Abstract
Streptococcus pneumoniae (pneumococci) adhere to human nasopharyngeal (NP) epithelial cells as a first step in pathogenesis and adherence of pneumococci to lung epithelia may be required to establish pneumonia. We sought to determine if PcpA can serve as an adhesin to human NP (D562) and lung (A549) epithelial cells and whether PcpA mediated adherence can be inhibited by human anti-PcpA antibodies. A PcpA isogenic mutant was prepared on a wild type pneumococcal TIGR4 background. When the mutant and wild type strains were compared for adherence to D562 and A549 cell lines a reduction in adherence by the mutant was observed (p= 0.0001 for both cell types). PcpA was ectopically expressed on the surface of minimally-adherent heterologous host E coli resulting in augmented adherence to D562 (p= 0.002) and A549 (p= 0.015) cells. Total IgG was purified from a pool of 6 human sera having high IgG titers of anti-pneumococcal proteins. The purified IgG reduced TIGR4 adherence to D562 cells but we determined that this effect was largely due to bacterial cell aggregation as determined by flow cytometry and confocal microscopy. Fab fragments were prepared from pooled IgG sera. Inhibition of TIGR4 adherence to D562 cells was observed using the Fab fragments without causing bacterial aggregation (p=0.0001). Depletion of PcpA-specific Fab fragments resulted in an increase in adherence of TIGR4 to D562 cells (p=0.028). We conclude that PcpA can mediate adherence of pneumococci to human NP and lung epithelial cells and PcpA mediated adherence can be inhibited by human anti-PcpA antibodies.
1. Introduction
Streptococcus pneumoniae (pneumococci) is a leading cause of sepsis, meningitis, pneumonia, and otitis media in adults and children [1, 2]. Adherence of pneumococci to NP epithelial cells is a primary step essential for its pathogenesis [2, 3] and adherence of pneumococci to lung epithelia may be required to establish pneumonia. Surface proteins, called adhesins, mediate attachment of bacteria to host cell surfaces [1, 4-6]. Several adhesins contribute to pneumococcal adherence including the lipoprotein pneumococcal surface adhesin (PsaA) [7], choline binding protein A (CbpA) [8], and proteins with LPxTG motifs [3]. Two related choline binding proteins pneumococcal surface protein A (PspA) and CbpA have previously been reported to elicit high IgG titers in a human experimental carriage model [9, 10]. Similarly, in vitro work has suggested that humans can raise functional antibodies against adhesin PsaA [11]. Induced immune responses to adhesins PsaA and CbpA have been demonstrated to prevent NP colonization in a mouse model of infection [12, 13]. Studying the role of human antibodies that can function to block pneumococcal adherence is a path forward for vaccine development [4, 5, 11].
PcpA is a choline binding protein of pneumococci expressed on the bacterial surface of nearly all virulent strains. PcpA is under the control of the manganese-dependent regulator psaR and RNA slot-blot analysis has shown that in vitro manganese concentrations of 50 μM (similar to that seen in NP secretions) results in repression of pcpA expression [14]. Hava et al. showed that pcpA is a pneumococcal gene necessary for lung infection [15]. Vaccination with rPcpA in mice elicits an antibody response that provides protection against lung and systemic infection [16] but does not impart protection against NP colonization [15, 17]. The lack of protection against colonization has been attributed to repression of expression of PcpA when pneumococci are in the NP where manganese concentrations are high.
We recently found that NP colonization of young children with pneumococci (without symptoms or signs of associated local or systemic infection) can elicit a strong systemic immune response [9]. Those findings suggest that PcpA could be expressed in the NP in children sufficiently to be highly antigenic or that pneumococci are locally invasive in the NP during colonization without causing clinically apparent inflammation. Manganese concentrations in secretions of children may be different from mice, especially during a viral URI when a dilution of secretions occurs due to transudation of water into the NP.
Here we demonstrate that PcpA mediates adherence of pneumococci to human NP and lung epithelial cells and that anti-PcpA human antibody can reduce pneumococcal adherence to NP epithelial cells. Compared to wild type TIGR4 pneumococci, a PcpA isogenic mutant had decreased bacterial adherence to human NP and lung epithelial cells. Also, a minimally-adherent heterologous host E coli ectopically expressing PcpA on its surface dramatically increased E coli binding to D562 and A549 cells compared to the parent strain. We also show that IgG purified sera of adults (having high IgG titers of anti-pneumococcal antigens) reduces adherence of pneumococci to epithelial cells due to bacterial cell aggregation. Fab fragments prepared from total IgG did not cause aggregation but were able to directly block pneumococcal adherence.
2. Material and Methods
2.1. Bacterial strains, pneumococcal proteins, cell lines and antibodies
The TIGR4 strain of pneumococci was obtained from ATCC. For growth in low manganese conditions (0.1μM), bacteria were grown in manganese depleted Todd Hewitt Yeast (THY) Broth. THY medium was prepared according to the manufacturer’s directions, with Chelex-100 (2% [wt/Vol]) (Sigma Aldrich, St Louis, MO) being added prior to autoclaving. After autoclaving, the THY’s-Chelex mixture was stirred overnight at room temperature and then filter sterilized. Prior to use, media was supplemented with 1mM FeSO4, CaCl2, MgCl2, and ZnCl2, and 0.1 μM MnSO4 [16]. Pneumococci were grown at 37°C in the above explained media in the presence of 5% CO2 and harvested at OD600 of 0.6. Pneumococcal surface exposed choline binding protein, PcpA, and a derivative of pneumolysin (PlyD1) were provided as a gift from Sanofi Pasteur. PlyD1 has point mutations that genetically detoxify the protein but maintain the structural integrity of Ply. Recombinant choline binding protein A (CbpA) was a gift from Dr Elaine Tuomanen (St Jude Medical Center). pDUMP vector was a gift by Dr Ben Adler (Monash University, Australia). Detroit 562 (D562) nasopharyngeal and lung epithelial cells (A549) were purchased from ATCC and maintained in Minimum Essential Medium (EMEM), with 10% FBS and Penicillin-Streptomycin according to the ATCC instructions. Anti-PcpA monoclonal antibody was obtained as a gift from Sanofi Pasteur; it is an IgG1 antibody. Blood from healthy adult volunteers, presumed to have prior natural exposure to pneumococci, was obtained by venipuncture. Written informed consent was obtained in association with a protocol approved by the Rochester General Hospital Institutional Review Board.
2.2. Cloning and expression of PcpA and construction of mutant
An N-terminally truncated 1.3 kb PcpA gene, that retains immunogenicity and protective efficacy, was PCR amplified, digested, gel purified and ligated in a pDUMP vector. pDUMP is a 4389 bp lipoprotein expression vector that expresses proteins on the surface of E coli in the correct orientation. This novel vector was derived from pET 9 and maintains all of the features of the original pET9c vector with the exception of the T7 tag [18]. The ligated mix was transformed in E coli DH5 alpha and plated on LB/Kan+ plates. Cloning of PcpA was confirmed by restriction digestion followed by DNA sequencing. The recombinant pDUMP containing PcpA was transformed in E coli BL21 (DE3), grown to mid log phase (OD ~0.6) and induced with 5mM IPTG (Sigma) for 4 hrs at 370C under vigorous shaking. The expression analysis was performed by SDS-PAGE. The PcpA mutant was generated by allelic-exchange mutagenesis. In short, the PCR product of PcpA flanking the 5′ and 3′ regions of the target gene was fused to an antibiotic resistance marker by overlap extension PCR. The resulting PCR product was then cloned and transformed into pneumococcal TIGR4 by methods previously described [19], followed by selection for the appropriate antibiotic resistance. Mutants were confirmed by PCR and sequencing of genomic DNA encompassing the targeted mutation site (including sequencing beyond the 5′ and 3′ flanks). The mutant was also characterized by RT-PCR and SDS-PAGE (data not shown).
2.3. Expression analysis of PcpA by Flow Cytometry and Western Blotting
Wild type pneumococcal TIGR4 and the PcpA isogenic mutant of TIGR4 were grown to log phase (OD~0.6) at 370C. Approximately 1×107 bacteria were incubated with PcpA-specific monoclonal antibody at 1:1000 dilution in a total volume of 500 μl for 1 hour at 40C. Similarly, E coli BL21 (DE3) cultures were grown for 4 hours at 370C and washed twice with PBS at 5000 x g for 10 mins. PcpA expressing and non-expressing E coli (1×107) were incubated with anti-PcpA monoclonal antibody. Monoclonal antibody bound E coli or pneumococci were washed twice with PBS and finally incubated with goat anti-mouse FITC labeled secondary antibody (Biolegend) at a dilution of 1:1000. The samples were read on a LSR II flow cytometer (BD Biosciences). For western blotting, Bacterial cultures were grown in the above mentioned medium to mid-log phase (OD600 of 0.6). Equivalent amounts of each strain were washed twice with PBS, resuspended in PBS with SDS-PAGE sample buffer, and boiled for 5 min. Samples and a pre stained protein standard (Biorad) were loaded onto a 10% precasted gels (Biorad) and separated by electrophoresis in SDS running buffer (Biorad) in accordance with the manufacturer’s instructions. The proteins were then transferred to a nitrocellulose membrane with the mini wet transfer cell (Bio-Rad, Hercules, CA). After transfer nitrocellulose membrane was blocked with 1% BSA in TBS (Biorad) and washed extensively before probing with anti-PcpA monoclonal antibody diluted to 1:1,000 in TBS+0.5% BSA. Goat anti-mouse IgG (H+L)-HRP conjugate was used as the secondary antibody. Colorimetric detection was performed with DAB kit as per manufacturer’s instructions (Pierce).
2.4. Labeling of Bacterial cells and Adherence assays
Pneumococci and E coli bacterial cells were fluorescently labeled with a PKH2 Green Fluorescent Cell Linker Kit (Sigma-Aldrich, PKH2GL), as described elsewhere [20]. Briefly, mid-log cultures (OD~0.6 at 600 nm) were pelleted by centrifugation and washed two times with PBS (5000 x g, 8 min). Bacterial cells were labeled with 7.5 μM PKH2 at a concentration of about 5 × 109 CFU/ml in the diluent for 5 minutes. The reaction was stopped by adding 10% FBS, at a 1:1 (v/v) in PBS for 2 minutes and labeled bacteria were washed three times with PBS. After the final wash, pneumococci and E coli were re-suspended in PBS and used for the assay. Viability of pneumococci, and E coli post-labeling was ascertained by growth on blood agar media. Labelling of bacteria and a flow cytometry-based adherence assay described by Hara–Kaonga et al [20] was adapted with some modifications. In our assay, we used mono fluorescence just to label bacteria, leaving the epithelial cells unlabelled. For use in adherence assays, cultures were harvested using 0.25% trypsin-EDTA (Sigma-Aldrich, St. Louis). Cells were washed three times with EMEM + 10% FBS, counted, and re-suspended to 1×106 cells/ml in antibiotic free media. PKH2 labeled pneumococci (TIGR4 or TIGR4 PcpA mutant) or E coli were added to D562 or A549 cells (1.0×105) at various multiplicity of infection (MOI) and incubated for 45 minutes, at 37°C in 96-well plates . Based on the curve, a MOI of 200 was chosen for the adherence assays. Non-adherent bacterial cells were removed by three washes with PBS (500 x g) before acquiring them on a LSR II flow cytometer (BD Biosciences). For adherence inhibition studies, varying concentrations of purified IgG or Fab samples were incubated with pneumococci. For depletion of antigen specific Fab fragments, 1μg of IgG Fab was pre-incubated with 100-500 ng of protein antigens (CbpA, PcpA, or Ply) for 30 minutes, at 37°C in a total volume of 10μl. Depletion of indivi dual antigen specific IgG Fabs were analyzed by ELISA. Acquired cells were analyzed using Flow Jo software (Tree Star).
2.5. ELISA
Six serum samples from healthy adults were selected for pooling. PcpA and CbpA specific IgG antibody titers in the pooled sera were determined by ELISA as previously described [9]. Briefly, recombinant proteins PcpA and CbpA were coated on 96-well plate with the concentration of 0.25 μg/mL each in coating buffer. After blocking with 3% skim milk, diluted serum samples were added to the wells, and the mixture was incubated at room temperature for 1 h. Affinity purified goat anti human IgG antibody conjugated to horseradish–peroxidase was used as a secondary antibody (Bethyl laboratory). The reaction products were developed using TMB Microwell peroxidase substrate system, stopped by the addition of 1.0 M phosphoric acid, and read by ELISA reader at 450 nm. Serum dilutions that gave OD as 0.2 were considered to be an end point titer and results are expressed as End point titer (log2).
2.6. Purification of total IgG from serum and preparation of IgG Fab fragments
Sera were pooled and IgG titers were confirmed by ELISA. Total IgG from the pooled serum samples was purified using a melon Gel IgG purification kit (Pierce), according to the manufacturer’s instructions. Purified IgG was concentrated using an Amicon Ultra Centrifugal Filter (100 kda cutoff, Millipore). Purity of IgG was confirmed on a 4-15% gradient SDS-PAGE gel (Bio-Rad) quantitated by nano drop (Thermo fisher) before using it for IgG Fab preparation. IgG Fab fragments were prepared as published earlier with slight modification [21]. Papain (2mg/ml, Sigma) was pre-activated in Fab Digestion Buffer (Pierce) + 20mM L-cysteine, for 10 minutes at 37°C. IgG was then added to the mixture at a concentration of 2mg/ml [digestion with 0.1% (w/w) papain] and incubated at 37°C, for 6 hou rs. Undigested IgG and Fc fragments were removed by passing the final product through a Protein A column (Pierce). The Fab product was concentrated using an Amicon ultra centrifugal filter (30kDa; Millipore) and purity was confirmed on a 4-15% gradient SDS-PAGE gel (Data not shown). Samples were stored at -20°C until used.
2.7. Bacterial Aggregation
Pneumococci (TIGR4 and PcpA-isogenic mutant) were incubated with varying concentrations of purified IgG or Fab preparations depending on the experiment. In a 96-well flat bottom plate, various MOI of TIGR4, or PcpA isogenic mutant strains were incubated with varying concentrations of IgG or Fab fragments, at 370C for 45 min. Samples were then analyzed for the formation of bacterial aggregates by flow cytometry as well as confocal microscopy. Flow cytometry forward and side scatter parameters were used to assess bacterial aggregation as previously described [22].
2.8. Statistical analysis
Data are reported as the mean of 9 results occurring in three experiments with triplicate samples in each experiment. One way ANOVA was used in multiple comparisons. For paired comparisons a t test was used. A p value of <0.05 was considered significant.
3. Results
3.1. TIGR4 PcpA- mutant exhibits reduced pneumococcal adherence to human epithelial cells
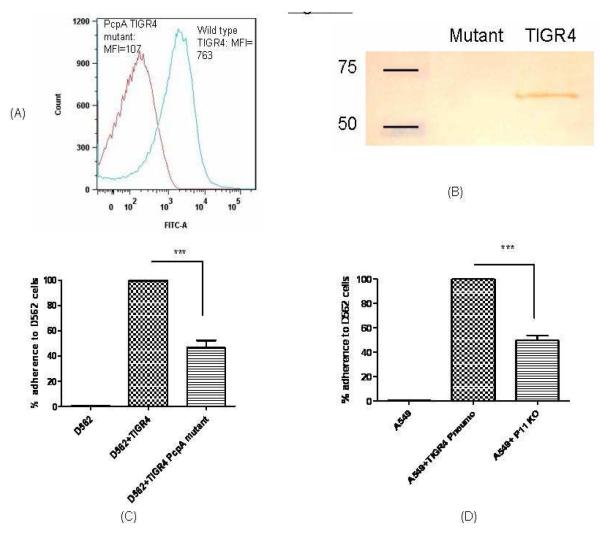
We constructed a PcpA deletion mutant from TIGR4 pneumococci to study the contribution of PcpA in adherence to D562 and A549 cells. No difference in growth rates between the wild type and isogenic mutant strains was identified (data not shown), suggesting that the disruption of PcpA did not affect major metabolic pathways under a nutrient- enriched condition. To further clarify if the mutagenesis strategy affected the expression of other surface proteins, we determined the expression of PspA, PhtD, PhtE and CbpA by real time PCR and found that the expression of these genes was not affected as a result of the PcpA deletion (data not shown). The expression analysis of PcpA was studied by both flow cytometry and western blot. Fig 1a demonstrates the expression of PcpA on the surface of TIGR4 pneumococci; however, PcpA mutant had no expression of PcpA. Similarly, western blot reveals the expression of PcpA in the TIGR4 wild type pneumococci (around 62 kDa) as demonstrated earlier (16) however; there was no expression of PcpA in isogenic mutant (Fig 1b).
Fig 1. Surface expression analysis of PcpA and pneumococcal adherence.
A: Equal bacterial counts (2×107) were taken, washed twice and incubated with PcpA specific monoclonal antibodies in the dilution of 1:1000 for 1 h at 40C. Cultures were washed twice and incubated with anti mouse goat secondary antibodies in the dilution of 1:500 and incubated for 30 mins at room temperature. The surface expression was studied by flow cytometry by taking 20000 events. Histogram shows the surface expression of PcpA wild type TIGR4 and lack of expression on TIGR4 PcpA mutant.
B: PcpA expression analysis by western blotting. After bacteria were cultured until mid-log phase, total cellular protein samples were prepared and separated by SDS-PAGE, transferred to nitrocellulose, and probed with anti-PcpA monoclonal antibody. Lane 1: marker, lane 2: pcpA mutant and lane 3: Wild type TIGR4.
C: D562 cell line was infected with 200 MOI of wild type TIGR4 pneumococci and PcpA isogenic mutant. *** represents the difference in adherence of TIGR4 pneumococci with PcpA isogenic mutant as highly significant (p=0.002). The adherence of wild type pneumococci on D562 cells was normalized to 100% and reduction in the adherence of mutant is the representation of % reduction in adherence as compared to wild type adherence. The data represents the mean with Standard error (SEM) of three experiments in triplicates.
(D): Adherence of TIGR4 wild type and PcpA isogenic mutants on A549 cell line. *** represents the difference in adherence of TIGR4 pneumococci with PcpA isogenic mutant as highly significant (p=0.002). The adherence of wild type pneumococci on A549 cells was normalized to 100% and reduction in the adherence of mutant is the representation of % reduction in adherence as compared to wild type adherence. The data represents the mean with Standard error (SEM) of three experiments in triplicates.
Compared to the wild type TIGR4 strain, the PcpA mutant had a 46% and 50% reduction in adherence to D562 and A549 cells respectively (Fig 1 b & c, p= 0.0001, p= 0.0001). Flow cytometry based adherence assays need cells in suspension. In our study, trypsinization was not found to affect the bacterial adherence to epithelial cells. After the incubation of pneumococci in different Multiplicity of infection (MOI) with epithelial cells an exponential increase in bacterial adherence was observed (supplement figure data).
3.2. An E. coli strain that is minimally adherent to human epithelial cells becomes adherent when expressing PcpA
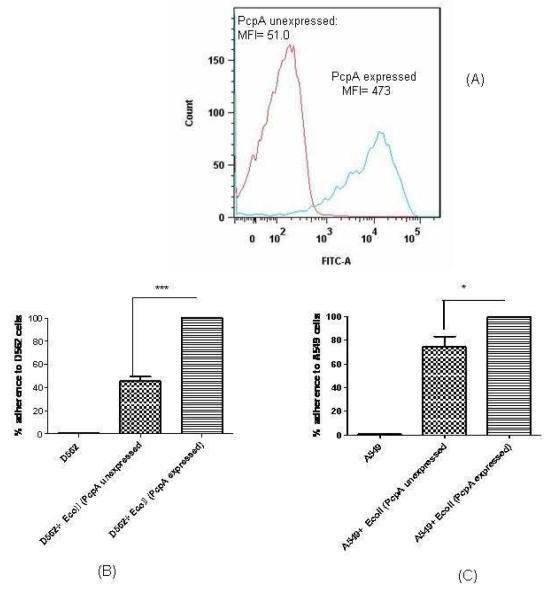
To understand the direct role of PcpA in pneumococcal adherence and to negate the contribution of other pneumococcal surface adhesins, we expressed PcpA (N-terminal truncated; 41 kDa) on the surface of a minimally-adherent strain of E. coli BL-21 (DE3). Lipoprotein expression vector (pDUMP) that results in the production of fusion proteins containing the E. coli major outer membrane lipoprotein (Lpp) signal sequence, lipoprotein signal peptidase recognition site, and the +2 outer membrane sorting signal at the N termini was used to express PcpA in E coli[18]. pDUMP has been designed in the past to express proteins in E coli as lipoproteins in the correct orientation on the cell surface as demonstrated in the case of pneumococcal PsaA. [18]. To confirm that PcpA expression on the E coli surface was in the correct orientation we used the same anti-PcpA monoclonal antibody to detect PcpA on the pneumococcal and E coli surface (Fig 2a). Expression analysis of PcpA was also accomplished by SDS-PAGE and a prominent band of 41 kDa was identified in IPTG induced cultures. No band was seen in un-induced cultures (data not shown). PcpA expression augmented the adherence of E coli significantly to both D562 (Fig 2 b; p= 0.002) and A549 (Fig 2 c; p= 0.015) cells. Using the E coli expressing PcpA on the surface demonstrates the increase in adherence is directly attributable to the surface expressed protein.
Fig 2. Surface expression analysis of PcpA on E coli and adherence.
A: Equal bacterial counts (PcpA expressed and non expressed E coli) (2×107) were taken, washed twice and incubated with PcpA specific monoclonal antibodies in the dilution of 1:1000 for 1 h at 40C. Cultures were washed twice and incubated with anti mouse goat secondary antibodies in the dilution of 1:500 and incubated for 30 mins at room temperature. The surface expression was studied by flow cytometry by taking 20000 events. Histogram shows the surface expression of PcpA on IPTG induced E coli and lack of expression on uninduced E coli harboring recombinant plasmid.
B: D562 cell line was infected with 200 MOI of PcpA expressed and non expressed E coli represents the difference in adherence of adherence between the PcpA expressed and non expressed E coli strains (p=0.002). *** represents the difference in adherence of PcpA surface expressed E coli and PcpA unexpressed E coli. The adherence of surface PcpA expressed E coli on D562 cells was normalized to 100% and reduction in the adherence of PcpA unexpressed E coli is the representation of % reduction in adherence as compared to PcpA expressing E coli. The data represents the mean with Standard error (SEM) of three experiments in triplicates.
C: A549 cell line was infected with 200 MOI of PcpA expressed and nonexpressed E coli * represents the difference in adherence of adherence between the PcpA expressed and non expressed E coli strains (p=0.015).* represents the difference in adherence of PcpA surface expressed E coli and PcpA unexpressed E coli. The adherence of surface PcpA expressed E coli on A549 cells was normalized to 100% and reduction in the adherence of PcpA unexpressed E coli is the representation of % reduction in adherence as compared to PcpA expressing E coli. The data represents the mean with Standard error (SEM) of three experiments in triplicates.
3.3. Anti-pneumococcal human IgG blocks pneumococcal adherence by inducing aggregation of bacterial cells
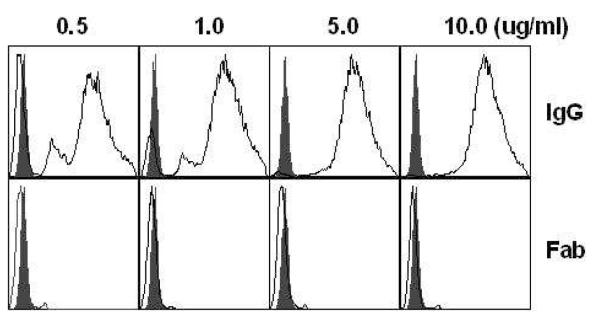
We used purified IgG from human sera for inhibition of pneumococcal adherence to D562 and A549 cells. However at various concentrations (0.5-10μg), IgG treatment caused bacterial cell aggregation as demonstrated by the flow cytometry histograms (Fig 3). Of note, these aggregates were present even in the presence of the lowest concentration of IgG tested (Fig 3). The presence of bacterial aggregates was confirmed with confocal microscopy (data not shown). Using the same pool of IgG, we prepared IgG Fab fragments and found that Fab preparations did not result in pneumococcal aggregation at concentrations of 0.5-10.0 μg/ml (Fig 3). Negative controls containing only papain or PBS (without IgG Fab) did not show inhibition in the adherence assay (data not shown). The mean fluorescence index (MFI) of IgG treated (1 μg IgG) pneumococcal aggregates (MFI: 19,257) was significantly higher than Fab treated (1 μg Fab) pneumococci (MFI: 6827) (p<0.05). The significant difference in MFI was demonstrable at all IgG and Fab conc. tested ranging from 05.-10 μg (Fig 3). There was no difference in MFI between Fab treated and control (Pneumococci+PBS) pneumococci (data not shown). As a result of IgG induced bacterial aggregation, pneumococcal adherence on NP cells was dropped non-specifically. Therefore, in order to understand the role of antigen specific antibodies in preventing pneumococcal adherence, we ascertained the use of Fab fragments to be a prerequisite. Adult pooled sera that had high and equivalent serum IgG titers (end point, 25620) for PcpA and CbpA were selected for the purification of IgG, preparation of Fabs and subsequently for the Fab depletion assays.
Figure 3. Aggregation of labeled pneumococci in the presence of purified human IgG and Fab fragments.
Pneumococci were incubated with varying concentrations (0.5-10 μg) of IgG and Fab fragments for 30 minutes at 37°C. 10,000 events were run for each sample, yielding three main populations of pneumococcal bacterial cells. Forward scatter of histograms: IgG treatment (upper panel, open histograms) caused bacterial aggregation at different concentrations whereas Fab treatment (lower panel, open histograms) did not. Solid (filled) histograms show negative control where pneumococci were treated with PBS only. Mean fluorescence index (MFI) of IgG and Fab treated pneumococci was compared using Non parametric Mann Whitney test (p<0.05). The mean fluorescence index (MFI) of IgG treated (1 μg IgG) pneumococcal aggregates (MFI: 19,257) was significantly higher than Fab treated (1 μg Fab) pneumococci (MFI: 6827) (p<0.05).
3.4. PcpA elicits functional antibodies in adults as demonstrated by an antigen specific Fab depletion assay
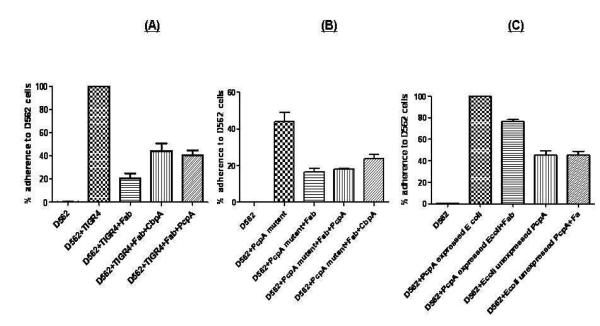
In order to understand the role of antigen-specific antibody in blocking pneumococcal adherence, we developed a competitive inhibition assay whereby we selectively depleted antigen-specific antibodies from a total human Fab preparation. To demonstrate that Fab fragments bind to pneumococci and the drop in pneumococcal adherence to NP cells is a result of blocking adhesins on pneumococcal surface, we incubated pneumococci with human Fab preparations in a dose dependent manner (0.5-5 μg) and found that at 1 μg of Fab, there was a maximum drop of 79% in pneumococcal adherence on NP cells (Fig 4, p<0.0001). Increasing the Fab concentration did not impact pneumococcal adherence further. Therefore, 1 μg Fab was used subsequently for the assays. To rule out the possibility of human Fab-mediated indirect inhibition of pneumococcal adherence by blocking epithelial cells receptors, we incubated epithelial cells with 1 μg Fab and did not find any difference in pneumococcal adherence (data not shown). To assess the contribution of anti- PcpA IgG in blocking pneumococcal adherence to human epithelial cells, antigen specific (PcpA, CbpA ) Fab fragments were depleted from the total Fab preparation by incubation with respective individual proteins. Depletion of antigen-specific Fab fragments from total Fab preparation was confirmed by ELISA. We also determined that the incubation of Fab with pneumococci did not affect the viability of the bacteria. We used CbpA depleted Fab as a positive control in our study. Depleting PcpA-specific IgG Fab resulted in a significant increase in pneumococcal adherence to D562 cells (p=0.026), implying a contribution of anti PcpA Fab in preventing pneumococcal adherence. Similarly, the removal of CbpA-specific IgG Fab resulted in a significant increase in TIGR4 adherence to D562 cells (p=0.015, Figure 4a). In a similar way, we treated the PcpA TIGR4 isogenic mutant with the Fab pool and found that 1 μg of Fab reduced the adherence of mutant TIGR4 to D562 cells to 16.6 % (p=0.006). Unlike wild type TIGR4, the depletion of PcpA did not result in an increase in the bacterial adherence (p=0.40). As a control anti- CbpA antibodies were also depleted and this resulted in a marginal but not significant increase in adherence (p=0.067, Fig 4b)”. Increased adherence after depletion of anti-CbpA antibodies was not of the same magnitude as with wild type TIGR4. In order to further ascertain whether the increase in adherence of PcpA expressed on E coli was a result of surface expressed PcpA, E coli were also treated with 1 ug of Fabs as explained above for TIGR4 pneumococci. Treatment of Fabs marginally dropped the adherence of E coli expressing PcpA (p=0.1), however there was no effect on the adherence of PcpA unexpressed E coli after Fab treatment (p=, 0.97, Fig 4 c). It is noteworthy that treatment of PcpA expressed on E coli with Fabs did not result in complete reversal of adherence to D562 cells which potentially indicates that the Fab pool did not have enough antigen specific Fab to neutralize all the PcpA on the E coli surface.
Figure 4. Adherence inhibition with Fab anti-PcpA depleted fragments.
A: PcpA and CbpA specific Fab fragments were depleted from the total IgG Fab samples by incubating with 100ng of recombinant PcpA or CbpA. The data was first analysed by one way ANNOVA due to multiple comparisons and was found to be significant (p=0.024). Individual comparisons were made by non parametric Mann Whitney test. The depletion of PcpA and CbpA specific IgG Fab resulted in a significant increase in the pneumococcal adherence (p = 0.026, 0.015) on D562 cells. The adherence of wild type pneumococci on D562 cells was normalized to 100% and the decrease or increase in the adherence as a result of Fab treatment or the depletion of antigen specific Fab was normalized accordingly. The data represents the mean with Standard error (SEM) of three experiments in triplicates.
B: The PcpA isogenic mutant was treated with total and PcpA, CbpA depleted Fab fragments. The data were analysed as explained above. The depletion of PcpA specific Fab did not affect adherence (p=0.40); however, CbpA depleted Fabs resulted in an increase in adherence of PcpA mutant pneumococci to D562 Cells.
C: E coli expressing PcpA or control E coli (PcpA unexpressed) were treated with Fab fragments as described above and subsequently incubated with D562 cells in the MOI of 1:200. The adherence of E coli expressing PcpA decreased marginally (p=0.1) while the adherence E coli that did not express PcpA remained unchanged after Fab treatment (p=0.97).
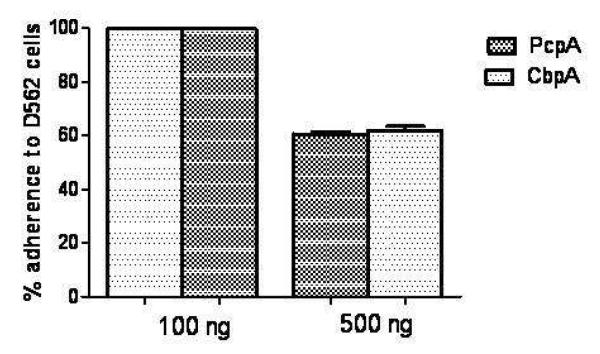
To rule out the possibility that soluble remnants of proteins might have persisted in the reaction mixture (Fab+proteins) and thereby may have modulated the adherence by direct binding to bacterial receptors on the human epithelial cells we conducted experiments using varying concentrations of proteins (PcpA and CbpA) incubated with D562 cells in the absence of Fab fragments. PcpA and CbpA concentrations of 100 ng were enough to deplete all antigen specific Fab fragments from the total Fab preparation. At the protein concentration used in the adherence assays (100 ng), PcpA or CbpA did not directly inhibit pneumococcal adherence. However at 5-fold higher concentrations of PcpA and CbpA, a significant inhibition in pneumococcal adherence to D562 cells was observed (p=0.011) (Fig. 5).
Figure 5.
Effect of recombinant PcpA on pneumococcal adherence blocking: Pneumococcal adherence was observed in the presence of pneumococcal proteins (PcpA, CbpA). At the concentration used in the adherence assays (100 ng), proteins alone did not inhibit adherence. Incubation of the D562 cells with a higher concentration (500 ng) of PcpA or CbpA did inhibit pneumococcal adherence significantly (p<0.05).
4. Discussion
This study provides the first direct evidence demonstrating PcpA-mediated adherence of pneumococci to human NP as well as lung epithelial cells. Although the activation of CodY (a nutritional gene regulator that controls the expression of PcpA) has been reported to be required for pneumococcal adherence to D562 cells [23], that study did not provide direct evidence that PcpA is an adhesin to D562 cells. The role of PcpA in pneumococcal adherence was confirmed by constructing a PcpA mutant as well as by an alternate method where PcpA was expressed ectopically on a non-adherent E coli strain. We observed that the PcpA mutant had significantly reduced adherence to D562 and A549 epithelial cell lines. Moreover, ectopic expression of PcpA on the heterologous bacterial strain of E. coli facilitated adherence to D562 and A549 epithelial cells. The later approach was used rather than complementing mutants to reduce the potential interference of other factors on the surface of pneumococci. The findings of our experiments clearly indicate that PcpA is able to facilitate pneumococcal adherence to human NP and lung epithelial cells. We also demonstrate that IgG purified sera of adults (having high anti pneumococcal IgG titers) reduces adherence of pneumococci to human D562 NP epithelial cells in vitro but the inhibition was largely due to bacterial cell aggregation. Therefore by using Fab fragments instead of IgG, we eliminated antibody-mediated aggregation and thereby directly demonstrated that pneumococcal adherence to D562 human NP epithelial cells can be significantly reduced using PcpA-specific antibody derived from human serum. Since, there may not be enough antigens specific IgG as a result of colonization, vaccination may lead to the generation of high affinity quantitatively enough antigen specific antibodies to prevent the pneumococcal colonization.
NP colonization is essential for pneumococci to cause systemic (sepsis and meningitis) or local (pneumonia, otitis media and sinusitis) diseases. Prior studies have established several adhesin proteins of pneumococci that facilitate adherence to a broad range of human epithelial cells (D562, A549 and Hep-2) including a lipoprotein (PsaA) [7], choline binding protein A (CbpA) [24], and proteins with LPxTG motifs [3]. Most of those studies used the same cell lines as used in our study. However, our study is significantly different in terms of the design of an adherence assay and the use of Fab fragments instead of IgG to evaluate the role of antigen specific antibody in the pneumococcal adherence process. Human antibodies to PsaA and CbpA have also been previously shown to block pneumococcal adherence in vitro to NP cells [11, 15]. However the usage of IgG in those studies may not have precisely defined the functional role of antigen specific antibody in pneumococcal adherence blocking because it is likely that the reduction in adherence observed was due to bacterial aggregation as shown in our study.
Our observation that exposure of pneumococci to whole serum or purified IgG resulted in bacterial cell aggregation is consistent with an earlier report of pneumococcal aggregation caused by anti-polysaccharide IgM [25]. Schlievert et al [26] recently showed anti-polysaccharide IgG mediated aggregation of Enterococcus faecalis in a rabbit model of endocarditis but aggregation did not have a therapeutic impact. However, Fab fragments prepared from IgG were more efficient at reducing adherence of the bacteria to host cells and did provide a therapeutic benefit. Antibody-mediated bacterial aggregation could be a naturally occurring defense mechanism for bacteria to evade the innate immunity during NP colonization. Here we propose that the role of a protein as an adhesin can best be established by study of specific antigen-antibody interactions that demonstrate specific blocking of the adhesin/receptor cell interaction [27]. Earlier reports demonstrating serum or IgG mediated inhibition of pneumococcal adherence to host cells lack information on whether the inhibition was caused by aggregation of the pneumococci versus a specific blocking of a putative adhesin protein on the bacterial surface [10, 11, 13, 28, 29].
Flow cytometry based analysis is being increasingly adapted as a new method for the study of host-microbe interactions, including its application to bacterial adherence assays [30-34]. This method has the advantage of evaluation of multiple samples to provide better quantitation with more precision than conventional methods such as colony forming unit counts and/or fluorescence microscopy detection [6]. D562 and A549 cells were chosen as a model to study pneumococcal adherence since these cell lines have been used extensively in the past to study host-pneumococcal interactions [4, 5].
Johnston et al (2006) have reported in the past that the expression of PcpA in the NP is suppressed due to high Mn++ (50 μM) concentrations in nasal secretions [14]. The expression of PcpA is driven by PsaR, a metal dependent regulator that negatively affects the expression of various surface proteins including PcpA in the presence of high Mn++ environment [16]. However, McDevitt et al (2011) have recently demonstrated that Mn++ conc. was rather low (<1μM) in the nasopharynx of mice and there is a complex interplay between Mn++ and Zn++ and an utmost necessity of these metals for the growth and virulence of pneumococci [35]. The regulation of PsaA metal transport system also needs Mn++ and help in the growth and virulence of pneumococci. One prior study using one strain of pneumococci showed no effect of PcpA on pneumococcal carriage (16). We are pursuing further studies with additional strains of pneumococci and mice to understand the role of PcpA in pneumococcal carriage more accurately. In young children, neither the concentration of Mn++ in nasopharyngeal secretions nor the expression of PcpA in the nasopharynx has been reported. We have recently found that children with viral upper respiratory infections express PcpA in the NP (Kaur et al, manuscript in preparation). Furthermore, our previous clinical studies discovered that young children[9] and adults (data not shown) develop high levels of serum antibody to PcpA in response to NP colonization by pneumococci. Therefore, the expression of PcpA might take place in human nasopharynx. The concentration of manganese may vary in children and adults as a consequence of a viral upper respiratory infection (since there is a copious production of mucus and water in the NP during a viral cold thereby potentially diluting the manganese concentration) and may allow the expression of PcpA to take place. Our findings support the notion that PcpA antibodies could protect against NP colonization and lung infection by the generation of serum anti PcpA antibodies that would transudate from serum to the NP and lung. Such antibodies could function to block the adherence of pneumococci to NP epithelial cells if transudation of antibody to the NP occurred in sufficient quantity. An induced mucosal antibody response might also play a pivotal role in containing pneumococcal colonization.
Supplementary Material
Supplement Figure: D562 cells were incubated with various MOI of PKH-2 stained TIGR4 pneumococci at 370C for 45 minutes. Cells were read at LSRII and an optimum MOI 200 was derived. A consistent increase in % PKH-2 positive cells with increasing MOI on D562 cells was found. The MOI was subsequently used for the experiment.
Acknowledgments
Supported by NIH NIDCD RO1 08671 and Sanofi Pasteur
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Murphy TF, Yi K. Mechanisms of recurrent otitis media: importance of the immune response to bacterial surface antigens. Ann.N.Y.Acad.Sci. 1997;830:353–60. doi: 10.1111/j.1749-6632.1997.tb51907.x. [DOI] [PubMed] [Google Scholar]
- [2].Tuomanen EI, Austrian R, Masure HR. Pathogenesis of pneumococcal infection. N.Engl.J.Med. 1995;332:1280–4. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- [3].Frolet C, Beniazza M, Roux L, et al. New adhesin functions of surface-exposed pneumococcal proteins. BMC.Microbiol. 2010;10:190. doi: 10.1186/1471-2180-10-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jensch I, Gamez G, Rothe M, et al. PavB is a surface-exposed adhesin of Streptococcus pneumoniae contributing to nasopharyngeal colonization and airways infections. Mol.Microbiol. 2010;77:22–43. doi: 10.1111/j.1365-2958.2010.07189.x. [DOI] [PubMed] [Google Scholar]
- [5].Pracht D, Elm C, Gerber J, et al. PavA of Streptococcus pneumoniae modulates adherence, invasion, and meningeal inflammation. Infect.Immun. 2005;73:2680–9. doi: 10.1128/IAI.73.5.2680-2689.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rajam G, Jackson D, Pilishvili T, et al. An in vitro model to assess pneumococcal adherence to nasopharyngeal cells under competition conditions. J.Microbiol.Methods. 2007;70:219–26. doi: 10.1016/j.mimet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- [7].Rajam G, Anderton JM, Carlone GM, Sampson JS, Ades EW. Pneumococcal surface adhesin A (PsaA): a review. Crit Rev.Microbiol. 2008;34:163–73. doi: 10.1080/10408410802383610. [DOI] [PubMed] [Google Scholar]
- [8].Luo R, Mann B, Lewis WS, et al. Solution structure of choline binding protein A, the major adhesin of Streptococcus pneumoniae. EMBO J. 2005;24:34–43. doi: 10.1038/sj.emboj.7600490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kaur R, Casey JR, Pichichero ME. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr.Infect.Dis.J. 2011;30:645–50. doi: 10.1097/INF.0b013e31821c2d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McCool TL, Cate TR, Tuomanen EI, Adrian P, Mitchell TJ, Weiser JN. Serum immunoglobulin G response to candidate vaccine antigens during experimental human pneumococcal colonization. Infect.Immun. 2003;71:5724–32. doi: 10.1128/IAI.71.10.5724-5732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Romero-Steiner S, Caba J, Rajam G, et al. Adherence of recombinant pneumococcal surface adhesin A (rPsaA)-coated particles to human nasopharyngeal epithelial cells for the evaluation of anti-PsaA functional antibodies. Vaccine. 2006;24:3224–31. doi: 10.1016/j.vaccine.2006.01.042. [DOI] [PubMed] [Google Scholar]
- [12].Pimenta FC, Miyaji EN, Areas AP, et al. Intranasal immunization with the cholera toxin B subunit-pneumococcal surface antigen A fusion protein induces protection against colonization with Streptococcus pneumoniae and has negligible impact on the nasopharyngeal and oral microbiota of mice. Infect.Immun. 2006;74:4939–44. doi: 10.1128/IAI.00134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rosenow C, Ryan P, Weiser JN, et al. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol.Microbiol. 1997;25:819–29. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- [14].Johnston JW, Briles DE, Myers LE, Hollingshead SK. Mn2+-dependent regulation of multiple genes in Streptococcus pneumoniae through PsaR and the resultant impact on virulence. Infect.Immun. 2006;74:1171–80. doi: 10.1128/IAI.74.2.1171-1180.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hava DL, Camilli A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol.Microbiol. 2002;45:1389–406. [PMC free article] [PubMed] [Google Scholar]
- [16].Glover DT, Hollingshead SK, Briles DE. Streptococcus pneumoniae surface protein PcpA elicits protection against lung infection and fatal sepsis. Infect.Immun. 2008;76:2767–76. doi: 10.1128/IAI.01126-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sanchez-Beato AR, Lopez R, Garcia JL. Molecular characterization of PcpA: a novel choline-binding protein of Streptococcus pneumoniae. FEMS Microbiol.Lett. 1998;164:207–14. doi: 10.1111/j.1574-6968.1998.tb13087.x. [DOI] [PubMed] [Google Scholar]
- [18].Cullen PA, Lo M, Bulach DM, Cordwell SJ, Adler B. Construction and evaluation of a plasmid vector for the expression of recombinant lipoproteins in Escherichia coli. Plasmid. 2003;49:18–29. doi: 10.1016/s0147-619x(02)00150-6. [DOI] [PubMed] [Google Scholar]
- [19].Kloosterman TG, Bijlsma JJ, Kok J, Kuipers OP. To have neighbour’s fare: extending the molecular toolbox for Streptococcus pneumoniae. Microbiology. 2006;152:351–9. doi: 10.1099/mic.0.28521-0. [DOI] [PubMed] [Google Scholar]
- [20].Hara-Kaonga B, Pistole TG. A dual fluorescence flow cytometric analysis of bacterial adherence to mammalian host cells. J.Microbiol.Methods. 2007;69:37–43. doi: 10.1016/j.mimet.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Boguslawski SJ, Ledden DJ, Fredrickson RA. Improved procedure for preparation of F(ab’)2 fragments of mouse IgGs by papain digestion. J.Immunol.Methods. 1989;120:51–6. doi: 10.1016/0022-1759(89)90288-3. [DOI] [PubMed] [Google Scholar]
- [22].Pitz AM, Perry GA, Jensen-Smith HC, Gentry-Nielsen MJ. A flow cytometric assay to quantify in vivo bacterial uptake by alveolar macrophages. J.Microbiol.Methods. 2010;81:194–6. doi: 10.1016/j.mimet.2010.02.005. [DOI] [PubMed] [Google Scholar]
- [23].Hendriksen WT, Bootsma HJ, Estevao S, et al. CodY of Streptococcus pneumoniae: link between nutritional gene regulation and colonization. J.Bacteriol. 2008;190:590–601. doi: 10.1128/JB.00917-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Luo R, Mann B, Lewis WS, et al. Solution structure of choline binding protein A, the major adhesin of Streptococcus pneumoniae. EMBO J. 2005;24:34–43. doi: 10.1038/sj.emboj.7600490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fabrizio K, Manix C, Guimaraes AJ, Nosanchuk JD, Pirofski LA. Aggregation of Streptococcus pneumoniae by a pneumococcal capsular polysaccharide-specific human monoclonal IgM correlates with antibody efficacy in vivo. Clin.Vaccine Immunol. 2010;17:713–21. doi: 10.1128/CVI.00410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schlievert PM, Chuang-Smith ON, Peterson ML, Cook LC, Dunny GM. Enterococcus faecalis endocarditis severity in rabbits is reduced by IgG Fabs interfering with aggregation substance. PLoS.One. 2010;5 doi: 10.1371/journal.pone.0013194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Anderton JM, Rajam G, Romero-Steiner S, et al. E-cadherin is a receptor for the common protein pneumococcal surface adhesin A (PsaA) of Streptococcus pneumoniae. Microb.Pathog. 2007;42:225–36. doi: 10.1016/j.micpath.2007.02.003. [DOI] [PubMed] [Google Scholar]
- [28].Huelves L, Del Prado G, Rodriguez-Cerrato V, et al. Adherence of Streptococcus pneumoniae to polystyrene plates, effect of serum on adhesion, and virulence in the gerbil otitis media model. Microb.Pathog. 2007;43:114–9. doi: 10.1016/j.micpath.2007.05.005. [DOI] [PubMed] [Google Scholar]
- [29].Williamson YM, Gowrisankar R, Longo DL, et al. Adherence of nontypeable Streptococcus pneumoniae to human conjunctival epithelial cells. Microb.Pathog. 2008;44:175–85. doi: 10.1016/j.micpath.2007.08.016. [DOI] [PubMed] [Google Scholar]
- [30].Hara-Kaonga B, Pistole TG. A dual fluorescence flow cytometric analysis of bacterial adherence to mammalian host cells. J.Microbiol.Methods. 2007;69:37–43. doi: 10.1016/j.mimet.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pitz AM, Perry GA, Jensen-Smith HC, Gentry-Nielsen MJ. A flow cytometric assay to quantify in vivo bacterial uptake by alveolar macrophages. J.Microbiol.Methods. 2010;81:194–6. doi: 10.1016/j.mimet.2010.02.005. [DOI] [PubMed] [Google Scholar]
- [32].Raybourne RB, Bunning VK. Bacterium-host cell interactions at the cellular level: fluorescent labeling of bacteria and analysis of short-term bacterium-phagocyte interaction by flow cytometry. Infect.Immun. 1994;62:665–72. doi: 10.1128/iai.62.2.665-672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rodriguez ME, van der Pol WL, van de Winkel JG. Flow cytometry-based phagocytosis assay for sensitive detection of opsonic activity of pneumococcal capsular polysaccharide antibodies in human sera. J.Immunol.Methods. 2001;252:33–44. doi: 10.1016/s0022-1759(01)00329-5. [DOI] [PubMed] [Google Scholar]
- [34].Sethman CR, Doyle RJ, Cowan MM. Flow cytometric evaluation of adhesion of Streptococcus pyogenes to epithelial cells. J.Microbiol.Methods. 2002;51:35–42. doi: 10.1016/s0167-7012(02)00054-4. [DOI] [PubMed] [Google Scholar]
- [35].McDevitt CA, Ogunniyi AD, Valkov E, et al. A molecular mechanism for bacterial susceptibility to zinc. PLoS.Pathog. 2011;7:e1002357. doi: 10.1371/journal.ppat.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure: D562 cells were incubated with various MOI of PKH-2 stained TIGR4 pneumococci at 370C for 45 minutes. Cells were read at LSRII and an optimum MOI 200 was derived. A consistent increase in % PKH-2 positive cells with increasing MOI on D562 cells was found. The MOI was subsequently used for the experiment.