Abstract
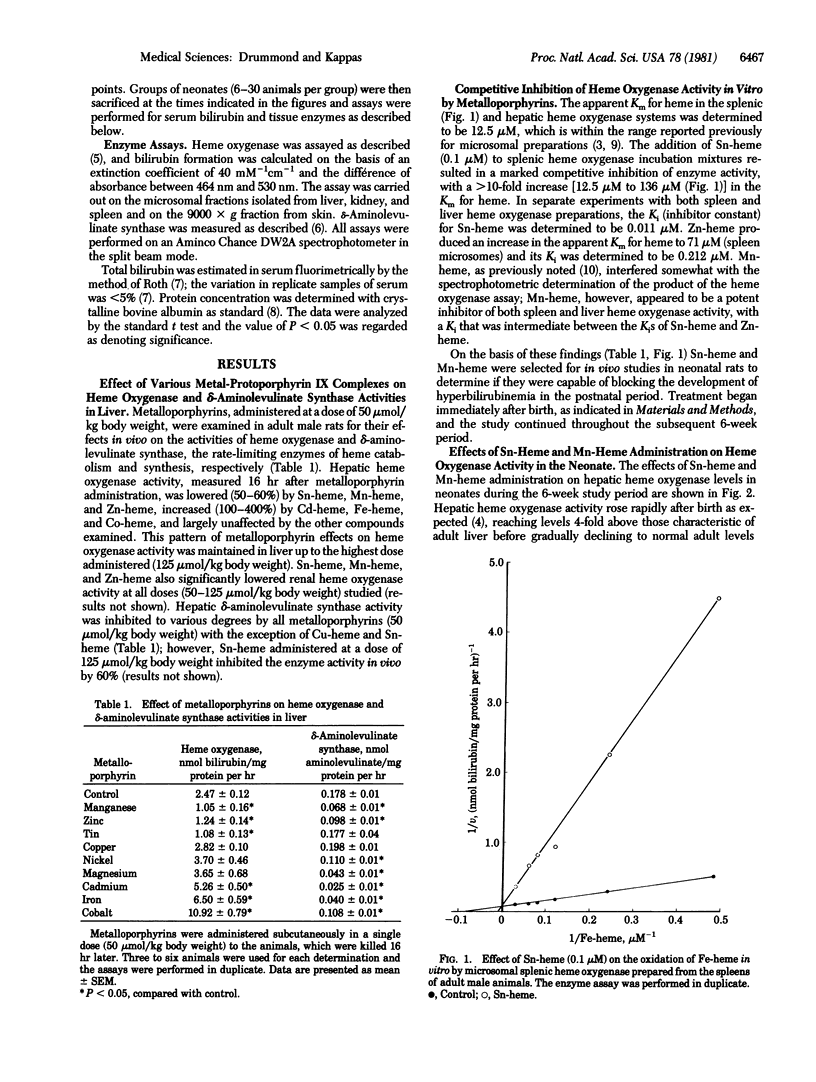
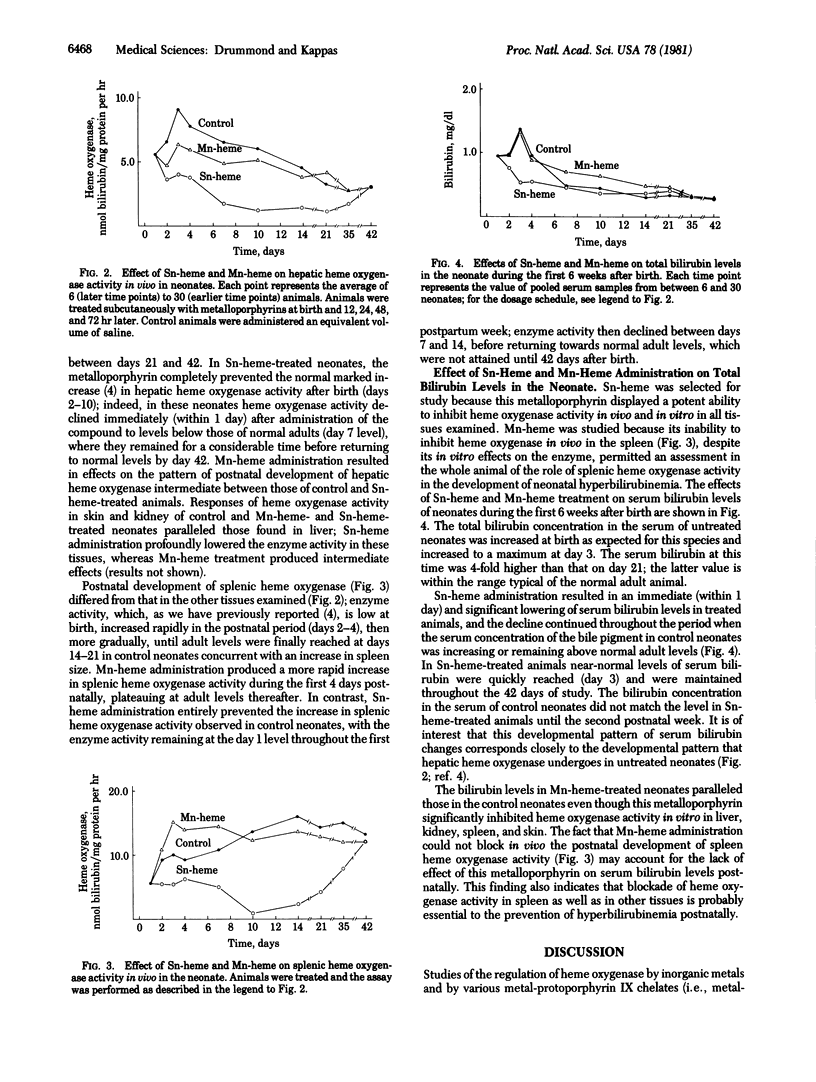
The effects of various metalloporphyrins on hepatic heme oxygenase (EC 1.14.99.3) activity were examined in order to identify compounds that could inhibit heme degradation to bile pigment and might therefore be utilized to suppress the development of hyperbilirubinemia in the newborn. Among nine metal-protoporphyrin IX chelates (i.e., metal-hemes) studied, Sn-heme, Mn-heme, and Zn-heme substantially diminished heme oxygenase activity in vivo in the rat. These metalloporphyrins act as competitive inhibitory substrates in the heme oxygenase reaction but are not themselves oxidatively degraded. Sn-heme was the most potent enzyme inhibitor (Ki = 0.011 microM) in liver, spleen, kidney, and skin. Sn-heme administered to newborn animals within the first 72 hr after birth blocked the postnatal increase in heme oxygenase activity that occurs in various tissues. Its effect on the enzyme levels was prompt and protracted. Sn-heme administration also entirely prevented the development of hyperbilirubinemia that normally occurs postnatally. The effect of the metalloporphyrin in lowering the increased concentrations of serum bilirubin in neonates was prompt (within 1 day) and persisted throughout the 42 days after birth. No deleterious effects of Sn-heme treatment of the newborn were observed. This demonstrates that a synthetic metalloporphyrin that is a potent competitive inhibitor of heme oxidation can, when administered to the newborn, also prevent the hyperbilirubinemia that normally develops postnatally. The potential clinical implications of these findings are evident, and it is suggested that the pharmacological properties of Sn-heme and related synthetic metalloporphyrins merit further study.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALAJOUANINE T., DEROBERT L., THIEFFRY S. Etude clinique d'ensemble de 210 cas d'intoxication par les sels organiques d'étain. Rev Neurol (Paris) 1958 Feb;98(2):85–96. [PubMed] [Google Scholar]
- Bonkowsky H. L., Tschudy D. P., Collins A., Doherty J., Bossenmaier I., Cardinal R., Watson C. J. Repression of the overproduction of porphyrin precursors in acute intermittent porphyria by intravenous infusions of hematin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2725–2729. doi: 10.1073/pnas.68.11.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba M., Kikuchi M. Effect of tin compounds on activity of 5-aminolevulinate dehydratase in blood. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1057–1061. doi: 10.1016/0006-291x(78)90294-2. [DOI] [PubMed] [Google Scholar]
- Conney A. H., Jacobson M. M., Levin W. Effects of drugs on the metabolism of bilirubin and other normal body constituents. Birth Defects Orig Artic Ser. 1976;12(2):275–292. [PubMed] [Google Scholar]
- De Groot A. P., Feron V. J., Til H. P. Short-term toxicity studies on some salts and oxides of tin in rats. Food Cosmet Toxicol. 1973 Feb;11(1):19–30. doi: 10.1016/0015-6264(73)90058-8. [DOI] [PubMed] [Google Scholar]
- Dhar G. J., Bossenmaier I., Cardinal R., Petryka Z. J., Watson C. J. Transitory renal failure following rapid administration of a relatively large amount of hematin in a patient with acute intermittent porphyria in clinical remission. Acta Med Scand. 1978;203(5):437–443. doi: 10.1111/j.0954-6820.1978.tb14903.x. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Manganese and zinc blockade of enzyme induction: studies with microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5331–5335. doi: 10.1073/pnas.76.10.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Metal ion interactions in the control of haem oxygenase induction in liver and kidney. Biochem J. 1980 Nov 15;192(2):637–648. doi: 10.1042/bj1920637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappas A., Maines M. D. Tin: a potent inducer of heme oxygenase in kidney. Science. 1976 Apr 2;192(4234):60–62. doi: 10.1126/science.1257757. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamon J. M., Frykholm B. C., Hess R. A., Tschudy D. P. Hematin therapy for acute porphyria. Medicine (Baltimore) 1979 May;58(3):252–269. doi: 10.1097/00005792-197905000-00005. [DOI] [PubMed] [Google Scholar]
- Lamon J. M., Frykholm B. C., Tschudy D. P. Hematin administration to an adult with lead intoxication. Blood. 1979 May;53(5):1007–1011. [PubMed] [Google Scholar]
- Lamon J. M., Poh-Fitzpatrick M. B., Lamola A. A. Hepatic protoporphyrin production in human protoporphyria. Effects of intravenous hematin and analysis of erythrocyte protoporphyrin distribution. Gastroenterology. 1980 Jul;79(1):115–125. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt stimulation of heme degradation in the liver. Dissociation of microsomal oxidation of heme from cytochrome P-450. J Biol Chem. 1975 Jun 10;250(11):4171–4177. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Enzymatic oxidation of cobalt protoporphyrin IX: observations on the mechanism of heme oxygenase action. Biochemistry. 1977 Feb 8;16(3):419–423. doi: 10.1021/bi00622a012. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Prematurely evoked synthesis and induction of delta-aminolevulinate synthetase in neonatal liver. Evidence for metal ion repression of enzyme formation. J Biol Chem. 1978 Apr 10;253(7):2321–2326. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Studies on the mechanism of induction of haem oxygenase by cobalt and other metal ions. Biochem J. 1976 Jan 15;154(1):125–131. doi: 10.1042/bj1540125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Study of the developmental pattern of heme catabolism in liver and the effects of cobalt on cytochrome P-450 and the rate of heme oxidation during the neonatal period. J Exp Med. 1975 Jun 1;141(6):1400–1410. doi: 10.1084/jem.141.6.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D. Zinc . protoporphyrin is a selective inhibitor of heme oxygenase activity in the neonatal rat. Biochim Biophys Acta. 1981 Mar 18;673(3):339–350. doi: 10.1016/0304-4165(81)90465-7. [DOI] [PubMed] [Google Scholar]
- Maurer H. M., Wolff J. A., Finster M., Poppers P. J., Pantuck E., Kuntzman R., Conney A. H. Reduction in concentration of total serum-bilirubin in offspring of women treated with phenobarbitone during pregnancy. Lancet. 1968 Jul 20;2(7560):122–124. doi: 10.1016/s0140-6736(68)90415-7. [DOI] [PubMed] [Google Scholar]
- McDonagh A. F., Palma L. A., Lightner D. A. Blue light and bilirubin excretion. Science. 1980 Apr 11;208(4440):145–151. doi: 10.1126/science.7361112. [DOI] [PubMed] [Google Scholar]
- Pierach C. A., Bossenmaier I., Cardinal R., Weimer M., Watson C. J. Hematin therapy in porphyric attacks. Klin Wochenschr. 1980 Aug 15;58(16):829–832. doi: 10.1007/BF01491103. [DOI] [PubMed] [Google Scholar]
- Rosenberg D. W., Drummond G. S., Cornish H. C., Kappas A. Prolonged induction of hepatic haem oxygenase and decreases in cytochrome P-450 content by organotin compounds. Biochem J. 1980 Aug 15;190(2):465–468. doi: 10.1042/bj1900465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. Dosage fluorimétrique de la bilirubine. Clin Chim Acta. 1967 Sep;17(3):487–492. doi: 10.1016/0009-8981(67)90225-2. [DOI] [PubMed] [Google Scholar]
- Sassa S., Kappas A., Bernstein S. E., Alvares A. P. Heme biosynthesis and drug metabolism in mice with hereditary hemolytic anemia. Heme oxygenase induction as an adaptive response for maintaining cytochrome P-450 in chronic hemolysis. J Biol Chem. 1979 Feb 10;254(3):729–735. [PubMed] [Google Scholar]
- Schacter B. A., Waterman M. R. Activity of various metalloporphyrin protein complexes with microsomal heme oxygenase. Life Sci. 1974 Jan 1;14(1):47–53. doi: 10.1016/0024-3205(74)90244-6. [DOI] [PubMed] [Google Scholar]
- Schwarz K., Milne D. B., Vinyard E. Growth effects of tin compounds in rats maintained in a trace element-controlled environment. Biochem Biophys Res Commun. 1970 Jul 13;40(1):22–29. doi: 10.1016/0006-291x(70)91040-5. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J Lab Clin Med. 1970 Mar;75(3):410–421. [PubMed] [Google Scholar]
- Watson C. J., Bossenmaier I., Cardinal R., Petryka Z. J. Repression by hematin of porphyrin biosynthesis in erythrocyte precursors in congenital erythropoietic porphyria. Proc Natl Acad Sci U S A. 1974 Feb;71(2):278–282. doi: 10.1073/pnas.71.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe S. J., Levy G., Matsuzawa T., Baliah T. Enhancement of glucuronide-conjugating capacity in a hyperbilirubinemic infant due to apparent enzyme induction by phenobarbital. N Engl J Med. 1966 Dec 29;275(26):1461–1466. doi: 10.1056/NEJM196612292752602. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Kikuchi G. Reaction of the microsomal heme oxygenase with cobaltic protoporphyrin IX, and extremely poor substrate. J Biol Chem. 1978 Dec 10;253(23):8479–8482. [PubMed] [Google Scholar]



