Abstract
We evaluated the role of vaccine candidate surface proteins, PhtD and PhtE as antigens with functional importance for Streptococcus pneumoniae (pneumococci) in adherence to nasopharyngeal (D562) and lung (A549) epithelial cell lines. Comparing TIGR4 to PhtD and PhtE-deleted isogenic mutants, a 40% (p=0.001) and 42% (p=0.002) drop in the number of epithelial cells with adherent pneumococci was observed to both cells lines with the mutants, as quantitated using flow cytometry. We expressed PhtD and PhtE on the surface of E coli and demonstrated that when PhtD and PhtE were surface expressed on E coli adherence increased to D562 and A549 cells, compared with the E coli parent strain (p = 0.005, 0.013 for D562 and p=0.034, p=0.035 for A549). Using flow cytometry and confocal microscopy we found that pneumococci aggregated in the presence of human serum IgG, leading to a non-specific drop in adherence. Therefore IgG Fab fragments were prepared to study the functional role of PhtD and PhtE-specific Fabs in blocking adherence. The addition of 1 μg of IgG Fab from adult sera led to a 34% reduction (p= 0.002) and from children a 20% (p= 0.023) reduction in D562 epithelial cells with adherent pneumococci. In purified IgG from adult sera, the depletion of PhtD and PhtE specific Fab from total IgG Fab resulted in a significant increase in the number of D562 epithelial cells with adherent pneumococci (p=0.005 for PhtD and p=0.024 for PhtE). We conclude that antibody directed to PhtD and PhtE are adhesins of pneumococci, if raised by vaccination, may function to prevent pneumococcal adherence to human airway epithelial cells.
Introduction
Streptococcus pneumoniae (pneumococci) is associated with disease in a variety of host sites including septicemia, meningitis, pneumonia, sinusitis and pneumonia, resulting in high levels of morbidity and mortality. Risk groups for disease include young children, the elderly, and those with immunodeficiencies (1). Nasopharyngeal colonization by pneumococci represents the first step in pathogenesis, allowing the potential to seed the blood, brain, lungs, sinuses and middle ear (2). Colonization of the nasopharynx by pneumococci requires adherence of the bacteria to the epithelial cells of the upper respiratory tract and this process is mediated by cell wall associated surface proteins of pneumococci such as PsaA, CbpA, PavA, PsrR and others (3, 4, 5, 6). When pneumococci have colonized the nasopharynx, subsequent viral upper respiratory infection and associated generation of proinflammatory cytokines dramatically upregulate bacterial adherence receptors such as PAF-r and polymeric IgG receptor on host epithelial cells (7, 8, 9). Since colonization is the initial step in pathogenesis of pneumococcal infections, vaccination to prevent colonization is being sought as a potential strategy to prevent pneumococcal infections.
The currently licensed pneumococcal conjugate vaccine has been successful in preventing pneumococcal colonization by strains of the organism expressing capsular polysaccharide specific to the serotypes in the vaccine. However replacement of strains expressing non-vaccine serotypes has occurred followed by the occurrence of disease associated with non-vaccine serotypes (10, 11, 12).
PhtD and PhtE belong to the well-conserved Pht protein family expressed by pneumococci (13). They are surface exposed proteins characterized mainly by a histidine triad motif. In animal models both of these proteins have been shown to afford protection against sepsis, pneumonia and colonization (14, 15, 16, 17). PhtD and PhtE elicit antibody in children and adults in response to natural infection (18, 19). The function of these proteins has not been explored in great detail; nevertheless, they are considered to play an instrumental role in the pneumococcal pathogenic process (20).
The present study was undertaken to better understand the role of PhtD and PhtE in pneumococcal adherence and the ability of human antibody directed to these proteins to prevent adherence to human airway epithelial cells. Since Pht protein family members are part of a complex operon system, it becomes difficult to assess their adherence attribute by merely comparing the binding of isogenic mutant strains to human epithelial cells (21). We have recently discovered that pneumococci tend to form bacterial aggregates in the presence of serum or purified IgG (Khan et al, under review). Therefore prior research showing function of antibody to adhesins likely was due in whole or in part to an aggregation of the bacteria and not a measure of adhesion blocking by antibodies against specific adhesins.
In the view of the challenges defined above, we have used a flow cytometry- based approach to define the role of pneumococcal PhtD and PhtE as adhesins. To accomplish our aims, isogenic mutants of wild type TIGR4 pneumococci lacking expression of PhtD and PhtE were constructed, and PhtD and PhtE were expressed on the surface of a heterologous E coli strain that was minimally adherent to human nasopharyngeal epithelial (D562) and human lung epithelial cell lines (A549). Adherence of the isogenic mutants and recombinant E coli to the human cells was then quantitated. IgG Fab fragments were prepared from serum IgG of adults and children and the impact of these functional antibodies against PhtD and PhtE adherence to respiratory epithelial cells was characterized without the effect of bacterial aggregation. Our study shows that human antibody to PhtD and PhtE, as adhesins of S. pneumoniae, can function to prevent adherence of the bacteria to nasal epithelial cells in vitro.
Material and Methods
Bacterial strains, reagents and media for cultivation
The recombinant proteins PhtD, PhtE, Pneumolysin (PlyD1) and monoclonal antibodies against PhtD and PhtE proteins were procured from Sanofi Pasteur (Toronto, Canada). The anti-PhtD (clone 9E11) and anti-PhtE (clone 10D12) monoclonal antibodies were specific for the proteins, and were non- cross-reactive. Both of these monoclonal antibodies were IgG1 subclass. They were generated using standard methods (22) and purified subsequently. PlyD1 has point mutations to genetically detoxify pneumolysin (Ply). For the purposes of this work, the experiments would not differentiate between PlyD1 and Ply. The WU2 type 3 wild type and isogenic pneumolysin mutant strains were provided by David Briles, University of Alabama (Birmingham). pDUMP vector was provided by Prof. Ben Adler (Monash University, Australia). E coli strains were grown in Luria Bertani Broth (LB Broth) with or without Kanamycin (50μg/ml) depending on the experiments. Pneumococcal strains were cultivated in depleted Todd Hewitt Broth media supplemented with 1mM FeSO4, CaCl2, MgCl2, and ZnCl2, and 0.1 μM MnSO4· WU2 strains (type 3) were grown in Todd Hewitt Broth. Written informal consent was obtained in association with a protocol approved by the Rochester General Hospital Institutional Review Board for collection of samples containing blood (for serum).
Construction of PhtD and PhtE mutants in wild type TIGR4 pneumococci
PhtD and PhtE mutants were generated by allelic-exchange mutagenesis. In short, PCR products of PhtD and PhtE genes flanking the 5′ and 3′ regions of the target genes were fused to an antibiotic resistance cassette by overlap extension PCR. The resulting PCR products were then cloned and transformed into TIGR4 pneumococci as described previously (23), followed by selection for the appropriate antibiotic resistance. Deletion of the locus was confirmed by PCR and sequencing of genomic DNA encompassing the targeted mutation site (including sequence beyond the 5′ and 3′ flanks). In addition to PCR and sequencing, the mutants were also characterized by RT-PCR and SDS-PAGE (data not shown).
Cloning and expression of PhtD and PhtE in E. coli
The full-length PhtD and PhtE genes were PCR amplified, digested (NcoI), gel purified and ligated in pDUMP vector. Ligated mix was transformed in E coli DH5 alpha and plated on LB/Kan+ plates. Cloning of PhtD and PhtE genes in pDUMP vector was confirmed by restriction digestion followed by DNA sequencing. E coli BL21 (DE3) transformed with PhtD and PhtE cloned and sequenced pDUMP plasmids were inoculated in LB media containing Kan+. Expression vector pDUMP was used since it has been previously reported to be a successful vector for surface expression of pneumococcal PsaA (24). The log phase cultures were induced with IPTG (4mM) and incubated at 37°C for 4 h. The IPTG induced and uninduced E coli cultures were pelleted washed twice and lysed in SDS-PAGE sample buffer. The lysed samples were run on 4–15% gradient SDS-PAGE gels.
Surface expression analysis of PhtD and PhtE proteins on E coli
IPTG induced and uninduced E coli BL21 (DE3) cultures were grown for 4 hours at 37°C and washed twice with PBS at 5000 × g for 10 mins. Approximately 1×107 Bacteria were incubated with either PhtD or PhtE specific monoclonal antibodies at 1:1000 dilution in total volume of 500 μl for 1 hour at 4°C. Monoclonal antibody bound E coli were washed twice with PBS and finally incubated with goat anti-mouse FITC labeled secondary antibodies (Biolegend) at a dilution of 1:500. The samples were read by LSR II flow cytometry (BD Biosciences) by acquiring 20000 events.
Fluorescent labeling of E coli, pneumococci and pneumococcal aggregation
E coli (PhtD and PhtE expressed and non expressed strains) and log phase cultures of pneumococci were stained with green fluorescent dye PKH2 (Sigma) using an adapted protocol (25). Cultures were washed twice with PBS (5000 × g, 10 mins) and stained with 7 μm of PKH2 (green fluorescent dye, Sigma) in diluent buffer (750 μl) according to the manufacturer’s instructions at about 1×109 CFU/ml of E coli and TIGR4 pneumococci at room temperature for 4 mins. The reaction was stopped by adding an equal volume of EMEM + 10% FBS. The stained bacteria were washed 3 times with PBS and used immediately for the assay. Viability of the stained bacteria was ascertained by plating on blood agar (pneumococci) and LB/Kan+ plates (PhtD, PhtE expressed and non expressed E coli). The same count of labeled pneumococci used in the assay was incubated with varying concentrations of purified human IgG (0.5 μg to 5 μg) at 37°C for 1 h. The samples for aggregate formation were studied by flow cytometry and confocal microscopy. The forward scatter attribute of flow cytometry was explored to study bacterial aggregation as described previously (26).
Confocal Microscopy
To visualize the formation of bacterial aggregate complexes, log phase pneumococci were washed and labeled as described above. The same quantity of pneumococci as used for flow cytometry was mixed with 1–5 μg of purified IgG and IgG Fab and incubated at room temp for 1 h. The bacteria were washed 3 times, resuspended and the final volume was brought up to 1 ml. The aggregation complexes were examined using confocal microscopy (Olympus FV 1000).
Adherence Assay
The adherence assay of bacteria to epithelial cells was adapted from a previously described method (27). In brief, D562 and A549 cells were grown and maintained as confluent monolayers in minimal essential media (EMEM) supplemented with 10% FBS and penicillin-streptomycin (1x) according to ATCC recommendations. Cells (D562 and A549) were grown in a humidified incubator at 37°C with 5% CO2 and harvested using 0.25% trypsin EDTA. After harvesting, D562 and A549 cells were centrifuged at 500×g for 10 min at room temperature, washed three times with fresh medium, and adjusted to 1×106 cells/ml. In 96 well plates, 1×105 cells (D562 or A549) were added/well and infected with 200 MOI (2×107) of PKH2 stained wild type TIGR4 pneumococci, or PhtD and PhtE TIGR4 isogenic mutants, or PhtD and PhtE expressed or non -expressed E coli depending upon the experiment. Cells and bacterial mixtures were incubated statically at 37°C for 1 hr, and then pelleted at 500×g for 10 minutes. After a final wash, each sample was resuspended in 200 μl of DPBS without Ca+2 or Mg+2. Experiments were performed in triplicate and repeated on 3 different days.
Measurement of serum antibodies in adults and children against pneumococcal proteins (PhtD, PhtE, Ply) by ELISA
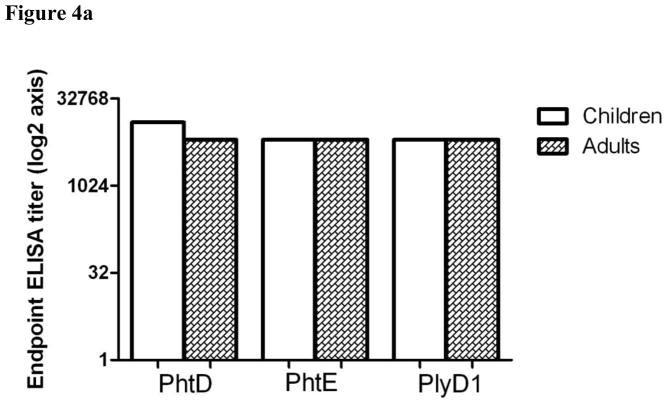
Six serum samples from healthy adults and from six healthy children (mean age = 11 months) with known high IgG titers to PhtD, PhtE and Ply based on previous analysis of the sera of children (18) and from available adult samples were selected for pooling. PhtD, PhtE, and Ply specific IgG antibody titers in the pooled sera were determined by ELISA as previously described (18). The end point ELISA titers for PhtD, PhtE and Ply were ca. 12800 in both adults and children (PhtD titers higher in children).
Purification of IgG and preparation of IgG Fab
IgG from serum samples of adults and children were purified using a Melon IgG purification kit (Pierce) according to the manufacturer’s instruction. The Fab fragments from purified IgG of adults and children were prepared using a papain based Fab preparation kit (Pierce) per the manufacturer’s instructions with slight modifications. The purity of purified IgG and IgG Fabs were assessed by SDS-PAGE.
Depletion of PhtD and PhtE specific Fab fragments from total IgG Fab and the contribution of PhtD and PhtE specific Fabs in adherence
In order to determine the optimum concentration of IgG Fab in preventing bacterial adherence, different concentrations of IgG Fabs (0.5–5 μg) from adults and children were incubated with the bacteria. To block the PhtD, PhtE and PlyD1 specific Fabs, Fabs from children and adults were incubated with varying concentrations of recombinant PhtD, PhtE and PlyD1 (50 ng - 5 μg). IgG Fab and protein mixes were incubated at room temperature for 30–60 mins to standardize the amount of protein and time required to completely deplete antigen specific Fabs from total IgG Fab. The experiments resulted in selection of 100 ng of proteins (PhtD, PhtE and Ply) at 37°C for 30 mins as optimal which led to the depletion of PhtD, PhtE and Ply specific IgG Fabs, confirmed by ELISA (data not shown). At 100ng of protein, PhtD and PhtE did not inhibit pneumococcal adherence on their own.
Statistics
Data are reported as the mean of three experiments with triplicate samples in each experiment. P values among experiments were calculated using a two-tailed T-test. A p value of <0.05 was considered significant. The data was analysed using Graph Pad Prism (Version 5).
Results
PhtD and PhtE proteins play a role in pneumococcal adherence
The wild type TIGR4 and isogenic mutants were characterized for the surface expression of PhtD and PhtE proteins before using them for adherence assays. No difference in growth rates between the wild type and isogenic mutant strains was identified (data not shown), suggesting that the disruption of PhtD and PhtE did not affect major metabolic pathways under a nutrient- enriched condition. To further clarify if the mutagenesis strategy affected other surface factors, we determined the expression of several other surface proteins of pneumococci including PspA, PcpA and CbpA and found that these genes were not affected as a result of mutation. These results suggest that the mutagenesis strategy did not influence other surface factors, and the PhtD and PhtE mutants have not compensated for the loss of these adhesins by altering expression profiles for surface proteins studied.
Eighty-two percent of D562 cells had adherent wild type TIGR4 and the adherence was significantly reduced to 40% (p=0.001) and 42% of cells (p=0.002) for PhtD and PhtE TIGR4 isogenic mutants, respectively [Figure 1(i)]. Similarly, 81% of A549 cells had adherent wild type TIGR4 and the proportion of A549 cells with adherent pneumococci was reduced to 40% (p= 0.002) and 42% (p=0.004) of cells for PhtD and PhtE isogenic mutants [Figure 1(ii)]. As a control, a comparison was made between the binding of WU2 wild type (type 3 pneumococci) and its isogenic Ply-deficient mutant at 200 MOI. The adherence to D562 and A549 cells of wild type WU2 and its Ply-deficient isogenic mutant were equivalent (data not shown), supporting a conclusion that the assay was specific since Ply is an intracellular protein toxin of pneumococci that does not mediate adherence.
Fig 1. Adherence of Pneumococcal TIGR4 and PhtD, PhtE isogenic mutants.
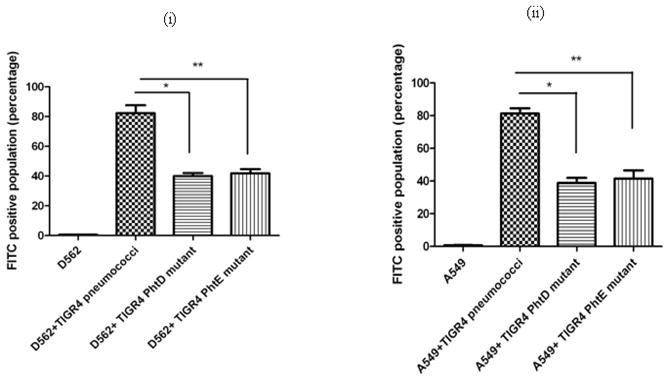
(i): D562 and A549 cell lines were infected with 200 MOI of wild type TIGR4 pneumococci, PhtD and PhtE isogenic mutants. (i): Adherence of TIGR4 wild type and PhtD, PhtE isogenic mutants on D562 cell line. * represents the difference in adherence of TIGR4 pneumococci with PhtD isogenic mutant (p=0.001). ** represents the difference in adherence between TIGR4 pneumococci with PhtE isogenic mutant (p=0.002).
(ii): Adherence of TIGR4 wild type and PhtD, PhtE isogenic mutants on A549 cell line. * represents the difference in adherence of TIGR4 pneumococci with PhtD isogenic mutant (p=0.002). ** represents the difference in adherence between TIGR4 pneumococci with PhtE isogenic mutant (p=0.004).
Characterization of adherence mediated by PhtD and PhtE of pneumococci using an E. coli strain expressing PhtD and PhtE
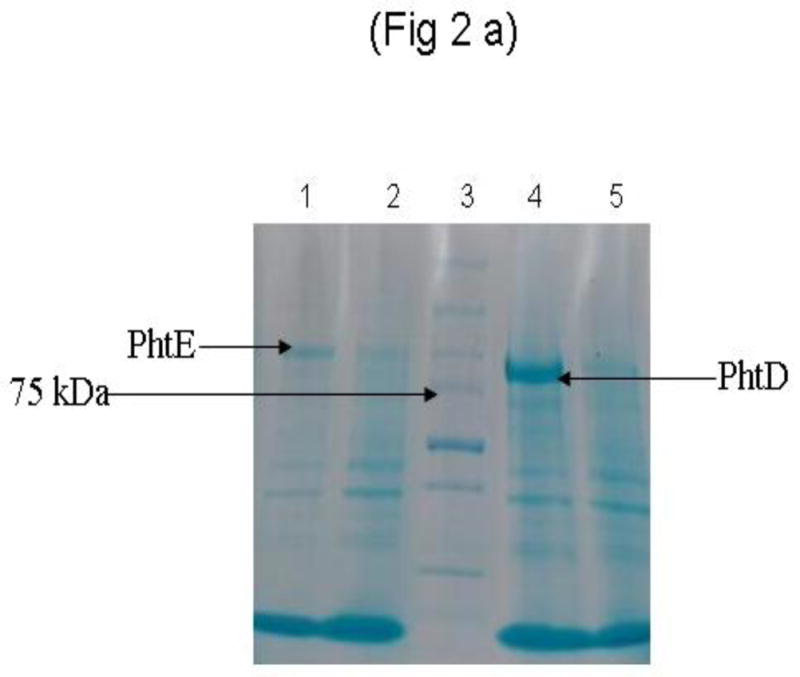
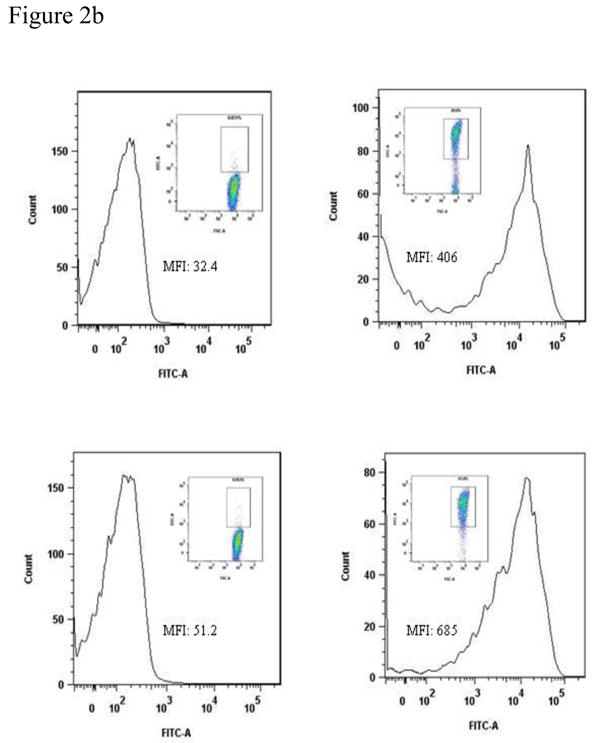
SDS-PAGE analysis demonstrated the expression of PhtD and PhtE proteins in induced E coli cultures (Fig 2a). The surface expression of PhtD and PhtE on the E coli surface was studied by using flow cytometry on whole bacteria. Comparisons were made using the same bacterial counts of induced (PhtD and PhtE expressed) and uninduced (non expressed) E coli. The histograms [Fig 2b (ii) and (iv)] demonstrate the binding of anti PhtD and PhtE monoclonal antibodies on the surface of E coli; however, uninduced E coli did not show any binding of monoclonal antibody to the E coli cell surface [Fig 2b(i) and (iii)].
Fig 2. Expression analysis of PhtD and PhtE andE coli adherence:
(a)
E coli (BL-21 DE3) harboring PhtD and PhtE cloned plasmids were grown in LB/ Kan+ media. The cultures were grown to log phase (OD600= 0.6) and induced with 4 mM of IPTG. Induced (IPTG) and uninduced cultures were incubated at 37°C under shaking. The cultures were pelleted, washed twice and lysed in SDS-PAGE sample buffer and run on 4–15% gradient SDS-PAGE. Lanes 2 and 5 represent uninduced PhtE and PhtD, while lanes 1 and 4 represent induced PhtE and PhtD cultures. Lane 3 represents molecular weight marker.
(b)
E coli BL21 (DE3) cultures were grown to log phase (OD ~0.6) in LB media containing Kanamycin (50 μg/ml) and induced with IPTG (4h). Equal counts (1×107) of uninduced and induced E coli BL21 (DE3) were taken, washed twice and incubated with PhtD and PhtE specific monoclonal antibodies in the dilution of 1:1000 for 1 h at 4°C. Cultures were washed twice and incubated with anti mouse goat secondary antibodies in the dilution of 1:500 and incubated for 30 mins at room temperature. The surface expression was studied by flow cytometry by taking 20000 events.
i) Histogram showing the surface expression of PhtD on uninduced E coli.
ii) Histogram showing the surface expression of PhtD on induced E coli.
iii) Histogram showing the surface expression of PhtE on uninduced E coli.
iv) Histogram showing the surface expression of PhtE on induced E coli
(c)
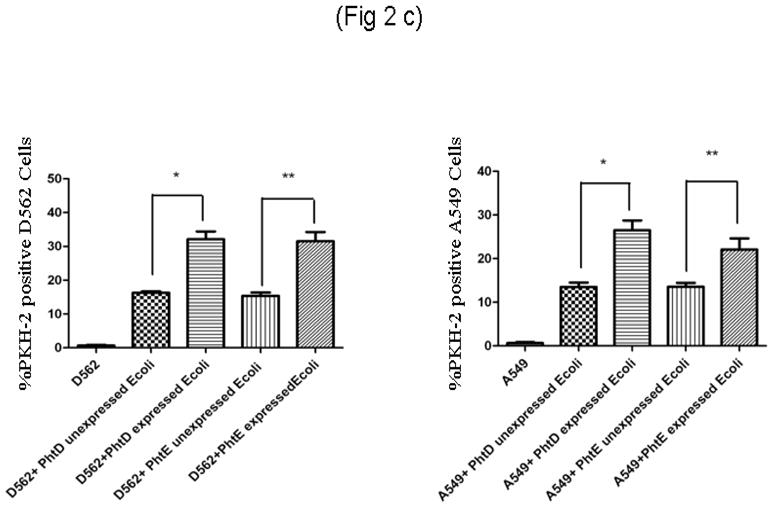
D562 and A549 cell lines were infected with 200 MOI of PhtD, PhtE expressed (induced) and unexpressed (uninduced) E coli BL21 (DE3), arrested in same growth phase.
(i): Adherence of PhtD, PhtE surface expressed and unexpressed E coli on D562 cell line. * represents the difference in adherence between PhtD expressed and unexpressed E coli (p=0.005). ** represents the difference in adherence between PhtE expressed and unexpressed E coli (p=0.013).
(ii): Adherence of PhtD, PhtE surface expressed and unexpressed E coli on A549 cell line. * represents the difference in adherence between PhtD expressed and unexpressed E coli (p=0.034). ** represents the difference in adherence between PhtE expressed and unexpressed E coli (p=0.035).
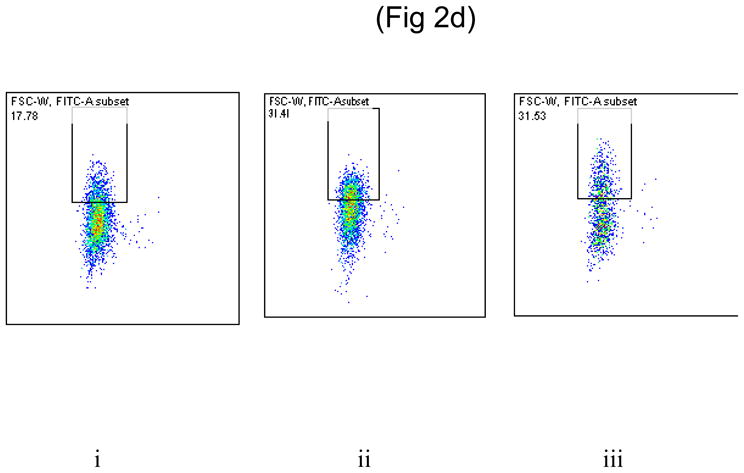
(d)
Dot plot showing the binding of uninduced E coli (i), PhtD expressed E coli (ii) and PhtE expressed E coli (iii) on D562 cells.
We studied the expression of PhtD and PhtE on the surface of heterologous E coli: a. to confirm the results observed with TIGR4 PhtD and PhtE-isogenic mutants, b. to exclude interference of other pneumococcal surface factors during adherence assays, and c. to directly test whether PhtD and PhtE were sufficient to allow adherence to human epithelial cells (D562 and A549).
The binding of the E coli strain constructed to express PhtD and PhtE was compared to the parent E coli strain to D562 and A549 cell lines. Adherence analysis demonstrated that both PhtD and PhtE surface expressed on E coli significantly increased adherence to D562 and A549 cells, compared with their E coli parent strain (PhtD and PhtE non expressed) (p = 0.005, 0.013 for D562) [Fig 2c(i)] (p=0.034, p=0.035 for A549) [Fig 2c(ii)].
Pneumococcal aggregation and limitations of using total IgG in adherence studies
Using purified IgG from human serum samples at different concentrations, we found adherence of pneumococci to D562 and A549 cells was inhibited. However, by flow cytometry and confocal microscopy we determined that the drop in adherence could be attributed to an indirect effect of serum IgG on blocking adherence of the bacteria through the formation of bacterial aggregates rather than adhesin blocking.
The forward scatter attribute of flow cytometry was exploited to identify pneumococcal aggregation. In the experimental setting of our work, an increase in autofluorescence and an accompanying shift of forward scatter is a measure of bacterial aggregation. We found that pneumococci formed bacterial aggregates at various IgG concentrations. The data in Figure 3a is representative of the bacterial aggregation in the presence of 1 μg IgG or Fab since the same amount of either IgG or Fab was taken for adherence blocking experiments. Purified IgG from adults and children had the same effect on IgG induced pneumococcal aggregation. An evident shift in forward scatter (reflecting the bigger size as a result of aggregation) was detected (Mean Fluorescence Index, MFI= 14300±405) with 1μg of IgG [Fig 3a(iii)]. Aggregation mediated by anti-polysaccharide IgM antibody has been demonstrated in the past (26); therefore, we tested anti- polysaccharide (type 4) IgG as a validation of our assay. Similar results were observed when anti-serotype 4 polysaccharide IgG was added to TIGR4 pneumococci [Fig 3a(iv) MFI= 13600±300)] indicating bacterial aggregation. In contrast, when Fabs (1 μg) were added to TIGR4 pneumococci, bacterial aggregation did not occur [Fig 3a(ii)]. The autofluorescence (MFI) of IgG Fab-treated pneumococci was significantly lower than IgG-treated pneumococci (p= 0.001). In Figure 3a(ii), flow cytometry forward scatter (MFI= 7273±190) was shown to be comparable to control [pneumococci with PBS, Fig 3a(i) (MFI= 6919±405)]. There was no difference between IgG Fab treated pneumococci and control (pneumococci and PBS, p= 0.5).
Fig 3. Demonstration of pneumococcal aggregate formation by Flow Cytometry and Confoccal microscope.
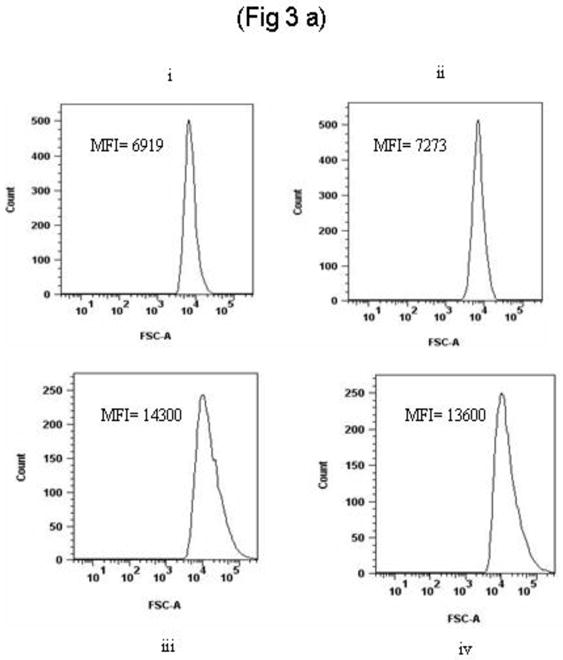
(a)
PKH2 labeled pneumococci (TIGR4) were incubated with varying concentrations (0.5–5 μg) of IgG and IgG Fab fragments for 30 minutes at 37°C. Ten thousand events were run for each sample. Forward scatter of histograms: IgG treatment [lower panel: iii (1 μg IgG) and iv (100 ng of anti polysaccharide 4 IgG)] caused bacterial aggregation whereas Fab treatment (upper panel, ii) did not. The first histogram of upper panel (upper panel i represents negative control where pneumococci were treated with PBS only. The mean fluorescence index (MFI) of IgG treated bacteria was significantly higher than Fab treated pneumococci (p= 0.001).
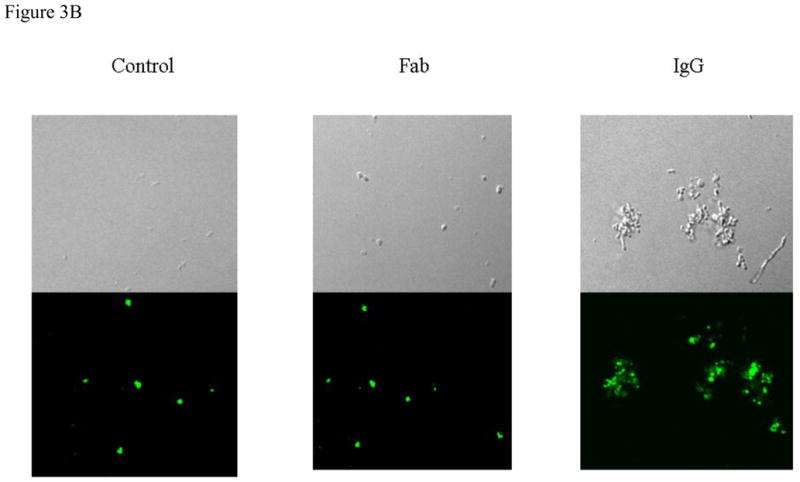
(b)
PKH2 labeled pneumococci (TIGR4) were treated with IgG and IgG Fab as explained above and imaged by laser scanning Confoccal microscopy. Total magnification, 60 x. Microscopic images represent; (i) TIGR4 with 1 μg IgG (ii) TIGR4 with 1 μg IgG Fab (iii) TIGR4 with PBS.
The results of flow cytometry experiments were confirmed by confocal microscopy (Fig 3b). We reasoned that to precisely understand the contribution of naturally acquired antibodies to PhtD and PhtE in preventing pneumococcal adherence, the usage of IgG Fab fragments was necessary.
PhtD and PhtE proteins elicit functional antibodies in adults
To overcome the bacterial aggregation induced by IgG, we prepared Fab fragments from purified IgG of adults and children sera pools. Fig 4a demonstrates the comparable IgG titers for PhtD, PhtE and Ply in the sera pool of adults and children. Interestingly, Fab fragments from purified IgG of adults and children (Figure 4b) unlike IgG did not lead to the formation of pneumococcal bacterial aggregates (Fig 3).
Figure 4. ELISA titers and preparation of IgG Fab fragments.
(a)
Six sera from children and adults were pooled and run ELISA. The IgG titers directed toward PhtD, PhtE and pneumolysin were determined. The titers are represented in the form of end point ELISA titer (Log 2) and both adults and children had equivalent PhtD, PhtE and Pneumolysin specific IgG titers (p>0.05).
(b)
Purified IgG was digested with 0.1% w/w of papain in Fab digestion buffer. The purity of papain digested IgG into Fab fragments was evaluated by 4–15% gradient SDS-PAGE. (i) Lane 1: Purified IgG; Lane 2: Protein molecular weight marker (ii) Lane 1: Protein molecular weight marker; Lane 2 & 3: Purified and concentrated Fab fragments respectively.
To assess the role of naturally acquired PhtD and PhtE antibodies in adherence, we performed competition assays using total IgG Fabs and recombinant PhtD, PhtE and Ply proteins. We used a range of dilutions of adults and children IgG Fab concentrations (100 ng-5 μg) to find the optimum concentration that led to a maximum drop in adherence by blocking PhtD and PhtE adhesins expressed on the bacterial surface. One μg IgG Fab led to a 34% drop in the number of D562 epithelial cells with adherent pneumococci using the adults sera pool (p= 0.002) (Fig 5a) and a 20% drop in D562 epithelial cells with adherent pneumococci using the sera pool from children (p= 0.023) (Fig 5b) compared to control (82% D562 cells had adherent TIGR4 pneumococci) with no IgG Fab. The IgG Fab mediated drop in adherence was significantly higher in adult sera than children (p=0.02) (Fig 5c).
Fig. 5. Adherence inhibition by IgG Fab of adults and children and a comparison with PhtD and PhtE depleted IgG Fabs on D562 cells.
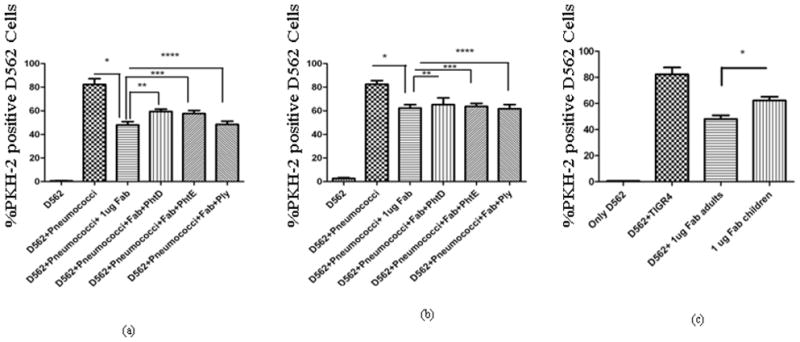
PhtD, PhtE and Ply specific Fab fragments were depleted from the total Fab samples by incubating with 100 ng of recombinant PhtD, PhtE or Ply.
(a). * represents the drop in adherence by incubation of pneumococci with total IgG Fabs of adults (p= 0.002). The depletion of PhtD and PhtE specific Fabs resulted in a significant increase in the adherence in the case of adult IgG Fabs (** p = 0.005, *** p= 0.024, ****p=0.59).
(b) * represents the drop in adherence by incubation pneumococci with total IgG Fabs of children (p= 0.023). Depletion of PhtD and PhtE specific Fab fragments did not result in significant increase in adherence (** p= 0.57, *** p=0.84). The depletion of pneumolysin specific IgG Fabs did not mediate any change in adherence in children (****p=0.57).
(c) Comparison of the drop in adherence: D562 cells were incubated with 1 μg of IgG from adults and children. The drop in adherence with adults IgG was significantly higher than children (p= 0.02).
In purified IgG from adults sera, the depletion of PhtD and PhtE specific Fab from total IgG Fab resulted in a significant increase in the number of D562 epithelial cells with adherent pneumococci (p=0.005 for PhtD and p=0.024 for PhtE), reflecting the prominent contribution of these antibodies in preventing adherence to D562 cells. The depletion of Ply specific Fab did not result in any increase of adherence (P=0.59) (Fig 5a). The increase in adherence as a result of the depletion of PhtD and PhtE specific Fabs was therefore significantly different than Ply depleted Fabs (p<0.05) (Fig 5a). In purified IgG from children sera, the depletion of PhtD (p=0.57) and PhtE specific Fabs (p=0.84) did not result in a significant change in the number of D562 epithelial cells with adherent pneumococci (Fig 5b). The depletion of Ply served as a negative control in the assay and Ply specific Fabs did not change the outcome of adherence in children sera pools (Fig 5b).
Discussion
In this study we demonstrate the role of human antibody directed to PhtD and PhtE to prevent adherence of pneumococcal to human NP epithelial cells. The results encourage further study of these antigens as vaccine ingredients in a third generation pneumococcal vaccine. Our experiments allowed the accumulation of several complimentary sets of data to justify our conclusions: (1) PhtD and PhtE-deficient TIGR 4 isogenic mutants were shown to bind to D562 and A549 cells significantly less well than the TIGR4 parent strain; (2) PhtD and PhtE when expressed individually on E coli surface showed significantly enhanced adherence to the studied human respiratory epithelial cells; (3) The functional role of human antibody in preventing pneumococcal adherence to D562 cells was established by preparing human Fab fragments of antibody directed to the putative adhesins and showing that human adults Fabs directed to either PhtD or PhtE caused a direct effect of blockage of pneumococcal adherence to the human epithelial cells studied. The complementation of mutants is another method to determine whether a mutant phenotype can be reversed. We used another alternative approach - expression of an adhesin on the surface of heterologous host E coli to corroborate the role of PhtD and PhtE as adhesins. The E coli approach of expressing an adhesin on heterologous host surface has been described in the past by Chen et al (2010) (28). However, in the absence of complementation we cannot be absolutely certain that the phenotypes observed in our mutants were a direct result of the mutation introduced. In addition, we demonstrated the functional role of anti-PhtD and anti-PhtE specific antibodies to block adherence of the pneumococci to human epithelial cells as a further validation of the role these proteins served as adhesins.
Extensive efforts are currently geared toward the development of effective alternative vaccination strategies against pneumococcal disease to address the shortcomings associated with capsule-based vaccines. Pneumococcal adherence to eukaryotic cells of the nasopharynx is a prerequisite for colonization and subsequent pathogenesis in the human host. These pathogen-host interactions are mediated by the binding of pneumococcal surface-exposed adhesins to specific cellular receptor molecules on human nasopharyngeal epithelial cells (29, 30, 31). Pneumococcal colonization of the nasopharynx precedes the development of invasive disease and is the main gateway of transmission of the pathogen between individuals. Pneumococcal colonization is considered to be an immunizing event (32, 33), but the presence of natural antibodies may not be quantitatively or qualitatively adequate to prevent colonization. Adhesins from pneumococci and Non typeable Haemophilus influenzae have been demonstrated to protect against infection by preventing the attachment of bacteria to host cells in animal models (6, 34) and could potentially translate in human upon vaccination. Antibodies directed against a pneumococcal adhesin protein might protect at the human mucosal surface by preventing pneumococcal attachment and subsequent colonization.
PhtD and PhtE are surface exposed, highly conserved proteins of the Pht family of pneumococcal proteins (21). All four Pht proteins (Pht A, B, D and E) contain a classical lipoprotein motif (LxxC) within their N-terminal hydrophobic-leader sequences similar to that recognized for processing by signal peptidase II. PhtD and PhtE are 2520 and 3120 bp long open reading frames (ORF) and encode polypeptides of 839 and 1039 amino acids. PhtD and PhtE contain five and six histidine triad motifs and also contain segments that are predicted by colis algorithm to adopt coiled coil information, a feature common among gram positive surface proteins, as well as proline rich regions (14). Signature tagged mutagenesis has suggested the involvement of these proteins in lung-specific virulence in mice (35). Among their other putative roles, neutralization of complement factor C3b through factor H binding has been suggested (36), though there are conflicting reports (17). Proteins of the Pht family have been found to be protective against colonisation, pneumonia and sepsis in mouse models and the degree of protection offered was shown to be greater than other pneumococcal vaccine candidates including PspA and PsaA (37). Isogenic mutants have shown a reduced binding in the case of pneumococcal PavA in the past but eventually it was found to be an indirect adhesin as PavA antisera could not reduce the binding of wild type pneumococci to cells (5).
Until now, the role of human functional antibodies in preventing pneumococcal adherence has either been studied by using the serum or purified antibodies from serum (38). Fabrizio et al (2010) recently reported that pneumococcal polysaccharide specific human IgM leads to pneumococcal aggregation (26). We also have recently found that pneumococci form bacterial aggregates when incubated with serum or purified IgG from human serum, thereby causing as impediment to pneumococcal adherence on host cells (Khan et al under review). In nature an IgG mediated drop in pneumococcal adherence to host epithelial cells might be attributable completely to the aggregative effect of IgG on adherence since IgG mediated aggregation will facilitate clearance of the bacteria by cilia in the nasopharynx, sweeping the organisms towards the gastrointestinal tract. Although antibody-mediated pneumococcal aggregation could be a naturally occurring protective phenomenon during NP colonization, the role of functional antibodies directed against adhesin proteins can only be established in vitro by overcoming pneumococcal aggregation. Thus in this report we used IgG Fab fragments from purified serum IgG of adults and children to avoid the complication of formation of pneumococcal aggregates in the interpretation of the findings. Our results suggest the need of using IgG Fabs as a prerequisite to study the specific adhesin-blocking role of antibodies in pneumococcal adherence in vitro.
We found that adults raise functional adhesion-blocking antibodies against PhtD and PhtE proteins. Similar findings have been recently reported by Godfroid et al (2011), where they have shown protection against pneumococcal colonisation in mice by vaccination with PhtD and PhtE against D39 pneumococci (37). They have also demonstrated that the passive transfer of adult-raised PhtD antibodies can protect mice against lethal infection. In our experiments sera from children depleted of PhtD and PhtE specific Fabs did not result in a statistically significant increase in adherence. Given that the ELISA titers of the adults and children sera pools were quantitatively comparable for PhtD and PhtE, we interpret this finding to suggest that the functional ability of children antibodies in response to natural infection at an early age may not be similar to that of adults. The disparity in functional antibody between adults and children has been described in the past in various contexts as adult antibodies typically have greater affinity for antigen, and are more often bactericidal compared to children (39, 40, 41).
Taken together, our findings clearly demonstrate that PhtD and PhtE are direct adhesins of Streptococcus pneumoniae, which elicit functional antibodies in adults. Further study of these antigens as vaccine candidates appears warranted.
Supplementary Material
Highlights.
PhtD and PhtE of Streptococcus pneumoniae (Spn) are adhesins.
PhtD and PhtE isogenic mutants were shown to have reduced adherence.
E coli expressing either PhtD or PhtE show increased adherence.
IgG Fab is a required to study the role of functional antibodies in Spn adherence.
PhtD and PhtE elicit functional antibodies in adults but not in children.
Acknowledgments
Supported by NIH NIDCD RO1 08671 and Sanofi Pasteur
We thank Dr Linda Callahan (School of Medicine and Dentistry, University of Rochester) for her kind help in performing confocal microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paton JC. New pneumococcal vaccines: basic science developments. In: Tuomanen EI, Mitchell TJ, Morrison DA, Spratt BG, editors. The pneumococcus. ASM Press; Washington, DC: 2004. pp. 382–402. [Google Scholar]
- 2.Bogaert D, de Grott R, Hermans P. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–54. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 3.Rajam G, Anderton JM, Carlone GM, Sampson JS, Ades EW. Pneumococcal surface adhesin A (PsaA): a review. Crit Rev Microbiol. 2008;34:163–73. doi: 10.1080/10408410802383610. [DOI] [PubMed] [Google Scholar]
- 4.Rosenow C, Ryan P, Weiser JN, Johnson S, Fontan P, Ortqvist A, Masure HR. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol. 1997;25(5):819–29. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 5.Pracht D, Elm C, Gerber J, Bergmann S, Rohde M, Seiler M, Kim KS, Jenkinson HF, Nau R, Hammerschmidt S. PavA of Streptococcus pneumoniae modulates adherence, invasion, and meningeal inflammation. Infection and Immunity. 2005;73(5):2680–9. doi: 10.1128/IAI.73.5.2680-2689.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rose L, Shivshankar P, Hinojosa E, Rodriguez A, Sanchez CJ, Orihuela CJ. Antibodies against PsrP, a novel Streptococcus pneumoniae adhesin, block adhesion and protect mice against pneumococcal challenge. J Infect Dis. 2008;198(3):375–83. doi: 10.1086/589775. [DOI] [PubMed] [Google Scholar]
- 7.Golda A, Malek N, Dudek B, Zeglen S, Wojarski J, Ochman M, Kucewicz E, Zembala M, Potempa J, Pyrc K. Infection with human coronavirus NL63 enhances streptococcal adherence to epithelial cells. J Gen Virol. 2011;92:1358–68. doi: 10.1099/vir.0.028381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang JR, Mostov KE, Lamm ME, Nanno M, Shimida S, Ohwaki M, Tuomanen E. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell. 2000;102(6):827–37. doi: 10.1016/s0092-8674(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 9.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19(3):571–82. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, Noyes J, Lewis E, Ray P, Hackell LJ. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21:810–816. doi: 10.1097/00006454-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, Takala A, Kayhty H, Karma P, Kohberger R, Siber G, Makela PH, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403–409. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 12.Obaro S, Adegbola R. The pneumococcus: carriage, disease and conjugate vaccines. J Med Microbiol. 2002;51:98–104. doi: 10.1099/0022-1317-51-2-98. [DOI] [PubMed] [Google Scholar]
- 13.Hamel J, Charland N, Pineau I, Ouellet C, Rioux S, Martin D, Brodeur BR. Prevention of pneumococcal disease in mice immunized with conserved surface-accessible proteins. Infect Immun. 2004;72:2659–2670. doi: 10.1128/IAI.72.5.2659-2670.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamou JE, Heinrichs JH, Erwin AL, Walsh W, Gayle T, Dormitzer M, Dagan R, Brewah YA, Barren P, Lathigra R, Langermann S, Koenig S, Johnson S. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect Immun. 2001;69:949–958. doi: 10.1128/IAI.69.2.949-958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beghetto E, Gargano N, Ricci S, Garufi G, Peppoloni S, Montagnani F, Oggioni M, Pozzi G, Felici F. Discovery of novel Streptococcus pneumoniae antigens by screening a whole-genome lambda-display library. FEMS Microbiol Lett. 2006;262:14–21. doi: 10.1111/j.1574-6968.2006.00360.x. [DOI] [PubMed] [Google Scholar]
- 16.Ogunniyi AD, Grabowicz M, Briles DE, Cook J, Paton JC. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect Immun. 2007;75:350–357. doi: 10.1128/IAI.01103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melin M, Di Paolo E, Tikkanen L, Jarva H, Neyt C, Kayhty H, Meri S, Poolman J, Vakevainen M. Interaction of pneumococcal histidine triad proteins with human complement. Infect Immun. 2010;78:2089–2098. doi: 10.1128/IAI.00811-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur R, Casey JR, Pichichero ME. Serum Antibody Response to Five Streptococcus pneumoniae Proteins During Acute Otitis Media in Otitis-prone and Non-otitis-prone Children. Pediatr Infect Dis J. 2011;30(8):645–50. doi: 10.1097/INF.0b013e31821c2d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmlund E, Quiambao B, Ollgren J, Jaakkola T, Neyt C, Poolman J, Nohynek H, Käyhty H. Antibodies to pneumococcal proteins PhtD, CbpA, and LytC in Filipino pregnant women and their infants in relation to pneumococcal carriage. Clin Vaccine Immunol. 2009;16(6):916–23. doi: 10.1128/CVI.00050-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panina EM, Mironov AA, Gelfand MS. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc Natl Acad Sci USA. 2003;100:9912–9917. doi: 10.1073/pnas.1733691100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rioux S, Neyt C, Di Paolo E, Turpin L, Charland N, Labbé S, Mortier MC, Mitchell TJ, Feron C, Martin D, Poolman JT. Transcriptional regulation, occurrence and putative role of the Pht family of Streptococcus pneumoniae. Microbiology. 2011;157:336–48. doi: 10.1099/mic.0.042184-0. [DOI] [PubMed] [Google Scholar]
- 22.Ragheb R, Abrahams S, Beecroft R, Hu J, Ni J, Ramakrishna V, Yu G, Gorczynski RM. Preparation and functional properties of monoclonal antibodies to human, mouse and rat OX-2. Immunol Lett. 1999;168:311–5. doi: 10.1016/s0165-2478(99)00060-7. [DOI] [PubMed] [Google Scholar]
- 23.Kloosterman TG, Bijlsma JJ, Kok J, Kuipers OP. To have neighbour’s fare: extending the molecular toolbox for Streptococcus pneumoniae. Microbiology. 2006;152:351–359. doi: 10.1099/mic.0.28521-0. [DOI] [PubMed] [Google Scholar]
- 24.Cullen PA, Lo M, Bulach DM, Cordwell SJ, Adler B. Construction and evaluation of a plasmid vector for the expression of recombinant lipoproteins in Escherichia coli. Plasmid. 2003;49(1):18–29. doi: 10.1016/s0147-619x(02)00150-6. [DOI] [PubMed] [Google Scholar]
- 25.Raybourne RB, Bunning VK. Bacterium-host cell interactions at the cellular level: fluorescent labeling of bacteria and analysis of short-term bacterium-phagocyte interaction by flow cytometry. Infect Immun. 1994;62:665–72. doi: 10.1128/iai.62.2.665-672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabrizio K, Manix C, Guimaraes AJ, Nosanchuk JD, Pirofski LA. Aggregation of Streptococcus pneumoniae by a pneumococcal capsular polysaccharide-specific human monoclonal IgM correlates with antibody efficacy in vivo. Clin Vaccine Immunol. 2010;17:713–21. doi: 10.1128/CVI.00410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hara-Kaonga B, Pistole TG. A dual fluorescence flow cytometric analysis of bacterial adherence to mammalian host cells. J Microbiological Methods. 2007;69:37–43. doi: 10.1016/j.mimet.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen SM, Tsai YS, Wu CM, et al. Streptococcal collagen-like surface protein 1 promotes adhesion to the respiratory epithelial cell. BMC Microbiology. 2010;10:320. doi: 10.1186/1471-2180-10-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timpe JM, Holm MM, Vanlerberg SL, Basrur V, Lafontaine ER. Identification of a Moraxella catarrhalis outer membrane protein exhibiting both adhesin and lipolytic activities. Infect Immun. 2003;71:4341–4350. doi: 10.1128/IAI.71.8.4341-4350.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensch I, Gamez G, Rothe M, et al. PavB is a surface-exposed adhesin of Streptococcus pneumoniae contributing to nasopharyngeal colonization and airways infections. Mol Microbiol. 2010;77:22–43. doi: 10.1111/j.1365-2958.2010.07189.x. [DOI] [PubMed] [Google Scholar]
- 31.Tuomanen EI, Austrian R, Masure HR. Pathogenesis of pneumococcal infection. N Engl J Med. 1995;332:1280–4. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- 32.Richards L, Ferreira DM, Miyaji EN, Andrew PW, Kadioglu A. The immunising effect of pneumococcal nasopharyngeal colonisation: protection against future colonisation and fatal invasive disease. Immunobiology. 2010;215(4):251–63. doi: 10.1016/j.imbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 33.McCool TL, Cate TR, Moy G, Weiser JN. The immune response to pneumococcal proteins during experimental human carriage. J Exp Med. 2002;195(3):359–65. doi: 10.1084/jem.20011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakaletz LO, Leake ER, Billy JM, Kaumaya PT. Relative immunogenicity and efficacy of two synthetic chimeric peptides of fimbrin as vaccinogens against nasopharyngeal colonization by nontypeable Haemophilus influenzae in the chinchilla. Vaccine. 1997;15(9):955–61. doi: 10.1016/s0264-410x(96)00298-8. [DOI] [PubMed] [Google Scholar]
- 35.Hava DL, Camilli A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol Microbiol. 2002;45:1389–1406. [PMC free article] [PubMed] [Google Scholar]
- 36.Ogunniyi AD, Grabowicz M, Mahdi LK, Cook J, Gordon DL, Sadlon TA, Paton JC. Pneumococcal histidine triad proteins are regulated by the Zn2+-dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. FASEB J. 2009;23:731–738. doi: 10.1096/fj.08-119537. [DOI] [PubMed] [Google Scholar]
- 37.Godfroid Fabrice, Hermand Philippe, Verlant Vincent, Denoël Philippe, Poolman Jan T. Preclinical Evaluation of the Pht Proteins as Potential Cross-Protective Pneumococcal Vaccine Antigens. Infect Immun. 2011;79(1):238–45. doi: 10.1128/IAI.00378-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero-Steiner S, Pilishvili T, Sampson JS, Johnson SE, Stinson A, Carlone GM, Ades EW. Inhibition of pneumococcal adherence to human nasopharyngeal epithelial cells by anti-PsaA antibodies. Clin Diagn Lab Immunol. 2003;10(2):246–51. doi: 10.1128/CDLI.10.2.246-251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris SL, King WJ, Ferris W, Granoff DM. Age-related disparity in functional activities of human group C serum anticapsular antibodies elicited by meningococcal polysaccharide vaccine. Infect Immun. 2003;71(1):275–86. doi: 10.1128/IAI.71.1.275-286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pollard AJ, Levin M. Production of low-avidity antibody by infants after infection with serogroup B meningococci. The Lancet. 2010;356:2065–66. doi: 10.1016/S0140-6736(00)03405-X. [DOI] [PubMed] [Google Scholar]
- 41.Usinger WR, Lucas AH. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect Immun. 1999;67:2366–2370. doi: 10.1128/iai.67.5.2366-2370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.