Abstract
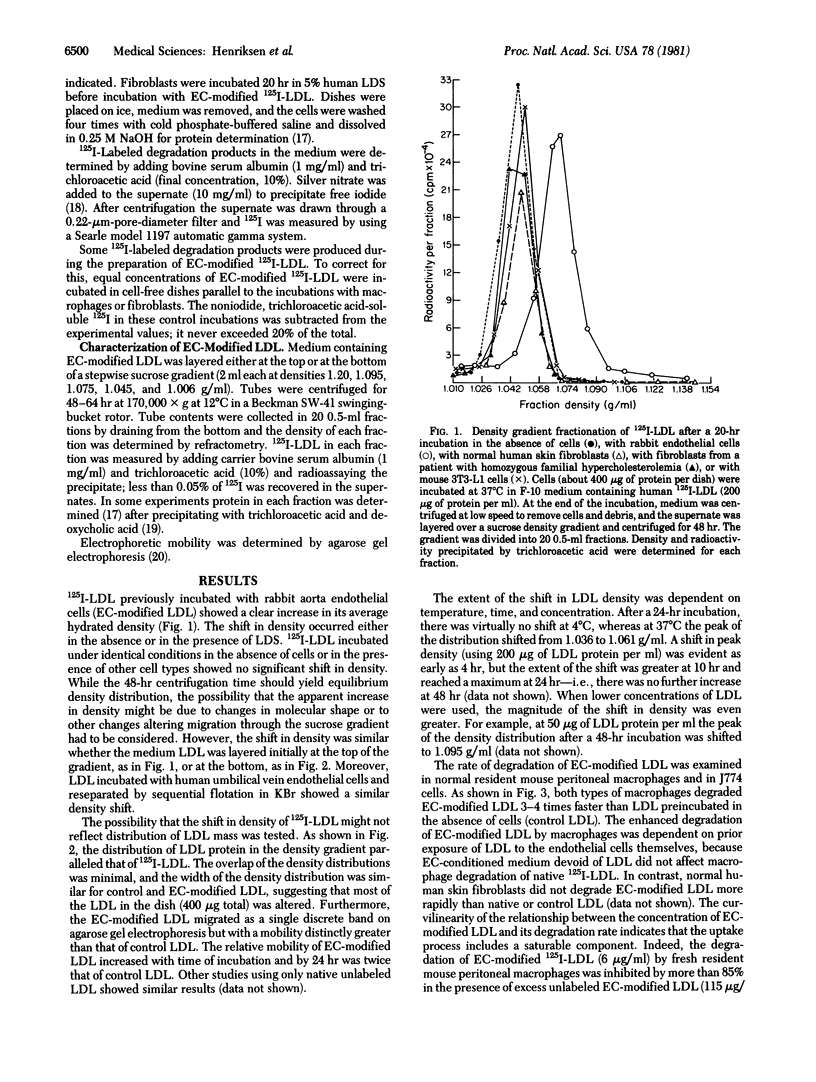
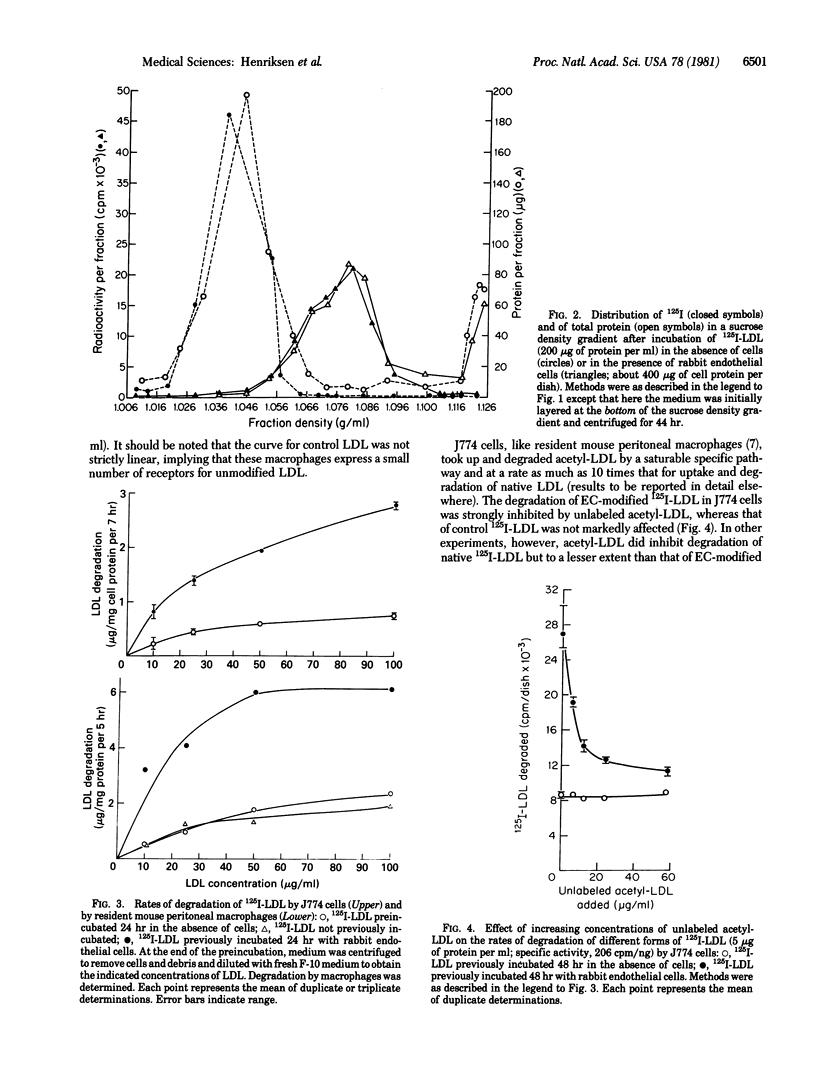
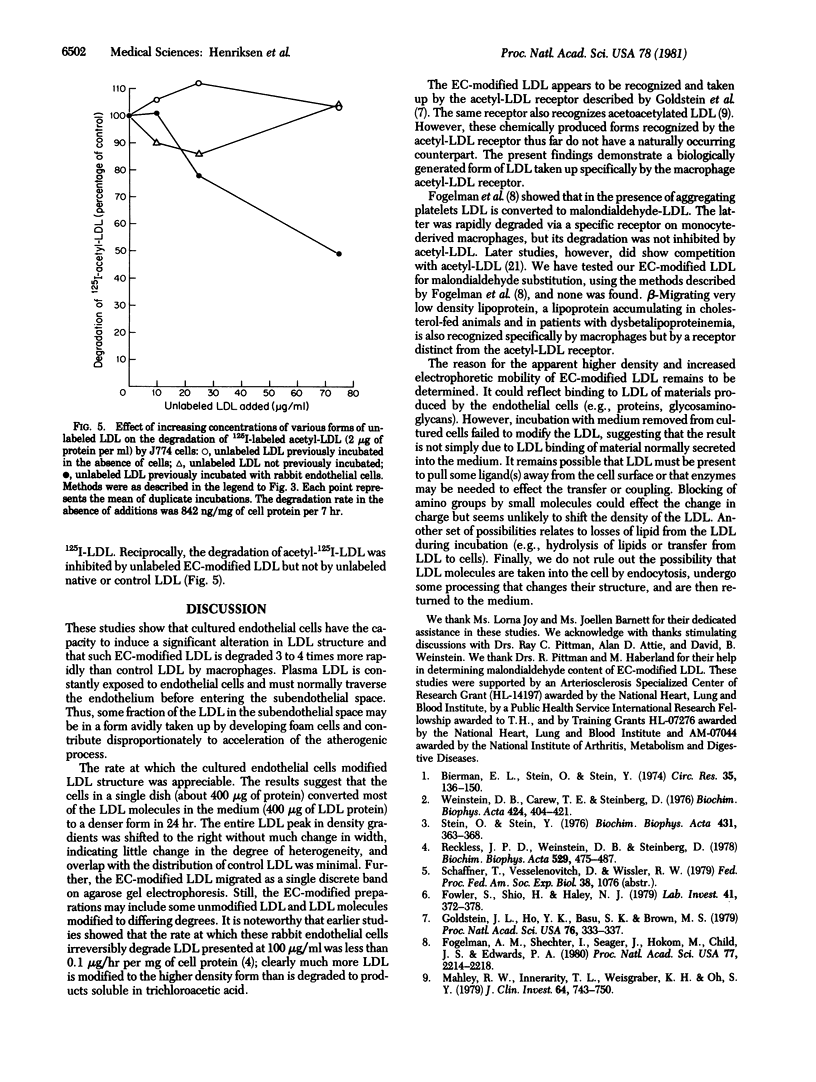
Human low density lipoprotein (LDL) was incubated with an established line of rabbit aortic endothelial cells. Density gradient fractionation showed a time-, concentration-, and temperature-dependent increase in the average density of the LDL (from about 1.036 to as high as 1.070 g/ml). Incubation without cells or with other types of cultured cells (fibroblasts, hepatocytes, 3T3-L1 cells) caused no significant change in density. 125I-Labeled LDL (125I-LDL) recovered after incubation with endothelial cells (EC-modified LDL) was taken up and degraded 3 to 4 times more rapidly than control LDL by resident mouse peritoneal macrophages and by an established tumor line of mouse macrophages (J774 cells). Macrophage degradation of EC-modified 125I-LDL exhibited saturation kinetics (greater than 85% inhibited by excess unlabeled EC-modified LDL). Degradation was also inhibited by unlabeled acetylated LDL and, conversely, unlabeled EC-modified LDL inhibited degradation of acetylated 125I-LDL. Incubation of LDL with conditioned medium-removed from endothelial cell cultures modified neither its density nor its rate of degradation by macrophages. These studies show that endothelial cells have the potential to metabolically modify the LDL molecule, generating a form that is more rapidly degraded by macrophages and that is recognized by the macrophage receptor for acetylated LDL. This process may play a significant role in the pathogenesis of atherosclerosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bierman E. L., Stein O., Stein Y. Lipoprotein uptake and metabolism by rat aortic smooth muscle cells in tissue culture. Circ Res. 1974 Jul;35(1):136–150. doi: 10.1161/01.res.35.1.136. [DOI] [PubMed] [Google Scholar]
- Buonassisi V., Root M. Enzymatic degradation of heparin-related mucopolysaccharides from the surface of endothelial cell cultures. Biochim Biophys Acta. 1975 Mar 14;385(1):1–10. doi: 10.1016/0304-4165(75)90067-7. [DOI] [PubMed] [Google Scholar]
- Buonassisi V., Venter J. C. Hormone and neurotransmitter receptors in an established vascular endothelial cell line. Proc Natl Acad Sci U S A. 1976 May;73(5):1612–1616. doi: 10.1073/pnas.73.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevon C. A., Attie A. D., Pangburn S. H., Steinberg D. Metabolism of homologous and heterologous lipoproteins by cultured rat and human skin fibroblasts. J Lipid Res. 1981 Jan;22(1):37–46. [PubMed] [Google Scholar]
- Fogelman A. M., Shechter I., Seager J., Hokom M., Child J. S., Edwards P. A. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S., Shio H., Haley N. J. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. IV. Investigation of macrophage-like properties of aortic cell populations. Lab Invest. 1979 Oct;41(4):372–378. [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M., Jensen D. F., Wancewicz E. V., Joy L. L., Khoo J. C., Steinberg D. Hormone-sensitive lipase in differentiated 3T3-L1 cells and its activation by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Feb;78(2):732–736. doi: 10.1073/pnas.78.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Weisgraber K. B., Oh S. Y. Altered metabolism (in vivo and in vitro) of plasma lipoproteins after selective chemical modification of lysine residues of the apoproteins. J Clin Invest. 1979 Sep;64(3):743–750. doi: 10.1172/JCI109518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney E. M., Hamill A. L., Scott W. A., Cohn Z. A. Response of endocytosis to altered fatty acyl composition of macrophage phospholipids. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4895–4899. doi: 10.1073/pnas.74.11.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N. E., Weinstein D. B., Carew T. E., Koschinsky T., Steinberg D. Interaction between high density and low density lipoproteins uptake and degradation by cultured human fibroblasts. J Clin Invest. 1977 Jul;60(1):78–88. doi: 10.1172/JCI108772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- Ralph P., Prichard J., Cohn M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J Immunol. 1975 Feb;114(2 Pt 2):898–905. [PubMed] [Google Scholar]
- Reckless J. P., Weinstein D. B., Steinberg D. Lipoprotein and cholesterol metabolism in rabbit arterial endothelial cells in culture. Biochim Biophys Acta. 1978 Jun 23;529(3):475–487. doi: 10.1016/0005-2760(78)90091-7. [DOI] [PubMed] [Google Scholar]
- Shechter I., Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. The metabolism of native and malondialdehyde-altered low density lipoproteins by human monocyte-macrophages. J Lipid Res. 1981 Jan;22(1):63–71. [PubMed] [Google Scholar]
- Stein O., Stein Y. High density lipoproteins reduce the uptake of low density lipoproteins by human endothelial cells in culture. Biochim Biophys Acta. 1976 May 27;431(2):363–368. doi: 10.1016/0005-2760(76)90157-0. [DOI] [PubMed] [Google Scholar]
- Weinstein D. B., Carew T. E., Steinberg D. Uptake and degradation of low density lipoprotein by swine arterial smoot muscle cells with inhibition of cholesterol biosynthesis. Biochim Biophys Acta. 1976 Mar 26;424(3):404–421. doi: 10.1016/0005-2760(76)90030-8. [DOI] [PubMed] [Google Scholar]



