Abstract
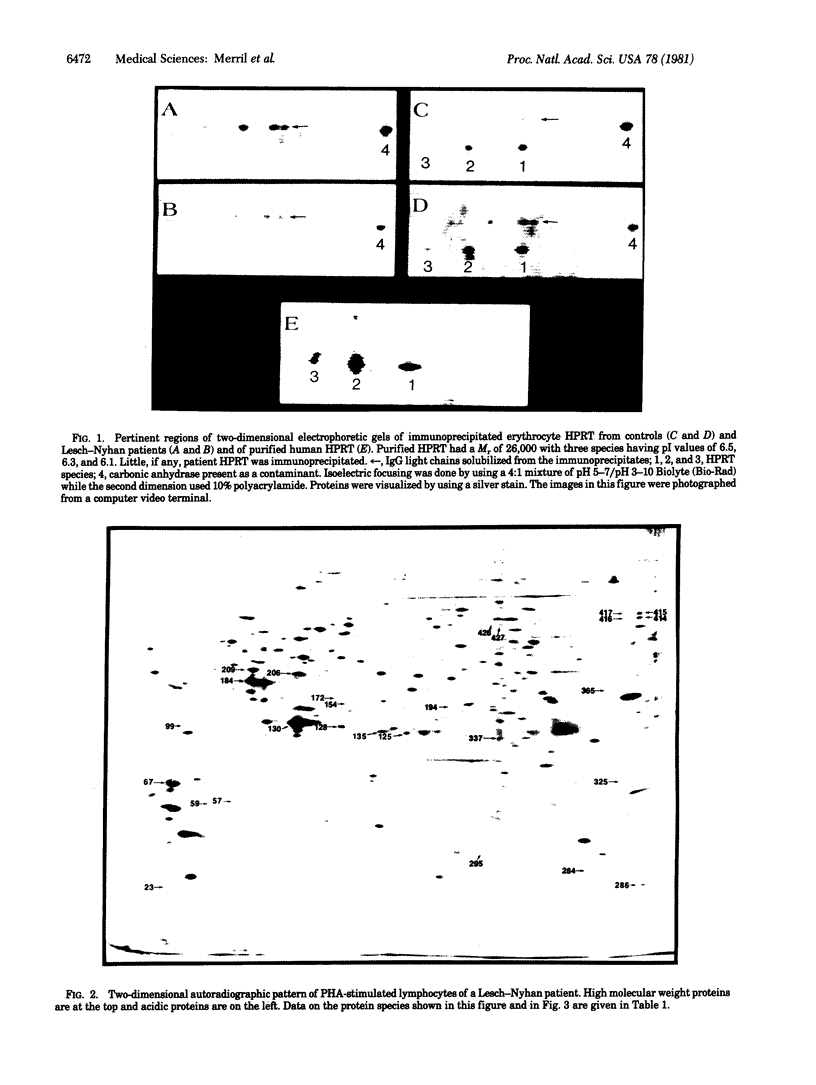
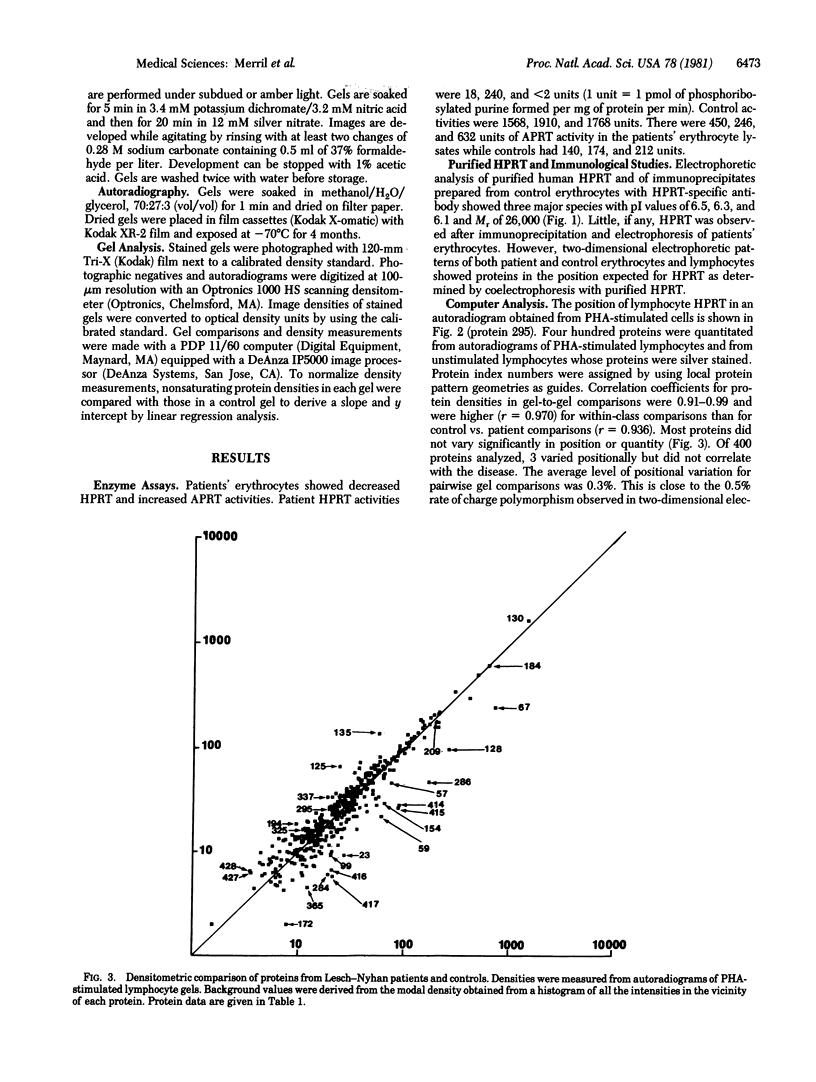
Patients having Lesch--Nyhan syndrome were studied by using enzymatic, immunologic, and two-dimensional electrophoretic techniques. Four hundred proteins were analyzed on each two-dimensional electrophoretogram for positional or quantitative variation. In autoradiograms of lymphocytes stimulated with phytohemagglutinin, there were 11 quantitative differences found in all patients that were significant at the 2P less than 0.01 level. A significant quantitative difference was also found in an analysis of silver-stained gels of unstimulated lymphocytes. Patients had trace amounts of erythrocyte hypoxanthine phosphoribosyl transferase (HPRT) activity and trace or no immunoprecipitable HPRT. However, HPRT was observed in silver-stained erythrocyte electrophoretograms and in autoradiograms from phytohemagglutinin-stimulated lymphocytes. Unstimulated lymphocytes contained 65% of the control HPRT concentration. Currently, the technology of two-dimensional electrophoresis detects a fraction of the total cellular proteins and defective proteins may not show electrophoretic alterations. However, specific secondary changes in other polypeptides may be observed and, when catalogued, will serve as an aid in the diagnosis and understanding of the pathophysiology of metabolic diseases.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. J., Meade J. C., Kelley W. N. Hypoxanthine-guanine phosphoribosyltransferase: characteristics of the mutant enzyme in erythrocytes from patients with the Lesch-Nyhan syndrome. J Clin Invest. 1972 Jul;51(7):1805–1812. doi: 10.1172/JCI106982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakay B., Becker M. A., Nyhan W. L. Reaction of antibody to normal human hypoxanthine phosphoribosyltransferase with products of mutant genes. Arch Biochem Biophys. 1976 Dec;177(2):415–426. doi: 10.1016/0003-9861(76)90454-9. [DOI] [PubMed] [Google Scholar]
- Bakay B., Nyhan W. L. Electrophoretic properties of hypoxanthine-guanine phosphoribosyl transferase in erythrocytes of subjects with Lesch-Nyhan syndrome. Biochem Genet. 1972 Apr;6(2):139–146. doi: 10.1007/BF00486398. [DOI] [PubMed] [Google Scholar]
- Bassermann R., Gutensohn W., Jahn H., Springmann J. S. Pathological and immunological observations in a case of Lesch-Nyhan-syndrome. Eur J Pediatr. 1979 Oct;132(2):93–98. doi: 10.1007/BF00447375. [DOI] [PubMed] [Google Scholar]
- Ghangas G. S., Milman G. Hypoxanthine phosphoribosyltransferase: two-dimensional gels from normal and Lesch-Nyhan hemolyzates. Science. 1977 Jun 3;196(4294):1119–1120. doi: 10.1126/science.870972. [DOI] [PubMed] [Google Scholar]
- Ghangas G. S., Milman G. Radioimmune determination of hypoxanthine phosphoribosyltransferase crossreacting material in erythrocytes of Lesch-Nyhan patients. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4147–4150. doi: 10.1073/pnas.72.10.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M. L., Boyle J. A., Seegmiller J. E. Substrate stabilization: genetically controlled reciprocal relationship of two human enzymes. Science. 1970 Feb 6;167(3919):887–889. doi: 10.1126/science.167.3919.887. [DOI] [PubMed] [Google Scholar]
- Holmes E. W., Pehlke D. M., Kelley W. N. Human IMP dehydrogenase. Kinetics and regulatory properties. Biochim Biophys Acta. 1974 Oct 17;364(2):209–217. doi: 10.1016/0005-2744(74)90006-0. [DOI] [PubMed] [Google Scholar]
- Kelley W. N., Arnold W. J. Human hypoxanthine-guanine phosphoribosyltransferase: studies on the normal and mutant forms of the enzyme. Fed Proc. 1973 Jun;32(6):1656–1659. [PubMed] [Google Scholar]
- Kelley W. N., Greene M. L., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. Hypoxanthine-guanine phosphoribosyltransferase deficiency in gout. Ann Intern Med. 1969 Jan;70(1):155–206. doi: 10.7326/0003-4819-70-1-155. [DOI] [PubMed] [Google Scholar]
- LESCH M., NYHAN W. L. A FAMILIAL DISORDER OF URIC ACID METABOLISM AND CENTRAL NERVOUS SYSTEM FUNCTION. Am J Med. 1964 Apr;36:561–570. doi: 10.1016/0002-9343(64)90104-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McConkey E. H., Taylor B. J., Phan D. Human heterozygosity: a new estimate. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6500–6504. doi: 10.1073/pnas.76.12.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Dunau M. L., Goldman D. A rapid sensitive silver stain for polypeptides in polyacrylamide gels. Anal Biochem. 1981 Jan 1;110(1):201–207. doi: 10.1016/0003-2697(81)90136-6. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Leavitt J., Van Keuren M. L., Ebert M. H., Caine E. D. Hypoxanthine guanine phosphoribosyltransferase (HGPRT) in Gilles de la Tourette syndrome. Neurology. 1979 Jan;29(1):131–134. doi: 10.1212/wnl.29.1.131. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Switzer R. C., Van Keuren M. L. Trace polypeptides in cellular extracts and human body fluids detected by two-dimensional electrophoresis and a highly sensitive silver stain. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4335–4339. doi: 10.1073/pnas.76.9.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Olsen A. S., Milman G. Human hypoxanthine phosphoribosyltransferase. Purification and properties. Biochemistry. 1977 May 31;16(11):2501–2505. doi: 10.1021/bi00630a029. [DOI] [PubMed] [Google Scholar]
- Pehlke D. M., McDonald J. A., Holmes E. W., Kelley W. N. Inosinic acid dehydrogenase activity in the Lesch-Nyhan syndrome. J Clin Invest. 1972 Jun;51(6):1398–1404. doi: 10.1172/JCI106935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom F. M., Henderson J. F., Caldwell I. C., Kelley W. N., Seegmiller J. E. Biochemical bases of accelerated purine biosynthesis de novo in human fibroblasts lacking hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1968 Mar 25;243(6):1166–1173. [PubMed] [Google Scholar]
- Rubin C. S., Dancis J., Yip L. C., Nowinski R. C., Balis M. E. Purification of IMP:pyrophosphate phosphoribosyltransferases, catalytically incompetent enzymes in Lesch-Nyhan disease. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1461–1464. doi: 10.1073/pnas.68.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Dancis J., Yip L. C., Nowinski R. C., Balis M. E. Purification of IMP:pyrophosphate phosphoribosyltransferases, catalytically incompetent enzymes in Lesch-Nyhan disease. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1461–1464. doi: 10.1073/pnas.68.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Sorensen L. B., Benke P. J. Biochemical evidence for a distinct type of primary gout. Nature. 1967 Mar 18;213(5081):1122–1123. doi: 10.1038/2131122b0. [DOI] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Upchurch K. S., Leyva A., Arnold W. J., Holmes E. W., Kelley W. N. Hypoxanthine phosphoribosyltransferase deficiency: association of reduced catalytic activity with reduced levels of immunologically detectable enzyme protein. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4142–4146. doi: 10.1073/pnas.72.10.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip L. C., Dancis J., Balis M. E. Immunochemical studies of AMP:pyrophosphate phosphoribosyltransferase from normal and Lesch-Nyhan subjects. Biochim Biophys Acta. 1973 Feb 15;293(2):359–369. doi: 10.1016/0005-2744(73)90344-6. [DOI] [PubMed] [Google Scholar]
- Zannis V. I., Gudas L. J., Martin D. W., Jr Characterization of the subunit composition of HGPRTase from human erythrocytes and cultured fibroblasts. Biochem Genet. 1980 Feb;18(1-2):1–19. doi: 10.1007/BF00504356. [DOI] [PubMed] [Google Scholar]