Abstract
Time-lapse imaging of dark-grown Arabidopsis (Arabidopsis thaliana) hypocotyls has revealed new aspects about ethylene signaling. This study expands upon these results by examining ethylene growth response kinetics of seedlings of several plant species. Although the response kinetics varied between the eudicots studied, all had prolonged growth inhibition for as long as ethylene was present. In contrast, with continued application of ethylene, white millet (Panicum miliaceum) seedlings had a rapid and transient growth inhibition response, rice (Oryza sativa ‘Nipponbare’) seedlings had a slow onset of growth stimulation, and barley (Hordeum vulgare) had a transient growth inhibition response followed, after a delay, by a prolonged inhibition response. Growth stimulation in rice correlated with a decrease in the levels of rice ETHYLENE INSENSTIVE3-LIKE2 (OsEIL2) and an increase in rice F-BOX DOMAIN AND LRR CONTAINING PROTEIN7 transcripts. The gibberellin (GA) biosynthesis inhibitor paclobutrazol caused millet seedlings to have a prolonged growth inhibition response when ethylene was applied. A transient ethylene growth inhibition response has previously been reported for Arabidopsis ethylene insensitive3-1 (ein3-1) eil1-1 double mutants. Paclobutrazol caused these mutants to have a prolonged response to ethylene, whereas constitutive GA signaling in this background eliminated ethylene responses. Sensitivity to paclobutrazol inversely correlated with the levels of EIN3 in Arabidopsis. Wild-type Arabidopsis seedlings treated with paclobutrazol and mutants deficient in GA levels or signaling had a delayed growth recovery after ethylene removal. It is interesting to note that ethylene caused alterations in gene expression that are predicted to increase GA levels in the ein3-1 eil1-1 seedlings. These results indicate that ethylene affects GA levels leading to modulation of ethylene growth inhibition kinetics.
Ethylene is a simple, unsaturated hydrocarbon that affects diverse processes throughout the lifetime of a plant, including seed germination, growth, formation of the apical hook, organ senescence, fruit ripening, abscission, gravitropism, and responses to various stresses (Mattoo and Suttle, 1991; Abeles et al., 1992). Of these many processes much attention has focused on the effects of ethylene on dark-grown seedlings. The Russian scientist Dimitry Neljubow discovered that ethylene is biologically active when he showed that ethylene was the active compound in illuminating gas that altered growth of etiolated pea (Pisum sativum) seedlings (Neljubow, 1901). The growth inhibition response from prolonged exposure to ethylene is a sensitive and easily quantified bioassay that has been used to screen for mutants to acquire information and generate models about the ethylene signaling pathway (Bleecker et al., 1988; Guzmán and Ecker, 1990).
The model plant Arabidopsis (Arabidopsis thaliana) contains five receptor isoforms localized to the membranes of the endoplasmic reticulum and Golgi apparatus that mediate responses to ethylene (Chang et al., 1993; Hua and Meyerowitz, 1998; Hua et al., 1998; Sakai et al., 1998; Chen et al., 2002, 2010; Dong et al., 2008; Grefen et al., 2008). The ethylene receptors form a complex with CONSTITUTIVE TRIPLE RESPONSE1 (CTR1; Clark et al., 1998; Cancel and Larsen, 2002; Gao et al., 2003; Huang et al., 2003; Mayerhofer et al., 2012). CTR1 negatively regulates the ethylene response pathway by either directly or indirectly inhibiting activity of ETHYLENE INSENSITIVE2 (EIN2; Kieber et al., 1993; Alonso et al., 1999; Huang et al., 2003). The EIN3, EIN3-LIKE1 (EIL1), and perhaps EIL2 transcription factors act downstream of EIN2 (Chao et al., 1997; Solano et al., 1998; Alonso et al., 2003b; Binder et al., 2004a, 2007). These, in turn, regulate other transcription factors such as ETHYLENE RESPONSE FACTOR1 (ERF1) and ETHYLENE RESPONSE DNA BINDING FACTOR1 to EDF4 (Chao et al., 1997; Solano et al., 1998; Alonso et al., 2003a; Stepanova et al., 2007). EIN3 and EIL1 are required for prolonged responses to ethylene but transient growth responses still occur in double ein3 eil1 loss-of-function mutants (Alonso et al., 2003b; Binder et al., 2004a). At least part of the regulation of EIN3 and EIL1 involves selective proteolysis via the ubiquitin/26S proteasome pathway using S-PHASE KINASE-ASSOCIATED1-CULLIN-F-BOX (SCF) E3 ubiquitin ligase complexes containing EIN3-BINDING F-BOX1 and EBF2 F-box proteins (Guo and Ecker, 2003; Potuschak et al., 2003; Yanagisawa et al., 2003; Gagne et al., 2004; Binder et al., 2007; An et al., 2010). This model posits that ethylene binding to the receptors reduces the activity of the receptors, leading to reduced activity of CTR1 and an increase in EIN2 protein levels along with subsequent signaling associated with it (Qiao et al., 2009). EIN2 acts in part to directly or indirectly reduce ubiquitination of EIN3 and EIL1 by SCFEBF1/2 leading to an increase in EIN3 and EIL1 levels and most ethylene responses. Similar genes have been described in other plant species such as rice (Oryza sativa), tomato (Solanum lycopersicum), and strawberry (Fragaria vesca; Rzewuski and Sauter, 2008; Ma et al., 2010; Klee and Giovannoni, 2011; Shulaev et al., 2011) suggesting similar signaling pathways are present across land plant species. Events downstream of transcriptional changes are more complicated and less understood. However, in cases where ethylene stimulates growth, ethylene treatment is modeled to cause an increase in GA levels and sensitivity leading to an increase in growth (Rijnders et al., 1997; Kende et al., 1998; Voesenek et al., 2003; Cox et al., 2004; Pierik et al., 2004, 2006). The role of GA in the ethylene growth inhibition response in dark-grown seedlings is less clear.
Although end-point analysis of seedling growth continues to be an informative bioassay, it has the limitation of only examining the long-term, end-point effects of ethylene on seedlings, so that transient events are overlooked. Time-lapse imaging has led to a more refined understanding about the ethylene receptors, feedback control mechanisms, signaling cross talk, and the roles that various proteins have in ethylene signaling in Arabidopsis (Binder et al., 2004a, 2004b, 2006, 2007; Potuschak et al., 2006; Gao et al., 2008; van Zanten et al., 2010; Vandenbussche et al., 2010; Žádníková et al., 2010; Kim et al., 2011; McDaniel and Binder, 2012). Even though many new details about ethylene signaling and responses have come out of studies using this approach, it is important to determine whether this information is specific to Arabidopsis or applicable to other plant species. With this in mind, we undertook a comparative study examining the growth response kinetics to the application and removal of ethylene in the shoots of several eudicot and monocot plant species.
Coupled with analyses of Arabidopsis mutants, these experiments revealed EIN3/EIL1-dependent and -independent regulation of GA levels, leading to modulation of both ethylene-induced growth inhibition kinetics and growth recovery kinetics after removal of ethylene.
RESULTS
Ethylene Growth Responses in Eudicots
In this study, Arabidopsis Columbia (Col) seedling hypocotyls grew in air with a rate of 0.32 ± 0.01 mm h−1 that diminished to a steady-state rate of 0.04 ± 0.01 mm h−1 upon application of 10 μL L−1 ethylene (Supplemental Fig. S1). This is consistent with our prior results (Binder et al., 2004a, 2004b). We examined the rate of growth in air and ethylene for a variety of other eudicot plant species including kale (Brassica oleracea ‘Red Russian’), canola (Brassica napus), two cultivars of tomato (Solanum lycopersicum ‘Floradade’ and ‘German Queen’), poppy (Papaver orientale ‘Oriental Scarlet’), and beetberry (Chenopodium capitatum). The growth rates in air for these species ranged from 0.15 ± 0.01 to 0.76 ± 0.06 mm h−1. The growth rate of each of these eudicots was inhibited by the application of ethylene and ranged from 0.04 ± 0.01 to 0.20 ± 0.01 mm h−1 (Supplemental Fig. S1).
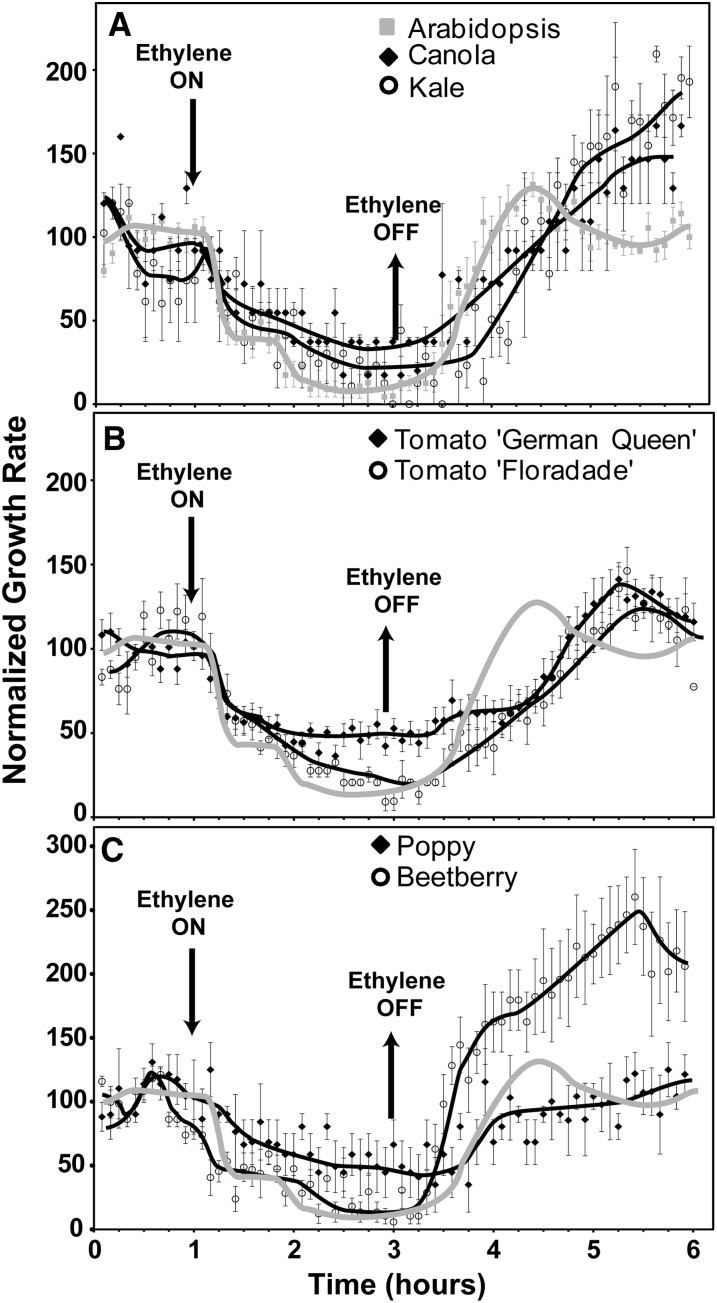
Our earlier studies on Arabidopsis seedlings uncovered two phases of hypocotyl growth inhibition when 10 μL L−1 ethylene was applied (Binder et al., 2004a, 2004b). The first phase of inhibition starts about 10 min after ethylene is added and lasts approximately 15 min when it reaches a new steady state. This new steady state lasts approximately 20 min when the second phase of growth inhibition occurs that further inhibits growth. A new steady-state growth rate is reached after approximately 20 min. This second, prolonged phase of growth inhibition requires the EIN3 and EIL1 transcription factors and lasts for as long as saturating concentrations of ethylene are present (Binder et al., 2004a, 2006, 2007). Similar onset kinetics are seen in both the Col and Wassilewskija ecotypes (Binder et al., 2004b). When ethylene is removed after a 2-h treatment, hypocotyl growth recovers to pretreatment rates in approximately 85 min in Col and 120 min in Wassilewskija seedlings. A dampened oscillation in growth rate often occurs during this recovery phase that involves a growth rate overshoot where the growth rate increases over the rate initially observed prior to ethylene treatment (Binder et al., 2004b, 2007). The growth response kinetics of Col hypocotyls to a 2-h application of 10 μL L−1 ethylene (Fig. 1) were indistinguishable from our previous results (Binder et al., 2004a, 2006, 2007).
Figure 1.
Ethylene growth response kinetics in dark-grown shoots of various eudicots. Seedlings were grown in air 1 h before 10 µL L−1 ethylene was applied (downward arrow). Ethylene was removed 2 h later (upward arrow), and seedlings allowed to grow another 3 h. The growth rate was normalized to the growth rate in the 1-h air pretreatment. A, Arabidopsis, canola, and kale. B, Two cultivars of tomato. C, Poppy and beetberry. In B and C, responses of Arabidopsis seedlings are shown in gray for comparison. Data represent the average ± sem of at least five seedlings.
We examined kale and canola as two other members of the Brassicaceae family. The onset of the second phase of growth inhibition was less distinct in these species than Arabidopsis (Fig. 1A). Additionally, after the removal of ethylene, kale and canola seedlings had a growth rate overshoot that continued to increase rather than the dampened oscillation in growth rate observed with Arabidopsis. By 5 h after the removal of ethylene, the growth rates of both species reached a plateau but had not yet started to return to pretreatment growth rates (data not shown). It is possible that this is part of a dampened oscillation in growth rate that had a longer period beyond the time frame of these experiments. The shoots of both cultivars of tomato showed a measurable decrease in growth rate approximately 10 min after application of ethylene (Fig. 1B). cv German Queen reached steady-state growth inhibition within approximately 60 min whereas cv Floradade reached maximum growth inhibition approximately 90 min after ethylene was added. Both took approximately 1 h longer than Col to recover to pretreatment growth rates after the removal of ethylene. To gain a better understanding about the variability of ethylene growth response kinetics in eudicots, we examined ethylene growth responses in poppy and beetberry to represent two other plant families (Fig. 1C). Poppy seedling shoots showed growth inhibition kinetics very similar to cv German Queen seedlings (Fig. 1, B and C). However, after the removal of ethylene, poppy had growth recovery kinetics that were more similar to Col seedlings with one difference being the lack of a growth rate overshoot. In contrast, beetberry seedling shoots showed ethylene growth inhibition kinetics very similar to Col seedlings. Of the eudicots observed, beetberry seedlings had the shortest delay in the onset of growth recovery and the fastest rate of growth recovery after ethylene removal (Fig. 1C; Supplemental Table S1). These seedlings also had a growth rate overshoot that rose to approximately 2.5-fold over the growth rate initially observed in air before treatment with ethylene (Fig. 1C).
These results show that eudicots vary in their ethylene growth response and recovery kinetics even within the same species. The underlying mechanisms for these differences are unknown. From the above data, five growth parameters were measured including growth rate in air, steady-state growth rate in ethylene, time to maximum growth inhibition after addition of ethylene, delay in onset of recovery after ethylene removal, and recovery time after ethylene removal (Supplemental Table S1). To determine if any of these parameters might be mechanistically linked, we compared them pairwise and calculated the correlation coefficient (r2). Most have an r2 value well below 0.5 indicating a weaker correlation between the traits (Supplemental Table S2). The steady-state growth rate in air correlated with the steady-state growth rate in ethylene with an r2 = 0.51 whereas the delay in the onset of growth recovery correlated with the recovery time with an r2 = 0.49. These low correlations suggest that these traits are not tightly linked mechanistically.
Ethylene Growth Responses in Monocots
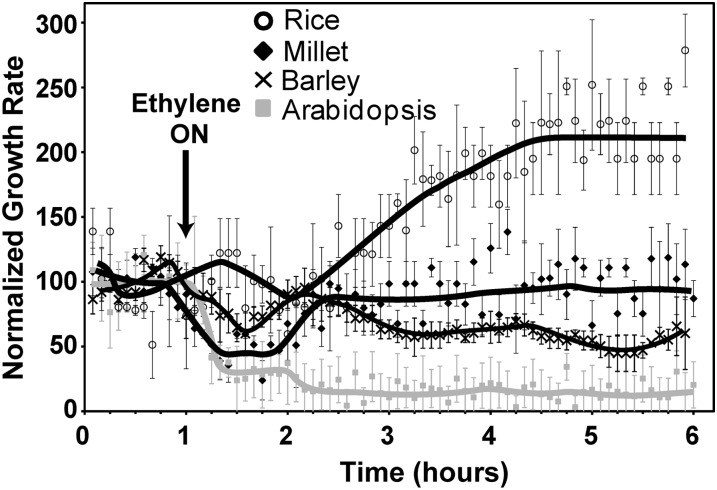
To gain more insights into the action of ethylene, we examined the ethylene rapid growth response kinetics of the coleoptiles of seedlings from rice ‘Nipponbare’, white millet (Panicum miliaceum), and barley (Hordeum vulgare). In air, under these conditions, rice coleoptiles grew at 0.16 ± 0.02 mm h−1, millet coleoptiles at 0.44 ± 0.05 mm h−1, and barley coleoptiles at 0.71 ± 0.03 mm h−1. Application of 10 μL L−1 ethylene inhibited coleoptile growth in millet, although the growth rate began to recover prior to ethylene removal 2 h later (data not shown). This prompted us to examine the growth response kinetics of millet with prolonged exposure to ethylene. For comparison, we also measured the growth inhibition kinetics of Arabidopsis seedlings with prolonged ethylene treatment. Consistent with our prior research (Binder et al., 2004a, 2006), Col seedling hypocotyls had prolonged growth inhibition in the continued presence of 10 μL L−1 ethylene (Fig. 2). Ethylene treatment of millet seedlings resulted in a transient growth inhibition response that corresponded in timing to the first phase of growth inhibition observed in Arabidopsis hypocotyls (Fig. 2). Millet showed maximum growth inhibition approximately 20 min after ethylene was added. This growth rate stabilized for approximately 30 min at 0.19 ± 0.04 mm h−1 after which the growth rate increased, returning to pretreatment growth rates approximately 90 min after ethylene was added.
Figure 2.
Ethylene growth response kinetics in dark-grown shoots of several monocots. Seedlings were grown in air 1 h before 10 µL L−1 ethylene was applied (arrow). The growth rate was normalized to the growth rate in the 1-h air pretreatment. The growth response kinetics of Arabidopsis are shown for comparison. Data represent the average ± sem of at least five seedlings.
We obtained very different growth response kinetics with rice seedlings. Application of ethylene caused no measurable growth inhibition of rice coleoptiles (Fig. 2). Approximately 70 min after the addition of ethylene, the growth rate started to increase, reaching a maximum about 3.5 h after ethylene was applied. The maximum growth rate was approximately double the air pretreatment growth rate. This growth stimulation response to ethylene is consistent with prior studies on rice (Ku et al., 1970; Suge, 1972, 1985; Suge et al., 1971; Raskin and Kende, 1984; Rose-John and Kende, 1985; Satler and Kende, 1985; Keith et al., 1986; Hoffmann-Benning and Kende, 1992; Watanabe et al., 2007). It is interesting to note that whereas adult cv Nipponbare rice is considered submergence intolerant, at the seedling stage this cultivar is flooding tolerant and has increased coleoptile growth upon flooding (Lee et al., 2009).
In contrast to millet and rice, application of ethylene to barley resulted in a small, transient growth inhibition response that was followed, after a delay, with a prolonged growth inhibition response (Fig. 2). These results show that the ethylene growth response kinetics in these monocots are more variable and distinct from what are seen in the eudicots studied.
Ethylene Alters Transcript Levels of OsEIL2 and OsFBL7 in Rice Coleoptiles
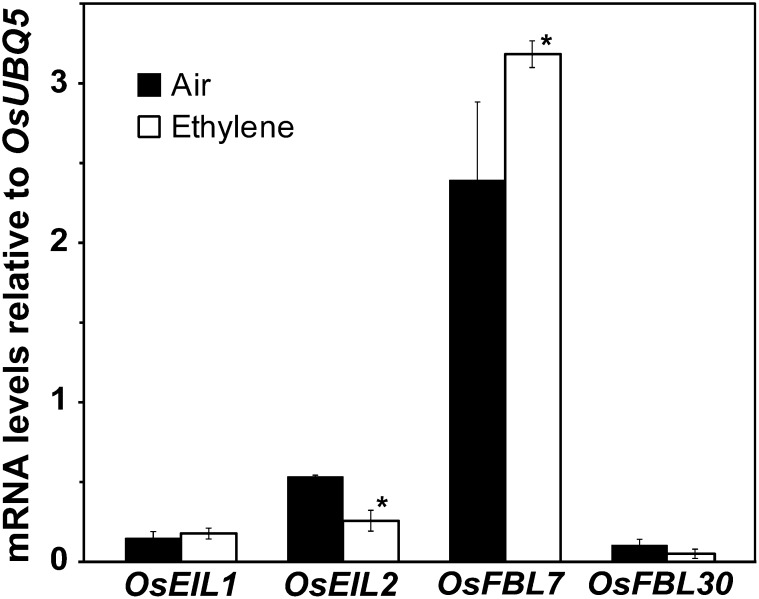
The EIN3 and EIL1 transcription factors are required for long-term growth inhibition responses to ethylene in Arabidopsis (Chao et al., 1997; Solano et al., 1998; Alonso et al., 2003b; Binder et al., 2004a, 2007). Because of this, we were interested to examine the levels of the homologous genes, OsEIL1 and OsEIL2, from rice. These genes have been linked to ethylene responses in rice (Mao et al., 2006; Hiraga et al., 2009). In Arabidopsis, growth inhibition caused by application of ethylene correlates with increases in EIN3 and EIL1 levels (Gao et al., 2003; Yanagisawa et al., 2003; Gagne et al., 2004). In rice, coleoptile growth is stimulated by ethylene. We therefore predicted that the transcript levels for OsEIL1 or OsEIL2 or both would decrease in coleoptiles treated with ethylene. The levels of these transcripts relative to OsUBIQUITIN5 (OsUBQ5) were analyzed using quantitative real-time reverse transcriptase (qRT)-PCR. This revealed that in coleoptiles of dark-grown rice seedlings maintained in air, the transcript levels of OsEIL2 were approximately 2.5-fold higher than transcript levels of OsEIL1 (Fig. 3). Upon treatment with 10 μL L−1 ethylene for 3 h, the OsEIL2 transcript levels decreased 50% but no significant change (P = 0.4) was observed in the transcript levels of OsEIL1 (Fig. 4). Thus, ethylene treatment may lead to lower levels of OsEIL2 because of decreased transcript abundance.
Figure 3.
Transcript levels for select ethylene signaling genes in rice coleoptiles. The levels of transcript for OsEIL1, OsEIL2, OsFBL7, and OsFBL30 were normalized relative to OsUBQ5. Seedlings were grown in the dark in air for 3 d, RNA was extracted from coleoptiles, and transcript levels were analyzed with qRT-PCR. Transcript levels in untreated seedlings were compared with seedlings treated with 10 µL L−1 ethylene for 3 h before RNA extraction. Data show the average ± sem for three biological replicates with two to three technical replicates each. *Statistical difference compared with untreated seedlings (P ≤ 0.05).
Figure 4.
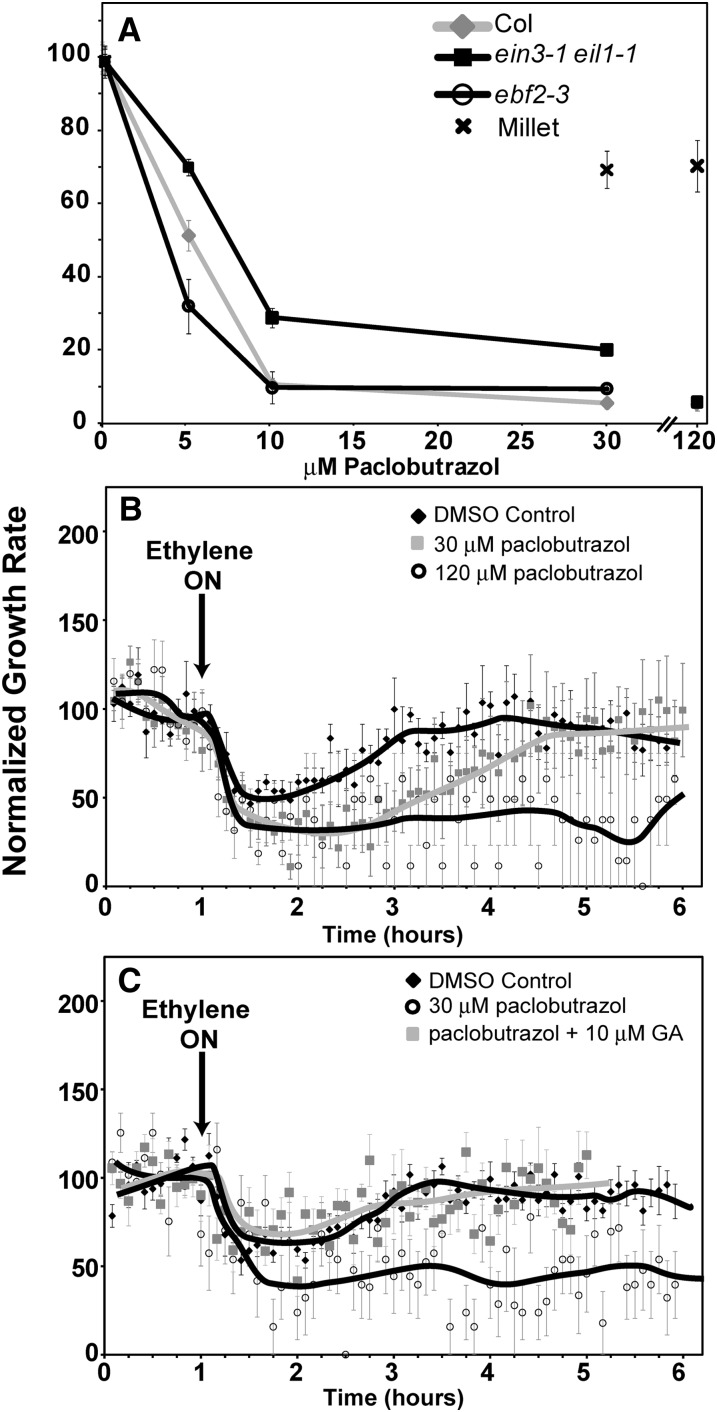
Effects of paclobutrazol on growth in air and ethylene growth response kinetics. Seedlings of millet, Col, ein3-1 eil1-1 double mutants, and ebf2-3 single mutants were treated with paclobutrazol. A, Paclobutrazol dose dependency on growth in air. The responses of millet shoots at several doses of paclobutrazol are shown for comparison. Data represent the average ± sem of at least four seedlings. B, Effects of paclobutrazol on ethylene growth response kinetics of millet. C, Effects of paclobutrazol and paclobutrazol plus GA on ein3-1 eil1-1 mutants. In B and C, seedlings were grown 1 h in air prior to application of 10 µL L−1 ethylene (arrow). Data represent the average ± sem of at least five seedlings.
In Arabidopsis, EIN3 and EIL1 levels are controlled by SCF E3 complexes containing the EBF1 and EBF2 F-box proteins (Guo and Ecker, 2003; Potuschak et al., 2003; Yanagisawa et al., 2003; Gagne et al., 2004; Binder et al., 2007; An et al., 2010). The rice genome contains two related genes, rice F-BOX DOMAIN AND LRR CONTAINING PROTEIN7 (OsFBL7) and OsFBL30 (Wang et al., 2009). We therefore examined the transcript levels of these two genes in coleoptiles of dark-grown rice seedlings maintained in air versus 10 μL L−1 ethylene for 3 h. We found that in air, OsFBL7 transcript levels were approximately 24-fold higher than OsFBL30 (Fig. 3). Application of ethylene caused OsFBL7 transcript levels to increase but no significant alterations (P = 0.13) in OsFBL30 transcript levels occurred. These observations suggest that application of ethylene results in lower levels of OsEIL1 and OsEIL2 due to higher levels of the OsFBL7 F-box protein, resulting in higher rates of ubiquitination and proteolysis.
Paclobutrazol Prolongs the Ethylene Growth Inhibition Response of Millet Seedlings
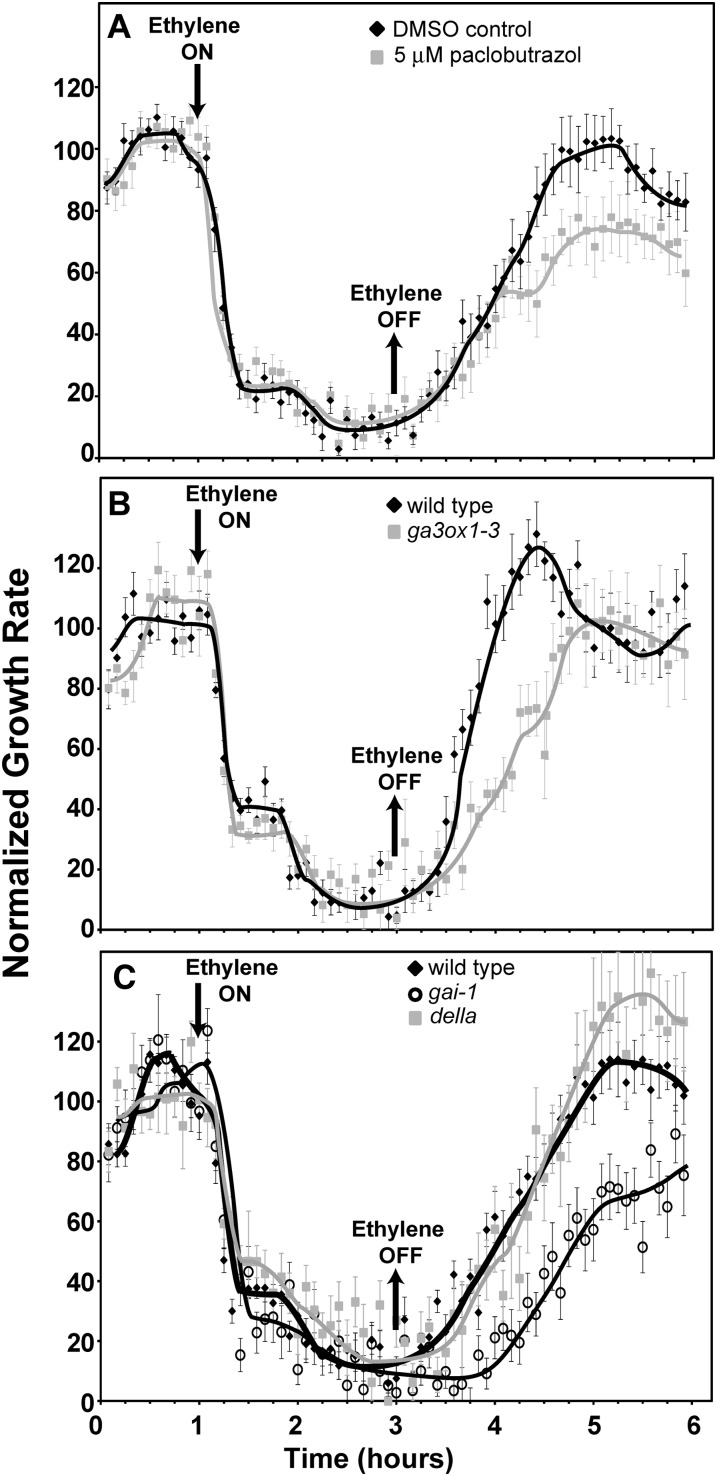
A model has developed that posits that when rice is submerged, ethylene levels increase that in turn lead to an increase in GA levels, causing increased growth of aerial tissues (Kende et al., 1998; Bailey-Serres and Voesenek, 2008). Similarly, GA is modeled to be involved in ethylene stimulated growth of Arabidopsis hypocotyls that occurs under specific growth conditions (Vandenbussche et al., 2007). This raises the possibility that the transient growth response in millet seedlings is caused by an increase in GA levels in ethylene-treated seedlings that counteracts the growth inhibition initially caused by application of ethylene. Inhibition of GA biosynthesis has previously been shown to reduce flood-induced and ethylene-stimulated growth of rice (Raskin and Kende, 1984; Setter and Laureles, 1996; Das et al., 2005; Hattori et al., 2009). Therefore, to test this hypothesis, we germinated millet seedlings on paclobutrazol-containing medium to block GA biosynthesis. Both 30 and 120 μm paclobutrazol reduced the growth rate of millet coleoptiles in air approximately 30% (Fig. 4A) from 0.39 ± 0.02 mm h−1 to 0.27 ± 0.01 mm h−1. Dimethyl sulfoxide (DMSO), solvent-control seedlings had a transient growth inhibition response (Fig. 4B) similar to what we observed above in the absence of DMSO (Fig. 2). Millet seedlings treated with 30 μm paclobutrazol still had a transient growth inhibition response, but took longer than control seedlings to recover to pretreatment growth rates whereas 120 μm paclobutrazol blocked the growth rate reversal so that the growth rate remained inhibited while ethylene was applied (Fig. 4B). Thus, GA appears to be important for the reversal in growth inhibition when millet seedlings are treated with ethylene.
Paclobutrazol Prolongs and Constitutive GA Signaling Attenuates the Ethylene Growth Inhibition Response of ein3 eil1 Seedlings
Unlike wild-type Col seedlings, ein3-1 eil1-1 double mutant seedlings only have a transient growth inhibition response to the application of ethylene (Binder et al., 2004a, 2007) resembling the growth kinetics observed with millet (this study). Therefore, we treated ein3-1 eil1-1 double mutant seedlings with paclobutrazol to determine whether GA biosynthesis is involved in the growth response kinetics of this mutant. The ein3-1 eil1-1 double mutants were more responsive to paclobutrazol than millet (Fig. 4A). Also, consistent with prior studies on Arabidopsis (Jacobsen and Olszewski, 1993; Peng et al., 1999; Wen and Chang, 2002), ein3-1 eil1-1 seeds failed to germinate well on paclobutrazol (data not shown). Therefore, ein3-1 eil1-1 seeds were germinated on media without paclobutrazol and then gently transferred onto plates containing paclobutrazol or DMSO and allowed to grow in air for 3 h prior to time-lapse imaging. Solvent control ein3-1 eil1-1 double mutants had a transient growth inhibition response with complete growth recovery taking approximately 2 h after the addition of ethylene (Fig. 4C) that was similar to our prior results with this double mutant (Binder et al., 2004a, 2007). Application of 30 μm paclobutrazol reduced the growth rate of these seedlings from 0.50 ± 0.01 mm h−1 to 0.10 ± 0.004 mm h−1 and completely blocked the growth reversal of these double mutants, so that ethylene caused prolonged growth inhibition (Fig. 4C). To determine whether the effect of paclobutrazol on the ein3-1 eil1-1 mutants was caused by GA, we treated the double mutants with both 30 μm paclobutrazol and 10 μm GA. The presence of GA slightly increased the growth rate in air to 0.13 ± 0.005 mm h−1 and restored the transient response to ethylene that was eliminated by paclobutrazol (Fig. 4C).
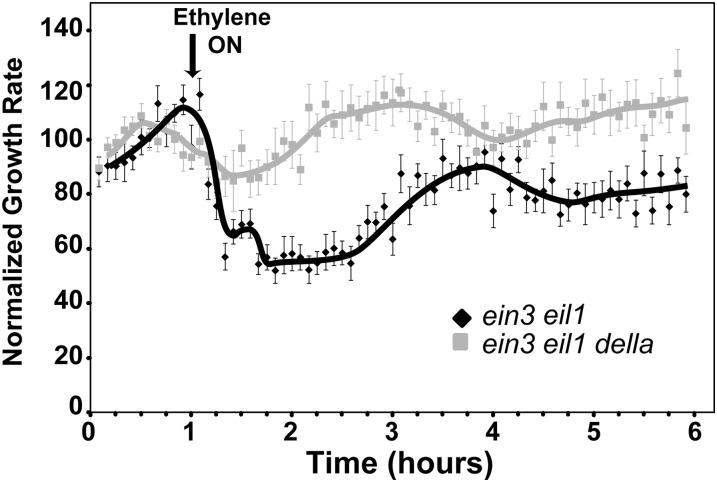
To confirm that GA is involved in reversing the first phase of growth inhibition, we compared the ethylene growth inhibition kinetics of ein3-1 eil1-1mutants with ein3-1 eil1-1 della mutants where all five DELLA genes have also been mutated (Feng et al., 2008; An et al., 2012). The quintuple della mutant seedlings have a constitutive GA response phenotype and hypocotyl growth in dark-grown della seedlings is insensitive to application of either GA or paclobutrazol (Feng et al., 2008). In air, the growth rate of the double mutants was indistinguishable from the septuple mutants (data not shown). The ein3-1 eil1-1 della mutants had a very attenuated or no growth inhibition response when ethylene was added compared with ein3-1 eil1-1 mutants (Fig. 5).
Figure 5.
Constitutive GA signaling attenuates the ethylene growth inhibition response of ein3 eil1 seedlings. Seedlings were grown 1 h in air prior to application of 10 µL L−1 ethylene (arrow). The growth response of ein3-1 eil1-1 versus ein3-1 eil1-1 della mutants are compared. Data represent the average ± sem of at least 11 seedlings.
Together, these data suggest that GA is involved in regulating the long-term growth inhibition response to ethylene. One possibility is that GA and ethylene are regulating long-term growth via parallel pathways. Alternatively, EIN3 and EIL1 could be negatively regulating GA levels, so that when ethylene levels are high, EIN3 and EIL1 levels increase and GA biosynthesis is reduced or catabolism is increased leading to reduced GA levels and long-term growth inhibition. The second hypothesis predicts that wild-type Arabidopsis seedlings should be more sensitive to paclobutrazol than the ein3-1 eil1-1 mutants because EIN3 and EIL1 in wild-type seedlings would also be negatively regulating GA levels. Consistent with the second hypothesis, Col seedlings grew slower than ein3-1 eil1-1 mutants in air (0.32 ± 0.01 versus 0.50 ± 0.01 mm h−1 respectively) and were also slightly more sensitive to paclobutrazol than the double mutants (Fig. 4A). We also examined the effects of paclobutrazol on an EIN3 overexpression line (EIN3-GFP) that has previously been shown to have a constitutive ethylene response phenotype (Guo and Ecker, 2003). Whereas paclobutrazol inhibited growth of these seedlings, we could not obtain clear dose-response measurements because these seedlings grew very slowly (0.09 ± 0.01 mm h−1) in the absence of paclobutrazol (data not shown). Therefore, we examined ebf2-3 mutants that have previously been shown to accumulate EIN3 protein to higher levels than wild-type seedlings (Gagne et al., 2004). Whereas ebf2-3 mutants grew at a similar rate in air as Col seedlings, the ebf2-3 seedlings were more sensitive to paclobutrazol than Col seedlings (Fig. 4A) providing more support for the idea that EIN3 and EIL1 negatively regulate GA levels.
Altered GA Levels or Signaling Affect Ethylene Growth Response Kinetics in Arabidopsis
These results prompted us to investigate whether GA is involved in regulating the ethylene growth kinetic responses of wild-type Arabidopsis seedlings. Addition of 1 μm GA had no obvious effect on the growth rate in air or ethylene growth response kinetics (data not shown). This is consistent with prior results showing that addition of GA had little effect on hypocotyl elongation in the dark suggesting that GA levels are saturated (Cowling and Harberd, 1999; Feng et al., 2008). Treating Col seedlings with 5 μM paclobutrazol had no measurable effect on the inhibition kinetics upon addition of 10 μL L−1 ethylene (Fig. 6A), although the growth rate in air was reduced from 0.29 ± 0.01 mm h−1 to 0.15 ± 0.01 mm h−1 when paclobutrazol was added. In contrast, the paclobutrazol-treated seedlings had altered growth recovery kinetics after the removal of ethylene. It is most notable that the paclobutrazol-treated seedlings failed to completely recover to pretreatment growth rates in the time scale of these measurements. These results indicate that GA levels may also modulate normal growth recovery after the removal of ethylene. To further address the role of GA in modulating these responses when EIN3 and EIL1 are present, we examined the ethylene growth response kinetics of ga3-oxidase1-3 (ga3ox1-3) that is mutated in the GA3ox1 gene and produces less GA (Mitchum et al., 2006). In air, the ga3ox1-3 mutants grew at 0.28 mm ± 0.01 mm h−1, which is slightly slower than the Col wild-type controls (P < 0.05). The ga3ox1-3 seedlings had similar growth inhibition kinetics compared with the wild type, but appeared to reach a slower growth rate during the plateau in growth prior to the onset of the second phase of growth inhibition response (Fig. 6B). As expected, based upon the results obtained with paclobutrazol-treated seedlings, these mutants also recovered more slowly after the removal of ethylene. We examined another mutant of GA3ox1 (ga4-1) that produces less GA (Talon et al., 1990; Chiang et al., 1995; Cowling et al., 1998) and obtained similar results (data not shown). Because reduced GA levels altered the ethylene growth response, we also examined the ethylene growth response kinetics of the ga insensitive-1 (gai-1) mutant that has reduced GA responses (Koorneef et al., 1985; Peng and Harberd, 1993, 1997; Wilson and Somerville, 1995; Peng et al., 1997). In air, hypocotyls of gai-1 seedlings grew at approximately 50% the rate of Landsberg erecta (Ler) control seedlings (0.14 ± 0.01 versus 0.27 ± 0.01 mm h−1 respectively) consistent with prior studies (Cowling and Harberd, 1999). Hypocotyls of Ler seedlings had growth inhibition kinetics similar to Col seedlings but took longer to recover after ethylene was removed (Fig. 6C; Supplemental Table S1). Like the ga3ox1-3 mutants, the gai-1 mutants appeared to reach a slower growth rate during the plateau in growth prior to the onset of the second phase of growth inhibition response (Fig. 6C). In addition, the mutants had slower growth recovery after removal of ethylene and never fully recovered to pretreatment growth rates in the timescale of these experiments (Fig. 6C). It is interesting to note that in ethylene the gai-1 mutants reached a steady-state growth rate of 0.02 ± 0.002 mm h−1, which is significantly slower (P < 0.001) than Ler seedlings that grew at 0.04 ± 0.004 mm h−1.
Figure 6.
Altered GA levels or signaling modulate ethylene growth response kinetics. Seedlings were then grown for 1 h in air, followed by the application of 10 µL L−1 ethylene (downward arrow). Ethylene was removed 2 h later (upward arrow). A, Col seedlings were treated with 5 µM paclobutrazol or DMSO as a solvent control for 3 h prior to time-lapse imaging. B, ga3ox1-3 compared with Col controls. Data for Col were taken from Figure 2. C, gai-1 and della mutants were compared with Ler controls. Data represent the average ± sem of at least eight seedlings.
We also examined quintuple della mutant seedlings where all five DELLA genes are mutated, and seedlings have a constitutive GA response phenotype (Feng et al., 2008). The hypocotyl growth rate of della seedlings in air was similar to wild-type seedlings consistent with prior research (Feng et al., 2008). The ethylene growth inhibition kinetics of the della seedlings were similar to the wild type except that they seemed to reach a slightly faster growth rate during the plateau in growth prior to the onset of the second phase of growth inhibition response (Fig. 6C). The della mutant seedlings had similar recovery kinetics to the wild type after the removal of ethylene, except that the mutants had a growth rate overshoot that was missing in the Ler controls (Fig. 6C). Both of these alterations are opposite to what we observed in the gai3ox1-3, ga4-1, and gai-1 mutants that have reduced GA levels and signaling (Fig. 6, B and C).
Transcript Levels for GA Metabolism Genes Are Altered in ein3 eil1 Seedlings
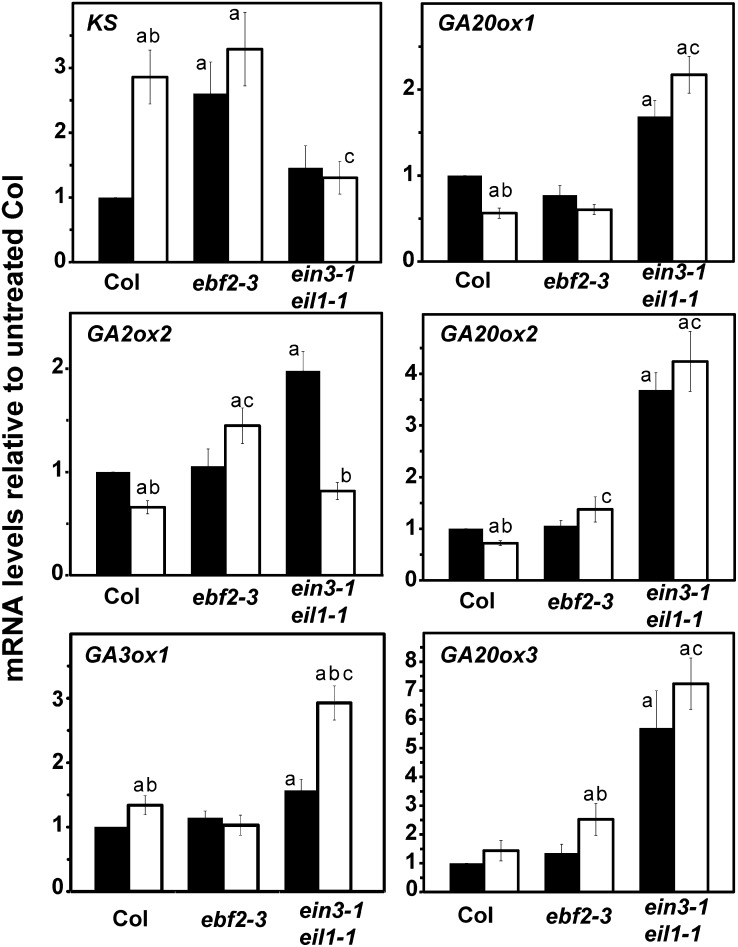
From the above, we were interested to know whether altered levels of EIN3 correlated with altered GA biosynthesis or degradation. To address this, RNA was extracted from 2-d-old Col, ebf2-3, and ein3-1 eil1-1 seedlings that were grown in the dark in hydrocarbon-free air. Some seedlings were treated with 1 μL L−1 ethylene for 2 h prior to RNA extraction. We used qRT-PCR to compare the transcript levels of select genes related to GA metabolism. We focused on several GA biosynthesis genes (ent-kaurene synthase [KS], GA20ox1–GA20ox3, and GA3ox1) and one gene coding for a GA degradation enzyme (GA2ox2). The levels of these transcripts relative to ACTIN2 (ACT2) were normalized to levels in untreated, Col seedlings (Fig. 7).
Figure 7.
Transcript levels for select GA metabolism genes in Arabidopsis seedlings. The levels of transcript for KS, GA2ox2, GA3ox1, GA20ox1, GA20ox2, and GA20ox3 relative to ACT2 in ebf2-3 and ein3-1 eil1-1 mutants were normalized to levels in untreated Col seedlings. Seedlings were grown in the dark in air for 2 d, RNA was extracted, and the transcript was analyzed with qRT-PCR. Transcript levels in untreated seedlings (closed bars) were compared with seedlings treated with 1 µL L−1 ethylene for 2 h before RNA extraction (open bars). Data shows the average ± sem for three biological replicates with two to three technical replicates each. aStatistical difference compared with untreated Col (P ≤ 0.05); bstatistical difference caused by treatment with ethylene compared with untreated seedlings of that background (P ≤ 0.05); cstatistical difference caused by ethylene compared with ethylene-treated Col seedlings (P ≤ 0.05).
In air, we found that the transcript levels for KS increased in the ebf2-3 background relative to Col seedlings, whereas the transcript levels for the other genes did not change. A different pattern was observed in transcripts from ein3-1 eil1-1 seedlings where the level of KS transcript was unchanged but the levels of GA2ox2, GA3ox1, GA20ox1, GA20ox2, and GA20ox3 transcripts increased (Fig. 7). Therefore, the levels of these transcripts in dark-grown seedlings maintained in air are affected by mutations that alter EIN3 levels.
When Col seedlings were treated with 1 μL L−1 ethylene for 2 h and examined for changes in gene expression, we observed increases in the transcript levels of KS and GA3ox1 and decreases in the levels of GA2ox2, GA20ox1, and GA20ox2 transcripts. The levels of KS transcript were similar between ethylene-treated Col and untreated ebf2-3 mutants. The pattern of ethylene-induced changes was different in the ebf2-3 mutants where application of ethylene led to a slight increase in the level of GA20ox3 transcripts. Other apparent differences in the ebf2-3mutants caused by ethylene were considered statistically insignificant (using a P ≤ 0.05). Surprisingly, application of ethylene to the ein3-1 eil1-1 double mutants caused a decrease in GA2ox2 and an increase in GA3ox1 transcript levels. Thus, ethylene may cause an increase in GA levels in the ein3-1 eil1-1 double mutants that is predicted to increase the growth rate of these seedlings.
It is also interesting to note that after treatment with ethylene, the ein3-1 eil1-1 double mutants had lower levels of KS transcript but higher levels of GA3ox1, GA20ox1, GA20ox2, and GA20ox3 compared with ethylene-treated Col that would likely result in higher GA levels in the double mutants compared with Col. By contrast, ethylene treatment of the ebf2-3 mutants resulted in an increase in the level of GA2ox2 transcript and a smaller increase in GA20ox2 transcripts compared with ethylene-treated Col. These differences would potentially lead to lower levels of GA in the ebf2-3 mutants and perhaps underlie the delay in growth recovery after removal of ethylene that has been observed in these mutants (Binder et al., 2007). These results show that ethylene affects GA metabolism genes both via EIN3/EIL1 and independently of these transcription factors.
DISCUSSION
It is believed that all land plants bind and respond to ethylene. However, the range and number of responses in dark-grown seedlings differs between species (Goeschl, 1975; Abeles et al., 1992). We used high-resolution, time-lapse imaging of dark-grown seedlings to compare the effects of ethylene on the growth of shoots from several eudicot and monocot species. All eudicots studied here had a growth inhibition response with a delay of between 10 and 15 min before the onset of growth inhibition upon application of 10 μL L−1 ethylene. Similar latent times for growth inhibition with ethylene have generally been observed in other eudicot species using other techniques (Warner and Leopold, 1971; Burg, 1973; Goeschl, 1975; Rauser and Horton, 1975; Jackson, 1983). Whereas the delay for growth inhibition was fairly constant between the eudicots studied, the time for full growth inhibition after ethylene was added and the time for full growth recovery after ethylene was removed were both more variable and differed by over 1 h between species. Similarly, the delay in the onset of growth recover after removal of ethylene varied up to approximately 50 min between the different eudicots. It remains to be seen whether the ethylene growth kinetics of shoots from other eudicots are similar to those observed in this study. The three monocots studied, millet, rice, and barley were more diverged in their growth kinetic responses to ethylene. Barley seedlings had growth inhibition kinetics that were somewhat similar to the eudicots, except that the initial growth inhibition response was attenuated, and there was a delay prior to prolonged growth inhibition. In contrast, millet seedlings had a rapid growth inhibition response when ethylene was added. However, whereas eudicots had a second phase of growth inhibition that was slower in onset and more prolonged, millet had a transient growth response to the continued application of 10 μL L−1 ethylene. The underlying mechanism for this first, transient growth inhibition response is unknown but in Arabidopsis occurs independently of EIN3 and EIL1 (Binder et al., 2004a). The transient response in millet is similar to our prior results with wild-type Arabidopsis seedlings treated with low, subsaturating dosages (≤ 0.01 μL L−1) of ethylene (Binder et al., 2004a). In this study, we used 1,000-fold higher ethylene concentrations suggesting that the transient growth response in millet is not caused by subsaturating dosages of ethylene. Rice seedlings lacked a growth inhibition response. Rather, rice seedlings had a slow onset of growth stimulation after ethylene was added. It is interesting to note that phase II growth inhibition in Arabidopsis is predicted to have a similar delay in onset and time for maximum response (Binder, 2007) as the growth stimulation response in rice (compared in Supplemental Fig. S2), indicating that the two responses may have common molecular components even though ethylene is having opposite effects.
Prior studies have shown that when ethylene treatment leads to stimulation of growth, GA levels increase to mediate this growth stimulation (Kende et al., 1998; Voesenek et al., 2003; Vandenbussche et al., 2007). We therefore explored the role of GA in ethylene growth response kinetics. Treatment of millet seedlings with paclobutrazol to block GA biosynthesis attenuated or prevented the growth reversal seen during continuous ethylene treatment. In other words, growth inhibition caused by application of ethylene was prolonged by paclobutrazol treatment. This suggests that ethylene treatment of millet leads to an increase in GA biosynthesis that in turn leads to an increase in the growth rate. In the case of millet, this overcomes the initial growth inhibition effect of ethylene whereas in rice there is no initial growth inhibition response, so ethylene only causes a stimulation of growth. Some links between ethylene and GA metabolism have been uncovered in light-grown seedlings (Achard et al., 2007; Vandenbussche et al., 2007). However, the role for GA metabolism in the ethylene growth inhibition response in the shoots of dark-grown eudicot seedlings remains largely unexplored. Whereas GA has been linked to growth stimulation caused by ethylene, here we show that regulation of GA levels also has a role in modulating growth inhibition caused by application of ethylene and recovery after ethylene removal. GA stimulates hypocotyl elongation in eudicots (Sun, 2008), which suggests a model where ethylene causes a decrease in GA levels in dark-grown eudicot seedlings to affect the second phase of growth inhibition. Support for this model comes from the observation that ethylene treatment causes a decrease in GA content in sunflowers (Helianthus annuus; Pearce et al., 1991), and ctr1 mutants in Arabidopsis with constitutive ethylene signaling have decreased GA content (Achard et al., 2007).
In Arabidopsis, the prolonged growth inhibition response to ethylene is controlled by EIN3, EIL1, and probably EIL2 (Chao et al., 1997; Binder et al., 2004a, 2007). EIN3 levels rise when Arabidopsis is treated with ethylene (Guo and Ecker, 2003; Yanagisawa et al., 2003; Gagne et al., 2004). Also, EIN3 overexpression in Arabidopsis leads to slower growth in air and slower recovery after ethylene is removed (Guo and Ecker, 2003; Binder et al., 2007). These observations are consistent with lower GA levels. It is interesting to note that mutants deficient in GA signaling or levels also recover slower after removal of ethylene. In contrast, ein3-1 eil1-1 loss-of-function mutants grow faster in air than wild-type plants and lack a prolonged growth inhibition response when ethylene is added (Binder et al., 2004a, 2007; this study) consistent with higher GA levels. This supports a model where ethylene-induced activation of the EIN3 and EIL transcription factors in eudicots leads to reduced GA levels to modulate growth inhibition (Fig. 8). Consistent with this, we found that sensitivity to paclobutrazol was inversely correlated to the levels of EIN3 indicating that paclobutrazol and EIN3 inhibit GA accumulation via independent mechanisms. Whereas ethylene altered the expression levels of genes for GA metabolic enzymes in Col seedlings, ethylene probably also modulates GA levels independently of EIN3 and EIL1 because ethylene treatment of ein3-1 eil1-1 seedlings resulted in decreased levels of GA2ox2 transcript and increased levels of GA3ox1 transcript. Both changes are predicted to lead to an increase in GA content. It is interesting to note that paclobutrazol prolonged the growth response of ein3-1 eil1-1 double mutants whereas coapplication of GA with paclobutrazol did not, suggesting that increased GA content in this double mutant leads to the transient ethylene growth inhibition response. It is also interesting to note that the ein3-1 eil1-1 della mutants had a highly attenuated growth inhibition response compared with ein3-1 eil-1 mutants. Because della mutants have a constitutive GA response phenotype, this further supports a role for GA signaling in modulating the growth inhibition response to ethylene.
Figure 8.
Models for modulation of ethylene growth responses by GA in Arabidopsis, rice, and millet. In Arabidopsis, this model proposes that in the absence of ethylene, EIN2 levels are low so there are no ethylene responses and growth is fast. Growth rate is modulated by feedback control of GA levels where a higher growth rate leads to lower levels. Ethylene leads to an accumulation of EIN2. One output of EIN2 reduces growth via an EIN3/EIL1-independent mechanism. On a slower time course, this reduced growth leads to an increase in GA levels resulting in an increase in growth. This pathway is proposed to underlie the transient first phase of growth inhibition response. On a slower time course, EIN2 also inhibits degradation of EIN3/EIL1 mediated by the EBF1 and EBF2 F-box proteins. Accumulation of EIN3 and EIL1causes a lower growth rate leading to the second phase of prolonged growth inhibition response. Our results indicate that this is occurring via a GA-dependent pathway where EIN3 and EIL1 inhibit GA accumulation. There also is likely to be a GA-independent pathway. Thus, when EIN3 and EIL1 are not present, ethylene initially causes growth inhibition, but this inhibition is reversed by the delayed feedback involving GA. The model predicts that in rice, OsEIN2 activation by ethylene has no growth inhibition effect. On a slower time scale, activation of OsEIN2 leads to enhanced degradation of OsEIL1 and OsEIL2 mediated by the OsFBL7 and OsFBL30 F-box proteins. This leads to lower levels of OsEIL1 and OsEIL2, resulting in higher levels of GA and enhanced growth. In millet, the model proposes that PmEILs are either absent or at very low levels. Thus, PmEIN2 activity initially leads to growth inhibition, but feedback resulting in higher GA levels reverses this inhibition leading to a transient growth response.
We have previously proposed two models invoking feedback mechanisms to explain the two phases of growth inhibition and the transient nature of the first phase of growth inhibition in Arabidopsis (Binder et al., 2004a). In both of these models, we proposed dual functions for EIN2. The results from this study suggest a third possible model involving GA (Fig. 8). In this model, EIN2 levels are low, so there are no ethylene responses and growth is fast; growth rate is modulated by feedback control of GA levels where higher growth rate leads to lower GA levels. Ethylene treatment of Arabidopsis seedlings leads to an initial growth inhibition response via EIN2 that is independent of EIN3 and EIL1. This is modeled to be independent of GA. After a delay, the reduced growth leads to an increase in GA levels or signaling that overcomes the initial growth inhibition response caused by EIN2. Also after a delay, EBF1 and EBF2 activity is inhibited resulting in higher levels of EIN3 and EIL1. In this model, we propose that this leads to decreased levels of GA resulting in the second, prolonged phase of growth inhibition. There also is a GA-independent inhibition of growth by EIN3 and EIL1. This model predicts that when EIN3 and EIL1 are removed, ethylene activation of EIN2 initially causes growth inhibition, but after a delay the feedback loop causes an increase in GA levels that overcomes this initial growth inhibition response leading to a transient growth inhibition response. Addition of paclobutrazol blocks or reduces this increase in GA, leading to prolonged growth inhibition. Similarly, mutants with reduced GA levels or signaling have a stronger growth inhibition response when ethylene is added and take longer to recover after removal of ethylene. This model also provides a framework to understand the variety of growth inhibition responses in the eudicots studied, as well as barley, where the timing and relative strengths of the proposed outputs from EIN2 and feedback varies between species.
This basic model needs to be modified to explain the results obtained with rice and millet. Six EIL genes have been identified in the rice genome (Mao et al., 2006). OsEIL1 and OsEIL2 are the most closely related to EIN3, EIL1, and EIL2 from Arabidopsis and have been linked to ethylene signaling in rice (Mao et al., 2006; Hiraga et al., 2009). Overexpression of OsEIL1 in rice leads to decreased growth (Mao et al., 2006) much like overexpression of EIN3 in Arabidopsis decreases growth (Guo and Ecker, 2003). Thus, these transcription factors have similar roles in Arabidopsis and rice, yet ethylene has the opposite effect on the growth of shoots of these species under the conditions used in this study. If these differences in the growth effects of ethylene are mediated by the EILs, it is likely that the levels of the EILs are controlled differently in these two species when ethylene is applied. Consistent with this, we found that ethylene caused a decrease in the mRNA levels of OsEIL2 in coleoptiles correlating with the growth stimulation caused by application of ethylene. Also, application of ethylene enhanced transcript levels of OsFBL7. Increased levels of this F-box protein would lead to decreased levels of OsEIL1 and OsEIL2. The Arabidopsis homologs of OsFBL7 and OsFBL30 are EBF2 and EBF1, respectively (Rzewuski and Sauter, 2008; Ma et al., 2010). EBF2 appears to have a larger role in long-term responses to ethylene than EBF1(Binder et al., 2007), paralleling the results in this study suggesting that FBL7 may have a larger role than FBL30 in the slow onset of growth stimulation caused by the application of ethylene. Several ethylene response factors have been identified that act downstream of the OsEILs to mediate ethylene responses in rice (Xu et al., 2000, 2006; Yang et al., 2002; Fukao and Bailey-Serres, 2008; Hu et al., 2008; Fukao et al., 2009, 2011; Hattori et al., 2009; Jung et al., 2010; Qi et al., 2011). At least some of these ethylene response factors act to modulate GA levels. The results from these prior studies coupled with results in this study indicate that the output from EIN2 in Arabidopsis that causes a rapid growth inhibition response is lacking from OsEIN2 in dark-grown cv Nipponbare rice coleoptiles (Fig. 8). Rather, increased activity from OsEIN2 leads to increases in OsFBL7, causing increased proteolysis of OsEIL1 and OsEIL2. The lower levels of these transcription factors are predicted to lead to increased GA biosynthesis and increased growth (Fig. 8). We cannot rule out that there is also an OsEIL1/OsEIL2-independent mechanism that leads to increased GA levels. Whereas ethylene stimulates coleoptile growth of dark-grown rice seedlings, it inhibits growth of roots (Ma et al., 2010). The differences in mechanisms by which ethylene causes growth stimulation versus inhibition has yet to be resolved. Arabidopsis EIN3 has been shown to be affected by factors other than ethylene (Yanagisawa et al., 2003; Lee et al., 2006; Zhu et al., 2011; An et al., 2012), suggesting that EIN3 and the EILs are part of a complex regulatory network that could underlie these opposite responses to ethylene. Although we have no data on the PmEILs from millet, we predict that PmEIL levels are low and ethylene causes little or no change in their levels (Fig. 8). Therefore, ethylene is predicted to initially cause growth inhibition via output from PmEIN2. On a slower timescale, this inhibition is counteracted by a feedback mechanism similar to what is predicted to occur in Arabidopsis.
Based upon the limited number of species studied, the shoots of eudicot species appear to have diverged only slightly in the kinetics of growth inhibition upon addition of ethylene. In contrast, shoots of monocots have evolved more divergent of ethylene growth responses. Even though the number of ethylene receptor isoforms can vary between species and receptor function within a species is not entirely overlapping (Binder et al., 2012), it is likely that much of this divergence in ethylene growth responses arises from differences in downstream signaling components. Our data indicate that the control of the EIN3 and EIL transcription factors by ethylene is one locus that differs between plant species and under different environmental and developmental conditions. This leads to differences in GA levels that affect growth responses to ethylene.
MATERIALS AND METHODS
A variety of eudicot plant species were used in this study including kale (Brassica oleracea ‘Red Russian’), canola (Brassica napus), two cultivars of tomato (Solanum lycopersicum ‘Floradade’ and ‘German Queen’), poppy (Papaver orientale ‘Oriental Scarlet’), and beetberry (Chenopodium capitatum). Three monocots species, rice (Oryza sativa ‘Nipponbare’), white millet (Panicum miliaceum), and barley (Hordeum vulgare) were also examined. Seeds for these different species were obtained from various sources (Supplemental Table S3). These species were chosen both to represent a variety of plant families as well as because of their small seed size, which made them compatible with the time-lapse imaging methods used with Arabidopsis (Arabidopsis thaliana) seedlings. The ein3-1 eil1-1 double mutant was originally obtained from Joseph Ecker and has previously been characterized (Alonso et al., 2003b; Binder et al., 2004a). The ga4-1, ga3ox1-3, and gai-1 mutants were from the Arabidopsis Biological Resource Center and have previously been characterized (Talon et al., 1990; Jacobsen and Olszewski, 1993; Chiang et al., 1995; Jacobsen et al., 1996; Mitchum et al., 2006), the quintuple della mutant seeds where all five DELLA genes are mutated (rga-t2 gai-t6 rgl1-1 rgl2-1 rgl3-1) were obtained from Xiuhua Gao and Xiangdong Fu (Feng et al., 2008), the EIN3-GFP overexpressing seeds and ein3 eil1 della septuple mutants were from Hongwei Guo (Guo and Ecker, 2003; An et al., 2012). The ebf2-3 loss-of-function mutant has been previously described and characterized (Gagne et al., 2004; Binder et al., 2007). The ein3-1 eil1-1, ebf2-3, and ga3ox1-3 mutants are in the Col background whereas the ga4-1, gai-1, and della mutants are in the Ler background. Gibberellic acid was from Fisher Scientific, and paclobutrazol was a gift from Elena Shpak (University of Tennessee–Knoxville).
Seed Preparation
Most seeds were surface-sterilized by treatment with 70% alcohol for 30 s. Rice and barley seeds were surface-sterilized with a solution containing 10% (v/v) bleach with 0.01% (v/v) Triton X-100 for 5 min prior to treatment with 70% (v/v) alcohol. Unless otherwise specified, surface sterilized seeds were placed on sterile filter paper to dry and then placed on agar plates containing 0.8% (w/v) agar, one-half-strength Murashige and Skoog basal salt mixture, (Murashige and Skoog, 1962), pH 5.7, fortified with vitamins and with no added sugar. Arabidopsis, kale, and canola seeds were cold treated at 4°C for 2 to 4 d prior to being light treated for 2 to 8 h at 21°C. Tomato seeds were plated and light treated without cold treatment. To ensure germination in the dark, the ga3ox1-3, ga4-1, gai-1, and della mutants were soaked in sterile, distilled water with 1 μm GA at 4°C in the dark for 1 d prior to being placed on agar plates and cold treated at 4°C for 2 d prior to being light treated. The ga3ox1-3 and ga4-1 seeds were light treated for 4 h, and gai-1 and della seeds for 12 to 24 h at 21°C. Light-treated seeds were then allowed to germinate in the dark at 21°C. Millet, poppy, and beetberry seeds were plated and allowed to germinate in the dark at 21°C without light treatment, and rice seeds were germinated in the dark at 28°C and then transferred to 21°C for 2 h prior to time-lapse imaging.
Paclobutrazol and GA Treatment of Seedlings
In some cases, seedlings were treated with paclobutrazol to block GA biosynthesis (Dalziel and Lawrence, 1984; Hedden and Graebe, 1985). Paclobutrazol was made as a 1,000× stock in DMSO, filter sterilized, and added to the agar after heat sterilization to the indicated final concentration. Millet seedlings were germinated on paclobutrazol-containing plates whereas solvent control millet seedlings were germinated and grown on agar plates containing DMSO. However, paclobutrazol inhibited Arabidopsis seed germination as has been previously been reported (Jacobsen and Olszewski, 1993; Peng et al., 1999; Wen and Chang, 2002). Therefore, we germinated Arabidopsis seeds on media without paclobutrazol, then gently transferred the seedlings onto plates containing the indicated concentration of paclobutrazol (or DMSO as a control) and allowed them to grow in air 3 h prior to time-lapse imaging. We have previously shown that this transfer protocol has no measurable effect on the ethylene growth inhibition response (Binder et al., 2006). These manipulations were done under dim green light. In some experiments, GA was also included in the agar at the indicated concentration.
Time-Lapse Imaging and Analyses
Seedlings were allowed to germinate in the dark until approximately 4 to 8 mm long as previously described (Binder et al., 2004a, 2004b, 2006). Images of growing shoots were captured in the dark with Marlin CCD cameras (Allied Vision Technology) using infrared radiation and at 21°C. All manipulations prior to imaging were done under dim green light.
Growth rate experiments used two different protocols. In one, seedlings were allowed to grow 1 h in air followed by 2 h with 10 μL L−1 ethylene. Ethylene was then removed and seedlings allowed to grow an additional 3 h in air to allow for growth recovery. Alternatively, seedlings were grown in air for 1 h followed by 10 μL L−1 ethylene treatment for 5 h. In both cases, images were captured every 5 min. To determine the growth rate of shoots, the height in pixels of each seedling in each frame was analyzed using custom software written by Edgar Spalding (Parks and Spalding, 1999; Folta and Spalding, 2001) as previously described (Binder et al., 2004a, 2004b). Growth rates in air were determined from the 1 h of air pretreatment prior to applying ethylene. Growth rates were normalized to the growth rate during the air pretreatment for each seedling to make response kinetic comparisons easier, and trend lines were drawn by hand based upon moving averages of two consecutive data points. To calculate the growth rate in ethylene, the average growth rate was determined after the growth rate had stabilized at a new rate after ethylene application.
RNA Isolation and qRT-PCR
The transcript levels of several Arabidopsis GA biosynthesis-related genes including: KS, GA3ox1, GA2ox2, GA20ox1, GA20ox2, and GA20ox3 were analyzed using qRT-PCR. Similar analysis was used to determine the transcript levels of OsEIL1, OsEIL2, OsFBL7, and OsFBL30 from rice. For qRT-PCR analysis of Arabidopsis genes, total RNA was extracted from 80 or more 2-d-old, dark-grown Arabidopsis seedlings using a TRIzol Reagent (Invitrogen). Some seedlings were treated with 1 μL L−1 ethylene for 2 h prior to RNA extraction. For qRT-PCR analysis of rice genes, total RNA was extracted from five coleoptiles of 3-d-old, dark-grown seedlings. Some seedlings were treated with 10 μL L−1 ethylene for 3 h prior to extractions. The quality and quantity of the extracted RNAs were examined on a 1.2% agarose gel and by using a Nanodrop (ND-1000) spectrophotometer (Thermo Fisher Scientific). One microgram of total RNA was used for complementary DNA (cDNA) synthesis using the ImProm-II Reverse Transcription System (Promega) according to the manufacturer’s instructions. The RNA was treated with one unit of DNase I (Invitrogen) prior to cDNA synthesis, and the cDNAs were diluted 10-fold. The qRT-PCR was performed with a Bio-Rad iQ5 Real-Time PCR Detection System (Bio-Rad) using SsoFast EvaGreen Supermix (Bio-Rad). Gene-specific primers (see below) were used in a total volume of 10 μL with 3 μL of cDNA, 0.5 μm of gene-specific primers and 4 μL of SsoFast EvaGreen Supermix (Bio-Rad). The qRT-PCR conditions were: 45 cycles consisting of 95°C for 15 s, 58°C for 30 s, 72°C for 10 s followed by the initial denaturation step at 95°C for 1 min. All qRT-PCR values are from three biological replicates with two to three technical replicates each. Data represents the average ± sem.
Arabidopsis transcript data were normalized to ACT2 as an internal control. The cycle threshold values for the ACT2 reference gene varied by no more than 1 between treatments and plant backgrounds. The primers used for qRT-PCR were: KS 5′-AATCAGCTCTACAAGCTCGTGAG-3′ (forward), KS 5′-ATTCCCTTGGAACCACACTTCCTT-3′ (reverse), GA2ox2 5′-CTGTGTGAAAGATGGAAGTTGGGT-3′ (forward), GA2ox2 5′-ATCTTGCTCAGGGACAAGGCAT-3′ (reverse); GA3ox1 5′-TCGCCTCTCAACGATTTCCGT-3′ (forward), GA3ox1 5′-ATTTAGCTGGAGAGCAGCTTGG-3′ (reverse); GA20ox1 5′-CATTCGTCCCAACCCCAA-3′(forward), GA20ox1 5′-TGATGCTGTCCAAAAGCTCTCT-3′ (reverse); GA20ox2 5′-TCGTTGTCAATATTGGTGACACTTTC-3′ (forward), GA20ox2 5′-TCTTCGGACACAAGAAAAACGC-3′ (reverse); GA20ox3 5′-TCGTGGTGAACATAGGCGACA-3′ (forward), GA20ox3 5′-CGTTTACTAGTTCTTCTGGTGGCTTC-3′ (reverse). ACT2 primers were from Cartieaux et al. (2008). Data were normalized to transcript levels in untreated Col seedlings. Rice transcript data were normalized OsUBQ5. The cycle threshold values for the OsUBQ5 reference gene varied by no more than 1 between treatments. The primers used were: OsFBL30 5′-ACTTGAACGGCTGCGAGAA-3′ (forward), 5′-ATCCTGCTGCAACCTTCGAGA-3′ (reverse); OsFBL7 5′-AGATAACCGATGCTAGCCTCTTT-3′ (forward), 5′-CAGGGTAGCAACACCATTGTCA-3′ (reverse). Primers for OsEIL1 and OsEIL2 were from Hiraga et al. (2009) and for OsUBQ5 was from Jain et al. (2006).
Ethylene Concentration Measurements
Ethylene concentrations were determined using a Hewlett-Packard 6890 gas chromatograph with an HP Plot/Q column (Agilent Technologies) or an ETD-300 ethylene detector (Sensor Sense).
Statistical Analysis
Five growth parameters (growth rate in air, growth rate in ethylene, time to maximum growth inhibition after addition of ethylene, delay in the onset of growth recovery after ethylene removal, recovery time after ethylene removal) were measured in this study (Supplemental Table S1) and compared pairwise. The square of r2 was determined using Microsoft Excel. These values are shown in Supplemental Table S2. qRT-PCR data were analyzed with Student’s t tests and considered statistically different with a P value of ≤ 0.05.
Arabidopsis Genome Initiative accession numbers for Arabidopsis genes described in this article are: EBF1, At2g25490; EBF2, At5g25350; EIN3, At3g20770; EIL1, At2g27050; EIL2, At5g21120; CTR1, At5g03730; EIN2, At5g03280; KS, At1g79460; GA3ox1 (GA4), At1g15550; GA2ox2, At1g30040; GA20ox1, At4g25420; GA20ox2, At5g51810; GA20ox3, At5g07200; GAI, At1g14920. The Gene Loci for rice genes described in this article are: OsEIL1, Os03g20780; OsEIL2, Os07g48630; OsFBL7, Os02g10700; OsFBL30, Os06g40360.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Growth rates of dark-grown eudicot seedling shoots in air and ethylene.
Supplemental Figure S2. Comparison of ethylene growth response kinetics of rice shoots and predicted phase II growth inhibition kinetics of Arabidopsis.
Supplemental Table S1. Growth parameter measurements for eudicots studied.
Supplemental Table S2. Correlations between growth parameters in eudicots.
Supplemental Table S3. List of species used and source for seeds.
Supplementary Material
Acknowledgments
The authors thank Joe Williams, Simon Gilroy and members of the Binder lab for helpful discussions, and Amanda Wehner for technical assistance. We thank Joseph Ecker, Xiangdong Fu, Hongwei Guo, Harry Klee, Sara Patterson, Amber Smith, and Albrecht von Arnim who kindly supplied us with seeds.
Glossary
- Col
Columbia
- r2
correlation coefficient
- qRT
quantitative real-time reverse transcriptase
- DMSO
dimethyl sulfoxide
- Ler
Landsberg erecta
- cDNA
complementary DNA
- sem
se of mean
References
- Abeles F, Morgan P, Saltveit MJ. (1992) Ethylene in Plant Biology, Ed 2. Academic Press, San Diego, CA [Google Scholar]
- Achard P, Baghour M, Chapple A, Hedden P, van Der Straeten D, Genschik P, Moritz T, Harberd NP. (2007) The plant stress hormone ethylene controls floral transition via DELLA-dependent regulation of floral meristem-identity genes. Proc Natl Acad Sci USA 104: 6484–6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen HM, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003a) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR. (2003b) Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci USA 100: 2992–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An F, Zhang X, Zhu Z, Ji Y, He W, Jiang Z, Li M, Guo H. (2012) Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell Res 22: 915–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An F, Zhao Q, Ji Y, Li W, Jiang Z, Yu X, Zhang C, Han Y, He W, Liu Y, et al. (2010) Ethylene-induced stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-box 1 and 2 that requires EIN2 in Arabidopsis. Plant Cell 22: 2384–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Voesenek LACJ. (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59: 313–339 [DOI] [PubMed] [Google Scholar]
- Binder BM. (2007) Rapid kinetic analysis of ethylene growth responses in seedlings: new insights into ethylene signal transduction. J Plant Growth Regul 26: 131–142 [Google Scholar]
- Binder BM, Chang C, Schaller GE. (2012) Perception of ethylene by plants: ethylene receptors. McManus MT, , Annual Plant Reviews, The Plant Hormone Ethylene, Vol 44. Wiley-Blackwell, Hoboken, NJ, pp 117–145 [Google Scholar]
- Binder BM, Mortimore LA, Stepanova AN, Ecker JR, Bleecker AB. (2004a) Short-term growth responses to ethylene in Arabidopsis seedlings are EIN3/EIL1 independent. Plant Physiol 136: 2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, O’Malley RC, Wang W, Moore JM, Parks BM, Spalding EP, Bleecker AB. (2004b) Arabidopsis seedling growth response and recovery to ethylene. A kinetic analysis. Plant Physiol 136: 2913–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, O’Malley RC, Wang W, Zutz TC, Bleecker AB. (2006) Ethylene stimulates nutations that are dependent on the ETR1 receptor. Plant Physiol 142: 1690–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, Walker JM, Gagne JM, Emborg TJ, Hemmann G, Bleecker AB, Vierstra RD. (2007) The Arabidopsis EIN3 binding F-Box proteins EBF1 and EBF2 have distinct but overlapping roles in ethylene signaling. Plant Cell 19: 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241: 1086–1089 [DOI] [PubMed] [Google Scholar]
- Burg SP. (1973) Ethylene in plant growth. Proc Natl Acad Sci USA 70: 591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancel JD, Larsen PB. (2002) Loss-of-function mutations in the ethylene receptor ETR1 cause enhanced sensitivity and exaggerated response to ethylene in Arabidopsis. Plant Physiol 129: 1557–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartieaux F, Contesto C, Gallou A, Desbrosses G, Kopka J, Taconnat L, Renou J-P, Touraine B. (2008) Simultaneous interaction of Arabidopsis thaliana with Bradyrhizobium Sp. strain ORS278 and Pseudomonas syringae pv. tomato DC3000 leads to complex transcriptome changes. Mol Plant Microbe Interact 21: 244–259 [DOI] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. (1993) Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science 262: 539–544 [DOI] [PubMed] [Google Scholar]
- Chao QM, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Chen Y-F, Gao Z, Kerris RJ, III, Wang W, Binder BM, Schaller GE. (2010) Ethylene receptors function as components of high-molecular-mass protein complexes in Arabidopsis. PLoS ONE 5: e8640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-F, Randlett MD, Findell JL, Schaller GE. (2002) Localization of the ethylene receptor ETR1 to the endoplasmic reticulum of Arabidopsis. J Biol Chem 277: 19861–19866 [DOI] [PubMed] [Google Scholar]
- Chiang HH, Hwang I, Goodman HM. (1995) Isolation of the Arabidopsis GA4 locus. Plant Cell 7: 195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KL, Larsen PB, Wang XX, Chang C. (1998) Association of the Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS ethylene receptors. Proc Natl Acad Sci USA 95: 5401–5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling RJ, Harberd NP. (1999) Gibberellins control Arabidopsis hypocotyl growth via regulation of cellular elongation. J Exp Bot 50: 1351–1357 [Google Scholar]
- Cowling RJ, Kamiya Y, Seto H, Harberd NP. (1998) Gibberellin dose-response regulation of GA4 gene transcript levels in Arabidopsis. Plant Physiol 117: 1195–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MCH, Benschop JJ, Vreeburg RAM, Wagemaker CAM, Moritz T, Peeters AJM, Voesenek LACJ. (2004) The roles of ethylene, auxin, abscisic acid, and gibberellin in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiol 136: 2948–2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel J, Lawrence DK. (1984) Biochemical and biological effects of kaurene oxidase inhibitors, such as paclobutrazol. Menhenett R, Lawrence DK, , Biochemical Aspects of Synthetic and Naturally Occurring Plant Growth Regulators, Monograph No. 11. British Plant Growth Regulator Group, Wantage, UK, pp 43–57 [Google Scholar]
- Das KK, Sarkar RK, Ismail AM. (2005) Elongation ability and non-structural carbohydrate levels in relation to submergence tolerance in rice. Plant Sci 168: 131–136 [Google Scholar]
- Dong C-H, Rivarola M, Resnick JS, Maggin BD, Chang C. (2008) Subcellular co-localization of Arabidopsis RTE1 and ETR1 supports a regulatory role for RTE1 in ETR1 ethylene signaling. Plant J 53: 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Martinez C, Gusmaroli G, Wang Y, Zhou J, Wang F, Chen L, Yu L, Iglesias-Pedraz JM, Kircher S, et al. (2008) Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451: 475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta KM, Spalding EP. (2001) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26: 471–478 [DOI] [PubMed] [Google Scholar]
- Fukao T, Bailey-Serres J. (2008) Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proc Natl Acad Sci USA 105: 16814–16819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Harris T, Bailey-Serres J. (2009) Evolutionary analysis of the Sub1 gene cluster that confers submergence tolerance to domesticated rice. Ann Bot (Lond) 103: 143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Yeung E, Bailey-Serres J. (2011) The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell 23: 412–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JM, Smalle J, Gingerich DJ, Walker JM, Yoo SD, Yanagisawa S, Vierstra RD. (2004) Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc Natl Acad Sci USA 101: 6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Wen C-K, Binder BM, Chen Y-F, Chang J, Chiang Y-H, Kerris RJ, III, Chang C, Schaller GE. (2008) Heteromeric interactions among ethylene receptors mediate signaling in Arabidopsis. J Biol Chem 283: 23801–23810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZY, Chen YF, Randlett MD, Zhao XC, Findell JL, Kieber JJ, Schaller GE. (2003) Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem 278: 34725–34732 [DOI] [PubMed] [Google Scholar]
- Goeschl JD. (1975) Concentration dependencies of some effects of ethylene on etiolated pea, peanut, bean, and cotton seedlings. Plant Physiol 55: 670–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefen C, Städele K, Růzicka K, Obrdlik P, Harter K, Horák J. (2008) Subcellular localization and in vivo interactions of the Arabidopsis thaliana ethylene receptor family members. Mol Plant 1: 308–320 [DOI] [PubMed] [Google Scholar]
- Guo HW, Ecker JR. (2003) Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 115: 667–677 [DOI] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Nagai K, Furukawa S, Song X-J, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H, et al. (2009) The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460: 1026–1030 [DOI] [PubMed] [Google Scholar]
- Hedden P, Graebe JE. (1985) Inhibition of gibberellin biosynthesis by paclobutrazol in cell-free homogenates of Cucurbita maxima endosperm and Malus pumila embryos. J Plant Growth Regul 4: 111–122 [Google Scholar]
- Hiraga S, Sasaki K, Hibi T, Yoshida H, Uchida E, Kosugi S, Kato T, Mie T, Ito H, Katou S, et al. (2009) Involvement of two rice ETHYLENE INSENSITIVE3-LIKE genes in wound signaling. Mol Genet Genomics 282: 517–529 [DOI] [PubMed] [Google Scholar]
- Hoffmann-Benning S, Kende H. (1992) On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiol 99: 1156–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Zhao L, Chong K, Wang T. (2008) Overexpression of OsERF1, a novel rice ERF gene, up-regulates ethylene-responsive genes expression besides affects growth and development in Arabidopsis. J Plant Physiol 165: 1717–1725 [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM. (1998) EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 10: 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YF, Li H, Hutchison CE, Laskey J, Kieber JJ. (2003) Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. Plant J 33: 221–233 [DOI] [PubMed] [Google Scholar]
- Jackson MB. (1983) Regulation of root growth and morphology by ethylene and other externally applied growth substances. In M Jackson, A Stead, eds, Growth Regulators in Root Development, Monograph No. 10. British Plant Growth Regulator Group, London, pp 103–116
- Jacobsen SE, Binkowski KA, Olszewski NE. (1996) SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proc Natl Acad Sci USA 93: 9292–9296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SE, Olszewski NE. (1993) Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell 5: 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Nijhawan A, Tyagi AK, Khurana JP. (2006) Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun 345: 646–651 [DOI] [PubMed] [Google Scholar]
- Jung K-H, Seo Y-S, Walia H, Cao P, Fukao T, Canlas PE, Amonpant F, Bailey-Serres J, Ronald PC. (2010) The submergence tolerance regulator Sub1A mediates stress-responsive expression of AP2/ERF transcription factors. Plant Physiol 152: 1674–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith KA, Raskin I, Kende H. (1986) A comparison of the submergence response of deepwater and non-deepwater rice. Plant Physiol 80: 479–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H, van der Knaap E, Cho HT. (1998) Deepwater rice: a model plant to study stem elongation. Plant Physiol 118: 1105–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Kim H, Helmbrecht EE, Stalans MB, Schmitt C, Patel N, Wen C-K, Wang W, Binder BM. (2011) Ethylene receptor ETHYLENE RECEPTOR1 domain requirements for ethylene responses in Arabidopsis seedlings. Plant Physiol 156: 417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ. (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45: 41–59 [DOI] [PubMed] [Google Scholar]
- Koorneef M, Elgersma A, Hanhart CJ, van Loenen-Martinet EP, van Rijn L, Zeevaart JAD. (1985) A gibberellin insensitive mutant of Arabidopsis thaliana. Physiol Plant 65: 33–39 [Google Scholar]
- Ku HS, Suge H, Rappaport L, Pratt HK. (1970) Stimulation of rice coleoptile growth by ethylene. Planta 90: 333–339 [DOI] [PubMed] [Google Scholar]
- Lee J-H, Deng XW, Kim WT. (2006) Possible role of light in the maintenance of EIN3/EIL1 stability in Arabidopsis seedlings. Biochem Biophys Res Commun 350: 484–491 [DOI] [PubMed] [Google Scholar]
- Lee K-W, Chen P-W, Lu C-A, Chen S, Ho T-HD, Yu S-M. (2009) Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Sci Signal 2: ra61. [DOI] [PubMed] [Google Scholar]
- Ma B, Chen SY, Zhang JS. (2010) Ethylene signaling in rice. Chin Sci Bull 55: 2204–2210 [Google Scholar]
- Mao C, Wang S, Jia Q, Wu P. (2006) OsEIL1, a rice homolog of the Arabidopsis EIN3 regulates the ethylene response as a positive component. Plant Mol Biol 61: 141–152 [DOI] [PubMed] [Google Scholar]
- Mattoo AK, Suttle JC. (1991) The Plant Hormone Ethylene. CRC Press Inc., Boca Raton, FL [Google Scholar]
- Mayerhofer H, Panneerselvam S, Mueller-Dieckmann J. (2012) Protein kinase domain of CTR1 from Arabidopsis thaliana promotes ethylene receptor cross talk. J Mol Biol 415: 768–779 [DOI] [PubMed] [Google Scholar]
- McDaniel BK, Binder BM. (2012) Ethylene Receptor 1 (ETR1) is sufficient and has the predominant role in mediating inhibition of ethylene responses by silver in Arabidopsis thaliana. J Biol Chem 287: 26094–26103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchum MG, Yamaguchi S, Hanada A, Kuwahara A, Yoshioka Y, Kato T, Tabata S, Kamiya Y, Sun TP. (2006) Distinct and overlapping roles of two gibberellin 3-oxidases in Arabidopsis development. Plant J 45: 804–818 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Neljubow DN. (1901) Uber die horizontale Nutation der Stengel von Pisum sativum und einiger anderer Pflanzen. Beitr Bot Centralbl 10: 128–138 [Google Scholar]
- Parks BM, Spalding EP. (1999) Sequential and coordinated action of phytochromes A and B during Arabidopsis stem growth revealed by kinetic analysis. Proc Natl Acad Sci USA 96: 14142–14146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce DW, Reid DM, Pharis RP. (1991) Ethylene-mediated regulation of gibberellin content and growth in Helianthus annuus L. Plant Physiol 95: 1197–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP. (1997) The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev 11: 3194–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Harberd NP. (1993) Derivative alleles of the Arabidopsis gibberellin-insensitive (gai) mutation confer a wild-type phenotype. Plant Cell 5: 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Harberd NP. (1997) Gibberellin deficiency and response mutations suppress the stem elongation phenotype of phytochrome-deficient mutants of Arabidopsis. Plant Physiol 113: 1051–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Richards DE, Moritz T, Caño-Delgado A, Harberd NP. (1999) Extragenic suppressors of the Arabidopsis gai mutation alter the dose-response relationship of diverse gibberellin responses. Plant Physiol 119: 1199–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Cuppens MLC, Voesenek LACJ, Visser EJW. (2004) Interactions between ethylene and gibberellins in phytochrome-mediated shade avoidance responses in tobacco. Plant Physiol 136: 2928–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek LACJ. (2006) The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci 11: 176–183 [DOI] [PubMed] [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P. (2003) EIN3-dependent regulation of plant ethylene hormone signaling by two arabidopsis F box proteins: EBF1 and EBF2. Cell 115: 679–689 [DOI] [PubMed] [Google Scholar]
- Potuschak T, Vansiri A, Binder BM, Lechner E, Vierstra RD, Genschik P. (2006) The exoribonuclease XRN4 is a component of the ethylene response pathway in Arabidopsis. Plant Cell 18: 3047–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Sun F, Wang Q, Chen M, Huang Y, Feng Y-Q, Luo X, Yang J. (2011) Rice ethylene-response AP2/ERF factor OsEATB restricts internode elongation by down-regulating a gibberellin biosynthetic gene. Plant Physiol 157: 216–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Chang KN, Yazaki J, Ecker JR. (2009) Interplay between ethylene, ETP1/ETP2 F-box proteins, and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes Dev 23: 512–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Kende H. (1984) Role of gibberellin in the growth response of submerged deep water rice. Plant Physiol 76: 947–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauser WE, Horton RF. (1975) Rapid effects of indoleacetic acid and ethylene on the growth of intact pea roots. Plant Physiol 55: 443–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnders JGHM, Yang Y-Y, Kamiya Y, Takahashi N, Barendse GWM, Blom CWPM, Voesenek LACJ. (1997) Ethylene enhances gibberellin levels and petiole sensitivity in flooding-tolerant Rumex palustris but not in flooding-intolerant R. acetosa. Planta 203: 20–25 [Google Scholar]
- Rose-John S, Kende H. (1985) Short-term growth response of deep-water rice to submergence and ethylene. Plant Sci 38: 129–134 [Google Scholar]
- Rzewuski G, Sauter M. (2008) Ethylene biosynthesis and signaling in rice. Plant Sci 175: 32–42 [Google Scholar]
- Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM. (1998) ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc Natl Acad Sci USA 95: 5812–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satler SO, Kende H. (1985) Ethylene and the growth of rice seedlings. Plant Physiol 79: 194–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter TL, Laureles EV. (1996) The beneficial effect of reduced elongation growth on submergence tolerance of rice. J Exp Bot 47: 1551–1559 [Google Scholar]
- Shulaev V, Sargent DJ, Crowhurst RN, Mockler TC, Folkerts O, Delcher AL, Jaiswal P, Mockaitis K, Liston A, Mane SP, et al. (2011) The genome of woodland strawberry (Fragaria vesca). Nat Genet 43: 109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao QM, Ecker JR. (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19: 2169–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suge H. (1972) Mesocotyl elongation of japonica rice: effect of high temperature pre-treatment and ethylene. Plant Cell Physiol 13: 401–405 [Google Scholar]
- Suge H. (1985) Ethylene and gibberellin: regulation of internodal elongation and nodal root development in floating rice. Plant Cell Physiol 26: 607–614 [Google Scholar]
- Suge H, Katsura N, Inada K. (1971) Ethylene-light relationship in the growth of the rice coleoptile. Planta 101: 365–368 [DOI] [PubMed] [Google Scholar]
- Sun T-P. (2008) Gibberellin metabolism, perception and signaling pathways in Arabidopsis. The Arabidopsis Book 6: e0103, doi/10.199/tab.0103
- Talon M, Koornneef M, Zeevaart JAD. (1990) Accumulation of C19-gibberellins in the gibberellin-insensitive dwarf mutant gai of Arabidopsis thaliana (L.) Heynh. Planta 182: 501–505 [DOI] [PubMed] [Google Scholar]
- van Zanten M, Basten Snoek L, van Eck-Stouten E, Proveniers MCG, Torii KU, Voesenek LACJ, Peeters AJM, Millenaar FF. (2010) Ethylene-induced hyponastic growth in Arabidopsis thaliana is controlled by ERECTA. Plant J 61: 83–95 [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Petrásek J, Zádníková P, Hoyerová K, Pešek B, Raz V, Swarup R, Bennett M, Zazímalová E, Benková E, et al. (2010) The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development 137: 597–606 [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Vancompernolle B, Rieu I, Ahmad M, Phillips A, Moritz T, Hedden P, Van Der Straeten D. (2007) Ethylene-induced Arabidopsis hypocotyl elongation is dependent on but not mediated by gibberellins. J Exp Bot 58: 4269–4281 [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Benschop JJ, Bou J, Cox MCH, Groeneveld HW, Millenaar FF, Vreeburg RAM, Peeters AJM. (2003) Interactions between plant hormones regulate submergence-induced shoot elongation in the flooding-tolerant dicot Rumex palustris. Ann Bot (Lond) 91(Spec No): 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kong H, Ma H. (2009) F-box proteins regulate ethylene signaling and more. Genes Dev 23: 391–396 [DOI] [PubMed] [Google Scholar]
- Warner HL, Leopold AC. (1971) Timing of growth regulator responses in peas. Biochem Biophys Res Commun 44: 989–994 [DOI] [PubMed] [Google Scholar]
- Watanabe H, Hase S, Saigusa M. (2007) Effects of the combined application of ethephon and gibberellin on growth of rice (Oryza sativa L.) seedlings. Plant Prod Sci 10: 468–472 [Google Scholar]
- Wen C-K, Chang C. (2002) Arabidopsis RGL1 encodes a negative regulator of gibberellin responses. Plant Cell 14: 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RN, Somerville CR. (1995) Phenotypic suppression of the gibberellin-insensitive mutant (gai) of Arabidopsis. Plant Physiol 108: 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ. (2006) Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442: 705–708 [DOI] [PubMed] [Google Scholar]
- Xu K, Xu X, Ronald PC, Mackill DJ. (2000) A high-resolution linkage map of the vicinity of the rice submergence tolerance locus Sub1. Mol Gen Genet 263: 681–689 [DOI] [PubMed] [Google Scholar]
- Yanagisawa S, Yoo SD, Sheen J. (2003) Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425: 521–525 [DOI] [PubMed] [Google Scholar]
- Yang H-J, Shen H, Chen L, Xing Y-Y, Wang Z-Y, Zhang J-L, Hong M-M. (2002) The OsEBP-89 gene of rice encodes a putative EREBP transcription factor and is temporally expressed in developing endosperm and intercalary meristem. Plant Mol Biol 50: 379–391 [DOI] [PubMed] [Google Scholar]
- Žádníková P, Petrásek J, Marhavý P, Raz V, Vandenbussche F, Ding Z, Schwarzerová K, Morita MT, Tasaka M, Hejátko J, et al. (2010) Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development 137: 607–617 [DOI] [PubMed] [Google Scholar]
- Zhu Z, An F, Feng Y, Li P, Xue L, A M, Jiang Z, Kim JM, To TK, Li W, et al. (2011) Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc Natl Acad Sci USA 108: 12539–12544 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.