Abstract
Plants respond to insect herbivory through the production of biochemicals that function as either direct defenses or indirect defenses via the attraction of natural enemies. While attack by closely related insect pests can result in distinctive levels of induced plant defenses, precise biochemical mechanisms responsible for differing responses remain largely unknown. Cowpea (Vigna unguiculata) responds to Fall armyworm (Spodoptera frugiperda) herbivory through the detection of fragments of chloroplastic ATP synthase γ-subunit proteins, termed inceptin-related peptides, present in larval oral secretions (OS). In contrast to generalists like Fall armyworm, OS of the legume-specializing velvetbean caterpillar (VBC; Anticarsia gemmatalis) do not elicit ethylene production and demonstrate significantly lower induced volatile emission in direct herbivory comparisons. Unlike all other Lepidoptera OS examined, which preferentially contain inceptin (Vu-In; +ICDINGVCVDA−), VBC OS contain predominantly a C-terminal truncated peptide, Vu-In−A (+ICDINGVCVD−). Vu-In−A is both inactive and functions as a potent naturally occurring antagonist of Vu-In-induced responses. To block antagonist production, amino acid substitutions at the C terminus were screened for differences in VBC gut proteolysis. A valine-substituted peptide (Vu-InΔV; +ICDINGVCVDV−) retaining full elicitor activity was found to accumulate in VBC OS. Compared with the native polypeptide, VBC that previously ingested 500 pmol of the valine-modified chloroplastic ATP synthase γ-subunit precursor elicited significantly stronger plant responses in herbivory assays. We demonstrate that a specialist herbivore minimizes the activation of defenses by converting an elicitor into an antagonist effector and identify an amino acid substitution that recovers these induced plant defenses to a level observed with generalist herbivores.
Excluding microorganisms, plants and insects constitute over 75% of the total species on earth (Hawksworth and Kalin-Arroyo, 1995). At a numerical species level, insect herbivores significantly outweigh plants and together display exquisitely complex ecological interactions (Price, 2002). Herbivorous insects are often broadly categorized as either generalists or specialists to denote feeding preferences on either a wide variety or a select subset of plant species, respectively. It is widely envisioned that host plant defenses have less impact on specialists as compared with generalist herbivores. Comparatively, specialists often manipulate plants to their benefit and demonstrate improved tolerance of host-specific biochemicals. For example, sawfly larvae in the genus Pontania induce protective closed galls in numerous willow species (Salix spp.) that result in dramatically reduced interior tissue defenses (Nyman and Julkunen-Tiitto, 2000). Swallowtail caterpillars, such as Papilio polyxenes, utilize plants in the family Apiaceae through efficient cytochrome P450 monooxygenase enzymes that directly detoxify dietary furanocoumarins (Cohen et al., 1992; Berenbaum, 2002). Cabbage white butterfly (Pieris rapae) larvae prevent toxic isothiocyanate formation through the action of a gut nitrile-specifier protein that redirects typical hydrolysis of glucosinolates to instead form nitriles that are excreted in the frass (Wittstock et al., 2004). Although specialists often exhibit greater tolerance to specific biochemicals than generalists, in many cases they are still negatively impacted by high levels of these plant defenses (Adler et al., 1995; Zalucki et al., 2001; Berenbaum, 2002; Agrawal and Kurashige, 2003).
A hallmark of herbivore-inducible plant defense is variability. One method to nondestructively measure this variation is through the assessment of indirect defenses such as herbivore-induced volatile emissions that attract natural enemies of the offending pest. In some cases, different feeding habits, such as leaf herbivory versus stem boring, readily account for this variation; however, even similar patterns of leaf herbivory can result in different volatile emission patterns and parasitoid attraction (De Moraes et al., 1998; Turlings et al., 1998). Oral secretions (OS)-derived fatty acid amino acid conjugate (FAC) elicitors, such as N-(17-hydroxylinolenoyl)-l-Gln and related analogs, promote plant recognition of Lepidopteran herbivores and trigger induced volatile emission in diverse plant species, including maize (Zea mays), eggplant (Solanum melongena), and soybean (Glycine max; Alborn et al., 1997; Schmelz et al., 2009). A combination of mechanical damage and FAC elicitors can constitute the initial stimuli leading to production of the defensive phytohormones jasmonic acid (JA) and ethylene (ET); however, elicitation can be phylogenetically idiosyncratic and even absent from closely related plants (Schmelz et al., 2009). In receptive plants, a potential for specificity comes from differences in the biochemical content of herbivore OS. For example, in Nicotiana attenuata, herbivory and OS of the specialist Manduca sexta elicit greater production of JA and ET than comparable treatments with the generalist beet armyworm (Spodoptera exigua; Diezel et al., 2009). These phytohormone differences are coincident with markedly different levels of FAC elicitors and also salivary-derived Glc oxidase (GOX) in the herbivore species.
As a salivary secretion, GOX is one of the few herbivore-derived effectors implicated in the suppression of inducible defenses, namely reduced nicotine accumulation in tobacco (Nicotiana tabacum) and improved growth of corn earworm (Helicoverpa zea; Musser et al., 2002). The suppressive activity of GOX in Nicotiana spp. is in part through the formation of hydrogen peroxide, elicitation of induced salicylic acid (SA) production, and associated negative cross talk interactions that attenuate induced JA and ET signaling (Diezel et al., 2009). Transcriptional evidence for similar SA-mediated suppression of JA signaling also exists in different feeding guilds. For example, in Arabidopsis (Arabidopsis thaliana), whitefly (Bemisia tabaci) feeding appears to repress the accumulation JA-regulated defense transcripts such as PDF1.2 (Zarate et al., 2007). Although the effector remains unknown, B. tabaci suppression of JA-regulated resistance is associated with strong up-regulation of the SA signaling pathway and associated gene transcript levels (Zarate et al., 2007). Regardless of diet breadth, herbivores cause responses in their hosts that are variable, and more recently, the paradigm of generalist and specialist herbivores inducing predictable plant responses has been challenged (Ali and Agrawal, 2012).
Improved utilization of host plants by any biotic attacker often involves a combination of detoxification and prevention of defense activation. The vast majority of mechanistic advances demonstrating the suppression of inducible defenses have come from plant-pathogen research (Jones and Dangl, 2006; Zhou and Chai, 2008; Hogenhout et al., 2009). A well-characterized component of plant basal immunity to pathogenic bacteria is recognition of microbe-associated molecular patterns (MAMPs) such as the flagellin peptide fragment, termed flg22. The FLS2 receptor binds flg22 to initiate defense; however, successful pathogens such as Agrobacterium tumefaciens and pathovar variants of Xanthomonas campestris pv campestris exhibit altered sequences of flg22 that neither associate with FLS2 nor activate defense (Felix et al., 1999; Zipfel et al., 2004; Sun et al., 2006). To counter elicitation, pathogenic bacteria, such as Pseudomonas syringae pv tomato DC3000, inject into plant cells a broad array of multifunctional effector proteins via the type III secretion system that suppress the activation of innate immunity (Jones and Dangl, 2006; Zhou and Chai, 2008). Moreover, P. syringae pv tomato DC3000 also secretes the jasmonoyl-Ile phytohormone mimic coronatine as a small-molecule effector that co-opts signaling by the E3 ubiquitin ligase Coronatine-Insensitive1 (COI1) and the transcription factor Jasmonate-Insensitive1 (JIN1)/MYC2 to suppress MAMP-mediated resistance responses (Millet et al., 2010). In a conceptually useful scheme detailing the probable evolutionary interplay of resistance and disease susceptibility, plants are hypothesized to have first evolved MAMP recognition systems to trigger innate immune responses. Subsequently, pathogens overcame recognition through effectors (virulence factors) that were able to suppress those immune responses (Bent and Mackey, 2007). Plant R proteins then evolved to either directly recognize pathogen effectors (avirulence factors) or products of their activities on guarded plant proteins to promote defense activation and resistance. In contrast to pathogens, relatively little is known about the mechanisms that Lepidopteran herbivores use to evade plant recognition and activation of defense responses (Bonaventure et al., 2011).
Cowpea (Vigna unguiculata) and common bean (Phaseolus vulgaris) recognize insect herbivory through the detection of trace amounts of cyclic disulfide-bridged inceptin-related peptides present in caterpillar OS, which are derived from proteolytic fragments of chloroplastic ATP synthase γ-subunit (cATPC) proteins (Schmelz et al., 2006, 2007). Inceptin-related peptides have thus far been described in the OS of Fall armyworm (FAW; Spodoptera frugiperda), which prefers grasses but is a confirmed generalist known to feed on over 60 different diverse plant species, including cowpea (Luginbill, 1928). To better understand the adaptations of a legume specialist, we examined the velvetbean caterpillar (VBC; Anticarsia gemmatalis), a devastating defoliator of tropical soybean and other legumes, whose host range includes Vigna spp. and Phaseolus spp. in the Americas (Buschman et al., 1977; Piubelli et al., 2005). Direct comparative assays of induced responses in cowpea to both natural herbivory and OS of both VBC and FAW revealed that VBC induced significantly lower plant defense responses. Compared with eight different Lepidoptera pest species examined, VBC demonstrates a unique preferential processing of inceptin-related peptides into a biologically inactive form, namely Vu-In−A (+ICDINGVCVD−). Moreover, Vu-In−A functions as a naturally occurring antagonist of plant defense responses triggered by inceptin (Vu-In; +ICDINGVCVDA−). Sequential screening of Vu-In peptide libraries with substituted C-terminal amino acids revealed a single conservative amino acid change that reduces VBC proteolysis of active inceptin-related peptides and recovers induced plant defense responses to this pest.
RESULTS
Decreased Defense Activation by a Legume Specialist Herbivore
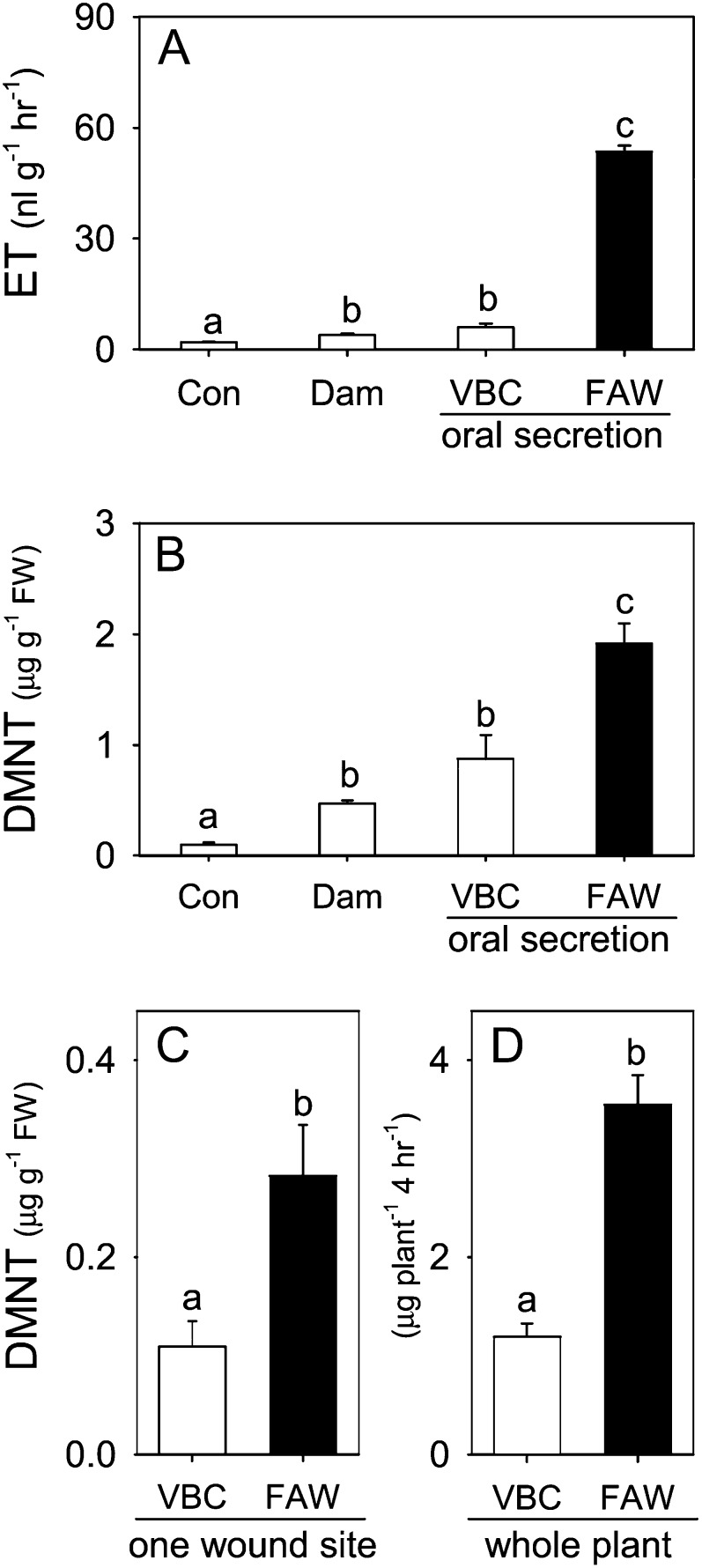
In cowpea, we previously established that FAW OS contain the elicitor Vu-In that drives the coordinated production of the defense-related phytohormones including JA, ET, and SA as well as increases in emission and leaf pools of the predominant volatile (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT; Schmelz et al., 2006, 2007; Carroll et al., 2008). Volatile DMNT is detected by numerous parasitoid wasps and attracts natural enemies of herbivorous pests (Gouinguené et al., 2005; Kappers et al., 2005). Cowpea leaves experiencing a FAW feeding bout from larvae devoid of Vu-In fail to elicit DMNT production (Schmelz et al., 2006). In a direct comparison with FAW OS, larval secretions from the legume specialist VBC elicited 8.8- and 2.2-fold lower levels of ET and DMNT, respectively, when applied to wounded cowpea leaves (Fig. 1, A and B). DMNT leaf pools present at 4 h following VBC OS treatment were not significantly different from those after mechanical damage + water alone (Fig. 1B). Quantified changes in leaf tissue pools of DMNT are used as an estimate for differences in volatile emission at the local site of treatment and herbivore attack. To examine cowpea defense responses to actual herbivory, we performed both short-term assays, which allowed single feeding bouts, and long-term assays, where plants were subjected to intensive sustained attack by either FAW or VBC. Analyses of cowpea leaf tissue surrounding short-term feeding sites that received equivalent damage (Fig. 1C) and long-term whole-plant volatile emissions (Fig. 1D) both demonstrate that VBC herbivory results in significantly lower levels of DMNT compared with FAW herbivory. Thus, cowpea plants produce quantitatively different defense responses to defoliation by these generalist and specialist Lepidopteran herbivores.
Figure 1.
The legume specialist VBC elicits weaker defense responses than the generalist FAW on cowpea leaves. A and B, Average (n = 4; +se) ET production (A) at 1 h and DMNT tissue pools (B) at 4 h after cowpea leaves were treated as undamaged controls (Con) or damaged and treated with 5 μL of water (Dam), VBC OS, or FAW OS. C, Average (n = 7; +se) cowpea leaf tissue pools of DMNT at 4 h after a single feeding bout by VBC and FAW larvae. D, Average (n = 4; +se) whole-plant cowpea volatile emission of DMNT during continuous herbivore feeding damage by eight early sixth instar VBC and FAW larvae. Different letters above the bars (a–c) represent significant differences (all ANOVA P values were P < 0.007; Tukey’s test corrections for multiple comparisons were at P < 0.05). FW, Fresh weight.
Unlike Other Lepidoptera, VBC Predominantly Contain an Inactive Inceptin-Related Peptide
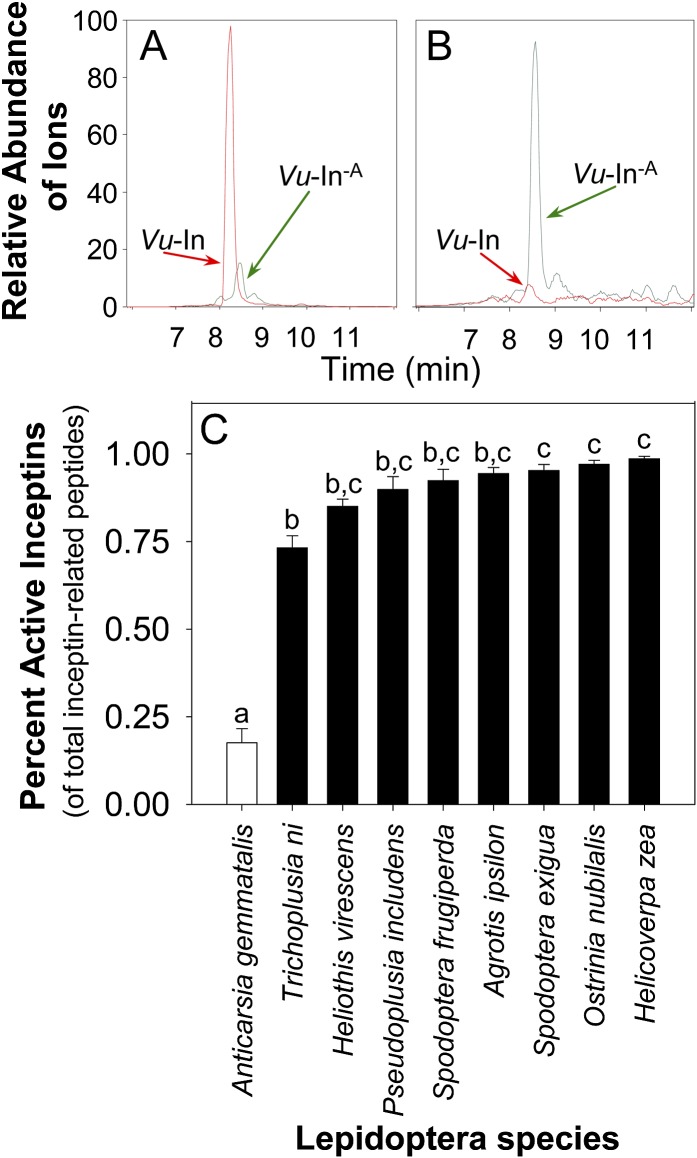
Large-scale purification of FAW OS previously revealed the presence of four inceptin-related peptides, consisting of predominantly Vu-In and lesser amounts of Vu-E+In (+EICDINGVCVDA−), Vu-GE+In (+GEICDINGVCVDA−), and Vu-In−A (+ICDINGVCVD−). Unlike the three inceptin-related peptides with additional N-terminal amino acids, the C-terminal truncated Vu-In−A lacked ET-, SA-, and DMNT-inducing activity (Schmelz et al., 2007). Consistent with our previous findings, FAW accumulates mainly Vu-In and only low amounts of Vu-In−A when fed peptide precursors containing the inceptin core sequence (Fig. 2A). In contrast, VBC OS contain largely the inactive Vu-In−A and only low levels of active Vu-In (Fig. 2B). To examine if the VBC pattern of inceptin-related peptide processing in OS is unique, we compared VBC, FAW, and seven additional Lepidopteran pests: cabbage looper (Trichoplusia ni), tobacco budworm (Heliothis virescens), soybean looper (Pseudoplusia includens), black cutworm (Agrotis ipsilon), S. exigua, European corn borer (Ostrinia nubilalis), and H. zea. After feeding on cATPC precursors containing the inceptin core sequence, only VBC exhibited significantly lower percentages (less than 25%) of active OS peptides (sum of Vu-In, Vu-E+In, and Vu-GE+In) compared with the total of all four inceptin-related peptides (Fig. 2C). Compared with the three highest accumulators of active inceptin-related peptides (S. exigua, O. nubilalis, and H. zea), T. ni larvae also exhibited a modest ability to accumulate Vu-In−A (Fig. 2C). While not a preferred host plant, in laboratory settings, T. ni is able to complete multiple instars on plants responsive to inceptin, such as common bean (Soo Hoo et al., 1984). Nonetheless, unlike the other eight Lepidopteran pests tested, VBC exhibits preferential processing of inceptin into the predominant inactive OS peptide Vu-In−A.
Figure 2.
In contrast with other Lepidopteran pests, VBC OS contain predominantly the inactive inceptin Vu-In−A. A and B, HPLC-MS selected [M+H]+ mass-to-charge ratio ion trace of inceptin-related peptides Vu-In (1,119.5) and Vu-In–A (1,048.5) in FAW OS (A) and VBC OS (B) following ingestion of the 19-mer-Vu-In peptide precursor (+KGEICDINGVCVDAAEDEF−). C, Average (n = 4; +se) percentage of active inceptins [(active = Vu-GE+In + Vu-E+In + Vu-In)/(total = Vu-GE+In + Vu-E+In + Vu-In + Vu-In−A)] quantified by HPLC-MS in the larval OS of eight different Lepidopteran species following ingestion of 4.5 nmol or less of 19-mer-Vu-In. Different letters above the bars (a–c) represent significant differences (ANOVA P < 0.0001; Tukey’s test correction for multiple comparisons was at P < 0.05).
Vu-In−A Is a Naturally Occurring Antagonist of Inceptin-Induced Responses
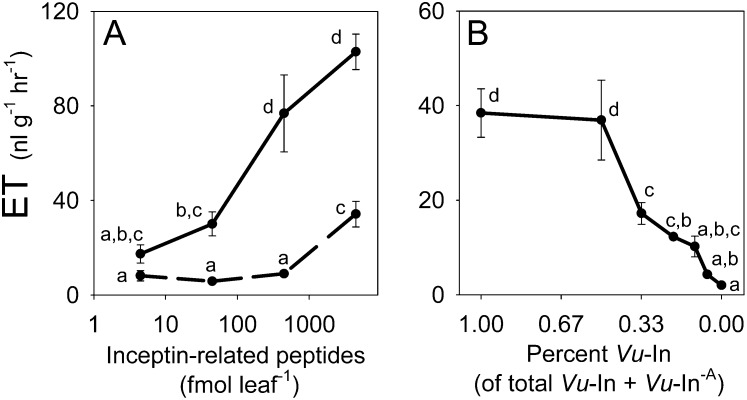
In plants, C-terminal amino acid deletions in synthetic peptide signals can result in the creation of competitive antagonists of binding and inhibitors of elicitor-induced plant responses (Pearce et al., 1993; Meindl et al., 1998, 2000). To examine if Vu-In−A functions as a naturally occurring antagonist in cowpea, leaves were wounded and first treated with either water or Vu-In−A and then subsequently retreated with Vu-In within 60 s. Prior treatment with 450 fmol leaf−1 Vu-In−A completely suppressed the plant response to subsequent elicitation with an equal level of Vu-In (Fig. 3A). At 4.5 pmol leaf−1, consecutive treatments with Vu-In−A and Vu-In resulted in ET responses identical to those of water followed by 45 fmol leaf−1 Vu-In. Thus, in the presence of Vu-In−A, a 100-fold greater level of Vu-In was required to produce an equivalent plant response. This general pattern of antagonism occurred over a wide range of concentrations (Fig. 3A). To function as an antagonist of plant defense responses during herbivory, Vu-In−A must also exhibit this activity in a mixture of compounds applied simultaneously. In a second experiment, Vu-In treatments were held constant (1 pmol leaf−1) and mixed with increasing amounts of Vu-In−A. At 33% Vu-In (of the total Vu-In + Vu-In−A), significant inhibition of elicitor-induced ET responses occurred, with continually increasing inhibition as the proportion of Vu-In−A increased (Fig. 3B). To examine the linkage between Vu-In−A-mediated antagonism of Vu-In-induced ET responses and subsequent metabolic leaf pools, a similar experiment was repeated to include sampling at 4 h. When leaves were treated with a mixture of 1 pmol of Vu-In and 4 pmol of Vu-In−A, significant ET inhibition at 1 h was again observed compared with an equivalent dose of Vu-In alone (Supplemental Fig. S1A). Significantly decreased pools of SA and DMNT at 4 h tracked this reduced ET production (Supplemental Fig. S1, C and E). While averages trended lower, significant Vu-In−A antagonism of Vu-In-induced JA and cinnamic acid (CA) pools was not detectable at 4 h (Supplemental Fig. S1, B and D). As expected, individual Vu-In−A treatments did not result in plant responses different from damage + water alone in any metabolite measured (Supplemental Fig. S1, A–E). Collectively, these results demonstrate that elevated ratios of Vu-In−A in larval OS can mediate antagonized defense elicitation triggered by active inceptin-related peptides.
Figure 3.
Vu-In−A is a natural antagonist of inceptin elicitation. A, Average (n = 4; ± se) ET production in damaged cowpea leaves treated first with either water (solid line) or Vu-In−A (dashed line) followed by a subsequent treatment 1 min later with Vu-In. Treatments involving both Vu-In−A and Vu-In utilized equivalent paired doses separated by time. B, Average (n = 4; ± se) ET production in damaged cowpea leaves treated simultaneously with a fixed amount of Vu-In (1 pmol leaf−1) and increasing levels of Vu-In−A in the mixture. Vu-In ranged from 100% to 5.8% and included a water control (0%). Different letters above the bars (a–d) represent significant differences (all ANOVA P values were P < 0.0001; Tukey’s test corrections for multiple comparisons were at P < 0.05).
Biochemical Screen of Substituted Peptide Precursors Recovers Active Elicitors in VBC OS
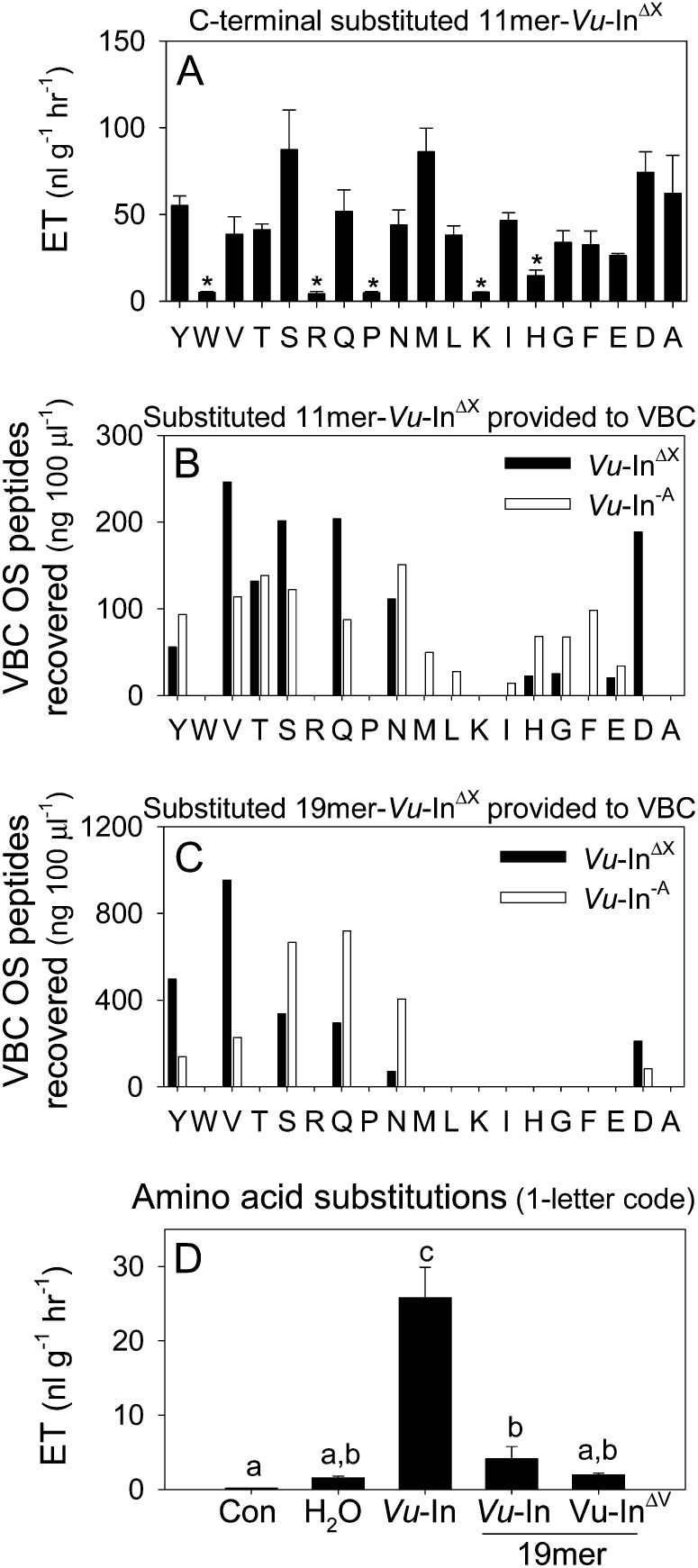
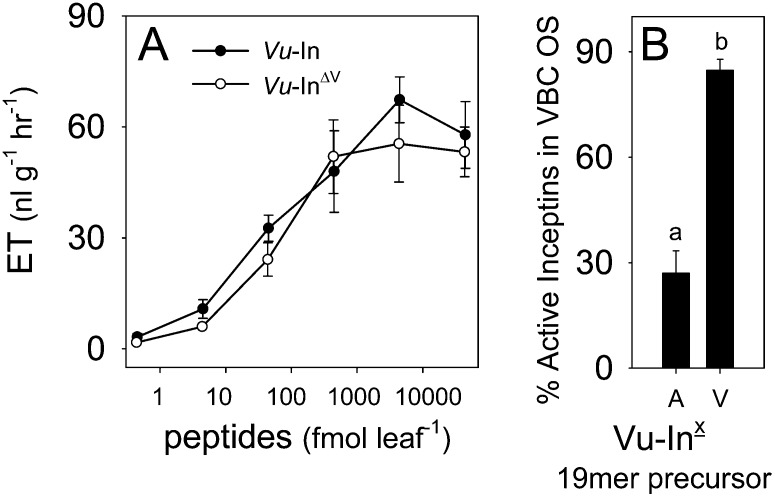
Given that amino acid sequences influence protease susceptibility (Poreba and Drag, 2010), one strategy to minimize the production of antagonists and restore plant defense elicitation following VBC attack is to modify the inceptin C terminal. Subtle sequence changes have the potential reduce VBC production of Vu-In−A relative to active elicitors. Toward this goal, we examined if the C-terminal Ala of Vu-In can withstand amino acid changes and still retain elicitor activity. As a first step, a Vu-InΔX substitution library was synthesized and assayed for induced ET production in cowpea leaves. With the exception of the basic amino acids, His, Lys, and Arg, as well as Pro and Trp, all other Vu-In C-terminal substitutions maintained significant elicitor activity (Fig. 4A). To estimate elicitor stability following larval ingestion, all active 11-mer-Vu-InΔX C-terminal substituted peptides Asp, Asn, Gln, Ser, Thr, Val, and Tyr were then fed to VBC larvae and recollected from the OS. The Vu-InΔX C-terminal substituted peptides were recoverable from VBC OS at equal or greater levels relative to Vu-In−A and thus predictably sufficient to maintain elicitation (Figs. 3B and 4B). Lepidopteran larvae generate inceptin-related peptides through both N- and C-terminal proteolysis of larger cATPC precursors. Given this complexity, we examined the larval production of six substituted peptide candidates from their respective model 19-mer-Vu-InΔX precursors requiring amide bond cleavage for activation. Following ingestion by VBC and subsequent OS collection, the precursor 19-mer-Vu-InΔV (+KGEICDINGVCVDVAEDEF−) displayed the highest recovery of the active peptide Vu-InΔV (+ICDINGVCVDV+) and a favorably low percentage of the Vu-In−A antagonist (Fig. 4C). Induced ET bioassays in cowpea leaves confirmed that both the native 19-mer-Vu-In and substituted 19-mer-Vu-InΔV precursors are inactive and thus require insect activation (Fig. 4D). Using a combination of plant and insect assays, serial analyses of peptide substitution libraries revealed a conservative amino acid change that retains elicitor activity and enables the preferential accumulation in VBC OS.
Figure 4.
cATPC amino acid substitutions alter inceptin activity and processing in VBC OS. A, Average (n = 3; +se) ET production in damaged cowpea leaves treated with 5 μL of water containing 10 pmol of C-terminally substituted peptides Vu-InΔX (+ICDINGVCVDX−). Capital letters (D through Y) denote the amino acid analogs of Vu-In. B and C, The 11-mer-Vu-InΔX peptides recovered from VBC OS (ng 100 μL−1) after ingestion of active C-terminally substituted 11-mer-Vu-InΔX (B) and 19-mer-Vu-InΔX (C) analogs (+KGEICDINGVCVDXAEDEF−). Black and white bars represent C-terminally substituted 11-mer-Vu-InΔX analogs and Vu-In−A, respectively. D, Average (n = 4; +se) ET production in undamaged cowpea leaves (Con) or those damaged and treated with 5 μL of water, Vu-In, 19-mer-Vu-In, or 19-mer-Vu-InΔV, all at 10 pmol leaf−1. Asterisks represent peptides with ET production significantly lower than Vu-In (i.e. C terminal in A). Different letters above the bars (a–c) represent significant differences (all ANOVA P values were P < 0.000; Tukey’s test correction for multiple comparisons was at P < 0.05).
A Single Amino Acid Substitution Recovers Plant Elicitation and Defense after Attack by a Legume Specialist Herbivore
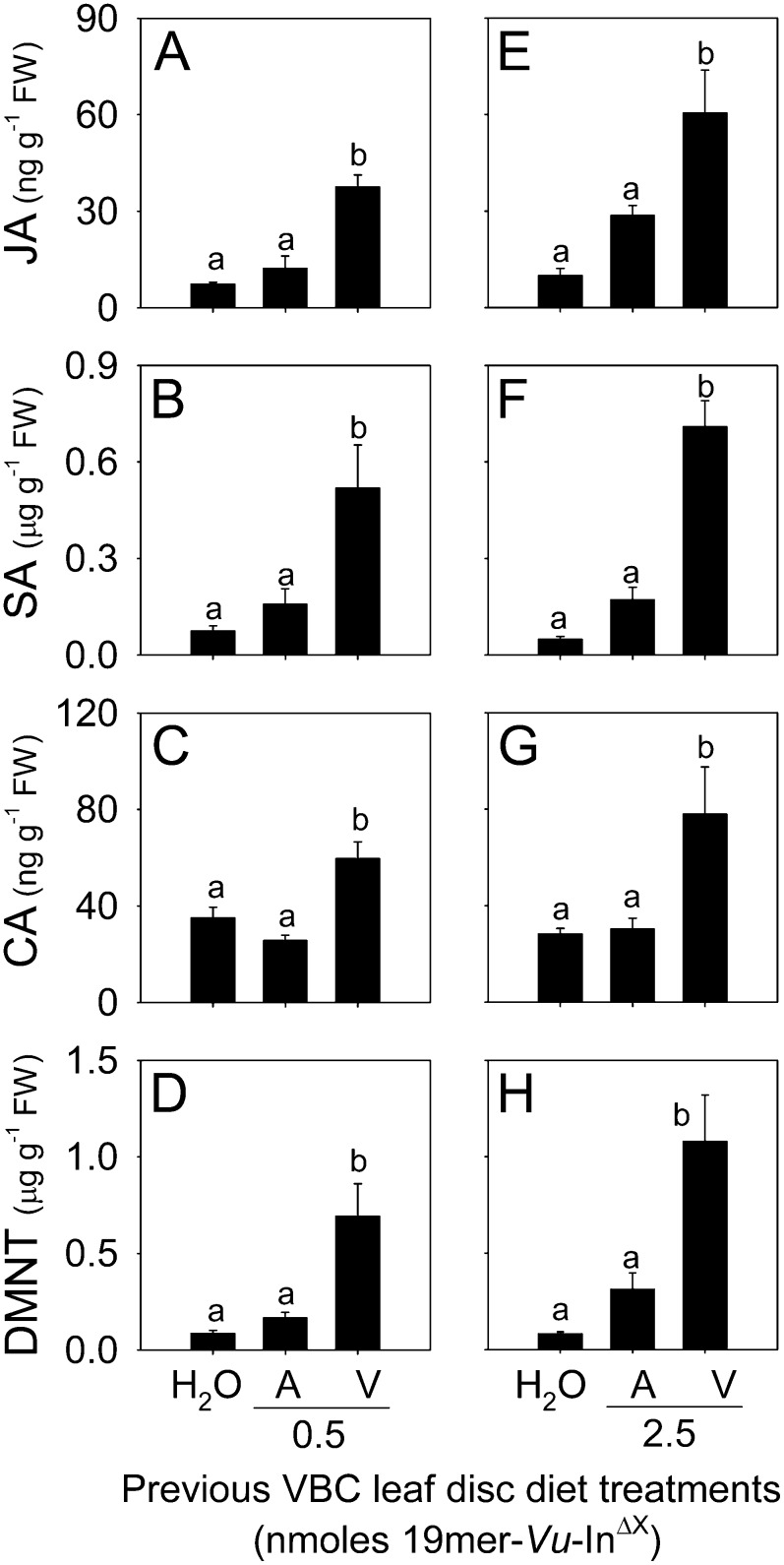
Ideally, a modified Vu-In analog would have either equivalent or greater activity than the native peptide signal. A detailed comparison of Vu-In and Vu-InΔV revealed identical ET-inducing activities in cowpea leaves over a range of concentrations (Fig. 5A). Further analysis of plant metabolite levels 4 h later confirmed the equivalent activity of Vu-InΔV and Vu-In (Supplemental Fig. S1, F–J). To quantitatively examine VBC peptide-processing patterns, larvae were allowed to feed upon leaf discs containing 4.9 nmol of either 19-mer-Vu-In or 19-mer-Vu-InΔV. Subsequent collections of OS revealed 24 ± 7 and 146 ± 13 pmol of total inceptin-related peptides, respectively. Thus, in addition to 6-fold greater concentrations of inceptin-related peptides, VBC that consumed the 19-mer-Vu-InΔV contained a significantly higher percentage of active elicitors, whereas the antagonist predominated in larvae that consumed the native 19-mer-Vu-In (Fig. 5B). Importantly, as demonstrated previously (Fig. 3B), this low proportion (27%) of active inceptin peptides is sufficient to enable Vu-In−A antagonism of Vu-In elicitation. To examine how trace amounts of substituted inceptin precursors affect plant recognition during herbivory, we fed individual VBC larvae cowpea leaf discs containing water, 0.5 nmol of the native 19-mer-Vu-In, or 0.5 nmol of 19-mer-Vu-InΔV. VBC larvae were then placed on intact cowpea plants and allowed to feed for 20 min. Individual herbivore damage sites 4 h later revealed significant increases in JA, SA, CA, and DMNT in plants attacked by VBC that had consumed the modified 19-mer-Vu-InΔV (Fig. 6, A−D). Plants attacked by VBC larvae that consumed the additional native 19-mer-Vu-In displayed defense-related metabolite levels statistically identical to those damaged by VBC from the water leaf disc treatment (Fig. 6, A−D).
Figure 5.
An inceptin Ala-to-Val C-terminal substitution maintains activity and enables the predominant accumulation of active elicitors in VBC OS. A, Dose response of average (n = 4; ± se) ET production in damaged cowpea leaves treated with either Vu-In or Vu-InΔV. B, Average (n = 4; +se) percentage of active inceptins recovered from VBC OS after larvae were fed 4.9 nmol of either 19-mer-Vu-In or 19-mer-Vu-InΔV. Different letters above the bars (a and b) represent significant differences (Student’s t test, P < 0.001).
Figure 6.
An Ala-to-Val substitution in cATPC polypeptides recovers induced plant defenses during VBC herbivory. A to D, Average (n = 6; +se) concentrations of JA (A), SA (B), CA (C), and DMNT (D) 4 h after cowpea leaves experienced a single feeding bout from VBC larvae that had previously ingested cowpea leaf discs containing water, 0.5 nmol of 19-mer-Vu-In (A) or 0.5 nmol of 19-mer-Vu-InΔV (V). E to H, Average (n = 6; +se) concentrations of JA (E), SA (F), CA (G), and DMNT (H) in a repeated experiment using 2.5 nmol of 19-mer-Vu-In (A) or 19-mer-Vu-InΔV (V). Within plots, different letters above the bars (a and b) represent significant differences (all ANOVA P values were P < 0.015; Tukey’s test corrections for multiple comparisons were at P < 0.05). FW, Fresh weight.
To examine both the reproducibility and importance of dose, this experiment was repeated by feeding VBC larvae 5-fold greater levels of the 19-mer peptide precursors. At 2.5 nmol of peptide, herbivory triggered 2.1-, 4.1-, 2.6-, and 3.5-fold greater levels of JA, SA, CA, and DMNT, respectively, when VBC larvae previously ingested the modified 19-mer-Vu-InΔV compared with the native 19-mer-Vu-In (Fig. 6, E−H). Plant responses to larvae that previously consumed cowpea leaves plus water or 19-mer-Vu-In were not statistically different (Fig. 6, E−H). On average, the 2.5-nmol dose of 19-mer-Vu-InΔV resulted in larvae that elicited slightly higher plant responses than those previously ingesting 0.5 nmol (Fig. 6, A−H). Collectively, these results demonstrate that an Ala-to-Val substitution in the cATPC polypeptide sequence is able to recover the elicitation of cowpea defense responses following VBC herbivory.
DISCUSSION
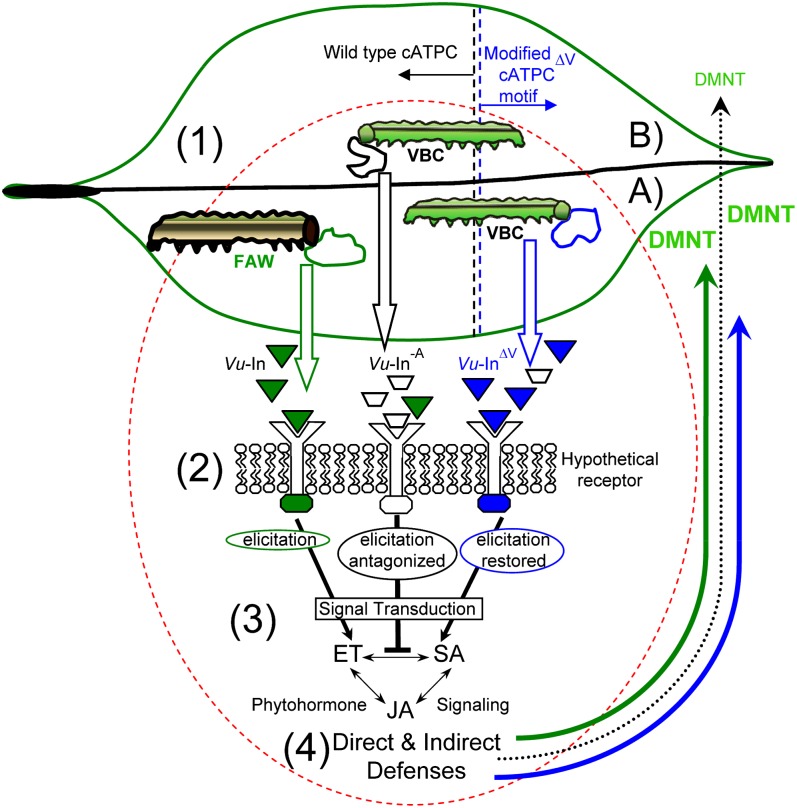
The identity and activity of herbivore-associated elicitors of plant defenses have been intensively studied over the past decade (Howe and Jander, 2008). In some cases, insects may avoid the production of active elicitors by feeding on tissues lacking essential precursors, while in other situations, individual herbivore elicitors within a class can vary greatly between conspecific larvae without significantly influencing defense activation (De Moraes and Mescher, 2004; Roda et al., 2004). While the outcome of pathogen and insect attack relies significantly upon their capacity for host plant utilization, the documentation of herbivore effectors that suppress elicitation is quite limited. A few examples come from aphids that consume phloem for many hours once a sieve element feeding site has been established. In broad bean (Vicia faba), secreted salivary proteins from the aphid Megoura viciae suppress sieve element occlusion, which typically occurs through the formation of dispersed forisomes (i.e. expanded proteinaceous inclusions), triggered in response to damage and increased local Ca2+ levels (Will et al., 2007). These salivary-derived Ca2+-binding proteins also promote dispersed forisomes to convert back into their original compact structure, thereby recovering the flow of phloem sap and enabling successful feeding. Similarly, a specific salivary protein of unknown function, designated as C002, has been demonstrated to be absolutely required for pea aphid (Acyrthosiphon pisum) feeding and survival (Mutti et al., 2008). Biochemical mechanisms that Lepidopteran larvae utilize to suppress plant responses during herbivory are less clear but can also involve salivary proteins. H. zea herbivory on tobacco results in secretion of the enzyme GOX onto the leaf surface and suppresses induced nicotine accumulation in tobacco (Musser et al., 2002). In cowpea, we here demonstrate a new mechanism of suppression, where legume specialist larvae exhibit the targeted removal of functional OS elicitors in part by converting the active signal into an antagonist of defense elicitation (Fig. 7).
Figure 7.
Simplified proposed model for generalist activation, specialist suppression, and engineered recovery of inceptin-elicited plant defenses in cowpea. 1A, FAW and VBC larvae consume cowpea leaves and produce the predominant cATPC digestive fragments Vu-In and Vu-In−A, respectively. 1B, VBC larvae that consume precursor proteins containing an altered cATPCΔV motif produce the active modified inceptin Vu-InΔV. 2, Plants indirectly perceive larval attack when Vu-In and Vu-InΔV peptides recontact the wounded leaf surface and bind a putative receptor. VBC that contain Vu-In−A at levels of 66% or greater of the total inceptin-related peptides mediate the antagonism of Vu-In activity. As a preliminary hypothesis, Vu-In−A antagonism potentially occurs at the receptor-ligand level through competition. 3, Dependent upon the proportions of inceptin-related peptides present, multiple signaling pathways can be elicited, including the phytohormones JA, ET, and SA, or antagonized (ET and SA). 4, Insect OS elicitors drive quantitatively different levels of induced biochemical defenses such as DMNT that can provide reliable cues to facilitate the attraction of natural enemies.
It is unclear if generalist and specialist herbivores consistently differ in their OS elicitors. The transcriptional response of N. attenuata to attack from two generalist herbivores, H. virescens and S. exigua, was more similar to each other than that of the specialist M. sexta. This difference was linked to OS-derived FACs (Voelckel and Baldwin, 2004). OS contents of the two generalists are virtually identical, whereas M. sexta lacks volicitin and predominantly harbors fatty acid-Glu conjugates (Pohnert et al., 1999; Alborn et al., 2003). FACs from M. sexta also have a role in suppressing induced nicotine accumulation in tobacco, yet they remain elicitors for indirect defense responses (Kahl et al., 2000). Unlike a number of solanaceous plants, defense responses in cowpea are not elicited by FACs (Schmelz et al., 2009). Instead, variation within inceptin-related peptides from herbivore OS mediates the activation or suppression of elicitor-induced defenses. The evidence here for specialist antagonism of elicitor-induced plant defenses is further supported by the lack of preferential Vu-In−A processing in all generalist species examined (Fig. 2C). A future survey of legume specialist Lepidoptera that include Vigna spp. and Phaseolus spp. as frequent host plants would reveal the breadth and scope of this mechanism in nature.
The theoretical existence of a naturally occurring peptide signal that functions as an antagonist to elicitor-induced plant responses dates back nearly two decades. In 1991, discovery of the 18-amino acid endogenous peptide from tomato (Solanum lycopersicum) foliage, termed systemin, marked the first bioactive peptide signal found in plants (Pearce et al., 1991). Shortly thereafter, structure-function studies with truncated peptides revealed that the successive removal of C-terminal amino acids inactivated the signal and resulted in peptides that blocked the subsequent binding and activity of native full-length systemin (Pearce et al., 1993; Meindl et al., 1998). Related studies with the bacterial peptide elicitor flg22 also found that C-terminal truncations resulted in competitive antagonist peptides and led to the “address-message” concept that peptide signal N- and C-terminal ends are important for receptor binding and activation, respectively (Meindl et al., 2000). With both systemin and flg22, numerous C-terminal amino acid deletions in synthetic peptides are required to achieve strong competitive antagonism. In this study, a single C-terminal amino acid loss resulting in Vu-In−A promotes the antagonism of elicitation when present at 66% or more of the total inceptin-related peptides. While comparative phylogenetic studies are consistent with the existence of specific plant receptors for insect-derived elicitors, the precise mechanism of Vu-In-mediated defense activation remains unknown (Schmelz et al., 2009). We identify Vu-In−A as a truncated elicitor derivative that antagonizes inceptin-induced responses, including ET, SA, and DMNT; however, the action of Vu-In−A as a competitive antagonist at the receptor level remains hypothetical and subject to future empirical examination (Fig. 7).
In contrast with plant-pathogen interactions, receptor-ligand pairs have yet to be identified to regulate plant resistance to insect attack. For aphid resistance, receptors including the Mi-1.2 gene in tomato and Vat genes in melon (Cucumis melo) protect against specific biotypes of Macrosiphum euphorbiae and Aphis gossypii, respectively (Rossi et al., 1998; Boissot et al., 2010). Currently, aphid-associated ligands that bind these receptors are unknown. A common long-term threat in crop breeding is the eventual shift in predominant insect biotypes that favor those able to overcome plant resistance genes. For example, in 1997, a virulent isolate M. euphorbiae that performs well on tomato plants harboring the Mi-1.2 gene was identified in California and currently appears to be predominant in the United States (Goggin et al., 2001). Of relevance to insect biotypes that overcome plant resistance, we demonstrate that VBC possesses a biochemical adaptation that minimizes the activation of plant defenses while feeding. The infrequency of predominant Vu-In C-terminal processing in other Lepidoptera species examined is likely due to the high level of protease resistance associated with cyclized peptides (Horton et al., 2002). While the precise mode of cleavage has not been described, VBC larvae may produce a specific gut carboxypeptidase that is capable of cleaving the C-terminal Ala from the largely cyclic Vu-In. Thus far, trypsin-like proteases have been predominantly described; however, the gut tissues of A. gemmatalis larvae also harbor bacteria potentially capable of further proteolysis (Oliveira et al., 2005; Visôtto et al., 2009).
In pathology terms, the presence of Vu-In in FAW OS is analogous to an “avirulence factor,” given its ability to trigger rapid plant defense responses in cowpea. Conversely, Vu-In−A in VBC OS functions as a “virulence factor” by suppressing elicitation and subsequent induced defenses triggered by trace amounts of Vu-In. Unfortunately, these designations are accurate only in the context of each individual system examined, given that virulence and avirulence factors often possess opposite activities in related plant species. Divergent activities of previously designated virulence and avirulence factors result in both confusion and conceptually restrictive usage. The term “effector” is now favored and broadly encompasses biochemicals that either positively or negatively modify plant defense signaling and expression (Hogenhout et al., 2009). The discovery of the elicitor antagonist activity of Vu-In−A compels us to view inceptin-related peptides not simply as a class of elicitors but more widely as herbivore-associated effectors capable of multiple activities.
In cowpea, we demonstrate that a conservative amino acid change in the cATPC precursor for Vu-In can block the production of an elicitor antagonist and recover elicitor-induced responses following VBC attack (Fig. 7). Curiously, potential mutations that convert precursors of Vu-In to Vu-InΔV may be subject to additional constraints. In an earlier analysis of the coding region of chloroplastic atpC spanning Vu-In, we observed perfect conservation of the C-terminal Ala in every plant species investigated (Schmelz et al., 2006). This might be due to interactions with the inhibitory ε-subunit that in part mediates chloroplastic ATP synthase activity (Hightower and McCarty, 1996). Thus, in legumes that respond to inceptin-related peptides, the transgenic manipulation of plants to improve defense responses to VBC attack may require the embedding of this modified sequence into proteins other than cATPC. An ultimate goal is to impart inceptin-mediated responses to crops that do not currently recognize the elicitor. While this approach has numerous daunting requirements, including the discovery of any putative inceptin receptor(s), successful examples of interfamily resistance transfer exist for bacterially mediated MAMP-triggered immunity (Lacombe et al., 2010). Encouragingly, the majority of Lepidoptera species examined accumulate inceptin-related peptides (Fig. 2C). Thus, while the utility of this perception system hinges partly on elicitor production, active signals already exist in a diversity of pests. Select legume specialists largely avoid inceptin-mediated defenses in cowpea; yet, an amino acid substitution, representing the addition of two methyl groups, can recover elicitor-induced responses. Clearly, little things mean a lot. Detailed biochemical knowledge of both herbivore-associated effectors and the respective plant receptors will remain essential for the directed improvement of crop resistance.
MATERIALS AND METHODS
Plant and Insect Materials
FAW (Spodoptera frugiperda) larvae were obtained from Dr. R. Meagher (Center of Medical, Agricultural, and Veterinary Entomology, U.S. Department of Agriculture, Agricultural Research Service, Gainesville, FL) and reared on a pinto bean (Phaseolus vulgaris)-based diet (Schmelz et al., 2006). Preliminary trials with VBC larvae utilized wild-caught individuals from outbreak areas on the surrounding margins of peanut (Arachis hypogaea) fields (Williston, FL), and these results were readily replicated using commercially obtained insects. All experiments utilized VBC (Anticarsia gemmatalis), Trichoplusia ni, Heliothis virescens, Pseudoplusia includens, Agrotis ipsilon, Spodoptera exigua, Ostrinia nubilalis, and Helicoverpa zea obtained on from Benzon Research. Cowpea (Vigna unguiculata var California Blackeye no. 5; The Wax Company) was germinated in MetroMix 200 (Sun Gro Horticulture) supplemented with 14-14-14 Osmocote (Scotts Miracle-Gro). All plants were maintained in a greenhouse with a 12-h photoperiod, a minimum of 300 μmol m−2 s−1 photosynthetically active radiation supplied by supplemental lighting, 70% relative humidity, and a temperature cycle of 24°C/28°C (night/day).
Synthetic Peptides
Inceptin-related sequences Vu-GE+In, Vu-E+In, Vu-In, and Vu-In−A were synthesized and purified (more than 95%) at the Protein Core Chemistry Facility of the University of Florida, Gainesville, as described previously (Schmelz et al., 2006, 2007). C-terminally substituted Vu-In 11-mer analogs were synthesized using the PEPscreen Peptides (Sigma-Genosys) service with an 85% average purity. Vu-InΔV and specific substituted 19-mers (+KGEICDINGVCVDXAEDEF−) utilized in this work were synthesized and received at more than 95% purity (Sigma-Genosys).
Cowpea Leaf Bioassays and Plant Metabolite Analysis
All experiments used 14- to 18-d-old plants containing two fully expanded pairs of trifoliate leaves. For ET induction assays, the adaxial sides of new fully expanded leaves were superficially scratched with a razor in three areas, removing approximately 5% of the total waxy cuticle. The damage sites (2 cm2 each) included the central leaf tip spanning both sides of the midrib and two midbasal sections on opposite sides of the midrib. Test solutions in 5 μL of water were immediately applied and dispersed over the damage sites. For ET quantification, leaves remained on the intact plants for 1 h, were excised, sealed in 13-mL tubes, and analyzed via head space sampling and gas chromatography as described previously (Schmelz et al., 2006). To quantify leaf tissue pools of DMNT, JA SA, and CA, a 4-cm2 section of leaf surrounding the treated site was weighed (50–100 mg), frozen in liquid N2, processed, and analyzed by gas chromatography isobutane-chemical ionization mass spectrometry as described elsewhere (Schmelz et al., 2004, 2006). The collection and quantification of leaf volatile emission from intact plants followed established protocols (Carroll et al., 2008).
Individual Insect Feeding Studies
Sixth instar VBC and FAW larvae were allowed to feed for 12 h on cowpea leaves and then individually isolated on polystyrene 12-well Cell Culture Plates (BD Falcon) for 1 h in the absence of plant tissue. The larvae were carefully transferred onto the leaf canopies of tightly spaced cowpea plants and allowed to feed for 20 min. At this time, all larvae were removed from plants, and only insect-damaged sites with comparable leaf area consumed (typically 50 mm2) were selected for analysis (n = 6). To ensure maximal uniformity of feeding time and surplus of larval wound sites with equivalent leaf area removed, each of these trials utilized groups of 48 insects on 16 plants for each insect species or treatment comparison. To control the elicitor content of larval OS, VBC on 12-well plates were allowed to consume a 65-mm2 cowpea leaf disc containing the native and substituted 19-mer cATPC peptides. Within 30 min of consumption, larvae where placed on leaves as described above.
Quantification of Inceptin-Related Peptides in Insect OS
Chemical verification and quantification of inceptin-related peptides from insect OS utilized HPLC-mass spectrometry (MS) as described previously (Schmelz et al., 2006). An isotopically labeled ([13C]Val and [15N]Val; +ICDING-V*-CVDA−) Vu-In analog was used as an internal standard for quantification. Aliquots of crude OS, typically 50 to 100 μL derived from a pool of 12 larvae, were sequentially spiked with 100 ng of the internal standard peptide and 5 μL of HCl, vortexed, and centrifuged at 12,000g for 5 min. The aqueous phase was mixed with an equal volume of ethanol, stored at –70°C for 30 min, and centrifuged at 12,000g for 2 min. Samples were diluted to 5% ethanol, loaded on 100-mg RP-C18 SPE columns, washed with 2 mL of water, and eluted with 9:1 CH3CN:water. Samples were then concentrated to dryness under vacuum and brought up in 50 μL of 5:95 CH3CN:water containing 10 mm NH4COOH. Using an HPLC system composed of a P4000 pump, an AS3000 autosampler, and a UV6000LP detector (Thermo Separation Products), 10-μL OS samples were injected onto a YMC ODS-AQ RP-C18 (250 × 4.6 mm, S-5 μm, 20 nm; Waters) analytical column heated to 60°C, using a flow rate of 1 mL min−1, with mobile phases A and B containing 95:5 water:CH3CN and 9:1 CH3CN:water, respectively, as well as 10 mm NH4COOH buffer. The postcolumn eluent was split, allowing 0.1 mL min−1 to enter the ion source for positive-ion MS using a LCQ Deca XPMAX ion trap (Thermo Electron) as described previously (Schmelz et al., 2006). Chemical verification was based on comparing retention times and MS2 daughter ion mass spectra of synthetic standards against natural products. Quantification was based on extracting [M+H]+ ions from full-scan spectra using the following common ion-analyte pairs: 1,125.5 (internal standard), 1,048.5 (Vu-In–A), 1,119.5 (Vu-In), 1,248.5 (Vu-E+In), 1,305.5 (Vu-GE+In), 1,147.5 (Vu-InΔV), 1,276.5 (Vu-E+InΔV), and 1,333.5 (Vu-GE+InΔV). A complete list of all amino acid-substituted peptides used in this study and analyzed for in insect OS via HPLC-MS is given in Supplemental Table S1.
Lepidoptera Species Comparison
The OS were collected and pooled from groups of six larvae (n = 4) for inceptin quantification. Early sixth instar larvae (n ≥ 48) were removed from diet and isolated for 1 h prior to feeding on leaf discs (65 mm2) containing 4.5 nmol of 19-mer-Vu-In. OS were collected and frozen immediately after the larvae consumed at least 50% of the treated leaf disc, which required between 30 and 120 min depending on the species. With the exception of A. ipsilon, O. nubilalis, and T. ni, which utilized either maize (Zea mays) or bok choy (Brassica rapa) leaves, all other species were fed cowpea leaves. Resulting levels of the four inceptin-related peptides Vu-In–A, Vu-In, Vu-E+In, and Vu-GE+In were then quantified by LC-MS as described.
Determination of Vu-In−A Inceptin Antagonist Activity
Four paired groups of cowpea leaves (n = 4; 32 total) were wounded and treated with either 5 μL of water or Vu-In−A at 0.0045, 0.045, 0.45, and 4.50 pmol leaf−1. One minute later, both leaves in each paired group were treated with a secondary application of Vu-In at 0.0045, 0.045, 0.45, and 4.50 pmol leaf−1. To assess the simultaneous application of Vu-In−A and Vu-In, all peptide-treated leaves (n = 4) received 10 μL of water containing 1 pmol of Vu-In in the presence of 0, 1, 2, 4, 8, or 16 pmol of Vu-In–A. A damage + water-only control group was included. All leaves were excised after 1 h and analyzed for ET production. To examine Vu-In−A antagonism of Vu-In activity in the context of leaf metabolites at 4 h, a subset of these treatments was reexamined. Cowpea leaves (n = 4; 48 total) were wounded and treated with 5 μL of water, 1 pmol of Vu-In, Vu-In + Vu-In−A (1:1; 1 pmol leaf−1 for each peptide), or Vu-In + Vu-In−A (1:4; 1 and 4 pmol, respectively), or 1 pmol of Vu-In−A alone. To address the similarity of Vu-InΔV activity to Vu-In, leaves were also treated with 1 pmol of Vu-InΔV. For each of the six treatments, one set of leaves (24) was analyzed at 1 h for ET confirmation and the other set (24) was analyzed at 4 h for JA, SA, CA, and DMNT pools.
Screening Activity and Stability of Substituted Inceptins in VBC OS
With the exception of Cys, which would have complicated peptide cyclization, a C-terminal substituted peptide of Vu-In library was first analyzed by LC-MS for purity, normalized for concentration, and tested at 10 pmol leaf−1 for E-inducing activity in cowpea. Vu-In C-terminal substituted peptides with activity similar to Vu-In were then fed to 14 groups of 12 VBC larvae (20 μg leaf disc−1), and OS were collected 6 h later and pooled for HPLC-MS analysis. To examine VBC proteolytic processing of precursors into inceptin-related peptides, six of the more promising Vu-InΔX C-terminal substituted peptides (where X = D, N, Q, S, V, Y) were synthesized as 19-mers (+KGEICDINGVCVDXAEDEF−). Similarly, the substituted 19-mers were fed to six groups of 12 VBC larvae (20 μg leaf disc−1), and within 1 h of consumption, the OS were collected and pooled for HPLC-MS analysis.
Data Analysis
ANOVAs were performed on quantified inceptin-related peptides, phytohormones, leaf tissue metabolites, and volatile emissions of DMNT. Significant treatment effects were investigated when the main effects of the ANOVAs were significant (P < 0.05). Where appropriate, Tukey’s tests were used to correct for multiple comparisons between control and treatment groups. Before statistical analysis, all nonpercentage data were subjected to square root transformation to compensate for elevated variation associated with larger mean values. The analysis was accomplished with JMP 4.0 statistical discovery software (SAS Institute).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Vu-In−A antagonism of inceptin-induced ET at 1 h parallels subsequently observed patterns for SA and DMNT at 4 h.
Supplemental Table S1. Native and modified inceptin-related peptides used for ethylene bioassays, larval feeding studies, and HPLC-MS-based quantification.
Supplementary Material
Acknowledgments
We thank J. Meredith, M. Legaspi, R. Harrison, and B. Forguson (all at the Center for Medical, Agricultural, and Veterinary Entomology at the U.S. Department of Agriculture, Agricultural Research Service [USDA-ARS]) for greenhouse and technical assistance. We thank A.Y. Chung (University of Florida, Gainesville) for peptide synthesis, R.L. Meagher (USDA-ARS) for encouragement in using of A. gemmatalis as an alternative model herbivore, and S.S. Walse (USDA-ARS), S. Christensen (USDA-ARS), and two anonymous reviewers for improving the manuscript.
Glossary
- OS
oral secretions
- FAC
fatty acid amino acid conjugate
- JA
jasmonic acid
- ET
ethylene
- GOX
glucose oxidase
- SA
salicylic acid
- MAMP
microbe-associated molecular pattern
- cATPC
chloroplastic ATP synthase γ-subunit
- FAW
Fall armyworm
- VBC
velvetbean caterpillar
- DMNT
(E)-4,8-dimethyl-1,3,7-nonatriene
- CA
cinnamic acid
- MS
mass spectrometry
References
- Adler LS, Schmitt J, Bowers MD. (1995) Genetic variation in defensive chemistry in Plantago lanceolata (Plantaginaceae) and its effect on the specialist herbivore Junonia coenia (Nymphalidae). Oecologia 101: 75–85 [DOI] [PubMed] [Google Scholar]
- Agrawal AA, Kurashige NS. (2003) A role for isothiocyanates in plant resistance against the specialist herbivore Pieris rapae. J Chem Ecol 29: 1403–1415 [DOI] [PubMed] [Google Scholar]
- Alborn HT, Brennan MM, Tumlinson JH. (2003) Differential activity and degradation of plant volatile elicitors in regurgitant of tobacco hornworm (Manduca sexta) larvae. J Chem Ecol 29: 1357–1372 [DOI] [PubMed] [Google Scholar]
- Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH. (1997) An elicitor of plant volatiles from beet armyworm oral secretion. Science 276: 945–949 [Google Scholar]
- Ali JG, Agrawal AA. (2012) Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci 17: 293–302 [DOI] [PubMed] [Google Scholar]
- Bent AF, Mackey D. (2007) Elicitors, effectors, and R genes: the new paradigm and a lifetime supply of questions. Annu Rev Phytopathol 45: 399–436 [DOI] [PubMed] [Google Scholar]
- Berenbaum MR. (2002) Postgenomic chemical ecology: from genetic code to ecological interactions. J Chem Ecol 28: 873–896 [DOI] [PubMed] [Google Scholar]
- Boissot N, Thomas S, Sauvion N, Marchal C, Pavis C, Dogimont C. (2010) Mapping and validation of QTLs for resistance to aphids and whiteflies in melon. Theor Appl Genet 121: 9–20 [DOI] [PubMed] [Google Scholar]
- Bonaventure G, VanDoorn A, Baldwin IT. (2011) Herbivore-associated elicitors: FAC signaling and metabolism. Trends Plant Sci 16: 294–299 [DOI] [PubMed] [Google Scholar]
- Buschman LL, Whitcomb WH, Neal TM, Mays DL. (1977) Winter survival and hosts of velvetbean caterpillar in Florida. Fla Entomol 60: 267–273 [Google Scholar]
- Carroll MJ, Schmelz EA, Teal PEA. (2008) The attraction of Spodoptera frugiperda neonates to cowpea seedlings is mediated by volatiles induced by conspecific herbivory and the elicitor inceptin. J Chem Ecol 34: 291–300 [DOI] [PubMed] [Google Scholar]
- Cohen MB, Schuler MA, Berenbaum MR. (1992) A host-inducible cytochrome P-450 from a host-specific caterpillar: molecular cloning and evolution. Proc Natl Acad Sci USA 89: 10920–10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moraes CM, Lewis WJ, Pare PW, Alborn HT, Tumlinson JH. (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393: 570–573 [Google Scholar]
- De Moraes CM, Mescher MC. (2004) Biochemical crypsis in the avoidance of natural enemies by an insect herbivore. Proc Natl Acad Sci USA 101: 8993–8997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diezel C, von Dahl CC, Gaquerel E, Baldwin IT. (2009) Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol 150: 1576–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18: 265–276 [DOI] [PubMed] [Google Scholar]
- Goggin FL, Williamson VM, Ullman DE. (2001) Variability in the response of Macrosiphum euphorbiae and Myzus persicae (Hemiptera: Aphididae) to the tomato resistance gene Mi. Environ Entomol 30: 101–106 [Google Scholar]
- Gouinguené S, Pickett JA, Wadhams LJ, Birkett MA, Turlings TCJ. (2005) Antennal electrophysiological responses of three parasitic wasps to caterpillar-induced volatiles from maize (Zea mays mays), cotton (Gossypium herbaceum), and cowpea (Vigna unguiculata). J Chem Ecol 31: 1023–1038 [DOI] [PubMed] [Google Scholar]
- Hawksworth DL, Kalin-Arroyo MT. (1995) Magnitude and distribution of biodiversity. In VH Heywood, RT Watson, eds, Global Biodiversity Assessment. United Nations Environment Program and Cambridge University Press, Cambridge, UK, pp 107–191 [Google Scholar]
- Hightower KE, McCarty RE. (1996) Proteolytic cleavage within a regulatory region of the gamma subunit of chloroplast coupling factor 1. Biochemistry 35: 4846–4851 [DOI] [PubMed] [Google Scholar]
- Hogenhout SA, Van der Hoorn RAL, Terauchi R, Kamoun S. (2009) Emerging concepts in effector biology of plant-associated organisms. Mol Plant Microbe Interact 22: 115–122 [DOI] [PubMed] [Google Scholar]
- Horton DA, Bourne GT, Smythe ML. (2002) Exploring privileged structures: the combinatorial synthesis of cyclic peptides. Mol Divers 5: 289–304 [DOI] [PubMed] [Google Scholar]
- Howe GA, Jander G. (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59: 41–66 [DOI] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL. (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Kahl J, Siemens DH, Aerts RJ, Gäbler R, Kühnemann F, Preston CA, Baldwin IT. (2000) Herbivore-induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta 210: 336–342 [DOI] [PubMed] [Google Scholar]
- Kappers IF, Aharoni A, van Herpen TW, Luckerhoff LLP, Dicke M, Bouwmeester HJ. (2005) Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science 309: 2070–2072 [DOI] [PubMed] [Google Scholar]
- Lacombe S, Rougon-Cardoso A, Sherwood E, Peeters N, Dahlbeck D, van Esse HP, Smoker M, Rallapalli G, Thomma BPHJ, Staskawicz B, et al. (2010) Inter-family transfer of a plant pattern recognition receptor confers broad-spectrum bacterial resistance. Nat Biotechnol 4: 365–369 [DOI] [PubMed] [Google Scholar]
- Luginbill P. (1928) The Fall Armyworm. USDA Technical Bulletin 34
- Meindl T, Boller T, Felix G. (1998) The plant wound hormone systemin binds with the N-terminal part to its receptor but needs the C-terminal part to activate it. Plant Cell 10: 1561–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl T, Boller T, Felix G. (2000) The bacterial elicitor flagellin activates its receptor in tomato cells according to the address-message concept. Plant Cell 12: 1783–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet YA, Danna CH, Clay NK, Songnuan W, Simon MD, Werck-Reichhart D, Ausubel FM. (2010) Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell 22: 973–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser RO, Hum-Musser SM, Eichenseer H, Peiffer M, Ervin G, Murphy JB, Felton GW. (2002) Herbivory: caterpillar saliva beats plant defences. Nature 416: 599–600 [DOI] [PubMed] [Google Scholar]
- Mutti NS, Louis J, Pappan LK, Pappan K, Begum K, Chen MS, Park Y, Dittmer N, Marshall J, Reese JC, et al. (2008) A protein from the salivary glands of the pea aphid, Acyrthosiphon pisum, is essential in feeding on a host plant. Proc Natl Acad Sci USA 105: 9965–9969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman T, Julkunen-Tiitto R. (2000) Manipulation of the phenolic chemistry of willows by gall-inducing sawflies. Proc Natl Acad Sci USA 97: 13184–13187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MGA, De Simone SG, Xavier LP, Guedes RNC. (2005) Partial purification and characterization of digestive trypsin-like proteases from the velvet bean caterpillar, Anticarsia gemmatalis. Comp Biochem Physiol B Biochem Mol Biol 140: 369–380 [DOI] [PubMed] [Google Scholar]
- Pearce G, Johnson S, Ryan CA. (1993) Structure-activity of deleted and substituted systemin, an 18-amino acid polypeptide inducer of plant defensive genes. J Biol Chem 268: 212–216 [PubMed] [Google Scholar]
- Pearce G, Strydom D, Johnson S, Ryan CA. (1991) A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 253: 895–897 [DOI] [PubMed] [Google Scholar]
- Piubelli GC, Hoffmann-Campo CB, Moscardi F, Miyakubo SH, de Oliveira MCN. (2005) Are chemical compounds important for soybean resistance to Anticarsia gemmatalis? J Chem Ecol 31: 1509–1525 [DOI] [PubMed] [Google Scholar]
- Pohnert G, Jung V, Haukioja E, Lempa K, Boland W. (1999) New fatty acid amides from regurgitant of lepidopteran (Noctuidae, Geometridae) caterpillars. Tetrahedron 55: 11275–11280 [Google Scholar]
- Poreba M, Drag M. (2010) Current strategies for probing substrate specificity of proteases. Curr Med Chem 17: 3968–3995 [DOI] [PubMed] [Google Scholar]
- Price PW. (2002) Resource-driven terrestrial interaction webs. Ecol Res 17: 241–247 [Google Scholar]
- Roda A, Halitschke R, Steppuhn A, Baldwin IT. (2004) Individual variability in herbivore-specific elicitors from the plant’s perspective. Mol Ecol 13: 2421–2433 [DOI] [PubMed] [Google Scholar]
- Rossi M, Goggin FL, Milligan SB, Kaloshian I, Ullman DE, Williamson VM. (1998) The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc Natl Acad Sci USA 95: 9750–9754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Carroll MJ, LeClere S, Phipps SM, Meredith J, Chourey PS, Alborn HT, Teal PEA. (2006) Fragments of ATP synthase mediate plant perception of insect attack. Proc Natl Acad Sci USA 103: 8894–8899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Engelberth J, Alborn HT, Tumlinson JH, III, Teal PEA. (2009) Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proc Natl Acad Sci USA 106: 653–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Engelberth J, Tumlinson JH, Block A, Alborn HT. (2004) The use of vapor phase extraction in metabolic profiling of phytohormones and other metabolites. Plant J 39: 790–808 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, LeClere S, Carroll MJ, Alborn HT, Teal PEA. (2007) Cowpea chloroplastic ATP synthase is the source of multiple plant defense elicitors during insect herbivory. Plant Physiol 144: 793–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo Hoo CR, Coudriet DL, Vail PV. (1984) Trichoplusia ni (Lepidoptera: Noctuidae) larval development on wild and cultivated plants. Environ Entomol 13: 843–846 [Google Scholar]
- Sun WX, Dunning FM, Pfund C, Weingarten R, Bent AF. (2006) Within-species flagellin polymorphism in Xanthomonas campestris pv campestris and its impact on elicitation of Arabidopsis FLAGELLIN SENSING2-dependent defenses. Plant Cell 18: 764–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlings TCJ, Bernasconi M, Bertossa R, Bigler F, Caloz G, Dorn S. (1998) The induction of volatile emissions in maize by three herbivore species with different feeding habits: possible consequences for their natural enemies. Biol Control 11: 122–129 [Google Scholar]
- Visôtto LE, Oliveira MGA, Ribon AOB, Mares-Guia TR, Guedes RNC. (2009) Characterization and identification of proteolytic bacteria from the gut of the velvetbean caterpillar (Lepidoptera: Noctuidae). Environ Entomol 38: 1078–1085 [DOI] [PubMed] [Google Scholar]
- Voelckel C, Baldwin IT. (2004) Generalist and specialist lepidopteran larvae elicit different transcriptional responses in Nicotiana attenuata, which correlate with larval FAC profiles. Ecol Lett 7: 770–775 [Google Scholar]
- Will T, Tjallingii WF, Thönnessen A, van Bel AJE. (2007) Molecular sabotage of plant defense by aphid saliva. Proc Natl Acad Sci USA 104: 10536–10541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittstock U, Agerbirk N, Stauber EJ, Olsen CE, Hippler M, Mitchell-Olds T, Gershenzon J, Vogel H. (2004) Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc Natl Acad Sci USA 101: 4859–4864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalucki MP, Brower LP, Alonso A. (2001) Detrimental effects of latex and cardiac glycosides on survival and growth of first-instar monarch butterfly larvae Danaus plexippus feeding on the sandhill milkweed Asclepias humistrata. Ecol Entomol 26: 212–224 [Google Scholar]
- Zarate SI, Kempema LA, Walling LL. (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JM, Chai J. (2008) Plant pathogenic bacterial type III effectors subdue host responses. Curr Opin Microbiol 11: 179–185 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JDG, Felix G, Boller T. (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428: 764–767 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.