Abstract
CATION EXCHANGERs CAX1 and CAX3 are vacuolar ion transporters involved in ion homeostasis in plants. Widely expressed in the plant, they mediate calcium transport from the cytosol to the vacuole lumen using the proton gradient across the tonoplast. Here, we report an unexpected role of CAX1 and CAX3 in regulating apoplastic pH and describe how they contribute to auxin transport using the guard cell’s response as readout of hormone signaling and cross talk. We show that indole-3-acetic acid (IAA) inhibition of abscisic acid (ABA)-induced stomatal closure is impaired in cax1, cax3, and cax1/cax3. These mutants exhibited constitutive hypopolarization of the plasma membrane, and time-course analyses of membrane potential revealed that IAA-induced hyperpolarization of the plasma membrane is also altered in these mutants. Both ethylene and 1-naphthalene acetic acid inhibited ABA-triggered stomatal closure in cax1, cax3, and cax1/cax3, suggesting that auxin signaling cascades were functional and that a defect in IAA transport caused the phenotype of the cax mutants. Consistent with this finding, chemical inhibition of AUX1 in wild-type plants phenocopied the cax mutants. We also found that cax1/cax3 mutants have a higher apoplastic pH than the wild type, further supporting the hypothesis that there is a defect in IAA import in the cax mutants. Accordingly, we were able to fully restore IAA inhibition of ABA-induced stomatal closure in cax1, cax3, and cax1/cax3 when stomatal movement assays were carried out at a lower extracellular pH. Our results suggest a network linking the vacuolar cation exchangers to apoplastic pH maintenance that plays a crucial role in cellular processes.
Stomata are pores at the surface of the leaves, gating water loss and gas exchange between plants and the atmosphere. One stoma is formed by two specialized guard cells that are able to modulate their size and shape to control stomatal aperture in response to various signals, including water status, hormonal stimuli, CO2 levels, light, or temperature (Kwak et al., 2008). These stomatal movements are regulated by ion fluxes in guard cells, the changes in the osmoticum status being compensated by water movement, which modifies the cell’s volume. Ion transport between the cell and ion stores (vacuole, apoplastic space) must be therefore tightly controlled, and any change in the guard cell’s ability to regulate this can compromise its faculty to trigger stomatal movement.
Calcium ion (Ca2+) is one ion that regulates stomatal movements, and its cytosolic concentration is controlled by both influx, via plasma membrane channels, and release from internal stores such as vacuoles and the endoplasmic reticulum. Calcium transport from the vacuole is ensured, at least in part, by members of the Cation Exchanger (CAX) family (Punshon et al., 2012). Six members of this family are found in Arabidopsis (Arabidopsis thaliana); all use a proton gradient generated by the vacuolar H+-ATPase (VHA) or the vacuolar pyrophosphatase (AVP1) to energize their activity. CAX1 and CAX3 are the closest homologs within the family and have been proposed to play similar roles in Ca2+ homeostasis (Zhao et al., 2008). However, biochemical characterization highlighted differences in their respective rates of Ca2+ transport, and they have been proposed to function as heterodimers, with unique properties associated with this structure (Cheng et al., 2005).
Among common phenotypes of cax1 and cax3, an increased sensitivity to abscisic acid (ABA; Zhao et al., 2008) suggests a function for these transporters in modulating hormone signaling. ABA is well known for its role in triggering stomatal closure, whereas auxin, ethylene, or cytokinins can counteract its effect. Auxin in particular is also essential in governing plant development, including root architecture, tropisms and polarity, apical dominance, tissue differentiation, and plant development. Tight control of its distribution throughout the plant is achieved via ubiquitous and specific expression of members of three transporter families, acting together in mediating indole-3-acetic acid (IAA) fluxes (Krecek et al., 2009).
The unique pattern of auxin distribution is predominately due to the asymmetrical localization of members of the PIN-FORMED (PIN) family of auxin exporters (Zazímalová et al., 2010). In Arabidopsis, this family comprises eight members, whose spatiotemporal expression is responsible for the auxin gradient observed in many plant tissues (Paponov et al., 2005). In addition, most members of the ATP-binding cassette (ABC)-type family of exporter ABCB (ABCB/multidrug resistance/phosphoglycoprotein) have been shown to mediate auxin export from the cell (Geisler and Murphy, 2006). Auxin import is mainly ensured by (1) active transport of IAA by members of the AUX1/LAX family proteins (Geisler and Murphy, 2006), and (2) passive diffusion across the plasma membrane. AUX1 activity was demonstrated to be pH-dependent (Yang et al., 2006), IAA transport being optimal at acidic pH (5.5–6), and dramatically reduced at higher values. It is interesting that passive, pH-dependent IAA diffusion across the plasma membrane also accounts for an important part of IAA transport and signaling. At apoplastic pH (5.5), between 10% and 25% of IAA is protonated (Yang et al., 2006), which allows for free diffusion of IAA through the membrane. In contrast, the ratio between protonated and deprotonated IAA (IAAH/IAA−) falls to 1% to 5% when pH exceeds 6.5, preventing it from being passively transported into the cytoplasm (Yang et al., 2006). These two aspects make control of the apoplastic pH crucial in the regulation of auxin signaling, as it modulates all the known routes of IAA import. Such a tight pH constraint is ensured by plasma membrane-localized Arabidopsis H+-ATPases (AHA; Haruta et al., 2010) that transport protons from the cytosol to the extracellular space.
Our work presents the characterization of two vacuolar transporters’ abilities to modulate the apoplastic pH, and therefore contribute to proper auxin transport and signaling. Our results highlight the effects of mutations in CAX1 and CAX3 in plant development and in stomatal functioning, providing new insights for understanding hormone signaling in plants as well as plant adaptation to stress conditions via hormone cross talk.
RESULTS
CAX1 and CAX3 Are Highly Expressed in Guard Cells
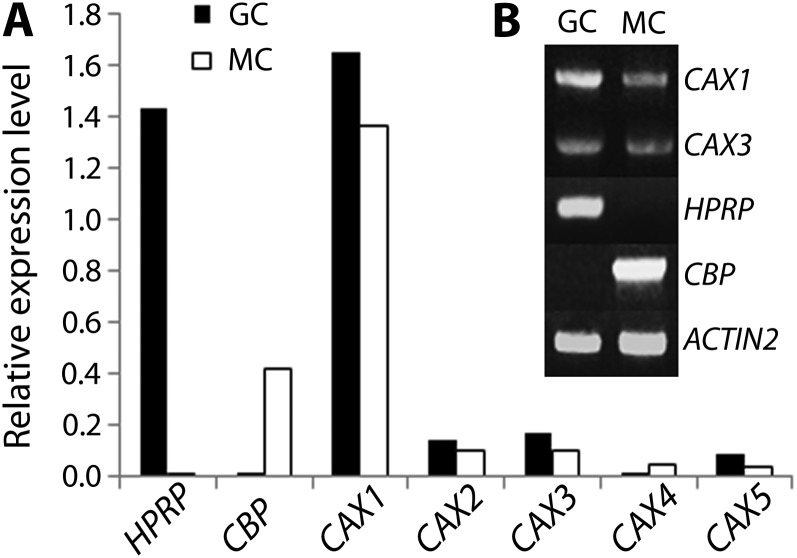
Based on the role of tonoplast transporters in regulating cytosolic calcium concentration, it was conceivable that Ca2+ transporters expressed in guard cells played a role in stomatal movements. We decided, therefore, to examine the CAX gene family and, as such, evaluated the expression of the family members in guard cells using microarray data of guard cell and mesophyll cell transcriptomes (Yang et al., 2008). Figure 1A shows the level of expression of the five CAX genes that are present in the ATH1 (Affymetrix) chip in guard cells and in mesophyll cells. Hydroxyproline-rich protein (HPRP; At2g21140) and calmodulin-binding protein (CBP; At4g33050) are shown as positive controls of guard cell- and mesophyll cell-specific expression, respectively (Jammes et al., 2009). CAX4 seems to be preferentially expressed in mesophyll cells, whereas CAX1, CAX2, CAX3, and CAX5 transcripts were present at a higher level in guard cells. Considering the very high expression of CAX1 in guard cells and its similarity to CAX3, we decided to focus on these two genes for further studies.
Figure 1.
CAX1 and CAX3 are expressed in guard cells. A, Expression levels of five CAX genes in guard cells (closed bar) compared with mesophyll cells (open bar) as assessed by microarray experiments (Yang et al., 2008). Expression levels were normalized to ACTIN2 (At4g33050). HPRP (At2g21140) and CBP (At4g33050) are shown as guard cell- and mesophyll cell-specific expression control (Jammes et al., 2009), respectively. B, RT-PCR analyses of CAX1 and CAX3. HPRP and CBP were amplified as cell-type specific marker genes.
To further verify the expression of CAX1 and CAX3 in guard cells, reverse transcription (RT)-PCR experiments were performed on highly pure protoplasts from both guard cells and mesophyll cells (Leonhardt et al., 2004; Fig. 1B), which confirmed that both CAX1 and CAX3 are relatively highly expressed in both cell types. Specific amplifications of the guard cell marker gene HPRP and of the mesophyll cell marker gene CBP (Jammes et al., 2009) indicate a high purity of the guard cell and mesophyll cell RNA.
cax1, cax3, and cax1/cax3 Mutants Are Impaired in Light-Induced Hyperpolarization of the Guard Cell Plasma Membrane
Guard cells primarily function in gas exchange but also in drought stress protection by closing stomata upon perception of the drought-induced hormone ABA. Thus, the implication of the CAX1 and CAX3 proteins was evaluated by analyzing the ABA response of guard cells in cax1, cax3, and cax1/cax3 (Cheng et al., 2005). Application of 1 µM ABA to stomata, previously open under light, led to a 20% reduction in stomatal apertures (Fig. 2A). When exposed to 5 µM ABA, stomatal apertures were reduced by about 30% in the wild type and cax1, cax3, and cax1/cax3 mutants (Fig. 2A). This result suggests that the cax1 and cax3 mutations did not alter stomatal response to ABA.
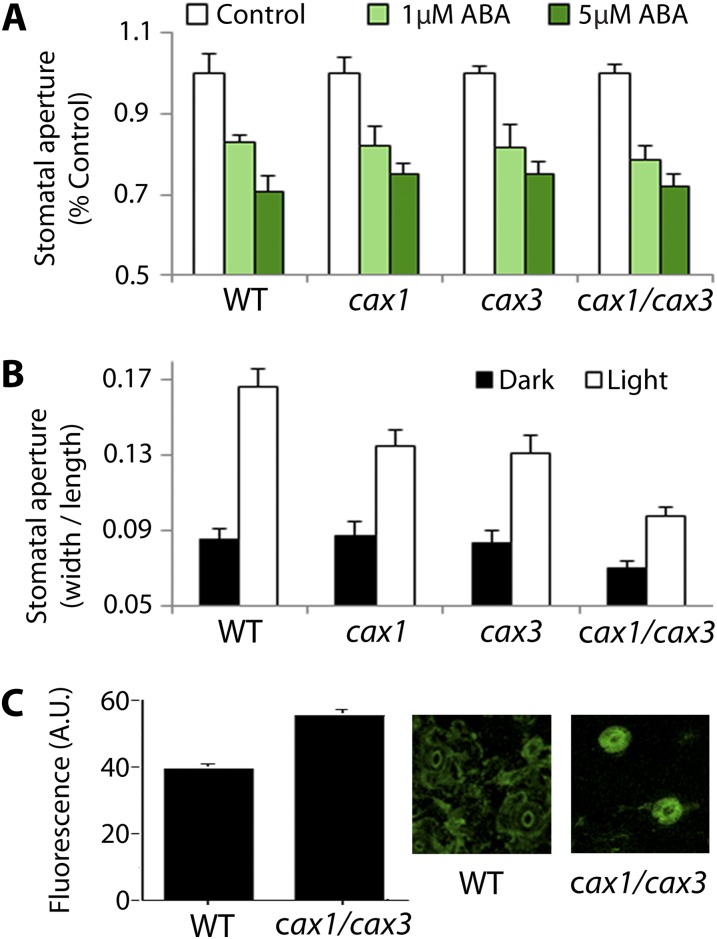
Figure 2.
cax1, cax3, and cax1/cax3 mutants are impaired in light-induced stomatal opening. A, Stomatal response to ABA is normal in cax1, cax3, and cax1/cax3. Epidermal strips were incubated 2 h in an opening buffer, and treated with 0, 1, and 5 µM ABA for 1 h prior to measurements of stomatal apertures. Data are mean ± sem (n = three independent experiments, >90 stomata for each data point). B, Light-induced stomatal opening is impaired in cax1, cax3, and cax1/cax3 compared with wild-type plants. Data are mean ± sem (n = five independent experiments, >100 stomata for each data point). C, cax1/cax3 guard cells have depolarized membrane potential compared with that of the wild type. Membrane potential was measured using the fluorescence dye DiBAC4 in the wild type and cax1/cax3. Higher fluorescence indicates depolarization of the plasma membrane. Average fluorescence from 685 (the wild type) and 917 (cax1/cax3) stomatal complexes is presented. Error bars = se.
It is interesting, however, that steady-state stomatal apertures, prior to ABA treatment, were significantly different in cax1, cax3, and cax1/cax3 from those in the wild type (Supplemental Fig. S1; 0.158 ± 0.007 for the wild type, 0.143 ± 0.005 for cax1, 0.149 ± 0.003 for cax3, 0.107 ± 0.002 for cax1/cax3; P < 0.01 compared with the wild type), which was later found to be consistent with the data in a recent study (Conn et al., 2011). These results suggested that the stomatal response to light might be impaired in the mutants. To test this hypothesis, light-induced stomatal opening analyses (Kwak et al., 2001) were carried out. After overnight incubation in the dark, stomatal apertures of cax1 and cax3 mutants appeared to be similar to that of wild-type plants, whereas stomatal aperture of cax1/cax3 was slightly less (Fig. 2B). Incubation in light for 2 h induced stomatal opening in wild-type plants, which was significantly impaired in cax1, cax3, and cax1/cax3 (0.167 ± 0.009 for the wild type, 0.135 ± 0.009 for cax1, 0.131 ± 0.009 for cax3, 0.097 ± 0.005 for cax1/cax3; P < 0.01 compared with the wild type). Because stomatal opening is largely controlled by membrane potential (Merlot et al., 2007), we examined whether there is a difference in the membrane polarization state of cax1/cax3 using the fluorescent dye bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4; Konrad and Hedrich, 2008). Figure 2C shows that wild-type guard cells exhibited relatively weaker fluorescence after 3 h of exposure to light compared with the cax1/cax3 double mutant, indicating the significantly more depolarized state of the plasma membrane (Fig. 2C) in the double mutant than in wild-type plants. Together, these results imply that the mutations in CAX1 and CAX3 lead to a shift of the membrane to more positive voltages (depolarization) and impaired stomatal response to light.
IAA, But Not 1-Naphthalene Acetic Acid, Fails to Inhibit ABA-Induced Stomatal Closure in cax1, cax3, and cax1/cax3
Auxin is known to regulate membrane polarization, and has been shown to induce hyperpolarization in tobacco (Nicotiana tabacum) guard cells and maize (Zea mays) protoplasts (Rück et al., 1993). At the guard cell level, auxin is also known to antagonize ABA-induced stomatal closure (Snaith and Mansfield, 1982; Tanaka et al., 2006). Because cax1/cax3 mutants displayed an altered response in membrane hyperpolarization, we examined auxin inhibition of ABA-induced stomatal closure in the cax1, cax3, and cax1/cax3 mutants. Epidermal strips of the plants were treated with 5 µM ABA in the presence or absence of 10 µM IAA and measured stomatal apertures. As shown in Figure 3A, IAA inhibited ABA-induced stomatal closure in wild-type plants (P < 0.05). In contrast, IAA failed to inhibit ABA-triggered stomatal closure in cax1, cax3, and cax1/cax3 mutant plants (P > 0.05), suggesting that CAX1 and CAX3 influence auxin signaling and/or transport in guard cells. Next, auxin inhibition of hypocotyl elongation in seedlings of cax1, cax3, and cax1/cax3 was examined. The mutants showed significantly reduced sensitivity to IAA in hypocotyl growth assays (P < 0.05; Supplemental Fig. S2), further indicating the effect of CAX1 and CAX3 on the auxin response.
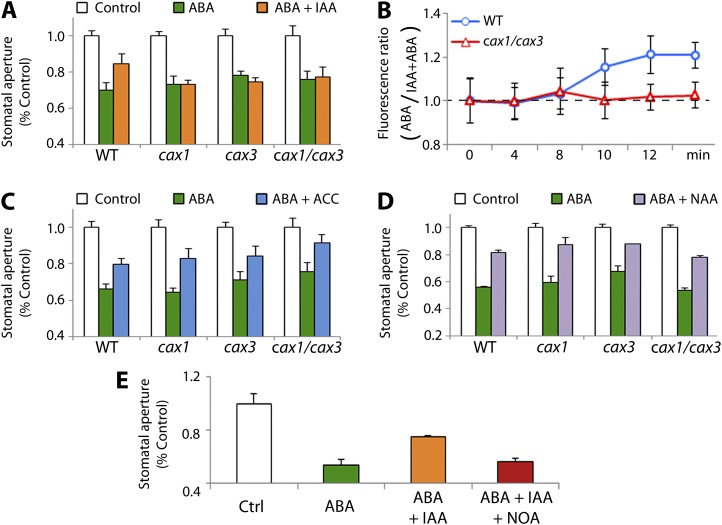
Figure 3.
cax1, cax3, and cax1/cax3 mutations impair IAA- but not NAA-inhibition of ABA-induced stomatal closure. A, IAA-inhibition of ABA-triggered stomatal closure is impaired in cax1, cax3, and cax1/cax3. Changes in stomatal aperture in response to 5 µM ABA were scored in the presence and absence of 10 µM IAA. Data are mean ± sem (n = 3 independent experiments, >90 stomata for each data point). B, IAA-inhibition of ABA-induced membrane depolarization is abolished in cax1/cax3 guard cells. Fluorescence ratio (ABA-treated/ABA + IAA-treated) in guard cells is shown for the purpose of clarity, because of the difference in steady-state membrane polarization between the wild type and cax1/cax3 (see Fig. 2C). Changes in guard cell-membrane polarization were assessed by DiBAC4 fluorescence in response to ABA in the presence and absence of 10 µM IAA. Cells were challenged with 5 µM ABA at 8 min. Data are mean ± sem (793 cells, the wild type; 129 cells, the wild type + IAA; 917 cells, cax1/cax3; 187 cells, cax1/cax3 + IAA). C, 10 µM ACC inhibits ABA-induced stomatal closure in cax1, cax3, and cax1/cax3 as well as in the wild type. Data are mean ± sem (n = three independent experiments, >90 stomata for each data point). D, NAA inhibits ABA-triggered stomatal closure in cax1, cax3, and cax1/cax3 as well as in the wild type (5 µM ABA; 10 µM NAA). Data are mean ± sem (n = three independent experiments, >90 stomata for each data point). E, The AUX1 transporter inhibitor NOA blocks IAA inhibition of ABA-induced stomatal closure in the wild type. Stomatal apertures were measured from epidermal strips treated with 5 µM ABA and 5 µM IAA in the presence or absence of 10 µM NOA. Data are mean ± sem (n = four independent experiments, >82 stomata for each data point). Error bars are smaller than symbols when not visible.
To determine whether the defective stomatal response to IAA in cax1, cax3, and cax1/cax3 was related to the auxin-induced hyperpolarization of the plasma membrane (Rück et al., 1993), we monitored the dynamic changes in guard cell membrane potential that IAA causes. Epidermal strips from the wild type and cax1/cax3 mutants were stained using DiBAC4, in the presence or absence of IAA, to which ABA was added 8 min after starting to monitor fluorescence. In ABA-treated wild-type guard cells, comparison between IAA- and mock-treated samples (Fig. 3B, blue circles) shows a significantly higher fluorescence in mock-treated epidermal strips (ABA/IAA + ABA ratio, 21.1 ± 8.5%, 4 min after ABA addition). This confirms that IAA is able to antagonize the ABA-induced depolarization of the plasma membrane. In contrast, fluorescence in cax1/cax3 guard cells (Fig. 3B, red triangles) was similar in IAA-treated samples compared with mock-treated ones (ABA/IAA + ABA ratio, 1.7 ± 6.0%, 4 min after ABA addition), consistent with the stomatal movement result (Fig. 3A).
To discriminate between IAA transport and IAA signaling impairment, the responses of these mutant plants to ethylene were evaluated because a functional ethylene biosynthetic and signaling pathway is required for proper IAA-mediated antagonism of ABA-induced stomatal closure (Tanaka et al., 2006; Acharya and Assmann, 2009). As shown in Figure 3C, wild-type plants treated with 5 µM ABA showed stomatal closure (66 ± 0.02% compared with the control), whereas epidermal strips pretreated with 10 µM 1-aminocyclopropane-1-carboxylic acid (ACC), a precursor of ethylene, displayed a reduction in ABA-triggered stomatal closure (80 ± 0.03% of the control; P < 0.05). This confirms the antagonistic effect of ethylene on ABA-triggered stomatal closure (Tanaka et al., 2006). ACC also inhibited ABA-triggered stomatal closure in the cax1, cax3, and cax1/cax3 mutants (Fig. 3C; P < 0.01 for all three cax mutants). This result shows that ethylene properly antagonizes ABA-triggered stomatal closure in the mutants and suggests that ethylene-dependent auxin signaling is functional, suggesting the defect is in mechanisms upstream of activation of the ethylene signaling pathway.
We then examined whether auxin transport was affected by the cax1 and cax3 mutations. To this end, stomatal movement analyses were conducted using epidermal strips treated with ABA together with 1-naphthaleneacetic acid (NAA). In contrast to IAA, which mainly requires auxin transporters to cross the plasma membranes and enter the cell, NAA is an IAA analog that can freely diffuse through the plasma membrane (Delbarre et al., 1996; Yamamoto and Yamamoto, 1998). As expected, 10 µM NAA significantly inhibited ABA-induced stomatal closure in wild-type plants (44.3 ± 0.6% closure with ABA alone versus 18.7 ± 2.1% with ABA + NAA, Fig. 3D; P < 0.05). NAA also antagonized ABA-triggered stomatal closure in cax1, cax3, and cax1/cax3 (46.2 ± 1.7% closure with ABA alone versus 21.9 ± 1% with ABA + NAA for cax1/cax3, Fig. 3D; P < 0.05), indicating that auxin signaling cascades are functional in these mutants. This result also suggested impairment of the auxin influx transporter AUX1. To evaluate this idea, we decided to pharmacologically inhibit the AUX1 transporter activity to examine whether this could copy the cax1, cax3, and cax1/cax3 mutations. The chemical 2-naphthoxyacetic acid (NOA), which inhibits auxin influx (Parry et al., 2001; Yang et al., 2006) without affecting AUX1 trafficking, was used for this purpose. Consistent with the results in Figure 3A, wild-type plants treated with 5 µM ABA exhibited typical stomatal closure (46.3 ± 4.6% closure compared with mock-treated plants), which was significantly inhibited by 10 µM IAA (24.9 ± 0.6% closure, Fig. 3E). When NOA was added together with IAA, ABA-induced stomatal closure was not inhibited by IAA (43.9 ± 3.1% closure, Fig. 3E; P < 0.05 compared with ABA + IAA, P > 0.05 compared with ABA alone), showing that the application of NOA to wild-type plants mimics the IAA-insensitive stomatal movements seen in cax1, cax3, and cax1/cax3.
CAX1 and CAX3 Contribute to the Regulation of Apoplastic pH and Accumulation of Plasma Membrane-Localized AHA
Previous studies have shown that auxin signaling can be modulated by pH in the apoplastic space, notably through AUX1 activity and IAA permeability of the plasma membrane (Li et al., 2005; Vieten et al., 2007). Our results showing that NAA but not IAA can inhibit ABA-induced stomatal closure in cax1, cax3, and cax1/cax3 (Fig. 3, A and D) imply a role for the AUX1 transporter. Knowing the function of CAX proteins in proton homeostasis and the effect of apoplastic pH in AUX1 activity, we hypothesized that pH regulation was affected in cax1, cax3, and cax1/cax3. Given the fact that all of our stomatal aperture measurements were conducted at pH 6.15 and IAA is a weak acid (pK = 4.8) that cannot be protonated at pH 6.15, we performed stomatal movement assays at a lower pH (5.6) to test whether pH could affect the outcome of stomatal movement analysis. Remarkably, IAA inhibition of ABA-induced stomatal closure was fully restored in cax1, cax3, and cax1/cax3 (Fig. 4A; P < 0.05 compared with ABA alone). However, we noticed that although we were able to completely restore the steady-state, stomatal aperture in cax1 and cax3, the cax1/cax3 double mutant still exhibited smaller stomatal apertures (Supplemental Fig. S4).
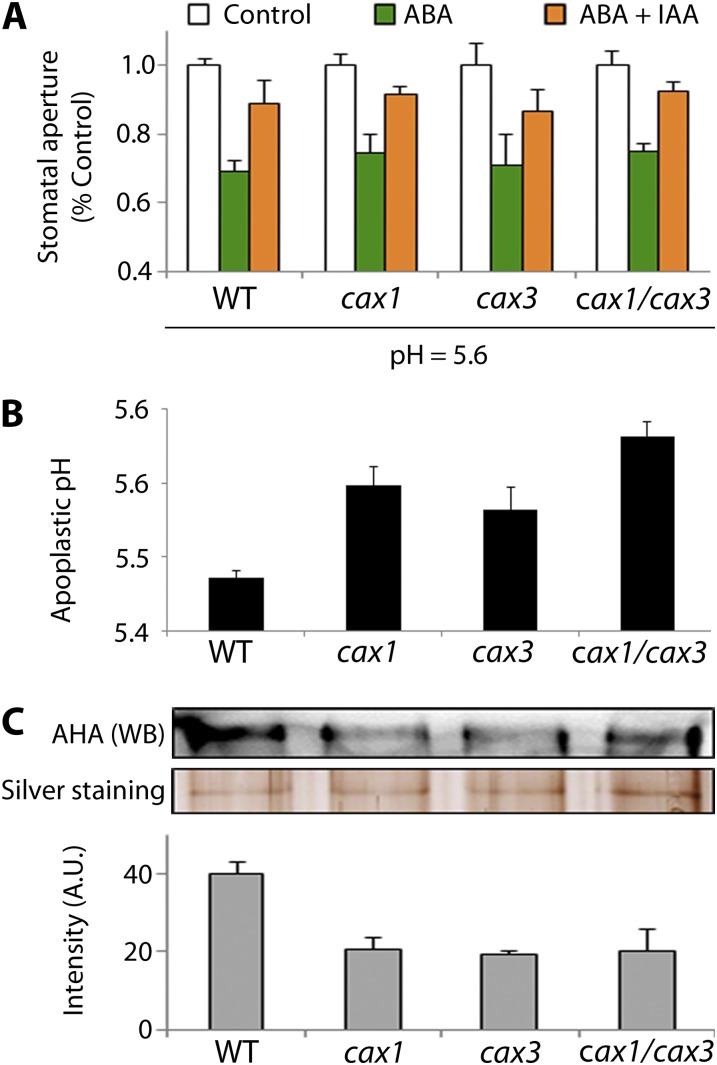
Figure 4.
Apoplastic pH is altered in cax1, cax3, and cax1/cax3. A, Lower extracellular pH (5.6) restores IAA-inhibition of ABA-induced stomatal closure in cax1, cax3, and cax1/cax3. Data are mean ± sem (n = 3 independent experiments, >86 stomata for each data point). B, Measurements of apoplastic pH reveal that cax1, cax3, and cax1/cax3 plants have a higher pH compared with the wild type. Emission wavelength of HPTS was collected at both 510 and 530 nm. Error bars in the graph represent sem of at least two independent experiments. At least 50 leaves were used for each experiment. C, Western-blot analysis of plasma membrane proteins shows that the P-type AHA protein level is significantly reduced in cax1, cax3, and cax1/cax3 compared with the wild type. Quantification of AHA protein level was normalized to the average protein amount from silver-stained gels. Error bars in the graph represent sem, n = three independent experiments.
This result strongly suggested that the pH of the apoplastic space might be affected in the cax mutants. Apoplastic fluids were retrieved by centrifugation from wild-type, cax1, cax3, and cax1/cax3 plants and then subjected to pH measurement using the fluorescent dye 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium (HPTS; Han and Burgess, 2010), whose fluorescence is pH-dependent (Supplemental Fig. S3). Figure 4B shows that the apoplastic pH in wild-type leaves was 5.4 ± 0.01 as previously reported (Zazímalová et al., 2010), whereas the apoplastic pH of cax1/cax3 mutants was significantly higher (5.7 ± 0.02; P < 0.01). Remarkably, cax1 and cax3 showed an intermediate apoplastic pH (5.9 ± 0.03 for cax1; 5.6 ± 0.03 for cax3). Because the pH of the apoplast has been suggested to be regulated by plasma membrane P-Type AHAs (Li et al., 2005), the levels of these proteins were examined. Western-blot analysis on the total membrane fraction of wild-type, cax1, cax3, and cax1/cax3 plants revealed that measure of AHAs in the membrane faction is significantly reduced in all three mutant plants by about 50% (Fig. 4C; P < 0.05). Together, these results suggest that the mutations in CAX1 and CAX3 influence the abundance of AHAs at the plasma membrane, resulting in the higher apoplastic pH in the cax mutants.
DISCUSSION
Vacuoles are very dynamic structures that can occupy up to 95% of the total volume in many types of plant cells, and play important roles in various cellular processes. Stomatal guard cells contain vacuoles whose structure and volume change during stomatal movements. Studies have shown that vacuole volume increases and decreases when stomata open and close, respectively (Fricker and White, 1990). In addition, vacuolar ion channels such as vacuolar K+ channels and slow vacuolar channels play important roles in regulation of stomatal movements (Kwak et al., 2008). To test the contribution of other vacuolar transporters to stomatal movements, expression pattern and levels in guard cells of the CAX genes that encode vacuolar cation exchangers were examined. Analysis of microarray data revealed the preferential expression, in guard cells over mesophyll cells, of CAX1 and CAX3, which prompted us to focus on these two genes for further experiments. Conn et al. (2011) have shown data suggesting a higher level of CAX1 and CAX3 expression in mesophyll cells than in the epidermal layer, which can most likely be attributed to extremely low expression in epidermal cells.
Cytosolic Ca2+ plays a significant role in signal transduction pathways, serving as a second messenger in a variety of cellular activities. Cytosolic Ca2+ concentration can change dramatically, which is achieved by Ca2+ influx from the extracellular space and Ca2+ release from internal stores, regulating subsequent cellular signal responses. In guard cells, ABA causes elevation in cytosolic Ca2+ concentration followed by stomatal closure (Schroeder et al., 2001). CAX1 and CAX3 have been shown to contribute to Ca2+ transport and homeostasis (Zhao et al., 2009; Conn et al., 2011; Manohar et al., 2011a, 2011b; Punshon et al., 2012). Thus, it was initially presumed that Ca2+ dyshomeostasis caused by the cax1 and cax3 mutations would affect ABA response in the mutants. It appeared, however, that cax1, cax3, and cax1/cax3 have normal ABA sensitivity in stomatal movement assays (Fig. 2A). Instead, auxin inhibition of ABA response in guard cells was found to be affected by the mutations in these genes (Fig. 3A). It is important to note that this result revealed a nonredundant phenotype in the cax1 and cax3 single mutants, suggesting that, at least in guard cells, both proteins are required to ensure their physiological function. Because stomatal closure in response to ABA was normal in the cax1, cax3, and cax1/cax3 mutants, it was speculated that CAX1 and CAX3 function in cross talk between ABA and auxin signaling or that auxin signaling/transport is impaired in the cax mutants. To test this hypothesis, an experiment that can bypass auxin transport was set up to evaluate solely auxin signaling cascades in the cax mutations. Considering that ethylene is required for proper auxin signaling in guard cells (Tanaka et al., 2006), epidermal strips were treated with ACC, a precursor of ethylene, and stomatal response to ABA was examined. Similar to IAA in wild-type plants, ACC successfully inhibited ABA-induced stomatal closure in the wild type, cax1, cax3, and cax1/cax3 (Fig. 3C). Unlike IAA inhibition of ABA-triggered stomatal closure, ACC inhibition of ABA-induced stomatal closure requires no IAA transport (Acharya and Assmann, 2009). This result implies that ethylene-dependent auxin signaling processes were not affected in cax1, cax3, and cax1/cax3, and the possibility of a reduced auxin transport was evaluated in these mutants. For this purpose, epidermal strips were treated with the membrane permeable auxin analog NAA (Delbarre et al., 1996). It is interesting that NAA led to full inhibition of the ABA-mediated stomatal closure in the cax1, cax3, and cax1/cax3 mutants (Fig. 3D). This result suggested that impairment of cross talk between auxin and ABA in the cax mutants was unlikely due to Ca2+ dyshomeostasis.
Differential stomatal responses to IAA and NAA in the cax mutants implied the involvement of AUX1 (Yamamoto and Yamamoto, 1998). This hypothesis was evaluated by the use of the AUX1 inhibitor NOA (Parry et al., 2001), which copied cax1, cax3, and cax1/cax3 phenotypes in the wild type (Fig. 3E), indicating that the IAA insensitivity of cax1, cax3, and cax1/cax3 is due to impaired IAA transport. Whereas such an effect of vacuolar cation exchangers on auxin influx was unexpected, proton transporters that can influence pH homeostasis in cells (Pittman et al., 2005) are known to play a role in auxin transport and signaling (Petrásek and Friml, 2009). Thus, it was conceivable that the cax1, cax3, and cax1/cax3 mutations could modify pH homeostasis, resulting in a defect in auxin transport. A previous study has shown that AVP1 contributes to regulation of apoplastic pH, which plays a role in auxin import, and therefore organ development (Li et al., 2005). Furthermore, previous studies demonstrated that CAX1 and CAX3 participate in the release of protons from the vacuole to the cytoplasm, thus contributing to regulation of cellular pH together with the VHA, the AVP1, and the plasma membrane AHA (Manohar et al., 2011). These proteins can modulate the pH of the vacuole and/or the cytosol, which can in turn affect the apoplastic pH (Li et al., 2005). Based on our results, we hypothesized that the cax1, cax3, and cax1/cax3 mutants have a higher pH in the apoplast, which was first assessed by performing IAA-mediated inhibition of ABA-triggered stomatal closure at a much lower pH (5.6) than is typically used (6.15), corresponding to that of the apoplast (Bibikova et al., 1998). Under these experimental conditions, guard cells exhibited restored IAA response (Fig. 4A). To directly determine whether the cax mutants have an altered apoplastic pH, the pH of apoplastic fluids from wild-type, cax1, cax3, and cax1/cax3 leaves was measured (François et al., 2002). This revealed that cax1/cax3 mutants have a significantly higher apoplastic pH than wild-type plants (Fig. 4B), a condition known to cause defective IAA influx. The cax1 and cax3 single mutants showed an intermediate phenotype. It is important to note that CAX1 and CAX3 do not physically interact with VHA or AVP1 (Zhao et al., 2009). This implies that the function of CAX1 and CAX3 is distinct from that of AVP1 in apoplastic pH regulation (Li et al., 2005). Overall, these results provide evidence supporting a previously suggested regulatory network linking vacuolar pH to apoplastic pH (Martinoia et al., 2007).
Apoplastic pH changes have long been considered as a means of regulating physiological processes. In maize, for instance, maximal elongation of the root tip is correlated to a minimal pH along a gradient (Felle, 1998). Fasano and colleagues reported changes in the apoplastic pH upon gravistimulation (Fasano et al., 2001), further supporting the role of pH in plant development and signal transduction in response to stimuli. Moreover, previous studies have also indicated a link between drought response and pH, and it is noteworthy that xylem sap pH increases upon water deficiency, which is correlated with a decrease in stomatal conductance in Commelina communis and maize (Wilkinson and Davies, 1997; Bahrun et al., 2002). In guard cells, auxin-mediated acidification of the apoplast has been shown to facilitate IAA transportation into the cell and plays a role in hyperpolarization of the plasma membrane. This hyperpolarization in turn triggers inward K+ channel-mediated currents, which lead to stomatal opening (Acharya and Assmann, 2009). Therefore, regulation of proton transport across the plasma membrane is crucial and involves members of the plasma membrane-localized P-type AHAs (Acharya and Assmann, 2009). Auxin has been shown to trigger synthesis and exocytosis of a high-turnover pool of the P-type AHAs (Hager et al., 1991), suggesting positive feedback regulation of auxin transport. Consistent with this finding, the level of P-type AHAs in the plasma membrane was affected in cax1 (Cheng et al., 2003), cax3, and cax1/cax3 mutants (Zhao et al., 2008). In both cax3 and cax1/cax3, the previously reported down-regulation of AHA activity correlates with our results, showing a significant decrease in the accumulation of these proteins (Fig. 4C). However, this result is not in agreement with the previous finding showing a wild-type level of the AHA proteins in the cax mutants (Zhao et al., 2008). This can be attributed to differences in specificity of the antibodies used. It should also be noted that whereas Cheng et al. (2003) have reported an increase in the AHA activity in cax1, we show a down-regulation in the amount of AHA protein in the cax1, cax3, and cax1/cax3 mutants compared with wild-type plants.
Altogether, our results indicate a fundamental role of CAX1 and CAX3 vacuolar transporters in modifying apoplastic pH, likely via the regulation of plasma membrane-localized AHA. Thus, the mutations in CAX1 and CAX3 result in altered IAA transport and defective stomatal response to IAA. Based on our findings, we propose the following model (see Fig. 5). In wild-type cells, CAX1 and CAX3 mediate proton export from the vacuole to the cytosol (Fig. 5a). In cax1, cax3, and cax1/cax3 mutants, this activity is disrupted, possibly causing compromised cytosolic proton homeostasis (Fig. 5b). To cope with this, the effect of P-Type AHAs, which acidify the apoplastic space (Fig. 5c), can be reduced via down-regulation of their activity and/or amount (Fig. 5d). This would cause a higher pH in the apoplastic space in the cax mutants, resulting in a lower activity of AUX1 in mediating IAA import, as well as a decreased propensity of IAA to be under its protonated, membrane-permeable form (Fig. 5, e and f).
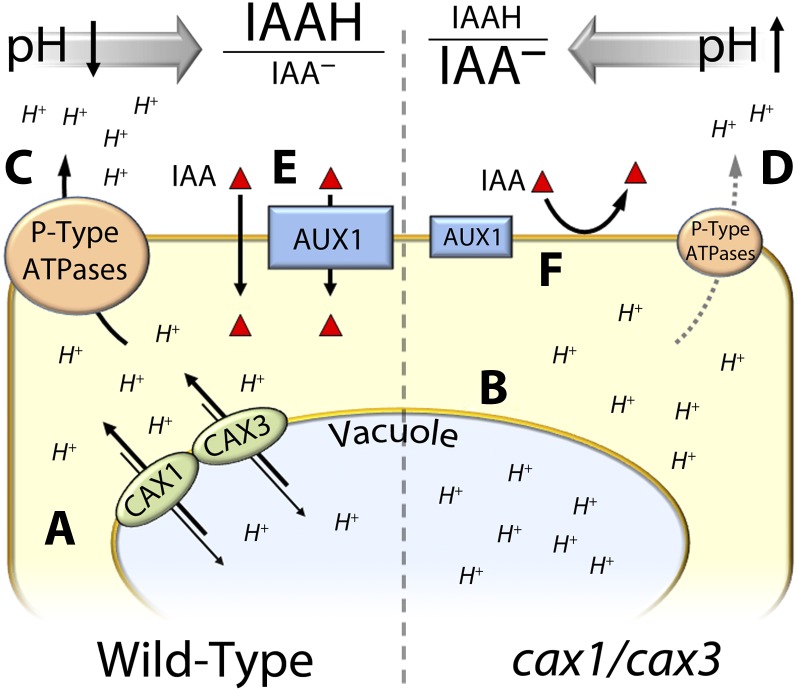
Figure 5.
A working model for the mode of IAA transport in the cax mutants. A, In guard cells, CAX1 and CAX3 transporters contribute to regulation of cytosolic pH in the cell by exporting H+ from vacuoles. B, This activity is reduced in cax1, cax3, and cax1/cax3. To maintain appropriate pH in the cytosol, H+ export into the apoplastic space that normally occurs in the wild type (C) is down-regulated due at least in part to the reduced level of AHA2 in the plasma membrane in the cax mutants (D). This results in a higher pH in the apoplast in the mutant, which in turn negatively affects AUX1 activity (E) and protonation of IAA– (F), thus reducing IAA transport and diffusion across the plasma membrane.
Because these data have been collected from epidermal strips, the extent to which this phenomenon applies in planta and actually affects photosynthesis remains to be determined. However, the lower IAA sensitivity of the cax1, cax3, and cax1/cax3 seedlings (Supplemental Fig. S2) and the known effect of pH on stomatal movements (Wilkinson and Davies, 1997; Bahrun et al., 2002; Acharya and Assmann, 2009) do suggest an effect of the mutations on in planta physiology. It should also be noted that these results do not contradict the conclusion by Conn et al. (2011), in which calcium was shown to be involved in the constitutive stomatal closure in cax1/cax3. Indeed, even though stomatal response to IAA is restored by lowering the apoplastic pH in all of the mutants, the steady-state aperture in the single mutants was found to be no different than in the wild type, whereas the stomata remained more closed in cax1/cax3 (Supplemental Fig. S4). Considering the effect of Ca2+ on stomatal movements and the fact that it acts downstream of IAA, it is not surprising that Conn et al. (2011) were able to fully restore stomatal opening by increasing the apoplastic Ca2+ concentration. This is also consistent with previous results suggesting that Ca2+ concentrations are not changed in the single cax1 and cax3 mutants, probably because of functional redundancy conferred by other Ca2+ transporters (Conn et al., 2011). Our data show that this redundancy might be sufficient enough to sustain an appropriate Ca2+ level, but not to maintain apoplastic pH. This is also suggested by the similar reduction in AHA protein accumulation in the single and double mutants (Fig. 4), further supporting the idea of a unique function of the heterodimeric CAX1/CAX3 in maintaining ion homeostasis and pH.
MATERIALS AND METHODS
Stomatal Movement Assays
Plants were grown on Sunshine Redi-earth soil (Sun Gro Horticulture) in a growth chamber at 22°C under 16 h of light and 8 h of dark conditions for 3 to 4 weeks. To measure stomatal apertures, Arabidopsis (Arabidopsis thaliana) rosette leaves were blended (Waring) and the resulting epidermal strips kept for 3 h under light (120 µE m−2 s−1) in opening solution (10 mm KCl, 10 mm MES, pH 6.15 or 5.6) to induce stomatal opening prior to stomatal closure assays (Kwak et al., 2001). Leaves were incubated for an additional 2 h in the presence of ABA (1 or 5 µM), IAA (10 µM), ACC (10 µM), NAA (10 µM), and/or NOA (10 µM). Photographs were taken with Scion software (Scion Corporation), and stomatal width/length ratio was measured using ImageJ software (National Institutes of Health).
Microarray Data and RT-PCR Analyses
Microarray data from Yang et al. (2008; guard cells versus mesophyll cells, without ABA treatment) were used as a primary means of evaluating the expression of CAX genes in guard cells compared with mesophyll cells. Arabidopsis guard cell and mesophyll cell protoplasts were prepared as previously described (Leonhardt et al., 2004), and 2 µg of total RNA was used to generate complementary DNAs. Primer sequences used in the study are shown in Supplemental Table S1. The guard cell-marker gene CBP (At4g33050) and the mesophyll cell-marker gene HPRP (At2g21140; Jammes et al., 2009) were used to verify the purity of guard cell and mesophyll cell protoplasts, respectively. Actin2 (At5g09810) was amplified as a control. A total of 32 PCR cycles were performed for CAX1 amplification and 35 cycles for CAX3 amplification.
GUS Histochemical Assay
Arabidopsis plants expressing proCAX1::GUS or proCAX3::GUS were grown on soil for 3 weeks before GUS staining. Epidermal peels were prepared and incubated in a GUS solution as described (Jammes et al., 2009) for 3 h (proCAX1::GUS) or 30 h (proCAX3::GUS).
IAA Inhibition of Hypocotyl Elongation
Inhibition of hypocotyl elongation by IAA was conducted. Seeds were planted on one-quarter-strength mass spectrometry media and kept for 2 d at 4°C in the dark for stratification. Plates were then transferred to continuous light (35 µE m−2 s−1, 22°C) filtered with yellow cellophane for 4.5 d before measuring hypocotyl lengths.
Measurements of Membrane Potential
To measure dynamic membrane potential changes in guard cells, epidermal peels of Arabidopsis rosette leaves were used. To prepare peels, the abaxial side of leaves of 4- to 5-week-old plants was fixed on coverslips with a silicone adhesive and peeled. Samples were then placed in buffer containing 30 mm KCl, 10 mm MES, pH 6. After 30 min in darkness, epidermal peels were incubated under light (250 µmol m−2 s−1) to promote stomatal opening. After 3 h under light, 2 µM of the fluorescent dye DiBAC4(3) (Sigma-Aldrich) was added to the KCl buffer for 10 min. Finally, the coverslip containing the epidermal peel was washed with KCl buffer and coated with a very thin film of vacuum grease (Dow Corning) to prevent strip movement. This coverslip formed the bottom of a microwell chamber, which was immediately filled with 200 µL of KCl buffer. The microwell chamber was mounted on an inverse confocal laser scanning microscope (Leica TCS SP2). Fluorescence measurements were conducted with Leica Confocal Software (LCS Lite version 2.61, Leica). The membrane potential dye was excited at 488 nm with an argon laser; fluorescence emission was filtered at 530 ± 15 nm and fluorescence intensity was captured through a 40× objective. To estimate dye fluorescence of whole guard cells, optical sections of 0.75 µm were made, and fluorescence was measured on an average image (LCS Lite version 2.61, Leica). Images were saved every 2 min. All measurements were corrected for background fluorescence that was recorded from reference regions close to the analyzed cells. Data shown with error bars represent mean values ± se.
Apoplastic pH Measurement
Apoplastic fluids were retrieved by the common infiltration-centrifugation technique. Fifty leaves from wild-type and cax1/cax3 plants were immersed in 50 mL of water and subjected to four, 5-min vacuum cycles, each followed by rapid vacuum release. The leaves were then quickly dried with a paper towel and placed in a syringe without a plunger. The exhaust was connected to a 1.5-mL conical tube. The whole mounting was placed in a 15-mL tube and centrifuged at 1,000g for 10 min at 4°C, and about 200 µL of apoplastic fluids were collected. On a 96-well plate, 160 µL of this apoplastic fluid was mixed with 40 µL of a 100 mg/mL HPTS solution (Invitrogen), and fluorescence was collected at 510 and 530 nm with a plate reader (Excitation = 460 nm, compare with manufacturer’s instructions). The pH of the solution was determined against a standard curve made with the Britton-Robinson universal buffer (0.05 m H3BO3, 0.05 m H3PO4, 0.05 m CH3COOH), adjusted to pHs ranging from 3 to 8.5 (by 0.5-unit increments) with 1 m NaOH.
Western-Blot Analyses
Leaves were ground in 2 mm EDTA, 5 mm dithiothreitol, 20% glycerol, 1 mm phenylmethylsulfonyl fluoride, 1 µg/mL pepstain, 10 µg/mL aprotinin, 10 µg/mL leupeptin, 50 mm Tris, pH 8.2. The protein extract was filtered through miracloth (Calbiochem) and centrifuged at 8,000g for 10 min to remove cell debris. The resulting supernatant was collected and centrifuged for 30 min at 100,000g to pellet membranes. Twenty micrograms of proteins from this membrane fraction were separated on a 10% acrylamide/Bis-acrylamide gel and transferred onto a nitrocellulose membrane. P-Type AHAs were detected after incubation of the membrane with anti-AHA antibody (1/2,000; Qiao et al., 2009). Loading control was assessed by silver staining of the gel.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Steady-state stomatal aperture in the wild type, cax1, cax3, and cax1/cax3.
Supplemental Figure S2. IAA inhibition of hypocothyl elongation in the wild type, cax1, cax3, and cax1/cax3.
Supplemental Figure S3. Fluorescence intensity of HPTS as a function of pH.
Supplemental Figure S4. Steady-state stomatal aperture in the wild type, cax1, cax3, and cax1/cax3 at pHapo = 5.6.
Supplemental Table S1. Primers used in this study.
Supplementary Material
Acknowledgments
The authors thank R. Bouten, M. Manohar, and J. Pittman for critical reading of the manuscript, and H. Qiao for providing the anti-H+-ATPase antibody. The authors are also grateful to Drs. H. Kazama, H. Sze, and S. Chanroj for technical help.
Glossary
- IAA
indole-3-acetic acid
- ABA
abscisic acid
- RT
reverse transcription
- DIBAC4
bis-(1,3-dibutylbarbituric acid) trimethine oxonol
- NAA
1-naphthalene acetic acid
- ACC
1-aminocyclopropane-1-carboxylic acid
- NOA
2-naphthoxyacetic acid
- HPTS
8-hydroxypyrene-1,3,6-trisulfonic acid trisodium
- AHA
H+-ATPase
- VHA
vacuolar H+-ATPase
- AHA:
Arabidopsis H+-ATPases
References
- Acharya BR, Assmann SM. (2009) Hormone interactions in stomatal function. Plant Mol Biol 69: 451–462 [DOI] [PubMed] [Google Scholar]
- Bahrun A, Jensen CR, Asch F, Mogensen VO. (2002) Drought-induced changes in xylem pH, ionic composition, and ABA concentration act as early signals in field-grown maize (Zea mays L.). J Exp Bot 53: 251–263 [DOI] [PubMed] [Google Scholar]
- Bibikova TN, Jacob T, Dahse I, Gilroy S. (1998) Localized changes in apoplastic and cytoplasmic pH are associated with root hair development in Arabidopsis thaliana. Development 125: 2925–2934 [DOI] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Barkla BJ, Shigaki T, Hirschi KD. (2003) The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 15: 347–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD. (2005) Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol 138: 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn SJ, Gilliham M, Athman A, Schreiber AW, Baumann U, Moller I, Cheng NH, Stancombe MA, Hirschi KD, Webb AA, et al. (2011) Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell 23: 240–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre A, Muller P, Imhoff V, Guern J. (1996) Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta 198: 532–541 [DOI] [PubMed] [Google Scholar]
- Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S. (2001) Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 13: 907–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle HH. (1998) The apoplastic pH of the Zea mays root cortex as measured with pH-sensitive microelectrodes: aspects of regulation. J Exp Bot 49: 987–995 [Google Scholar]
- François IE, De Bolle MF, Dwyer G, Goderis IJ, Woutors PF, Verhaert PD, Proost P, Schaaper WM, Cammue BP, Broekaert WF. (2002) Transgenic expression in Arabidopsis of a polyprotein construct leading to production of two different antimicrobial proteins. Plant Physiol 128: 1346–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker MD, White N. (1990) Volume measurement of guard cell vacuoles during stomatal movements using confocal microscopy. Transact Royal Microsc Soc London 1: 345–348 [Google Scholar]
- Geisler M, Murphy AS. (2006) The ABC of auxin transport: the role of p-glycoproteins in plant development. FEBS Lett 580: 1094–1102 [DOI] [PubMed] [Google Scholar]
- Hager A, Debus G, Edel HG, Stransky H, Serrano R. (1991) Auxin induces exocytosis and the rapid synthesis of a high-turnover pool of plasma-membrane H+-ATPase. Planta 185: 527–537 [DOI] [PubMed] [Google Scholar]
- Han J, Burgess K. (2010) Fluorescent indicators for intracellular pH. Chem Rev 110: 2709–2728 [DOI] [PubMed] [Google Scholar]
- Haruta M, Burch HL, Nelson RB, Barrett-Wilt G, Kline KG, Mohsin SB, Young JC, Otegui MS, Sussman MR. (2010) Molecular characterization of mutant Arabidopsis plants with reduced plasma membrane proton pump activity. J Biol Chem 285: 17918–17929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammes F, Song C, Shin D, Munemasa S, Takeda K, Gu D, Cho D, Lee S, Giordo R, Sritubtim S, et al. (2009) MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc Natl Acad Sci USA 106: 20520–20525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad KR, Hedrich R. (2008) The use of voltage-sensitive dyes to monitor signal-induced changes in membrane potential-ABA triggered membrane depolarization in guard cells. Plant J 55: 161–173 [DOI] [PubMed] [Google Scholar]
- Krecek P, Skupa P, Libus J, Naramoto S, Tejos R, Friml J, Zazímalová E. (2009) The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol 10: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mäser P, Schroeder JI. (2008) The clickable guard cell, version II: interactive model of guard cell signal transduction mechanisms and pathways. The Arabidopsis Book 6: e0114, doi/10.1199/tab.0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Murata Y, Baizabal-Aguirre VM, Merrill J, Wang M, Kemper A, Hawke SD, Tallman G, Schroeder JI. (2001) Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis. Plant Physiol 127: 473–485 [PMC free article] [PubMed] [Google Scholar]
- Leonhardt N, Kwak JM, Robert N, Waner D, Leonhardt G, Schroeder JI. (2004) Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell 16: 596–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yang H, Peer WA, Richter G, Blakeslee J, Bandyopadhyay A, Titapiwantakun B, Undurraga S, Khodakovskaya M, Richards EL, et al. (2005) Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 310: 121–125 [DOI] [PubMed] [Google Scholar]
- Manohar M, Shigaki T, Hirschi KD. (2011a) Plant cation/H+ exchangers (CAXs): biological functions and genetic manipulations. Plant Biol (Stuttg) 13: 561–569 [DOI] [PubMed] [Google Scholar]
- Manohar M, Shigaki T, Mei H, Park S, Marshall J, Aguilar J, Hirschi KD. (2011b) Characterization of Arabidopsis Ca2+/H+ exchanger CAX3. Biochemistry 50: 6189–6195 [DOI] [PubMed] [Google Scholar]
- Martinoia E, Maeshima M, Neuhaus HE. (2007) Vacuolar transporters and their essential role in plant metabolism. J Exp Bot 58: 83–102 [DOI] [PubMed] [Google Scholar]
- Merlot S, Leonhardt N, Fenzi F, Valon C, Costa M, Piette L, Vavasseur A, Genty B, Boivin K, Müller A, et al. (2007) Constitutive activation of a plasma membrane H(+)-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J 26: 3216–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paponov IA, Teale WD, Trebar M, Blilou I, Palme K. (2005) The PIN auxin efflux facilitators: evolutionary and functional perspectives. Trends Plant Sci 10: 170–177 [DOI] [PubMed] [Google Scholar]
- Parry G, Delbarre A, Marchant A, Swarup R, Napier R, Perrot-Rechenmann C, Bennett MJ. (2001) Novel auxin transport inhibitors phenocopy the auxin influx carrier mutation aux1. Plant J 25: 399–406 [DOI] [PubMed] [Google Scholar]
- Petrásek J, Friml J. (2009) Auxin transport routes in plant development. Development 136: 2675–2688 [DOI] [PubMed] [Google Scholar]
- Pittman JK, Shigaki T, Hirschi KD. (2005) Evidence of differential pH regulation of the Arabidopsis vacuolar Ca2+/H+ antiporters CAX1 and CAX2. FEBS Lett 579: 2648–2656 [DOI] [PubMed] [Google Scholar]
- Punshon T, Hirschi K, Yang J, Lanzirotti A, Lai B, Guerinot ML. (2012) The role of CAX1 and CAX3 in elemental distribution and abundance in Arabidopsis seed. Plant Physiol 158: 352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Chang KN, Yazaki J, Ecker JR. (2009) Interplay between ethylene, ETP1/ETP2 F-box proteins, and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes Dev 23: 512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rück A, Palme K, Venis MA, Napier RM, Felle HH. (1993) Patch-clamp analysis establishes a role for an auxin binding protein in the auxin stimulation of plasma membrane current in Zea mays protoplasts. Plant J 4: 41–46 [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Snaith PJ, Mansfield TA. (1982) Stomatal sensitivity to abscisic acid: can it be defined? Plant Cell Environ 5: 309–311 [Google Scholar]
- Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N, Hasezawa S. (2006) Cytokinin and auxin inhibit abscisic acid-induced stomatal closure by enhancing ethylene production in Arabidopsis. J Exp Bot 57: 2259–2266 [DOI] [PubMed] [Google Scholar]
- Vieten A, Sauer M, Brewer PB, Friml J. (2007) Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci 12: 160–168 [DOI] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ. (1997) Xylem sap pH increase: a drought signal received at the apoplastic face of the guard cell that involves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant Physiol 113: 559–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Yamamoto KT. (1998) Differential effects of 1-naphthaleneacetic acid, indole-3-acetic acid and 2,4-dichlorophenoxyacetic acid on the gravitropic response of roots in an auxin-resistant mutant of arabidopsis, aux1. Plant Cell Physiol 39: 660–664 [DOI] [PubMed] [Google Scholar]
- Yang Y, Costa A, Leonhardt N, Siegel RS, Schroeder JI. (2008) Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E. (2006) High-affinity auxin transport by the AUX1 influx carrier protein. Curr Biol 16: 1123–1127 [DOI] [PubMed] [Google Scholar]
- Zazímalová E, Murphy AS, Yang H, Hoyerová K, Hosek P. (2010) Auxin transporters—why so many? Cold Spring Harb Perspect Biol 2: a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Barkla BJ, Marshall J, Pittman JK, Hirschi KD. (2008) The Arabidopsis cax3 mutants display altered salt tolerance, pH sensitivity and reduced plasma membrane H+-ATPase activity. Planta 227: 659–669 [DOI] [PubMed] [Google Scholar]
- Zhao J, Shigaki T, Mei H, Guo YQ, Cheng NH, Hirschi KD. (2009) Interaction between Arabidopsis Ca2+/H+ exchangers CAX1 and CAX3. J Biol Chem 284: 4605–4615 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.