not altered in PMV or PMV+SPMV infections (data not shown), suggesting it to be less likely a transcriptional target of the WRKY factors misexpressed in PMV and PMV+SPMV infections (Fig. 5; Supplemental Table S4). Moreover, we did not find alterations in the expression of TGA factors in PMV or PMV+SPMV infections (data not shown). Together, these results suggest that PMV- and SPMV-induced defense responses may be NPR1 independent. Although we do not know if BdNPR1 activity or subcellular localization was affected, our results agree with reports in Arabidopsis compatible host-virus interactions, whereby expression of the majority of defense-related genes are NPR1 independent (Kachroo et al., 2000; Huang et al., 2005).
Whitham et al. (2003) conducted the most comprehensive analyses of profiling genome-wide expression changes in response to diverse RNA virus infections in Arabidopsis. Among the genes altered in several functional categories, five protein kinases, putatively involved in signal transduction, were differentially expressed in Arabidopsis infected with Cucumber mosaic virus (genus Cucumovirus), Oil seed rape mosaic virus (genus Tobamovirus), Turnip vein clearing virus (genus Tobamovirus), PVX (genus Potexvirus), and Turnip mosaic virus (genus Potyvirus; Whitham et al., 2003). In comparison to these compatible Arabidopsis viral responses, and other compatible dicot host:virus responses (Murphy et al., 1999; Kachroo et al., 2000; Whitham et al., 2003, 2006; Huang et al., 2005; García-Marcos et al., 2009; Hanssen et al., 2011), PMV and PMV+SPMV infections in Brachypodium altered expression of a higher proportion of protein kinase genes (27 kinases; Table III; SEA FDR < 0.05; Supplemental Table S2), predominantly in the RLK/Pelle family (Shiu et al., 2004). These RLKs possessed additional LRR, WAK/calcium-binding EGF, l-LEC, d-Man binding lectin, and DUF26 domains (Table III; Fig. 7), and several of them contained putative transmembrane domains and W-box elements in their promoter-proximal sequences (Supplemental Table S3). Together, we suggest that these kinases are likely candidates to function as transmembrane RLKs and are putative targets of WRKY transcription factors induced in PMV and PMV+SPMV infections.
To our knowledge, few compatible monocot host:virus responses have been studied using transcriptome tools compared with dicot host:virus responses. Our finding of several up-regulated kinases in PMV- and PMV+SPMV-infected Brachypodium are comparable to responses found in maize infected with Rice black-streaked dwarf virus (genus Phytoreovirus; Jia et al., 2012) and rice infected with Rice stripe virus (genus Tenuivirus; Satoh et al., 2010) but not to Rice dwarf virus (genus Phytoreovirus) infections in rice (Shimizu et al., 2007). Rice black-streaked dwarf virus and Rice stripe virus infections in maize and rice cause misexpression of 14 and 42 protein kinase genes, respectively (Satoh et al., 2010; Jia et al., 2012), although the proportion of RLKs and their characteristics are unclear. The rice genome encodes a substantially greater number of kinases (kinome size of approximately 1,454), compared with Brachypodium (kinome size of approximately 1,177) and Arabidopsis (kinome size of approximately 1,027 kinases; Shiu et al., 2004; Dardick and Ronald, 2006; International Brachypodium Initiative, 2010). Interestingly, the RLK/Pelle family is predicted to be larger even in the common ancestor of Arabidopsis and rice, which split approximately 150 to 200 million years ago and continued to undergo lineage-specific expansions in monocots and dicots alike (Shiu et al., 2004; International Brachypodium Initiative, 2010). The large number of RLKs among the kinases in general may explain the relatively higher abundance of RLKs (24 out of 27) in the PMV/SPMV kinome when compared with the other kinase subfamilies (Fig. 7). However, because the kinome sizes of Brachypodium and Arabidopsis are comparable, and because RLKs underwent parallel expansions in both dicots and monocots, mere differences in kinome size or composition do not explain why a higher proportion of kinases are misexpressed in PMV- and PMV+SPMV-infected Brachypodium when compared with other dicot:virus infections. Perhaps misexpression of these protein kinases is a unique feature of monocot host:virus interactions, with biological significance in antiviral defenses.
Plant RLKs are either cytosolic (e.g. RLCKs) or membrane-spanning (e.g. receptor His kinases) and mediate growth and development by serving as receptors to hormones such as ET (Bleecker and Kende, 2000), cytokinin (Higuchi et al., 2004), and brassinosteroids (Li and Chory, 1997). Some RLKs also recognize pathogen-associated molecular patterns, such as flagellin, chitin, or effector proteins involved in plant innate immune responses, commonly referred to as pathogen recognition receptors (PRRs; Afzal et al., 2008; Dodds and Rathjen, 2010). In Arabidopsis and rice, fewer than 20% of all RLKs constitute PRRs (e.g. Arabidopsis flagellin-sensitive FLS2 [Gómez-Gómez and Boller, 2002] and rice XA21 [Chen et al., 2010]). PRRs typically lack an Arg (R) residue preceding the invariant Asp (D) in the catalytic site and are referred to as non-RD kinases (Chen et al., 2010). In the PMV/SPMV kinome, Bradi5g02980, Bradi4g38690, Bradi4g21990, and Bradi2g47510 are non-RD kinases (Supplemental Table S3; data not shown), suggesting that they could function in a manner similar to PRRs. Despite the importance of PRRs in bacterial and fungal diseases (Afzal et al., 2008; Dodds and Rathjen, 2010), their function and that of the other RLKs in virus:host interactions are not known.
Unlike bacterial and fungal pathogens, plant viruses are mostly located within a cell and use symplastic connections (plasmodesmata) for cell-to-cell movement. Moreover, plant viruses do not produce pathogen-associated molecular patterns or encode effector-like proteins for cytoplasmic or cell surface-localized RLK recognition (Carr et al., 2010), yet RLKs could function in signal transduction in viral infection. Reflecting on this scenario, we suggest that RLKs (such as PRRs) could perceive virus-associated signals. As summarized in Figure 8, RLKs could recognize PMV and SPMV virions or their encoded proteins either at the plasmodesmata (via transmembrane RLKs) or in the cytoplasm (via cytosolic RLKs). In support of this, recent proteomic studies have identified several RLKs as an abundant class of membrane proteins enriched at the plasmodesmata (Fernandez-Calvino et al., 2011; Jo et al., 2011). Alternatively, and in a manner similar to WAKs, RLKs may recognize damage-associated molecular patterns, such as oligogalacturonides (Brutus et al., 2010), released by the damaged cells as a consequence of virus infection. Although these scenarios are not mutually exclusive, further studies to determine the localization and interactions between PMV- and SPMV-encoded proteins and the host protein kinases misregulated in the infection will test these hypotheses.
Synergism among viruses is generally described by enhanced host disease symptoms and enhanced movement of the viruses as a mixed complex versus movement of individual viruses (Rochow et al., 1955). The best known example of viral synergisms, the mixed infection of PVX and PVY, results in an increased accumulation of PVX by upwards of 40-fold in plants permissive for systemic infection compared with single infections (Rochow and Ross, 1955), primarily due to the effect of PVY-encoded silencing suppressor protein, HC-Pro (Pruss et al., 1997). Very little is known about the host processes affected by PVX-PVY synergism or viral synergisms in general. Using an orthologous potato cDNA array, García-Marcos et al. (2009) determined that PVX+PVY coinfection in N. benthamiana activated putative genes in JA biosynthesis pathway. In another study, expression of specific microRNAs, such as miR156, 171, 398, and 168, were found to be differentially expressed among single versus mixed infections of PVX, PVY, and Plum pox virus in N. benthamiana (Pacheco et al., 2012). However, the biological relevance of the microRNAs (Pacheco et al., 2012) and the misregulated JA components (García-Marcos et al., 2009) in the potyviral synergisms are not clear. Mechanistically, the SPMV synergism is unlike the potyviral synergism that involves the HC-Pro silencing suppressor, as SPMV (i.e. SPMV CP) has no known role in suppressing silencing (Qiu and Scholthof, 2001).
Although PMV accumulated to detectable levels earlier in the presence of SPMV in Brachypodium (Fig. 1) and in millets (Scholthof, 1999), the timing of the disease symptom appearance of PMV- and PMV+SPMV-infected plants was similar, suggesting that SPMV has only modest effect in altering the rate of infection or the disease progression. However, SPMV infection did exacerbate the disease symptoms (Figs. 1 and 2) and additively altered host gene expression (Tables IV and VI) in a manner similar to PVX-PVY synergistic interactions in N. benthamiana (García-Marcos et al., 2009). We hypothesize that the early detection of PMV and the exacerbation of symptoms could be a result of greater PMV accumulation per cell and/or more cells infected with PMV in the presence of SPMV, possibly due to SPMV enhancing PMV replication (Batten et al., 2006b) and/or PMV movement. In contrast with the additive effects on gene expression (Tables IV and VI), expression of multiple SA and JA signaling genes was relatively less misexpressed in PMV+SPMV coinfection, when compared with an infection of PMV alone (Table I; Fig. 6). Moreover, SPMV uniquely modulated expression of few transcription factors (Table VI). These observations suggest that at least some of the SPMV-induced effects are not direct consequences of enhancing PMV accumulation. Based on the altered gene expression data alone, it is difficult to ascertain if these additively or antagonistically altered genes are a direct cause or consequence of the exacerbated disease symptoms in PMV+SPMV infection, but they are indicative of the synergistic role imparted by SPMV at the molecular level.
SPMV uniquely altered the expression of putative nuclear-localized proteins in splicing processes, a pre-mRNA splicing factor 3A subunit 1, and a splicing factor 1/branch-point binding protein (Table VI), suggesting that SPMV may also function by affecting splicing-related processes. A preliminary analysis of the microarray data detected significant changes (FDR < 0.05) in expression of alternatively spliced transcripts in select genes, including an RNA polymerase II subunit in PMV+SPMV infections compared with PMV alone (Supplemental Table S5), although the fold change in expression of these transcripts was low. The alternatively spliced transcripts originated from intron retentions or alternate splice site preferences (Supplemental Table S5) and altered the primary protein sequence. Alternative splicing can influence plant defense responses, as demonstrated for fungal, bacterial, and viral infections (Dinesh-Kumar and Baker, 2000; Jordan et al., 2002; Zhang and Gassmann, 2003; Ayliffe et al., 2004; Reddy, 2007). For example, alternative splicing of eukaryotic translation initiation factor 4E was implicated in tolerance of tomato against two potyviruses, PVY and Pepper mottle virus, but not to Tobacco etch virus (also a potyvirus; Robaglia and Caranta, 2006; Piron et al., 2010). Although the observed effects of SPMV on alternative splicing may be a consequence of exacerbated stress inflicted by PMV/SPMV synergism, we suggest that SPMV CP may alter splicing-related processes; based on our previous findings, SPMV CP is a bona fide RNA-binding protein, stabilizes RNA virus gene inserts in cis and in trans, and translocates to the nucleolus (Qiu and Scholthof, 2004; Qi et al., 2008; Everett et al., 2010). Further studies are needed to clarify the exact mechanism(s) and biological significance of the alternatively spliced transcripts misexpressed in PMV+SPMV synergism.
Regardless of the exact mechanisms, the role of SPMV as a molecular effector to PMV offers an explanation for the exacerbated symptoms in plants infected with PMV+SPMV compared with PMV alone. By analogy to host RNA silencing processes that exert selective pressure on viruses to acquire viral suppressors like HC-Pro, an evolutionary pressure on PMV to acquire disease modulating effectors like SPMV may have primed the synergism. Taken together, our findings show that although exacerbated symptoms are a common phenotypic feature of viral synergisms, at the cellular level unique and defined molecular changes are elicited. To our knowledge, this is the first report of a global gene expression analysis of a plant virus synergism in Brachypodium and Poaceae in general.
MATERIALS AND METHODS
Plant Growth Conditions and Virus Inoculations
Brachypodium (Brachypodium distachyon) inbred line Bd21 seeds were sown in Metro-Mix 366 (SunGrow Horticulture), stratified at 4°C for 3 to 4 d, and subsequently transferred to diurnal growth conditions of 14 h of light (21°C)/10 h of dark (18°C) under a light intensity of approximately 250 to 280 µmol/m2 s with 60% relative humidity. To induce flowering, 6-week-old plants were vernalized at 4°C under continuous low light (20 to 30 µmol/m2 s) for 3 weeks and subsequently transferred to diurnal growth conditions. Alternatively, for viral infections, plants were vernalized at germination by cold-treating the seeds at 4°C for 7 to 10 d, followed by growth in diurnal conditions. For viral inoculations, full-length infectious clones of PMV and SPMV in pUC119 vectors were linearized using EcoICR1 and BglII restriction endonucleases, respectively (Turina et al., 1998). Linearized plasmid DNA (500 ng) was used for in vitro transcription reactions using T7 RNA polymerase (Fermentis) following the manufacturer’s instructions. After verification of the integrity of transcripts in agarose gels, approximately 100 ng of PMV and SPMV transcripts in RNA inoculation buffer (50 mm KH2PO4, 50 mm Gly, pH 9.0, 1% bentonite, and 1% celite) was used to rub-inoculate leaves of 1-week-old plants at two to three leaf stage as described previously (Qi et al., 2008). Inoculated plants were covered (to maintain humidity) and kept in the dark for 24 h. Subsequently, plants were transferred to diurnal growth conditions, and infection was determined by scoring for symptoms of chlorosis, necrosis, and stunting observed in a typical infection. During the course of our study, we noticed that the onset of the chlorosis symptoms on PMV/SPMV-infected Brachypodium varied in independent infections performed at different times. The first visual chlorosis symptoms on the noninoculated leaves typically appear by 10 dpi, but oftentimes appear earlier at 7 dpi or were delayed till 14 dpi. This broad range in the onset of PMV/SPMV disease symptoms could be due to inherent variation associated with the PMV/SPMV infectivity and/or influence of environmental factors, factors that are not uncommon in similar pathosystems (Cui et al., 2012). Nevertheless, to maintain consistency, we advise maintaining similar growth parameters, particularly light intensity, temperature, vernalization period, etc., all of which affected PMV/SPMV virulence (data not shown).
Chlorophyll Quantification
Chlorophyll was extracted, and measurements were performed as described previously (Goldberg and Brakke, 1987; Bedell and Hangarter, 2004). Briefly, 100 mg of fresh plant tissue was finely ground in liquid nitrogen, mixed with 2 mL of 80% acetone, and then centrifuged at 13,000g for 10 min at 4°C. The supernatant was separated and the pellet was resuspended in 1 mL of 80% acetone followed by centrifugation and separation of supernatant. The acetone extraction was repeated until the pellet was devoid of green chlorophyll. Finally, all the supernatants were combined and chlorophyll absorbance was measured using a spectrophotometer at 663 (chlorophyll a) and 646 nm (chlorophyll b) and converted to micrograms of chlorophyll/milligrams fresh weight of leaf tissue (Lichtenthaler and Wellburn, 1983).
Protein Extraction and Immunoblot Assays
Viral detection in infected plants was determined by immunoblot assays using rabbit polyclonal antibodies against PMV CP and SPMV CP, as described previously (Scholthof, 1999). Briefly, plant samples were harvested and homogenized directly in 2× Laemmli SDS sample buffer, as described previously (Martínez-García et al., 1999). The extracts were then boiled for 10 min and centrifuged at 10,000 rpm for 5 min. The supernatants were electrophoresed on SDS-PAGE gels (12.5%) and blotted onto nitrocellulose membranes (Amersham). The blots were blocked in 5% milk followed by incubation with primary anti-PMV CP (1:5,000) or anti-SPMV CP (1:1,000) serum and peroxidase-conjugated goat anti-rabbit IgG secondary antibodies (Rockland) at 1:10,000 dilutions. Visualization of the proteins was performed by using SuperSignal West Pico (Pierce) chemiluminescence detection reagents and x-ray film.
RNA Isolation, Microarray, and qRT-PCR Analysis
Total RNA was isolated from shoots (inoculated and noninoculated) of PMV and PMV+SPMV coinfected plants at stage II, with only mild chlorosis symptoms at 7 dpi, and with detectable PMV CP and SPMV CP (data not shown), along with mock-inoculated shoots, using the plant RNAeasy kit (Qiagen) following the manufacturer’s instructions. RNA from three independent biological replicates was submitted to Nottingham Arabidopsis Stock Centre Affymetrix Service for labeling and hybridization. Standard Affymetrix recommendations and quality controls were employed for microarray hybridization using a custom-designed Affymetrix Brachypodium tiling array (BradiAR1b520742), consisting of about 2.5 million probes representing approximately 27,000 genes, with >95% of exons and introns targeted by more than five probes. Mean probe size was about 42 bases, and the probes are spaced approximately 126 nucleotides apart. The gene predictions developed for this array are based on the Brachypodium (accession Bd21) genome sequence, Illumina, and EST-based transcriptome analyses (International Brachypodium Initiative, 2010) and are accessible through Phytozome (v7.0, released on April 8, 2011). For further information on the array design, refer to Fox et al. (2010). A robust multichip average sketch was employed for raw data normalization, background correction, and summarization of the chip signals. Transcript-level sense-target probe sets were filtered out for further analysis. Statistical significance among the normalized data sets was determined using Student’s t test and was corrected for FDR using the Benjamini and Hochberg method (q-value < 0.05; Benjamini and Hochberg, 1995). Assignment of putative functions to the misexpressed genes was performed using a combined GO using AgriGO resource (Du et al., 2010), as well as using Pfam (Punta et al., 2012) and PANTHER (Thomas et al., 2003) annotations through Phytozome (Goodstein et al., 2012). The annotations were trimmed for redundant GOs and multiple descriptions for similar biological processes and are summarized in Figure 3B. Enriched GO terms within the PMV and PMV+SPMV coinfection were subjected to SEA against a precalculated Brachypodium background genomic loci using the AgriGO tool (Du et al., 2010). χ2 test and FDR correction using the Hochberg method (FDR < 0.05) was employed for determining statistical significance. Significantly altered expression in specific functional classes of genes was represented as heat maps, generated using the Multiexperiment Viewer application (Saeed et al., 2003). For identifying Arabidopsis (Arabidopsis thaliana) orthologs of candidate Brachypodium genes, we used the peptide homolog tool available through Phytozome (Goodstein et al., 2012). The peptide homolog tool employs “all-against-all Smith-Waterman alignment” (Smith and Waterman, 1981), which is considered superior to BLAST- or FASTA-based alignments for recovery of orthologous peptides to a query sequence (Pearson, 1991; Shpaer et al., 1996). Reciprocal searches yielded similar results. However, because cross-species comparisons of orthologous genes (monocots and dicots split approximately 150 million years ago; Chaw et al., 2004) can be complicated, we took a conservative approach and named only those Brachypodium genes after an Arabidopsis ortholog (e.g. BdPR-1), only if they had unique and highest similarity scores. Others were assigned as “-like” genes (e.g. PR-1-like). Furthermore, for genes families such as WRKYs, protein kinases, ACO, AOX, FAD, LOX, MKK, OPR, and ERF proteins, multiple sequence alignment and phylogenetic analyses was performed using the predicted protein sequences of Brachypodium genes and their respective Arabidopsis orthologs retrieved from Phytozome using ClustalW2 (Larkin et al., 2007). The resulting neighbor-joining trees were displayed using NJplot (Perrière and Gouy, 1996) and/or Archeopterix (Han and Zmasek, 2009) display tools (Figs. 5 and 7; Supplemental Fig. S2, A–G). During the preparation of this article, Phytozome v8.0 was released (January 16, 2012), resulting in minor changes to a few gene loci. We manually screened for these changes and incorporated them in our analysis. Minimum Information About a Microarray Experiment-compliant data were deposited in ArrayExpress (Parkinson et al., 2011) with submission ID E-MEXP-3729.
Microarray data were further verified for reliability using qRT-PCR analysis of 20 genes using the same RNA used for the microarray analysis. Although qRT-PCR analysis only confirms the selected gene expression data, it is a commonly regarded independent laboratory-based approach to corroborate microarrays, as microarrays are often prone to several procedural and data-processing artifacts (Chuaqui et al., 2002). For an unbiased representation of the microarray data, we chose the 20 candidate genes in diverse functional categories with expression changes ranging from 2- to 50-fold in PMV and PMV+SPMV infections, when compared with mock-inoculated plants (Supplemental Table S1). Furthermore, temporal analysis of SA, JA, and ET responses was performed using qRT-PCR analysis of candidate genes in the respective signaling pathways, using plants at different stages of infection scored as: no symptoms (stage I), mild chlorosis symptoms (stage II), or severe chlorosis and necrosis symptoms (stage III), as well as mock-inoculated plants (Fig. 6). For all qRT-PCR analysis, three independent biological replicates consisting of three plant samples each were used for total RNA isolation using Trizol reagent (Ambion), following the manufacturer’s instructions. Two micrograms of the total RNA was used to make cDNA using SuperScript III first-strand cDNA synthesis kit (Invitrogen), as described previously (Mandadi et al., 2009), with minor modifications. Gene amplification by PCR was performed using Power SYBR Green Master-mix and the ABI Prism 7500 sequence detection system (Applied Biosystems). Gene-specific primers used for qRT-PCR analysis were designed using QuantPrime (Arvidsson et al., 2008) and are listed in Supplemental Tables S5 and S6. UBIQUITIN18 (Bradi4g00660) expression was used for normalization (Hong et al., 2008), and fold changes of gene expression in the infected plants were calculated following the comparative cycle threshold method (Livak and Schmittgen, 2001) and are relative to mock-inoculated plants set to 1. A Student’s t test was used to determine statistical significances between the control and infected plants, and Pearson correlation coefficient (r) and coefficient of determination (R2) were used to determine statistical correlation between the microarray and the qRT-PCR data (Supplemental Table S1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. PMV and SPMV genome maps and infection strategy.
Supplemental Figure S2. Phylogenetic analysis of putative SA, JA, and ET components altered in PMV and PMV+SPMV infection.
Supplemental Table S1. Validation of the microarray gene expression with qRT-PCR of selected up- or down-regulated Brachypodium genes.
Supplemental Table S2. GO and SEA analysis of PMV and PMV+SPMV misregulated genes.
Supplemental Table S3. Characteristics of PMV+SPMV modulated kinases.
Supplemental Table S4. PMV and SPMV alter expression of multiple transcriptional regulators in Brachypodium.
Supplemental Table S5. SPMV-associated alternatively spliced transcripts.
Supplemental Table S6. Primers used for validation of microarray data using qRT-PCR.
Supplemental Table S7. Primers used for temporal analysis of SA, JA, and ET responses using qRT-PCR.
Supplementary Material
Acknowledgments
We thank Todd Mockler (Danforth Plant Science Center) for statistical analysis of the microarray data and advice on gene functional annotations and Joshua Yuan and Julia Cope (Texas A&M University) for initial computer support. The generous gifts of Brachypodium seed and practical advice from John Vogel, Jennifer Bragg, and Ludmilla Tyler (U.S. Department of Agriculture-Agricultural Research Service, Albany, CA) made it possible for us to develop Brachypodium for our virus studies. We appreciate the helpful discussions of Herman Scholthof, Veria Alvarado, and Christopher Lyons (Texas A&M University) and their critical comments during this article preparation. We thank Jesse Pyle (Texas A&M University) for help with growth and maintenance of the Brachypodium plants.
Glossary
- SA
salicylic acid
- PVX
Potato virus X
- PVY
Potato virus Y
- PMV
Panicum mosaic virus
- SPMV
satellite Panicum mosaic virus
- dpi
days postinfection
- FDR
false discovery rate
- qRT
quantitative reverse transcription
- GO
gene ontology
- SEA
singular enrichment analysis
- JA
jasmonic acid
- ET
ethylene
- ACC
1-aminocyclopropane-1-carboxylic acid
- STK
Ser-Thr kinase
- RLK
receptor-like kinase
- LRR
Leu-rich repeat
- PRR
pathogen recognition receptor
- WAK
wall-associated kinase
- CP
capsid protein
- cDNA
complementary DNA
References
- Afzal AJ, Wood AJ, Lightfoot DA. (2008) Plant receptor-like serine threonine kinases: roles in signaling and plant defense. Mol Plant Microbe Interact 21: 507–517 [DOI] [PubMed] [Google Scholar]
- Alamillo JM, Saénz P, García JA. (2006) Salicylic acid-mediated and RNA-silencing defense mechanisms cooperate in the restriction of systemic spread of Plum pox virus in tobacco. Plant J 48: 217–227 [DOI] [PubMed] [Google Scholar]
- Alvarado V, Scholthof HB. (2009) Plant responses against invasive nucleic acids: RNA silencing and its suppression by plant viral pathogens. Semin Cell Dev Biol 20: 1032–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado VY, Scholthof HB. (2011) AGO2: a new Argonaute compromising plant virus accumulation. Front Plant Sci 2: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C, Mou Z. (2011) Salicylic acid and its function in plant immunity. J Integr Plant Biol 53: 412–428 [DOI] [PubMed] [Google Scholar]
- Arvidsson S, Kwasniewski M, Riaño-Pachón DM, Mueller-Roeber B. (2008) QuantPrime: a flexible tool for reliable high-throughput primer design for quantitative PCR. BMC Bioinformatics 9: 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascencio-Ibáñez JT, Sozzani R, Lee T-J, Chu T-M, Wolfinger RD, Cella R, Hanley-Bowdoin L. (2008) Global analysis of Arabidopsis gene expression uncovers a complex array of changes impacting pathogen response and cell cycle during geminivirus infection. Plant Physiol 148: 436–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayliffe MA, Steinau M, Park RF, Rooke L, Pacheco MG, Hulbert SH, Trick HN, Pryor AJ. (2004) Aberrant mRNA processing of the maize Rp1-D rust resistance gene in wheat and barley. Mol Plant Microbe Interact 17: 853–864 [DOI] [PubMed] [Google Scholar]
- Baebler Š, Stare K, Kovač M, Blejec A, Prezelj N, Stare T, Kogovšek P, Pompe-Novak M, Rosahl S, Ravnikar M, Gruden K. (2011) Dynamics of responses in compatible potato-Potato virus Y interaction are modulated by salicylic acid. PLoS ONE 6: e29009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban N, Larson SB, McPherson A. (1995) Structural comparison of the plant satellite viruses. Virology 214: 571–583 [DOI] [PubMed] [Google Scholar]
- Ban N, McPherson A. (1995) The structure of satellite panicum mosaic virus at 1.9 Å resolution. Nat Struct Biol 2: 882–890 [DOI] [PubMed] [Google Scholar]
- Batten JS, Desvoyes B, Yamamura Y, Scholthof K-BG. (2006a) A translational enhancer element on the 3′-proximal end of the Panicum mosaic virus genome. FEBS Lett 580: 2591–2597 [DOI] [PubMed] [Google Scholar]
- Batten JS, Turina M, Scholthof K-BG. (2006b) Panicovirus accumulation is governed by two membrane-associated proteins with a newly identified conserved motif that contributes to pathogenicity. Virol J 3: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell V, Hangarter R. (2004) Characterization of the Arabidopsis seedling plastid mutant spd2. BIOS 75: 65–73 [Google Scholar]
- Bell E, Creelman RA, Mullet JE. (1995) A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc Natl Acad Sci USA 92: 8675–8679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendahmane A, Kanyuka K, Baulcombe DC. (1999) The Rx gene from potato controls separate virus resistance and cell death responses. Plant Cell 11: 781–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300 [Google Scholar]
- Bevan MW, Garvin DF, Vogel JP. (2010) Brachypodium distachyon genomics for sustainable food and fuel production. Curr Opin Biotechnol 21: 211–217 [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Kende H. (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18 [DOI] [PubMed] [Google Scholar]
- Brkljacic J, Grotewold E, Scholl R, Mockler T, Garvin DF, Vain P, Brutnell T, Sibout R, Bevan M, Budak H, et al. (2011) Brachypodium as a model for the grasses: today and the future. Plant Physiol 157: 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutus A, Sicilia F, Macone A, Cervone F, De Lorenzo G. (2010) A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc Natl Acad Sci USA 107: 9452–9457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzen FG, Niblett CL, Hooper GR, Hubbard J, Newman MA. (1984) Further characterization of Panicum mosaic virus and its associated satellite virus. Phytopathology 74: 313–318 [Google Scholar]
- Cabrera O, Scholthof K-BG. (1999) The complex viral etiology of St. Augustine decline. Plant Dis 83: 902–904 [DOI] [PubMed] [Google Scholar]
- Cao S, Siriwardana CL, Kumimoto RW, Holt BF., III (2011) Construction of high quality Gateway™ entry libraries and their application to yeast two-hybrid for the monocot model plant Brachypodium distachyon. BMC Biotechnol 11: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr JP, Lewsey MG, Palukaitis P. (2010) Signaling in induced resistance. Adv Virus Res 76: 57–121 [DOI] [PubMed] [Google Scholar]
- Chaw S-M, Chang C-C, Chen H-L, Li W-H. (2004) Dating the monocot-dicot divergence and the origin of core eudicots using whole chloroplast genomes. J Mol Evol 58: 424–441 [DOI] [PubMed] [Google Scholar]
- Chen X, Chern M, Canlas PE, Jiang C, Ruan D, Cao P, Ronald PC. (2010) A conserved threonine residue in the juxtamembrane domain of the XA21 pattern recognition receptor is critical for kinase autophosphorylation and XA21-mediated immunity. J Biol Chem 285: 10454–10463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuaqui RF, Bonner RF, Best CJM, Gillespie JW, Flaig MJ, Hewitt SM, Phillips JL, Krizman DB, Tangrea MA, Ahram M, et al. (2002) Post-analysis follow-up and validation of microarray experiments. Nat Genet (Suppl) 32: 509–514 [DOI] [PubMed] [Google Scholar]
- Cui Y, Lee MY, Huo N, Bragg J, Yan L, Yuan C, Li C, Holditch SJ, Xie J, Luo M-C, et al. (2012) Fine mapping of the Bsr1 Barley stripe mosaic virus resistance gene in the model grass Brachypodium distachyon. PLoS ONE 7: e38333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmay T, Horsefield R, Braunstein TH, Baulcombe DC. (2001) SDE3 encodes an RNA helicase required for post-transcriptional gene silencing in Arabidopsis. EMBO J 20: 2069–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damirdagh IS, Ross AF. (1967) A marked synergistic interaction of potato viruses X and Y in inoculated leaves of tobacco. Virology 31: 296–307 [DOI] [PubMed] [Google Scholar]
- Dardick C, Ronald P. (2006) Plant and animal pathogen recognition receptors signal through non-RD kinases. PLoS Pathog 2: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvoyes B, Scholthof K-BG. (2000) RNA: protein interactions associated with satellites of Panicum mosaic virus. FEBS Lett 485: 25–28 [DOI] [PubMed] [Google Scholar]
- Dinesh-Kumar SP, Baker BJ. (2000) Alternatively spliced N resistance gene transcripts: their possible role in Tobacco mosaic virus resistance. Proc Natl Acad Sci USA 97: 1908–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds PN, Rathjen JP. (2010) Plant immunity: towards an integrated view of plant-pathogen interactions. Nat Rev Genet 11: 539–548 [DOI] [PubMed] [Google Scholar]
- Dong JG, Fernández-Maculet JC, Yang SF. (1992) Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proc Natl Acad Sci USA 89: 9789–9793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper J, Mur LA, Jenkins G, Ghosh-Biswas GC, Bablak P, Hasterok R, Routledge AP. (2001) Brachypodium distachyon. A new model system for functional genomics in grasses. Plant Physiol 127: 1539–1555 [PMC free article] [PubMed] [Google Scholar]
- Du Z, Zhou X, Ling Y, Zhang Z, Su Z. (2010) agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 38: W64–W70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunoyer P, Voinnet O. (2005) The complex interplay between plant viruses and host RNA-silencing pathways. Curr Opin Plant Biol 8: 415–423 [DOI] [PubMed] [Google Scholar]
- Elena SF, Carrera J, Rodrigo G. (2011) A systems biology approach to the evolution of plant-virus interactions. Curr Opin Plant Biol 14: 372–377 [DOI] [PubMed] [Google Scholar]
- Everett AL, Scholthof HB, Scholthof K-BG. (2010) Satellite panicum mosaic virus coat protein enhances the performance of plant virus gene vectors. Virology 396: 37–46 [DOI] [PubMed] [Google Scholar]
- Fernandez-Calvino L, Faulkner C, Walshaw J, Saalbach G, Bayer E, Benitez-Alfonso Y, Maule A. (2011) Arabidopsis plasmodesmal proteome. PLoS ONE 6: e18880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SE, Priest HD, Bryant DW, Wilhelm LJ, Michael TP, Mockler TC. (2010) Universal genome array and transcriptome atlas for Brachypodium distachyon (abstract no. W095). In Plant and Animal Genomes XVIII Conference. January 9–13, San Diego, CA
- García-Marcos A, Pacheco R, Martiáñez J, González-Jara P, Díaz-Ruíz JR, Tenllado F. (2009) Transcriptional changes and oxidative stress associated with the synergistic interaction between Potato virus X and Potato virus Y and their relationship with symptom expression. Mol Plant Microbe Interact 22: 1431–1444 [DOI] [PubMed] [Google Scholar]
- Garvin DF, McKenzie N, Vogel JP, Mockler TC, Blankenheim ZJ, Wright J, Cheema JJ, Dicks J, Huo N, Hayden DM, et al. (2010) An SSR-based genetic linkage map of the model grass Brachypodium distachyon. Genome 53: 1–13 [DOI] [PubMed] [Google Scholar]
- Goldberg K-B, Brakke MK. (1987) Concentration of Maize chlorotic mottle virus increased in mixed infections with Maize dwarf mosaic virus, strain B. Phytopathology 77: 162–167 [Google Scholar]
- Gómez-Gómez L, Boller T. (2002) Flagellin perception: a paradigm for innate immunity. Trends Plant Sci 7: 251–256 [DOI] [PubMed] [Google Scholar]
- González-Jara P, Tenllado F, Martínez-García B, Atencio FA, Barajas D, Vargas M, Díaz-Ruiz J, Díaz-Ruíz JR. (2004) Host-dependent differences during synergistic infection by potyviruses with Potato virus X. Mol Plant Pathol 5: 29–35 [DOI] [PubMed] [Google Scholar]
- Goodman RM, Ross AF. (1974) Enhancement of Potato virus X synthesis in doubly infected tobacco occurs in doubly infected cells. Virology 58: 16–24 [DOI] [PubMed] [Google Scholar]
- Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS. (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 40: D1178–D1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MV, Zmasek CM. (2009) phyloXML: XML for evolutionary biology and comparative genomics. BMC Bioinformatics 10: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen IM, van Esse HP, Ballester A-R, Hogewoning SW, Parra NO, Paeleman A, Lievens B, Bovy AG, Thomma BPHJ. (2011) Differential tomato transcriptomic responses induced by Pepino mosaic virus isolates with differential aggressiveness. Plant Physiol 156: 301–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Ton J. (2008) Long-distance signalling in plant defence. Trends Plant Sci 13: 264–272 [DOI] [PubMed] [Google Scholar]
- Higuchi M, Pischke MS, Mähönen AP, Miyawaki K, Hashimoto Y, Seki M, Kobayashi M, Shinozaki K, Kato T, Tabata S, et al. (2004) In planta functions of the Arabidopsis cytokinin receptor family. Proc Natl Acad Sci USA 101: 8821–8826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S-Y, Seo PJ, Yang M-S, Xiang F, Park C-M. (2008) Exploring valid reference genes for gene expression studies in Brachypodium distachyon by real-time PCR. BMC Plant Biol 8: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C-C, Hsu Y-H, Lin N-S. (2009) Satellite RNAs and satellite viruses of plants. Viruses 1: 1325–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Yeakley JM, Garcia EW, Holdridge JD, Fan J-B, Whitham SA. (2005) Salicylic acid-dependent expression of host genes in compatible Arabidopsis-virus interactions. Plant Physiol 137: 1147–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo N, Gu YQ, Lazo GR, Vogel JP, Coleman-Derr D, Luo MC, Thilmony R, Garvin DF, Anderson OD. (2006) Construction and characterization of two BAC libraries from Brachypodium distachyon, a new model for grass genomics. Genome 49: 1099–1108 [DOI] [PubMed] [Google Scholar]
- International Brachypodium Initiative (2010) Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature 463: 763–768 [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Masuda K, Naito S, Meshi T, Ishikawa M. (2007) An inhibitor of viral RNA replication is encoded by a plant resistance gene. Proc Natl Acad Sci USA 104: 13833–13838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubiec A, Yang SW, Chua N-H. (2012) Arabidopsis DRB4 protein in antiviral defense against Turnip yellow mosaic virus infection. Plant J 69: 14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AB, Raventos D, Mundy J. (2002) Fusion genetic analysis of jasmonate-signalling mutants in Arabidopsis. Plant J 29: 595–606 [DOI] [PubMed] [Google Scholar]
- Jia M-A, Li Y, Lei L, Di D, Miao H, Fan Z. (2012) Alteration of gene expression profile in maize infected with a double-stranded RNA fijivirus associated with symptom development. Mol Plant Pathol 13: 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y, Cho WK, Rim Y, Moon J, Chen X-Y, Chu H, Kim CY, Park Z-Y, Lucas WJ, Kim J-Y. (2011) Plasmodesmal receptor-like kinases identified through analysis of rice cell wall extracted proteins. Protoplasma 248: 191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan T, Schornack S, Lahaye T. (2002) Alternative splicing of transcripts encoding Toll-like plant resistance proteins: what’s the functional relevance to innate immunity? Trends Plant Sci 7: 392–398 [DOI] [PubMed] [Google Scholar]
- Kachroo P, Chandra-Shekara AC, Klessig DF. (2006) Plant signal transduction and defense against viral pathogens. Adv Virus Res 66: 161–191 [DOI] [PubMed] [Google Scholar]
- Kachroo P, Yoshioka K, Shah J, Dooner HK, Klessig DF. (2000) Resistance to Turnip crinkle virus in Arabidopsis is regulated by two host genes and is salicylic acid dependent but NPR1, ethylene, and jasmonate independent. Plant Cell 12: 677–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ryabov EV, Kalinina NO, Rakitina DV, Gillespie T, MacFarlane S, Haupt S, Brown JW, Taliansky M. (2007) Cajal bodies and the nucleolus are required for a plant virus systemic infection. EMBO J 26: 2169–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohm BA, Goulden MG, Gilbert JE, Kavanagh TA, Baulcombe DC. (1993) A Potato virus X resistance gene mediates an induced, nonspecific resistance in protoplasts. Plant Cell 5: 913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948 [DOI] [PubMed] [Google Scholar]
- Latham JR, Wilson AK. (2008) Transcomplementation and synergism in plants: implications for viral transgenes? Mol Plant Pathol 9: 85–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon AM, Neuenschwander UH, Ribas-Carbo M, Giles L, Ryals JA, Siedow JN. (1997) The effects of salicylic acid and Tobacco mosaic virus infection on the alternative oxidase of tobacco. Plant Physiol 115: 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chory J. (1997) A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90: 929–938 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Wellburn AR. (1983) Determinations of total carotenoids and chlorophylls A and B of leaf extracts in different solvents. Biochem Soc Trans 11: 591 [Google Scholar]
- Lim PO, Kim HJ, Nam HG. (2007) Leaf senescence. Annu Rev Plant Biol 58: 115–136 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-Δ Δ CT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sánchez-Serrano JJ, Solano R. (2003) ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 15: 165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandadi KK, Misra A, Ren S, McKnight TD. (2009) BT2, a BTB protein, mediates multiple responses to nutrients, stresses, and hormones in Arabidopsis. Plant Physiol 150: 1930–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García JF, Monte E, Quail PH. (1999) A simple, rapid and quantitative method for preparing Arabidopsis protein extracts for immunoblot analysis. Plant J 20: 251–257 [DOI] [PubMed] [Google Scholar]
- Masuta C, Zuidema D, Hunter BG, Heaton LA, Sopher DS, Jackson AO. (1987) Analysis of the genome of satellite panicum mosaic virus. Virology 159: 329–338 [DOI] [PubMed] [Google Scholar]
- Moore JW, Loake GJ, Spoel SH. (2011) Transcription dynamics in plant immunity. Plant Cell 23: 2809–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LAJ, Kenton P, Atzorn R, Miersch O, Wasternack C. (2006) The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol 140: 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AM, Chivasa S, Singh DP, Carr JP. (1999) Salicylic acid-induced resistance to viruses and other pathogens: a parting of the ways? Trends Plant Sci 4: 155–160 [DOI] [PubMed] [Google Scholar]
- Niblett CL, Paulsen AQ. (1975) Purification and further characterization of Panicum mosaic virus. Phytopathology 65: 1157–1160 [Google Scholar]
- Omarov RT, Qi D, Scholthof K-BG. (2005) The capsid protein of satellite panicum mosaic virus contributes to systemic invasion and interacts with its helper virus. J Virol 79: 9756–9764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco R, García-Marcos A, Barajas D, Martiáñez J, Tenllado F. (2012) PVX-potyvirus synergistic infections differentially alter microRNA accumulation in Nicotiana benthamiana. Virus Res 165: 231–235 [DOI] [PubMed] [Google Scholar]
- Padmanabhan MS, Dinesh-Kumar SP. (2010) All hands on deck: the role of chloroplasts, endoplasmic reticulum, and the nucleus in driving plant innate immunity. Mol Plant Microbe Interact 23: 1368–1380 [DOI] [PubMed] [Google Scholar]
- Pallas V, García JA. (2011) How do plant viruses induce disease? Interactions and interference with host components. J Gen Virol 92: 2691–2705 [DOI] [PubMed] [Google Scholar]
- Parkinson H, Sarkans U, Kolesnikov N, Abeygunawardena N, Burdett T, Dylag M, Emam I, Farne A, Hastings E, Holloway E, et al. (2011) ArrayExpress update: an archive of microarray and high-throughput sequencing-based functional genomics experiments. Nucleic Acids Res 39: D1002–D1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson WR. (1991) Searching protein sequence libraries: comparison of the sensitivity and selectivity of the Smith-Waterman and FASTA algorithms. Genomics 11: 635–650 [DOI] [PubMed] [Google Scholar]
- Perrière G, Gouy M. (1996) WWW-query: an on-line retrieval system for biological sequence banks. Biochimie 78: 364–369 [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. (2009) Networking by small-molecule hormones in plant immunity. Nat Chem Biol 5: 308–316 [DOI] [PubMed] [Google Scholar]
- Piron F, Nicolaï M, Minoïa S, Piednoir E, Moretti A, Salgues A, Zamir D, Caranta C, Bendahmane A. (2010) An induced mutation in tomato eIF4E leads to immunity to two potyviruses. PLoS ONE 5: e11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postnikova OA, Nemchinov LG. (2012) Comparative analysis of microarray data in Arabidopsis transcriptome during compatible interactions with plant viruses. Virol J 9: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AM. (1994) Grasses of the Trans-Pecos and Adjacent Areas. University of Texas Press, Austin, TX [Google Scholar]
- Pruss G, Ge X, Shi XM, Carrington JC, Bowman Vance V. (1997) Plant viral synergism: the potyviral genome encodes a broad-range pathogenicity enhancer that transactivates replication of heterologous viruses. Plant Cell 9: 859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss GJ, Lawrence CB, Bass T, Li QQ, Bowman LH, Vance V. (2004) The potyviral suppressor of RNA silencing confers enhanced resistance to multiple pathogens. Virology 320: 107–120 [DOI] [PubMed] [Google Scholar]
- Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J, et al. (2012) The Pfam protein families database. Nucleic Acids Res 40: D290–D301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi D, Omarov RT, Scholthof K-BG. (2008) The complex subcellular distribution of satellite panicum mosaic virus capsid protein reflects its multifunctional role during infection. Virology 376: 154–164 [DOI] [PubMed] [Google Scholar]
- Qiu W, Scholthof K-BG. (2004) Satellite panicum mosaic virus capsid protein elicits symptoms on a nonhost plant and interferes with a suppressor of virus-induced gene silencing. Mol Plant Microbe Interact 17: 263–271 [DOI] [PubMed] [Google Scholar]
- Qiu WP, Scholthof K-BG. (2001) Genetic identification of multiple biological roles associated with the capsid protein of satellite panicum mosaic virus. Mol Plant Microbe Interact 14: 21–30 [DOI] [PubMed] [Google Scholar]
- Rakhshandehroo F, Takeshita M, Squires J, Palukaitis P. (2009) The influence of RNA-dependent RNA polymerase 1 on Potato virus Y infection and on other antiviral response genes. Mol Plant Microbe Interact 22: 1312–1318 [DOI] [PubMed] [Google Scholar]
- Reddy ASN. (2007) Alternative splicing of pre-messenger RNAs in plants in the genomic era. Annu Rev Plant Biol 58: 267–294 [DOI] [PubMed] [Google Scholar]
- Robaglia C, Caranta C. (2006) Translation initiation factors: a weak link in plant RNA virus infection. Trends Plant Sci 11: 40–45 [DOI] [PubMed] [Google Scholar]
- Rochow WF, Ross AF. (1955) Virus multiplication in plants doubly infected by potato viruses X and Y. Virology 1: 10–27 [DOI] [PubMed] [Google Scholar]
- Rochow WF, Ross AF, Siegel BM. (1955) Comparison of local-lesion and electronmicroscope particle-count methods for assay of potato virus X from plants doubly infected by potato viruses X and Y. Virology 1: 28–39 [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Somssich IE, Ringler P, Shen QJ. (2010) WRKY transcription factors. Trends Plant Sci 15: 247–258 [DOI] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et al. (2003) TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34: 374–378 [DOI] [PubMed] [Google Scholar]
- Satoh K, Kondoh H, Sasaya T, Shimizu T, Choi I-R, Omura T, Kikuchi S. (2010) Selective modification of rice (Oryza sativa) gene expression by Rice stripe virus infection. J Gen Virol 91: 294–305 [DOI] [PubMed] [Google Scholar]
- Scholthof K-BG. (1999) A synergism induced by satellite panicum mosaic virus. Mol Plant Microbe Interact 12: 163–166 [Google Scholar]
- Scholthof K-BG, Jones RW, Jackson AO. (1999) Biology and structure of plant satellite viruses activated by icosahedral helper viruses. Curr Top Microbiol Immunol 239: 123–143 [DOI] [PubMed] [Google Scholar]
- Shimizu T, Satoh K, Kikuchi S, Omura T. (2007) The repression of cell wall- and plastid-related genes and the induction of defense-related genes in rice plants infected with Rice dwarf virus. Mol Plant Microbe Interact 20: 247–254 [DOI] [PubMed] [Google Scholar]
- Shiu S-H, Karlowski WM, Pan R, Tzeng Y-H, Mayer KFX, Li W-H. (2004) Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 16: 1220–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpaer EG, Robinson M, Yee D, Candlin JD, Mines R, Hunkapiller T. (1996) Sensitivity and selectivity in protein similarity searches: a comparison of Smith-Waterman in hardware to BLAST and FASTA. Genomics 38: 179–191 [DOI] [PubMed] [Google Scholar]
- Sill WH, Pickett RC. (1957) A new virus disease of switchgrass, Panicum virgatum L. Plant Dis Reptr 41: 241–249 [Google Scholar]
- Smith TF, Waterman MS. (1981) Identification of common molecular subsequences. J Mol Biol 147: 195–197 [DOI] [PubMed] [Google Scholar]
- Syller J. (2012) Facilitative and antagonistic interactions between plant viruses in mixed infections. Mol Plant Pathol 13: 204–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C, Zuo J, Dong X. (2008) Plant immunity requires conformational changes of NPR1 via S-nitrosylation and thioredoxins. Science 321: 952–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PD, Kejariwal A, Campbell MJ, Mi H, Diemer K, Guo N, Ladunga I, Ulitsky-Lazareva B, Muruganujan A, Rabkin S, Vandergriff JA, Doremieux O. (2003) PANTHER: a browsable database of gene products organized by biological function, using curated protein family and subfamily classification. Nucleic Acids Res 31: 334–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turina M, Desvoyes B, Scholthof K-BG. (2000) A gene cluster encoded by Panicum mosaic virus is associated with virus movement. Virology 266: 120–128 [DOI] [PubMed] [Google Scholar]
- Turina M, Maruoka M, Monis J, Jackson AO, Scholthof K-BG. (1998) Nucleotide sequence and infectivity of a full-length cDNA clone of Panicum mosaic virus. Virology 241: 141–155 [DOI] [PubMed] [Google Scholar]
- Vance VB. (1991) Replication of Potato virus X RNA is altered in coinfections with Potato virus Y. Virology 182: 486–494 [DOI] [PubMed] [Google Scholar]
- van der Fits L, Memelink J. (2001) The jasmonate-inducible AP2/ERF-domain transcription factor ORCA3 activates gene expression via interaction with a jasmonate-responsive promoter element. Plant J 25: 43–53 [DOI] [PubMed] [Google Scholar]
- van der Graaff E, Schwacke R, Schneider A, Desimone M, Flügge U-I, Kunze R. (2006) Transcription analysis of Arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiol 141: 776–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Valen L. (1973) A new evolutionary law. Evol Theory 1: 1–30 [Google Scholar]
- Verhage A, van Wees SCM, Pieterse CMJ. (2010) Plant immunity: it’s the hormones talking, but what do they say? Plant Physiol 154: 536–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF. (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47: 177–206 [DOI] [PubMed] [Google Scholar]
- Vogel J, Hill T. (2008) High-efficiency Agrobacterium-mediated transformation of Brachypodium distachyon inbred line Bd21-3. Plant Cell Rep 27: 471–478 [DOI] [PubMed] [Google Scholar]
- Vogel JP, Garvin DF, Leong OM, Hayden DM. (2006) Agrobacterium-mediated transformation and inbred line development in the model grass Brachypodium distachyon. Plant Cell Tissue Organ Cult 84: 199–211 [Google Scholar]
- Wang D, Amornsiripanitch N, Dong X. (2006) A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog 2: e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C. (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot (Lond) 100: 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham SA, Quan S, Chang H-S, Cooper B, Estes B, Zhu T, Wang X, Hou Y-M. (2003) Diverse RNA viruses elicit the expression of common sets of genes in susceptible Arabidopsis thaliana plants. Plant J 33: 271–283 [DOI] [PubMed] [Google Scholar]
- Whitham SA, Yang C, Goodin MM. (2006) Global impact: elucidating plant responses to viral infection. Mol Plant Microbe Interact 19: 1207–1215 [DOI] [PubMed] [Google Scholar]
- Xie Z, Fan B, Chen C, Chen Z. (2001) An important role of an inducible RNA-dependent RNA polymerase in plant antiviral defense. Proc Natl Acad Sci USA 98: 6516–6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Chen C, Chen Z. (2001) Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 13: 1527–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X-C, Gassmann W. (2003) RPS4-mediated disease resistance requires the combined presence of RPS4 transcripts with full-length and truncated open reading frames. Plant Cell 15: 2333–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Tootle TL, Tsui F, Klessig DF, Glazebrook J. (1998) PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell 10: 1021–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.