Abstract
Isopentenyl diphosphate (IPP), which is produced from mevalonic acid or other nonmevalonic substrates, is the universal precursor of isoprenoids in nature. Despite the presence of several isoprenoid compounds in plastids, enzymes of the mevalonate pathway leading to IPP formation have never been isolated or identified to our knowledge. We now describe the characterization of two pepper (Capsicum annuum L.) cDNAs, CapTKT1 and CapTKT2, that encode transketolases having distinct and dedicated specificities. CapTKT1 is primarily involved in plastidial pentose phosphate and glycolytic cycle integration, whereas CapTKT2 initiates the synthesis of isoprenoids in plastids via the nonmevalonic acid pathway. From pyruvate and glyceraldehyde-3-phosphate, CapTKT2 catalyzes the formation of 1-deoxy-xylulose-5-phosphate, the IPP precursor. CapTKT1 is almost constitutively expressed during the chloroplast-to-chromoplast transition, whereas CapTKT2 is overexpressed during this period, probably to furnish the IPP necessary for increased carotenoid biosynthesis. Because deoxy-xylulose phosphate is shared by the plastid pathways of isoprenoid, thiamine (vitamin B1), and pyridoxine (vitamin B6) biosynthesis, our results may explain why albino phenotypes usually occur in thiamine-deficient plants.
Because of the combined activities of mevalonate-synthesizing and -activating enzymes, IPP is known as the universal isoprenoid building block. How IPP is synthesized and channeled into plastid isoprenoids to support the production of carotenoids, chlorophylls, prenylquinones, and diterpenes is largely unknown. Two hypotheses have been proposed. One is that plastids operate autonomously, synthesizing plastid isoprenoids directly from carbon dioxide or from plastid glycolytic intermediates such as pyruvate (Goodwin, 1971; Moore and Shephard, 1978; Heintze et al., 1990, 1994; McCaskill and Croteau, 1995). However, the mechanism of carbon flow via a pyruvate intermediate is unknown for plants. A second hypothesis is that IPP is transported from the cytosol (Kleinig, 1989), which is based on the finding that hydroxymethylglutaryl CoA reductase and mevalonate-activating enzymes are absent in plastids (Gray, 1987). This view is reinforced by the fact that mevilonin, a specific inhibitor of 3-hydroxy-3-methylglutaryl-CoA reductase, drastically inhibits cytosolic sterol biosynthesis at moderate concentrations but does not affect isoprenoid synthesis in plastids (Bach and Lichtenthaler, 1983). This led to consideration of an alternative IPP-generation system. In fact, such a pathway is known for prokaryotes, in which IPP is formed via deoxy-xylulose phosphate rather than by mevalonate (Rohmer et al., 1993) in a transketolation reaction between pyruvate and glyceraldehyde-3-phosphate (Rohmer et al., 1996). In vivo precursor labeling indicates that a similar pathway operates for the synthesis of ginkgolides (Schwarz, 1994) and plastid isoprenoids (Schwender et al., 1996; Arigoni et al., 1997; Lichtenthaler et al., 1997a, 1997b).
In this study we identified and characterized from pepper (Capsicum annuum L.) fruits two plastid transketolases, CapTKT1 and CapTKT2, which are dedicated to specific functions. CapTKT1 is primarily engaged in plastid pentose phosphate and glycolytic cycle integration. CapTKT2 irreversibly converts pyruvate and glyceraldehyde-3-phosphate into deoxy-xylulose phosphate, which then behaves as the plastidial IPP precursor via the putative intermediate 2-C-methyl-d-erythritol (Duvold et al., 1997). This pathway has no steps in common with cytoplasmic IPP synthesis, which proceeds via the mevalonic acid pathway. Our data also suggest that plastid isoprenoid biosynthesis is tightly coupled to that of thiamine (vitamin B1) (David et al., 1981; Julliard and Douce, 1991) and pyridoxine (vitamin B6) (Hill et al., 1989; Julliard, 1992) biosynthesis.
MATERIALS AND METHODS
Plant Materials and Organelle Isolation
Pepper (Capsicum annuum L. cv Yolo Wonder) plants were grown under controlled greenhouse conditions. Chloroplasts and chromoplasts from pepper fruit were prepared as described previously (Camara, 1993). Mitochondria were isolated and purified by Percoll-gradient centrifugation (Neuburger et al., 1982). The purity of the subcellular fractions was monitored by electron-microscopic analysis (Deruère et al., 1994).
Antibody Preparation
A partially purified pepper chromoplast transketolase, obtained as described previously (Murphy and Walker, 1982), was used to prepare polyclonal antibodies against CapTKT1 (Harboe and Ingild, 1977). Anti-CapTKT1 was further affinity purified (Smith and Fisher, 1984) before immunoscreening or immunoblot analysis. Anti-CapTKT2 was prepared from the recombinant protein devoid of the plastid-targeting sequence.
cDNA Isolation and Analysis
Total RNA was isolated as described previously (Verwoerd et al., 1989) and poly(A+) RNA was purified using an mRNA purification kit (Qiagen, Chatsworth, CA) following the manufacturer's instructions. Following immunoscreening of the pepper cDNA library (Bouvier et al., 1996) according to standard methods (Huynh et al., 1985; Sambrook et al., 1989), three overlapping partial cDNAs designated as CapTKT1 and having 1040, 1030, and 550 bp were isolated. Sequence analysis revealed that these CapTKT1 lacked their 5′-prime ends, whereas the 550-bp fragment contained the 3′-prime end of the cDNA. The 5′-end of CapTKT1 was obtained by RACE. The 5′-RACE was performed using the 5′ RACE kit (GIBCO-BRL) according to the manufacturer's instructions.
First-strand cDNA synthesis used the specific CapTKT1 internal oligonucleotide GST1: CTCAAAGTTTTCAGGGTG. Following C-tailing the CapTKT1-specific oligonucleotide GST2, ATGTCCAGCGGAGAGAAC, was used in combination with the anchor primers (GIBCO-BRL) for PCR (30 cycles) according to the program: 94°C for 1 min, 55°C for 1 min, 72°C for 2 min, and 72°C for 7 min. Purified PCR products were cloned into pBluescript SK− vector and both strands were sequenced. For the second transketolase, a BLAST search for transketolase-homologous plant sequences allowed indentification of a cDNA clone, designated CLA1 for “altered chloroplasts” (Mandel et al., 1996), several EST clones from Arabidopsis (W43388), rice (D46713 and D46576), and castor bean (T14878), and an Arabidopsis genomic sequence (Z97339) displaying characteristic transketolase motifs (Hawkins et al., 1989; Schenk et al., 1997) but with a deduced sequence that was very different from CapTKT1.
The sense oligonucleotide CTTTGGGATGTTGGTCATCAG, coding for the sequence LWDVGHQ (Arabidopsis EST, W43388), and the antisense oligonucleotide AACGTGCGAGCCAAAACCTCC, coding for GGFGSHV (Arabidopsis genomic sequence, Z97339), were used to amplify a 1550-bp probe by reverse transcriptase-PCR, using pepper fruit poly(A+) mRNA, according to the program described above. After sequence verification, the 1550-bp probe was used to isolate a 600-bp fragment containing the 3′-prime end of the cDNA from the pepper cDNA library. The 5′-prime end of the cDNA was isolated by 5′-RACE, as described above using the gene-specific probe GST1, GTCGTTTAAAATAACAAT. After C-tailing the gene-specific oligonucleotide GST2, GATGGTGGTGGAACTGTGGCC, was used in combination with the anchor primers (GIBCO-BRL) for PCR according to the program described for CapTKT1 5′-RACE. The resulting fragment was cloned into plasmid pBluescript SK− and both strands were sequenced. Sequence data were analyzed using computer programs of the University of Wisconsin Genetics Computer Group (Madison) (Devereux et al., 1984).
Expression and Purification
To express CapTKT1 and CapTKT2, the corresponding open reading frames devoid of the transit peptide sequences were amplified by PCR according to the above- described program and cloned into the PKK223–3 expression vector (Pharmacia). To this end, sense oligonucleotide CCGGAATTCATGCGCACTCTTCCGTCCCCCGTCGCC (encoding the peptide sequence RTLPSPVA), containing an EcoRI site, and the antisense oligonucleotide CCCAAGCTTAACAACTTTGGTAGACACCAGTAA, containing a HindIII site, were used for CapTKT1. Similarly, the sense oligonucleotide TCCCCCCGGGATGACGGTTCAGGCTTCTTTGTCAGAA (encoding the peptide sequence TVQASLSE), containing a XmaI site, and the antisense oligonucleotide TCCCCCCGGGTAATATACATTCTTTTACAGTTCT, containing a XmaI site, were used to amplify the coding region of CapTKT2. In the latter case, the orientation of the insert was verified by PCR and restriction-endonuclease digestion. After sequence verification, PKK223–3 containing CapTKT1 or CapTKT2 was used to transform competent Escherichia coli JM109.
Site-directed mutagenesis was carried out as previously described (Bouvier et al., 1997) using a site-directed mutagenesis kit (QuickChange, Stratagene). For CapTKT1 the amino acid change (Glu-491 to Ala) was introduced using the sense mutagenic oligonucleotide TTTGGTGTTCGTGCACATGGTATGGGA and the corresponding antisense oligonucleotide. For CapTKT2 the change (Glu-449 to Ala) was introduced using the sense GTTGGAATAGCAGCACAACATGCAGTA and the corresponding antisense oligonucleotide. Mutants CapTKT1 and CapTKT2 were expressed as shown for the corresponding wild-type enzymes.
To purify the expressed proteins, E. coli harboring CapTKT1 or CapTKT2 open reading frames was grown at 28°C to A = 0.4 in Luria-Bertani medium (1 L) before induction with 0.25 mm IPTG for 4 h. The cells were pelleted by centrifugation at 5,000g for 10 min before resuspension in 50 mm Tris-HCl buffer, pH 7.6, containing 5 mm MgCl2, 50 μm thiamine diphosphate, and 2 mm DTT. After sonication on ice at 10-s intervals for a total of 2 min, the soluble supernatant obtained after 10,000g centrifugation for 10 min was used for protein purification. To this end, the supernatant was loaded directly to a Q-Sepharose Fast-Flow column (2 × 30 cm, Pharmacia) equilibrated with 50 mm Tris-HCl buffer, pH 7.6, containing 5 mm MgCl2, 50 μm thiamine diphosphate, and 2 mm DTT (buffer A). After washing with 50 mm NaCl in the same buffer, the column was developed with a linear gradient of 50 to 250 mm NaCl in buffer A. The active fractions were pooled and diluted 5-fold with buffer A before Mono-Q HR (Pharmacia) column chromatography. Active fractions were eluted with the same linear gradient and used for enzymatic assays as described below.
Enzyme Assay and Analysis of Products
Two procedures were used to evaluate the transketolase activities. In the first procedure, tranketolase activity was assayed by monitoring the formation of d-glyceraldehyde-3-phosphate during the C2 transfer from d-xylulose-5-phosphate to d-erythrose-4-phosphate or d-Rib-5-phosphate using a coupled assay. The standard 100-μL reaction volume contained 50 mm Tris-HCl buffer, pH 7.6, 5 mm of each substrate, 500 μm thiamine diphosphate, 10 mm MgCl2, 250 μm NADH, 15 units of triose phosphate isomerase (Sigma), 5 units of glycerol-3-phosphate dehydrogenase (Sigma), and a definite amount of recombinant CapTKT1 and CapTKT2 devoid of the plastid-targeting sequence. Following incubation at 30°C, the decrease of NADH concentration was monitored spectrophotometrically by the decrease in A340.
For the second procedure, transketolase activity was assayed by monitoring the C2 transfer of [2-14C] pyruvate (26 mCi mol−1, New England Nuclear) to d-glyceraldehyde-3-phosphate. The reaction was performed in a volume of 100 μL, and contained 50 mm Tris-HCl buffer, pH 7.6, 5 mm pyruvic acid (2 μCi), 5 mm d-glyceraldehyde-3-phosphate, 500 μm thiamine diphosphate, 10 mm MgCl2, and a definite amount of enzyme. Following incubation at 30°C, the reaction was stopped by the addition of 5% TCA. Insoluble proteins were removed by centrifugation for 5 min in a microfuge at 4°C. The supernatant was adjusted to pH 9.0 and treated with 10 units of bovine alkaline phosphatase before further incubation for 1 h at 37°C. Reactions were stopped by the addition of 4 volumes of cold acetone followed by centrifugation. After addition of authentic unlabeled deoxy-xylulose synthesized as described previously (Yokota and Sasajima, 1984, 1986) or kindly provided by Ian D. Spenser (MacMaster University, Hamilton, Ontario, Canada), the supernatant was spotted onto Silicagel 60 plates developed with the solvent system (acetone:ethylacetate; 1:1, v/v). Reaction products were identified by autoradiography and by spraying the plates with vanillin-perchloric reagent (MacLennan et al., 1959). Alternatively, disposable Silicagel 60 microcolumns eluted with the same solvent system were used to monitor the radioactivity incorporated into deoxy-xylulose. Incorporated radioactivity was determined by liquid-scintillation counting.
Other Methods
Protein concentrations were determined according to the dye-binding procedure (Bradford, 1976). Immunoblot and northern analyses of CapTKT1 and CapTKT2 were performed as described previously (Bouvier et al., 1996).
RESULTS
The Role of Transketolase in Generating Carbon-Carbon Condensations
Indirect evidence of a precursor-product relationship or carbon flow between carbohydrate and isoprenoid synthesis during chloroplast-to-chromoplast differentiation was suggested by the active pentose phosphate and glycolytic cycles (Thom et al., 1998) and the total disappearance of starch, which coincides with the intense accumulation of carotenoids that occurs in chromoplasts of ripening, nonmutant pepper fruits (Camara et al., 1995). More recently, incorporation of 13C-labeled Glc into plant cells (Eisenreich et al., 1996; Schwender et al., 1996; Arigoni et al., 1997; Knöss et al., 1997; Lichtenthaler et al., 1997b) followed by NMR analysis led to new insights about the conversion of carbohydrate precursors into IPP. The new pathway proposes the condensation between the C2 unit from pyruvate and the C3 acceptor glyceraldehyde-3-phosphate to yield deoxy-xylulose phosphate, the nonmevalonic precursor of IPP (Rohmer et al., 1996; Arigoni et al., 1997).
The above proposed pathway of carbon-carbon condensations is reminiscent of a transketolation reaction catalyzed by several enzymes from microorganisms (Yokota and Sasajima, 1984). This mechanism has been used to generate several classes of compounds (Villafranca and Axelrod, 1971; Bolte et al., 1987; Kobori et al., 1992; Hobbs et al., 1993). To identify putative transketolases involved in plastid isoprenoid biosynthesis we first raised antibodies against partially purified chromoplast transketolase from pepper, and selected from the National Center for Biotechnology Information (Bethesda, MD) appropriate ESTs encoding characteristic transketolase motifs (Hawkins et al., 1989; Reizer et al., 1993; Schenk et al., 1997).
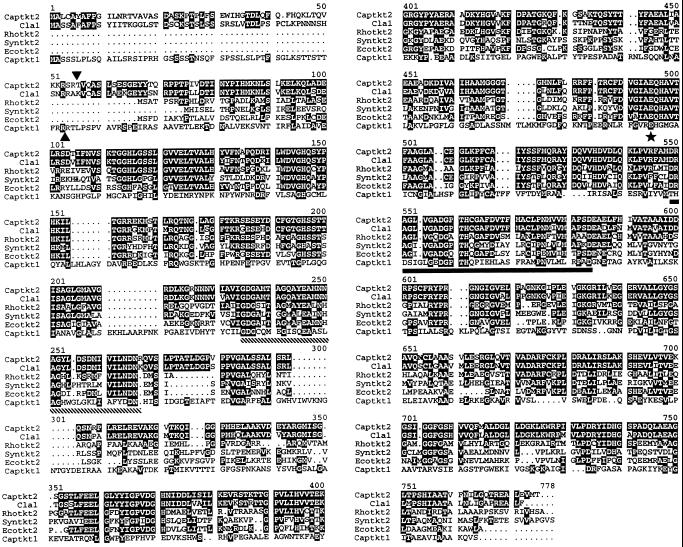
Two strategies, immunoscreening and DNA hybridization, were used to isolate two partial cDNAs, respectively designated CapTKT1 and CapTKT2, from a pepper fruit library. The longest CapTKT1 and CapTKT2 clones with ATG start codons isolated by the RACE procedure were 2232 and 2157 bp, respectively. CapTKT1 and CapTKT2 encode for 744 and 719 amino acid proteins with respective molecular masses of 80.1 and 77.5 kD. The predicted primary structure contains domains with potential functional significance, including a transketolase motif (Schenk et al., 1997) and a thiamine diphosphate-binding domain (Hawkins et al., 1989) (Fig. 1). Hydropathy plots of CapTKT1 and CapTKT2 did not reveal significant transmembrane-spanning domains, suggesting that both proteins are hydrophilic (results not shown).
Figure 1.
Sequence alignment of CapTKT1 and CapTKT2 proteins with related gene products. Black boxes indicate identity between CapTKT1 (Captkt1) (accession no. Y15781) and CapTKT2 (Captkt2) (accession no. Y15782) with ClaI protein from Arabidopsis (Cla1) (Mandel et al., 1996), putative Rhodobacter (Rhotkt2) (accession no. P26242), Synechocystis (Syntkt2) (accession no. D90903), and E. coli (Ecotkt2) (accession no. P77488) transketolases. The underlined domain fits to the consensus thiamin diphosphate-binding site (striped bar), which is well conserved among various thiamine-dependent enzymes (Hawkins et al., 1989; Schenk et al., 1997), and the transketolase motifs (Schenk et al., 1997) are indicated by the striped and solid bars. The invariant Glu residue thought to be specific for transketolase activity is indicated by a star. The arrowheads indicate the amino terminus of the recombinant proteins expressed in E. coli.
CapTKT1 and CapTKT2 displayed 21.6% amino acid identity with each other. However, CapTKT1 shared 89%, 87%, and 82.7% amino acid identity with potato (accession no. Q43848), Craterostigma plantagineum (Bernacchia et al., 1995), and spinach chloroplast (Flechner et al., 1996) transketolases, respectively. In addition, the identity with E. coli and yeast transketolase varied from 52.3% to 53.7%. CapTKT2 also showed at least 80% amino acid identity with Arabidopsis CLA1 (Mandel et al., 1996) (Fig. 1), the Arabidopsis cDNA clone (Y14333), Arabidopsis genomic clone sequence (Z97339), and several EST clones from Arabidopsis (W43388), pine (H75224), rice (D46713 and D46576), and castor bean (T14878). In addition, CapTKT2 showed 45.6% to 54.4% amino acid sequence identity with the gene product encoded by Synechocystis (D90903), E. coli (P77488), and Rhodobacter (P26242) open reading frames (Fig. 1).
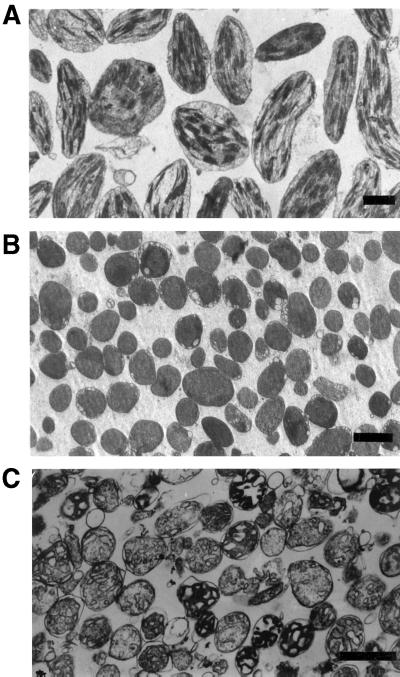
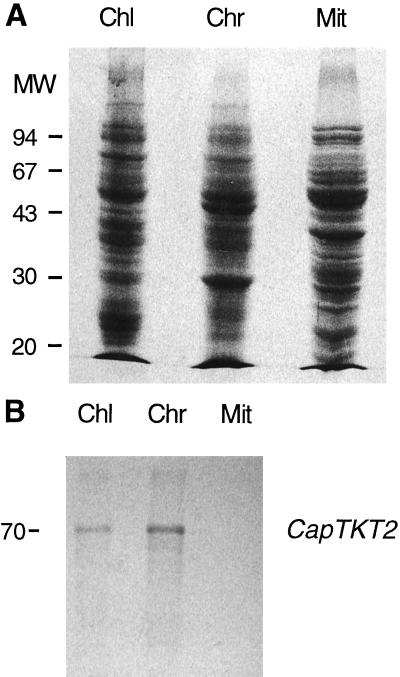
To localize CapTKT1 and CapTKT2 in pepper cells, antibodies raised against recombinant CapTKT1 and CapTKT2 were used to detect their presence in plastids and mitochondria. Intact and pure chloroplasts, chromoplasts, and mitochondria were prepared from pepper fruits (Fig. 2) and their total proteins were subjected to western blotting against anti-CapTKT1 and anti-CapTKT2 antibodies. Data displayed in Figure 3 reveal that CapTKT2 antibodies specifically react with plastidial polypeptides having approximatively a molecular mass of 67 to 70 kD, in good agreement with the mature size predicted from CapTKT2 cDNA. No reactive polypeptide bands were detected in mitochondria (Fig. 3). Similar results were obtained with CapTKT1 (results not shown). Thus, CapTKT1 and CapTKT2 exclusively colocalize in plastids, as suggested by the putative plastid-targeting signal (Von Heijne et al., 1991) observed at the amino terminus of CapTKT1 and CapTKT2 cDNAs (Fig. 1).
Figure 2.
Electron micrograph showing the purity of pepper cell organelles used for CapTKT2 localization in chloroplasts (A), chromoplasts (B), and mitochondria (C). Bars, 1 μm.
Figure 3.
SDS-PAGE and immunoblot analysis of CapTKT. A, Coomassie blue-stained gel of proteins isolated from chloroplasts (Chl), chromoplasts (Chr), and mitochondria (Mit). B, Immunoblot analysis of organelle proteins shown in A with anti-CapTKT2. For both A and B, molecular mass (MW) markers are shown on the left in kD.
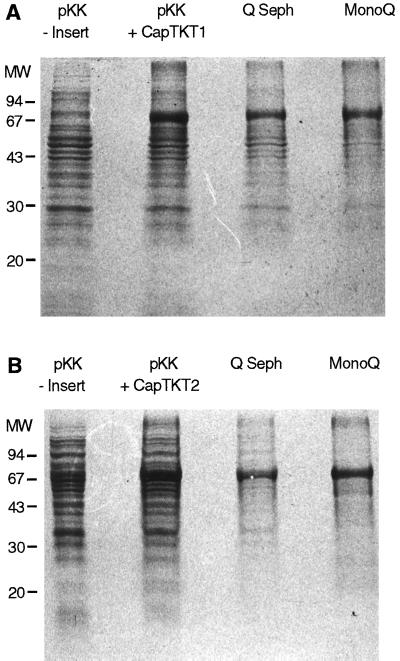
To determine the biological function of CapTKT1 and CapTKT2, the coding regions of both cDNAs were ligated to the bacterial vector pKK223–3 for expression in E. coli. Following IPTG induction and SDS-PAGE analysis of samples of bacterial cultures, prominent polypeptide bands having molecular masses of 74 kD (CapTKT1) and 71 kD (CapTKT2) were observed in extracts of transformed but not in nontransformed E. coli cells (Fig. 4). Following lysis of bacterial cells and centrifugation, recombinant CapTKT1 and CapTKT2 proteins recovered in the supernatant were subjected to two chromatographic purification steps using Q-Sepharose and Mono-Q columns (Fig. 4) before analyzing their transketolase activity according to the two enzymatic procedures described in the experimental section. Preliminary enzyme tests were performed on purified CapTKT1 and CapTKT2 by incubating each with d-xylulose-5-phosphate (a C2 donor) and d-Rib-5-phosphate (a C2 acceptor), the two usual substrates of transketolases. Under these conditions only CapTKT1 showed significant activity (Table I), behaving as previously characterized plastid transketolases (Murphy and Walker, 1982; Flechner et al., 1996).
Figure 4.
Purification of recombinant CapTKT1 and CapTKT2. A and B, Coomassie blue-stained gel of proteins representative of recombinant CapTKT1 and CapTKT2 purification after IPTG induction. Lanes from left to right include: total proteins from E. coli harboring control vector (pKK-Insert); vector plus insert (pKK+CapTKT1 in A and pKK+CapTKT2 in B); and pooled active fractions from the Q-Sepharose Fast-Flow (Q Seph) and Mono-Q HR (MonoQ) columns. For both A and B, molecular mass (MW) markers are shown on the left in kD.
Table I.
Substrate specificity of CapTKT1 and CapTKT2
| Enzyme Assayed | Activity
|
||
|---|---|---|---|
| d-Rib-5-Paplus d-Xylulose-5-P | d-Erythrose-4-P plus d-Xylulose-5-P | d-Glyceraldehyde-3-P plus Pyruvate | |
| nmol min−1 mg−1 protein | |||
| CapTKT1 | 30.103 | 25.103 | 35 |
| CapTKT2 | 8 | 10 | 500 |
Combined C2 acceptors and donors were used as substrates for monitoring the transketolase activity of CapTKT1 and CapTKT2.
P, Phosphate.
Additional studies using pyruvic acid (a C2 donor) and d-glyceraldehyde-3-phosphate (a C2 acceptor), the two putative substrates predicted for use by the nonmevalonic acid pathway of IPP synthesis (Rohmer et al., 1996; Arigoni et al., 1997), revealed that CapTKT2 alone catalyzed a transketolation condensation (Table I), yielding 1-deoxy-xylulose-5-phosphate, which after dephosphorylation migrated with authentic unlabeled 1-deoxy-xylulose to a RF of 0.45. The Km of CapTKT1 for d-xylulose-5-phosphate, d-Rib-5-phosphate, and d-erythrose-4-phosphate was 95, 750, and 200 μm, respectively. The Km of CapTKT2 for pyruvate and d-glyceraldehyde-3-phosphate was 500 and 750 μm, respectively. As shown in Table I, CapTKT1 utilized preferentially products of the plastid pentose phosphate pathway, whereas CapTKT2 had a preference for pyruvate as 2-carbon ketol donors. This confirmed that each enzyme possessed distinctly different roles. The role of CapTKT2 is reminiscent of a partially purified E. coli pyruvate dehydrogenase (Yokota and Sasajima, 1984, 1986).
As this manuscript was being prepared, a report of a previously uncharacterized E. coli gene (P77488) was shown to encode a deoxy-xylulose synthase (Sprenger et al., 1997). This likely catalyzes the aforementioned reactions (Yokota and Sasajima, 1984, 1986), since other previously characterized E. coli transketolases, A and B, are not involved in deoxy-xylulose synthesis (Zhao and Winkler, 1994). Thus, CapTKT2 could be designated as a deoxy-xylulose synthase, as shown for E. coli (Sprenger et al., 1997).
A mandatory requirement for transketolation is the presence of the prosthetic group TPP (Lindqvist and Schneider, 1993). In fact, during transketolase catalysis, fission of the ketol group of the donor is initiated by the C2 atom of the thiazolium ring, which is activated by deprotonation via an invariant Glu residue that has been characterized for yeast transketolase (Wikner et al., 1994; Kern et al., 1997). The sequence alignment of CapTKT1 and CapTKT2 suggests that Glu-491 and Glu-449 fulfill this role in CapTKT1 and CapTKT2, respectively (Fig. 1). When Glu-491 and Glu-449 were mutated into Ala, the electrophoretic behavior of wild-type and mutant CapTKT1 or CapTKT2 was not modified (results not shown). However, transketolase activity was nearly abolished in CapTKT1 and CapTKT2 (Table II).
Table II.
Enzymatic activity of wild-type and mutant CapTKT1 and CapTKT2
| Enzyme | Activity |
|---|---|
| % | |
| Wild-type CapTKT1 | 100 |
| CapTKT1 (Glu-491 to Ala) | 0.8 |
| Wild-type CapTKT2 | 100 |
| CapTKT2 (Glu-449 to Ala) | 0.1 |
Mono-Q-purified wild-type and mutant CapTKT1 and CapTKT2 were used for the different assays. CapTKT1 activity was determined using d-Rib-5-phosphate and d-xylulose-5-phosphate as substrates. CapTKT2 activity was determined using d-glyceraldehyde-3-phosphate and [2-14C]pyruvate as substrates. The indicated Glu residue was mutated into Ala as shown by the arrow.
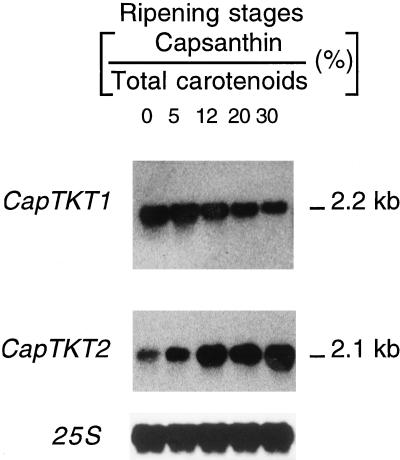
Collectively, these findings suggest that plastids possess two types of transketolases with distinct specificities. Further confirmation of these different roles for CapTKT1 and CapTKT2 is supported by the fact that during the chloroplast-to-chromoplast transition in pepper, expression of CapTKT1 gene is nearly constitutive, whereas expression of CapTKT2 gene is up-regulated when carotenoid accumulation is at its greatest (Fig. 5). This coincides with the period when plastidial demand for IPP is at its highest level.
Figure 5.
Analysis of CapTKT1 and CapTKT2 mRNAs during chloroplast-to-chromoplast differentiation in pepper fruit. Identical amounts of total RNA (20 μg) were blotted onto each lane and hybridized with the specified probes. Top to bottom, CapTKT1 cDNA, CapTKT2 cDNA, and pepper fruit probe coding for 25S rRNAs used as a standard probe. Chloroplast-to-chromoplast differentiation stages were characterized by the level of the chromoplast-specific carotenoid capsanthin.
DISCUSSION
Dedicated Roles of Plastid Transketolases
It was widely believed that the synthesis of isoprenoids in plastids involved the formation of mevalonic acid and its conversion into IPP, the building block of all isoprenoids. Despite a long debate, a conclusive demonstration of mevalonic acid pathway reactions in plastids was still lacking (Gray, 1987). In vivo studies using 13C-Glc labeling subsequently revealed the existence of an alternative nonmevalonic acid pathway for IPP synthesis in prokaryotes (Flesch and Rohmer, 1988; Rohmer et al., 1993, 1996) and in plants (Schwender et al., 1996; Arigoni et al., 1997; Knöss et al., 1997; Lichtenthaler et al., 1997a, 1997b), which is initiated by a transketolation mechanism. We have characterized two pepper plastid transketolases, CapTKT1 and CapTKT2, and showed that they possess distinct specificities.
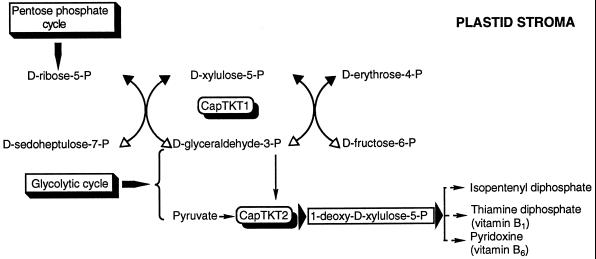
CapTKT1, like previously characterized plastid transketolases (Murphy and Walker, 1982; Bernacchia et al., 1995), links the plastidial pentose phosphate and the glycolytic cycles under physiological conditions. In contrast, CapTKT2 catalyzes an irreversible reaction between glyceraldehyde phosphate and pyruvate to yield deoxy-xylulose phosphate, the IPP precursor (Fig. 6). As a consequence, CapTKT2 probably initiates the nonmevalonic acid pathway for isoprenoid synthesis in plastids. The fact that similar open reading frames exist in E. coli, Rhodobacter, and Synechocystis also reinforces the cyanobacterial endosymbiotic origin of plastids.
Figure 6.
Metabolic specificities of plastid CapTKT1 and CapTKT2. Due to its flexibility, CapTKT1 integrates the plastid stroma pentose phosphate and glycolytic cycles, whereas CapTKT2 catalyzes an irreversible condensation between d-glyceraldehyde-3-phosphate and pyruvate to yield 1-deoxy-xylulose-5-phosphate. The latter is further channeled to the formation of IPP, thiamine, or pyridoxine. P, Phosphate.
Interaction between Plastid Isoprenoid and Vitamin Biosynthesis
As deoxy-xylulose phosphate, an IPP precursor (Schwarz, 1994; Schwender et al., 1996; Arigoni et al., 1997), is involved in thiamine (David et al., 1981; Julliard and Douce, 1991) and pyrydoxine (Hill et al., 1989; Julliard, 1992) synthesis, CapTKT2 appears to play a key role in the previously unexpected pathways that link isoprenoid and thiamine and pyridoxine synthesis in plastids (Fig. 6). Several indirect lines of evidence support this. Thiamine deficiency generally leads to the development of albino phenotypes in Arabidopsis (Li and Redei, 1969; Komeda et al., 1988), tobacco (McHale et al., 1988), pea (Proebsting et al., 1990), tomato (Boynton, 1966), and Plantago insularis (Murr and Stebbins, 1971). Although albinism in these plants could be caused by impairment of other thiamine-dependent enzymes, it is noteworthy that most of these mutants are rescued following the addition of thiamine or its precursors. Furthermore, it has been recently observed that in ripening citrus the thiamine-biosynthesis gene c-thi1, which is homologous to the yeast thi4 gene (Praekelt et al., 1994), is strongly induced during carotenoid accumulation (Jacob-Wilk et al., 1997). Finally, the albino phenotype of an Arabidopsis mutant altered in the cla1 gene (Mandel et al., 1996), which is highly homologous to CapTKT2, points to a similar conclusion, that the CapTKT2 is involved in isoprenoid, thiamine, and pyridoxine biosynthesis.
Interaction between Plastid and Cytosol Compartments during Isoprenoid Biosynthesis
One may postulate that plants possess at least two pathways for IPP synthesis. This is supported by in vivo studies of mevalonate-synthesis inhibitors (Bach and Lichtenthaler, 1983) and by newly characterized substrate precursors that contribute to IPP formation (Rohmer et al., 1996; Schwender et al., 1996; Arigoni et al., 1997). From previous in vivo data (Schwender et al., 1996; Arigoni et al., 1997) and our enzymatic analysis, one may suggest that the deoxy-xylulose pathway operates within plastids to yield carotenoids and geranylgeranyl derivatives, whereas the “classical” mevalonic acid pathway operates in the cytosol and is primarily responsible for the synthesis of IPP units that form sesquiterpenes, sterols, and various polyprenols in addition to mitochondrial ubiquinones (Disch et al., 1998). However, a general conclusion as to the metabolic compartmentation of plant isoprenoid synthesis is still unclear.
In unicellular alga the nonmevalonic acid pathway of IPP synthesis is known to be involved in sterol synthesis (Schwender et al., 1996); however, in higher plants IPP for sterol synthesis is prominently formed by the mevalonic pathway. One intriguing feature is that despite the fact that parallel pathways appear to occur in different compartments, isoprenoid substrates formed in the cytosol can still apparently also be transported into the plastid and vice versa (Schwarz, 1994; Nabeta et al., 1995; Arigoni et al., 1997). It has also been suggested that two mevalonic acid pathways can operate in cell cultures of mulberry tree cells, one that is sensitive to mevinolin and another that is not (Hano and Nomura, 1995). Obviously, plant cells possess complex features with regard to IPP synthesis, and additional studies are needed to clarify the steps leading from deoxy-xylulose to IPP formation to determine how the metabolic flux of isoprenoid precursors is regulated at the molecular level.
ACKNOWLEDGMENTS
We thank Dr. J. Schäeffer for electron microscopy and Professor Ian D. Spenser for the kind gift of deoxypentulose. We also thank Professor M. Rohmer for critical reading of the manuscript.
Abbreviations:
- EST
expressed sequence tag
- IPP
isopentenyl diphosphate
- IPTG
isopropylthio-β-galactoside
- RACE
rapid amplification of cDNA ends
Footnotes
LITERATURE CITED
- Arigoni D, Sagner S, Latzel C, Eisenreich W, Bacher A, Zenk MH. Terpenoid biosynthesis from 1-deoxy-d-xylulose in higher plants by intramolecular skeletal rearrangement. Proc Natl Acad Sci USA. 1997;94:10600–10605. doi: 10.1073/pnas.94.20.10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach TJ, Lichtenthaler HK. Inhibition by mevilonin of plant growth, sterol formation and pigment accumulation. Physiol Plant. 1983;59:50–60. [Google Scholar]
- Bernacchia G, Schwall G, Lottspeich F, Salamini F, Bartels D. The transketolase gene family of the resurrection plant Craterostigma plantagineum: differential expression during the rehydratation phase. EMBO J. 1995;14:610–618. doi: 10.1002/j.1460-2075.1995.tb07037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte J, Demuynck C, Samaki H. Utilization of enzymes in organic chemistry: transketolase catalyzed synthesis of ketoses. Tetrahedron Lett. 1987;28:5525–5528. [Google Scholar]
- Bouvier F, d'Harlingue A, Camara B. Molecular analysis of carotenoid cyclase inhibition. Arch Biochem Biophys. 1997;346:53–64. doi: 10.1006/abbi.1997.0278. [DOI] [PubMed] [Google Scholar]
- Bouvier F, d'Harlingue A, Hugueney P, Marin E, Marion-Poll A, Camara B. Xanthophyll biosynthesis: cloning, expression, functional reconstitution, and regulation of β-cyclohexenyl carotenoid epoxidase from pepper (Capsicum annuum) J Biol Chem. 1996;271:28861–28867. doi: 10.1074/jbc.271.46.28861. [DOI] [PubMed] [Google Scholar]
- Boynton JE. Chlorophyll-deficient mutants in tomato requiring vitamin B1. I. Genetics and physiology. Hereditas. 1966;56:171–199. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Camara B. Plant phytoene synthase complex: component enzymes, immunology, and biogenesis. Methods Enzymol. 1993;214:352–365. [Google Scholar]
- Camara B, Hugueney P, Bouvier F, Kuntz M, Monéger R. Biochemistry and molecular biology of chromoplast development. Int Rev Cytol. 1995;163:175–247. doi: 10.1016/s0074-7696(08)62211-1. [DOI] [PubMed] [Google Scholar]
- David S, Estramareix B, Fischer JC, Thérisod M. 1-deoxy-d-threo-2-pentulose: the precursor of the five carbon chain of the thiazole of thiamine. J Am Chem Soc. 1981;103:7341–7342. [Google Scholar]
- Deruère J, Römer S, d'Harlingue A, Backhaus RA, Kuntz M, Camara B. Fibril assembly and carotenoid over accumulation: a model for supramolecular lipoprotein structures. Plant Cell. 1994;6:119–133. doi: 10.1105/tpc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disch A, Hemmerlin A, Bach TJ, Rohmer M. Mevalonate-derived isopentenyl diphosphate is the biosynthetic precursor of ubiquinone prenyl side chain in tobacco BY-2 cells. Biochem J. 1998;331:615–621. doi: 10.1042/bj3310615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvold T, Bravo JM, Pale-Grosdemange C, Rohmer M. Biosynthesis of 2-C-methyl-d-erythritol, a putative C5 intermediate in the mevalonate independent pathway for isoprenoid biosynthesis. Tetrahedron Lett. 1997;33:4769–4772. [Google Scholar]
- Eisenreich W, Menhard B, Hylands P, Zenk MH, Bacher A. Studies on the biosynthesis of taxol: the taxane carbon skeleton is not of mevalonoid origin. Proc Natl Acad Sci USA. 1996;93:6431–6436. doi: 10.1073/pnas.93.13.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechner A, Dressen U, Westhoff P, Henze K, Schnarrenberger C, Martin W. Molecular characterization of transketolase (EC 2.2.1.1) active in the Calvin cycle of spinach chloroplasts. Plant Mol Biol. 1996;32:475–484. doi: 10.1007/BF00019099. [DOI] [PubMed] [Google Scholar]
- Flesch G, Rohmer M. Prokaryotic hopanoids: the biosynthesis of the bacteriohopane skeleton—formation of isoprene units from two distinct acetate pools and a novel type of carbon/carbon linkage between a triterpene and d-ribose. Eur J Biochem. 1988;175:405–411. doi: 10.1111/j.1432-1033.1988.tb14210.x. [DOI] [PubMed] [Google Scholar]
- Goodwin TW (1971) Biosynthesis. In O Isler, ed, Carotenoids. Birkhäuser Verlag, Basel, Switzerland, pp 577–636
- Gray JC. Control of isoprenoid biosynthesis in higher plants. Adv Bot Res. 1987;14:25–91. [Google Scholar]
- Hano Y, Nomura T. Alternative response of two isoprenoid biosynthetic pathways to compactin in Morus alba cell cultures. Naturwissenchaften. 1995;82:376–378. [Google Scholar]
- Harboe N, Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. In: Axelsen HH, Kroll NH, Weeks B, editors. Quantitative Immunoelectrophoresis. Oxford, UK: Blackwell Scientific Publications; 1977. pp. 161–164. [DOI] [PubMed] [Google Scholar]
- Hawkins CF, Borges A, Perham RN. A common structural motif in thiamin pyrophosphate-binding enzymes. FEBS Lett. 1989;255:77–82. doi: 10.1016/0014-5793(89)81064-6. [DOI] [PubMed] [Google Scholar]
- Heintze A, Görlach J, Leuschner C, Hoppe P, Hagelstein P, Schulze-Siebert D, Schultz G. Plastidic isoprenoid synthesis during chloroplast development. Change from metabolic autonomy to a division-of-labor stage. Plant Physiol. 1990;93:1121–1127. doi: 10.1104/pp.93.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintze A, Riedel A, Aydogdu S, Schultz G. Formation of chloroplast isoprenoids from pyruvate and acetate by chloroplasts from young spinach plants: evidence for a mevalonate pathway in immature chloroplasts. Plant Physiol Biochem. 1994;32:791–797. [Google Scholar]
- Hill RE, Sayer BG, Spenser ID. Biosynthesis of vitamin B6: incorporation of d-1-deoxyxylulose. J Am Chem Soc. 1989;111:1916–1917. [Google Scholar]
- Hobbs GR, Lilly MD, Turner NJ, Ward JM, Willets AJ, Woodley JM. Enzyme-catalysed carbon-carbon bond formation: use of transketolase from Escherichia coli. J Chem Soc Perkin Trans. 1993;I:165–166. [Google Scholar]
- Huynh TV, Young RA, Davies RW. Construction and screening cDNA libraries in λgt10 and λgt11. In: Glover DM, editor. DNA Cloning: a Practical Approach. Oxford, UK: IRL Press; 1985. pp. 49–78. [Google Scholar]
- Jacob-Wilk D, Goldschmidt EE, Riov J, Sadka A, Holland D. Induction of a Citrus gene highly homologous to plant and yeast thi genes involved in thiamine biosynthesis during natural and ethylene-induced fruit maturation. Plant Mol Biol. 1997;35:661–666. doi: 10.1023/a:1005833724582. [DOI] [PubMed] [Google Scholar]
- Julliard JH. Biosynthesis of the pyridoxal ring (vitamin B6) in higher plant chloroplasts and its relationship with the biosynthesis of the thiazole ring (vitamin B1) C R Acad Sci Ser III. 1992;314:285–290. [Google Scholar]
- Julliard JH, Douce R. Biosynthesis of the thiazole moiety of thiamin (vitamin B1) in higher plant chloroplasts. Proc Natl Acad Sci USA. 1991;88:2042–2045. doi: 10.1073/pnas.88.6.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern D, Kern G, Neef H, Tittmann K, Killenberg-Jabs M, Wilner C, Schneider G, Hübner G. How thiamine diphosphate is activated in enzymes. Science. 1997;275:67–70. doi: 10.1126/science.275.5296.67. [DOI] [PubMed] [Google Scholar]
- Kleinig H. The role of plastids in isoprenoid biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:39–59. [Google Scholar]
- Knöss W, Reuter B, Zapp J. Biosynthesis of the labdane diterpene marrubium in Marrubium vulgare via a non-mevalonate pathway. Biochem J. 1997;326:449–454. doi: 10.1042/bj3260449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori Y, Myles DC, Whitesides GM. Substrate specificity and carbohydrate synthesis using transketolase. J Org Chem. 1992;57:5899–5907. [Google Scholar]
- Komeda Y, Tanaka M, Nishimune T. A th-1 mutant of Arabidopsis thaliana is defective for a thiamine-phosphate-synthesizing enzyme: thiamine phosphate pyrophosphorylase. Plant Physiol. 1988;88:248–250. doi: 10.1104/pp.88.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange BM, Wildung MR, McCaskill D, Croteau R. A family of transketolases that directs isoprenoid biosynthesis via a mevalonate-independent pathway. Proc Natl Acad Sci USA. 1998;95:2100–2104. doi: 10.1073/pnas.95.5.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SL, Redei GP. Thiamine mutants of the crucifer, Arabidopsis. Biochem Genet. 1969;3:163–170. doi: 10.1007/BF00520351. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Rohmer M, Schwender J. Two independent biochemical pathways for isopentenyl diphosphate and isoprenoid biosynthesis in higher plants. Physiol Plant. 1997a;101:643–652. [Google Scholar]
- Lichtenthaler HK, Schwender J, Disch A, Rohmer M. Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett. 1997b;400:271–274. doi: 10.1016/s0014-5793(96)01404-4. [DOI] [PubMed] [Google Scholar]
- Lindqvist Y, Schneider G. Thiamin diphosphate dependent enzymes: transketolase, pyruvate oxidase and pyruvate decarboxylase. Curr Opin Struct Biol. 1993;3:896–901. [Google Scholar]
- MacLennan AP, Randall HM, Smith DW (1959) Detection and identification of deoxysugars on paper chromatograms. Analyt Chem 31, 2020–2022
- Mandel MA, Feldmann KA, Herrera-Estrella L, Rocha-Sosa M, Léon P. CLA1, a novel gene required for chloroplast development, is highly conserved in evolution. Plant J. 1996;9:649–658. doi: 10.1046/j.1365-313x.1996.9050649.x. [DOI] [PubMed] [Google Scholar]
- McCaskill D, Croteau R. Monoterpene and sesquiterpene biosynthesis in glandular trichomes of peppermint (Mentha × piperita) rely exclusively on plastid-derived isopentenyl diphosphate. Planta. 1995;197:49–56. [Google Scholar]
- McHale NA, Hanson KR, Zelitch I. A nuclear mutation in Nicotiana sylvestris causing a thiamine reversible defect in synthesis of chloroplast pigments. Plant Physiol. 1988;88:930–935. doi: 10.1104/pp.88.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore FD, Shephard DC. Chloroplast autonomy in pigment synthesis. Protoplasma. 1978;94:1–17. [Google Scholar]
- Murphy DJ, Walker DA (1982) The properties of transketolase from photosynthetic tissue. Planta 155, 316–320 [DOI] [PubMed]
- Murr SM, Stebbins GL. An albino mutant in Plantago insularis requiring thiamine pyrophosphate. I. Genetics. 1971;68:231–243. doi: 10.1093/genetics/68.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeta K, Ishikawa T, Okuyama H. Sesqui- and di-terpene biosynthesis from 13C-labelled acetate and mevalonate in cultured cells of Heteroscyphus planus. J Chem Soc Perkin Trans. 1995;I:3111–3115. [Google Scholar]
- Neuburger M, Journet EP, Bligny R, Carde JP, Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of percoll. Arch Biochem Biophys. 1982;217:312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Praekelt UM, Byrne KL, Meacock PA. Regulation of thi4 (mol1), a thiamine-biosynthetic gene of Saccharomyces cerevisiae. Yeast. 1994;10:481–490. doi: 10.1002/yea.320100407. [DOI] [PubMed] [Google Scholar]
- Proebsting WM, Maggard SP, Guo WW. The relationship of thiamine to the Alt locus of Pisum sativum L. J Plant Physiol. 1990;13:231–235. [Google Scholar]
- Reizer J, Reizer A, Bairoch A, Saier JMH. A diverse transketolase family that includes the RecP protein of Streptococcus pneumoniae, a protein implicated in genetic recombination. Res Microbiol. 1993;144:341–347. doi: 10.1016/0923-2508(93)90191-4. [DOI] [PubMed] [Google Scholar]
- Rohmer M, Knanni M, Simonin P, Sutter B, Sahm H. Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J. 1993;295:517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmer M, Seemann M, Horbach S, Bringer-Meyer S, Sahm H. Glyceraldehyde 3-phosphate and pyruvate as precursors of isoprenic units in an alternative non-mevalonate pathway for terpenoid biosynthesis. J Am Chem Soc. 1996;118:2564–2566. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schenk G, Layfield R, Candy JM, Duggleby RG, Nixon PF. Molecular evolutionary analysis of the thiamine-diphosphate-dependent enzyme, transketolase. J Mol Evol. 1997;44:552–572. doi: 10.1007/pl00006179. [DOI] [PubMed] [Google Scholar]
- Schwarz MK (1994) Terpen-Biosynthese in Ginkgo biloba:eine überraschende Geschichte. PhD thesis. Eidgenössische Technische Hochschule, Zürich
- Schwender J, Seemann M, Lichtenthaler HK, Rohmer M. Biosynthesis of isoprenoids (carotenoids, sterols, prenyl side chain of chlorophylls and plastoquinone) via a novel pyruvate/glyceraldehyde 3-phosphate non-mevalonate pathway in the green Scenedesmus obliquus. Biochem J. 1996;316:73–80. doi: 10.1042/bj3160073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Fisher PA. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984;99:20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger GA, Schörken U, Wiegert T, Grolle S, de Graaf AA, Taylor SV, Begley TP, Bringer-Meyer S, Sahm H. Identification of a thiamin-dependent synthase in Escherichia coli required for the formation of the 1-deoxy-d-xylulose 5-phosphate precursor to isoprenoids, thiamin, and pyridoxol. Proc Natl Acad Sci USA. 1997;94:12857–12862. doi: 10.1073/pnas.94.24.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom E, Möhlmann T, Quick WP, Camara B, Neuhaus E. Enzymic components of plastids from sweet pepper fruits, characterisation of the plastidic oxidative pentose phosphate pathway and transport processes across the envelope membrane. Planta. 1998;204:226–233. [Google Scholar]
- Verwoerd TC, Dekker BMM, Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafranca JJ, Axelrod B. Heptulose synthesis from nonphosphorylated aldoses and ketoses by spinach transketolase. J Biol Chem. 1971;246:3126–3131. [PubMed] [Google Scholar]
- Von Heijne G, Hirai T, Klösgen RB, Steppuhn J, Bruce B, Keegstra K, Herrmann RG. CHLPEP: a database of chloroplast transit peptides. Plant Mol Biol. 1991;9:104–126. [Google Scholar]
- Wikner C, Meshalkina L, Nilsson U, Nikkola M, Lindqvist Y, Sundström M, Schneider G. Analysis of an invariant cofactor-protein interaction in thiamin diphosphate-dependent enzymes by site-directed mutagenesis-glutamic acid 418 in transketolase is essential for catalysis. J Biol Chem. 1994;269:32144–32150. [PubMed] [Google Scholar]
- Yokota A, Sasajima K. Formation of 1-deoxy-d-threo-pentulose and 1-deoxy-l-threo-pentulose by cell-free extracts of microorganisms. Agric Biol Chem. 1984;149:149–158. [Google Scholar]
- Yokota A, Sasajima KI. Formation of 1-deoxy-ketoses by pyruvate dehydrogenase and acetoin dehydrogenase. Agric Biol Chem. 1986;50:2517–2524. [Google Scholar]
- Zhao G, Winkler ME. An Escherichia coli K-12 tktA tktB mutant deficient in transketolase activity requires pyridoxine (vitamin B6) as well as the aromatic amino acids and vitamins for growth. J Bacteriol. 1994;176:6134–6138. doi: 10.1128/jb.176.19.6134-6138.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]