Abstract
Studies relating sensory hearing impairment to lead (Pb) exposure in children have presented inconsistent results. The objective of this study was to measure distortion product otoacoustic emissions (DPOAE), sounds emanating from the outer hair cells of the inner ear, in Pb-exposed children to determine the effects of Pb poisoning on the inner ear. DPOAE were recorded for 9 f2 frequencies from 1187 to 7625 Hz on 102 ears of 53 Pb-exposed children (aged 6–16 years) residing in Pb-contaminated environments in the Andes Mountains of Ecuador where Pb-glazing of ceramics is the primary livelihood. Blood lead (PbB) levels ranged from 4.2 to 94.3 µg/dl (mean: 37.7; SD: 25.7; median: 36.4). The median PbB level was significantly higher than the CDC and WHO’s 10 µg/dl action level. Spearman rho correlation analyses of the relation between PbB level and DPOAE amplitude, and between PbB level and DPOAE signal-to-noise ratio revealed no significant associations at any of the f2 frequencies tested. In addition, no significant correlation (Spearman rho) between PbB level and hearing sensitivity for 6 pure-tone test frequencies from 1000–8000 Hz was found. Although the study group was found to have abnormally elevated PbB levels, in contrast to some earlier reports, the results of the current study showed no consistent Pb-induced sensory effects on the cochlea of Pb-intoxicated children.
Keywords: ototoxicity, cochlea, sensory-neural hearing loss, neurotoxicity
INTRODUCTION
Lead (Pb) exposure has been associated with adverse neurocognitive and neurobehavioral outcomes in children (Bellinger, 1996; Cole & Winsler, 2010), as well as with pediatric auditory neurosensory impairment (Holdstein et al., 1986; Schwartz & Otto, 1987, 1991; Dietrich et al., 1992; Osman et al., 1999; Lin et al., 2008). A number of recent review articles on the developmental consequences of Pb exposure, and a policy statement by the American Academy of Pediatrics conclude that Pb exposure induces hearing impairment or subclinical auditory sensory deficits (Markowitz, 2000; Lidsky & Schneider, 2003; American Academy of Pediatrics, 2005; Olympio et al., 2009; Rosin, 2009). However, studies of Pb exposure and auditory sensory function have shown mixed results. Some studies report an association between Pb exposure and cochlear (inner ear) hearing impairment in both children and adults (Schwartz & Otto, 1987; 1991; Farahat, et al., 1997; Forst et al., 1997; Osman, et al., 1999; Wu et al., 2000; Chuang et al., 2007; Lin et al., 2008; Hwang, et al., 2009; Park et al., 2010), leading some investigators to suggest that Pb-induced hearing loss in children may contribute to the neurodevelopmental learning disabilities that are associated with pediatric Pb exposure (Schwartz & Otto, 1987; Anderson et al., 1996; Bellinger, 1996). Other studies found no unequivocal association between Pb exposure and sensory hearing loss or any change in auditory sensitivity (Baloh et al., 1979; Otto et al., 1985; Counter et al., 1997a, 1997b; Buchanan et al., 1999; Counter & Buchanan, 2002; Alvarenga et al., 2003; Martins et al., 2007). Our previous investigations of children living in highly Pb-contaminated environments in rural Andean villages of Ecuador revealed extremely elevated blood lead (PbB) levels (median: 40–52 µg/dl; range: 6–128 µg/dl), but the results showed no statistical association between PbB levels and auditory sensory function (Counter et al., 1997a, 1997b; Buchanan et al., 1999; Counter & Buchanan, 2002). These disparate findings on the effects of Pb poisoning on the auditory system suggest that Pb exposure is not invariably associated with hearing impairment, and that behavioral auditory sensitivity measures may not be a reliable marker of Pb toxicity.
A more direct, and objective technique for examining inner ear integrity involves the physiologic measurement of otoacoustic emissions, a noninvasive procedure that is routinely used to screen infants’ hearing as part of universal newborn hearing screening programs. Otoacoustic emissions refer to sounds generated by the active mechanoelectric transduction processes of intact cochlear sensory cells, the outer hair cells of the inner ear. These low intensity level sounds are transmitted in a retrograde manner from the inner ear to the ear canal, where they are recorded in a noninvasive manner via a miniature microphone placed at the entrance to the ear canal. (Kemp, 1978; Brownell, 1990; Lonsbury-Martin et al., 1991; Trautwein et al., 1996). The outer hair cells are quite susceptible to acoustic trauma or certain toxic agents resulting in a reduction or absence of otoacoustic emissions (Lonsbury-Martin et al., 1991). In fact, otoacoustic emissions were found to be diminished in individuals treated with ototoxic medications, such as aminoglycoside antibiotics or cisplatin, even before changes in behavioral auditory thresholds become apparent (Mulheran & Degg, 1997; Lonsbury-Martin & Martin, 2001; Stavroulaki et al., 2001, 2002). Thus, if Pb exposure exerts and adverse effect on the inner ear, otoacoustic emissions may be a more sensitive index of Pb-induced auditory sensory impairment than behavioral pure-tone thresholds, which were used in most previous Pb-exposure studies to assess hearing status. A preliminary otoacoustic emissions study by Buchanan et al. (1999) on 14 Pb-exposed children found no unequivocal associations of Pb-exposure with hearing impairment. However, in light of more recent investigations (Wu, et al., 2000; Chuang, et al., 2007; Lin, et al., 2008; Hwang et al., 2009; Park et al., 2010) that continue to implicate Pb-exposure as a cause of hearing impairment, it was thus scientifically germane to conduct an otoacoustic emissions study of inner ear function on a larger sample, and a different group of Pb-exposed children.
The clinically advanced otoacoustic emissions technique for measuring the status of outer hair cells of the inner ear has rarely been used to examine Pb-exposed ears, especially in children. The primary aim of the current study was to evaluate the impact of Pb exposure at the cochlear level by examining possible subclinical effects through observation of distortion product otoacoustic emissions (DPOAE) in a large sample of children with chronic exposure to Pb.
METHODS
Study Area
The results in this field study were obtained from participants who resided in two rural ceramic production and Pb-glazing Ecuadorian Andean villages, La Victoria and Racar. La Victoria is a village of several neighborhoods in Pujilí County, Cotopaxi Province, and is located about 125 km south of Quito, Ecuador at an altitude of approximately 2,800 m in the Andes Mountains. The village of Racar is located approximately 15 km west of the southern Ecuadorian city of Cuenca in Azuay Province at an altitude of approximately 2,500 m above sea level. The inhabitants of these villages were exposed to Pb from a ceramics Pb-glazing cottage industry that produces artisan crafts and roofing tiles. The Pb-glazing process involves extracting the Pb acid from used automobile storage batteries, as well as utility batteries, churning the extracted Pb into a slurry, and manually applying the suspension to the ceramic objects. The Pb-coated items are then baked at a high temperature in open kilns or ovens. The Pb-laden, thick smoke resulting from the baking process pollutes the immediate environment, and contributes to the high Pb contamination of the air, soil, dust, and food produce. As a consequence, the inhabitants of the study area have chronically elevated PbB levels (Counter et al., 1997a, 2000, 2008, 2009a, 2009b; Vahter et al., 1997). The children and adult inhabitants of the study area are essentially a homogeneous population in that they reside in rural communities in the Andes Mountains of Ecuador, are of low socioeconomic and educational status, engage in similar Pb-glazing occupational practices, and have identical pathways of environmental Pb exposure.
Study Participants
Informed consent was obtained from the parents of the children who participated in the study. The study was conducted under the auspices of Universidad San Francisco de Quito, and was approved by the Comité de Bioética (Human Studies Committee) of Universidad San Francisco de Quito. Audiologic testing was performed on both ears of 60 children as part of a comprehensive medical examination at our temporary field clinics. The children had no known family history of hereditary hearing loss, and showed no apparent evidence of vestibular dysfunction. Children with ear canal or middle ear pathology, as determined by otoscopy, acoustic immittance of the middle ear, and pure-tone threshold measurements, were not included in the data analysis for the current study. Seven of the 60 children tested were not included in the data analysis because of ceruminosis, conductive hearing loss, abnormal tympanometric results bilaterally, unreliable pure-tone threshold data, or unreliable otoacoustic emissions recordings. Data included in this study are from 102 ears (52 right and 50 left ears) of 53 Pb-exposed children. The children ranged in age from 6–16 years (median: 10.8 years.; mean: 10.8 years.; SD: 2.8), and included 28 males (mean age: 10.7 years.; SD: 3; age range: 6.5–16) and 25 females (mean age: 10.9 years; SD: 2.7; age range: 6–15). The Pb-exposed children are mainly of Ecuadorian indigenous Quechua Indian and Mestizo (mixture of Spanish and indigenous Indian ethnicity) backgrounds. The results for the Pb-exposed children were compared to 33 ears of a DPOAE reference group (age range: 7.3–30 years.; median: 12.9 yrs.; mean: 15.6 years.; SD: 6.5) with normal hearing (hearing level ≤ 20 dB) as determined by pure-tone threshold testing, and a negative history of exposure to noise, Pb or other neurotoxic or ototoxic agents. The non-Pb-exposed group consisted of U.S. and Ecuadorian children and young adults.
Blood Lead Analysis
Blood samples were collected by trained Ecuadorian medical personnel. Following a thorough cleaning of the skin using isopropanol swabs, 2–4 ml of blood were drawn from the antecubital vein of each participant and stored in 4 ml-Vacutainer tubes with Li-heparin. All blood samples were stored in a refrigerated container and later analyzed for Pb concentrations by graphite furnace atomic absorption spectrometry (GFAAS) with Zeeman background correction at Boston Children’s Hospital Department of Laboratory Medicine, or by inductively coupled plasma mass spectrometry (ICP-MS) at the Channing Trace Metals Laboratory of the Harvard School of Public Health. Previous investigations found that these two PbB analysis procedures are comparable (Counter et al., 1998, 2000). In the current study, PbB levels were determined for 29 of the participants using both the ICP-MS and GFAAS procedures. The high correlation coefficients obtained using both Spearman rho (rho = 0.944, p = < 0.0001) and Pearson r (r = 0.961, p = < 0.0001) correlation analyses on the PbB levels for the 29 participants showed that the two PbB analysis techniques provided comparable results for the current study. The PbB data for the children were summarized by grouping them in accordance with the classification system of the Centers for Disease Control and Prevention (CDC, 1991),which is as follows: Classes I (< 10 µg/dl : not considered Pb-poisoned), IIA (10–14.9 µg/dl: abnormally elevated PbB level; frequent re-screening and community Pb prevention activities recommended), IIB (15–19.9 µg/dl : nutritional, educational and environmental interventions and frequent screening recommended), III (20–44.9 µg/dl : environmental evaluation and remediation, and medical evaluation recommended; pharmacological treatment may be indicated), IV (45–69.9 µg/dl : environmental and medical interventions, including chelation therapy recommended), and V (≥ 70 µg/dl : medical emergency; immediate medical and environmental management recommended).
Audiologic Test Procedures
Otoscopy, and acoustic immittance testing were performed to rule out ear canal, tympanic membrane or middle ear pathology. Pure-tone bone conduction thresholds were obtained only in cases where air conduction thresholds were abnormal. All audiologic test data were obtained concurrently with the collection of blood samples from the study participants, thus precluding any inadvertent audiologic assessment bias.
Pure-Tone Thresholds
Participants were instructed in Spanish to respond to the tonal stimuli no matter how faint. Pure-tone air conduction threshold data were collected in the field in a quiet location using the Interacoustics (Model AD 12, Copenhagen, Denmark) and the Tegner (Model PTA-8, Stockholm, Sweden) portable audiometers with standard TDH 39 and TDH 49 earphones (calibrated to ANSI S3.6–1996/ISO-1998). In addition to the electroacoustic calibration of the audiometers in the lab, biological calibration checks of the audiometers were performed in the lab, and on a daily basis in the field on a staff member whose thresholds had been determined in the lab prior to field deployment. Pure-tone threshold data were obtained using a conventional descending-ascending threshold crossing procedure in which the stimulus intensity was decreased in 10-dB steps, and increased in 5-dB steps. The pure-tone threshold was defined as the minimum hearing level (HL) at which the participant responded accurately at least two times on ascending trials. The a priori intra-test reliability was set at 5 dB at 1000 Hz.
Distortion Product Otoacoustic Emissions
DPOAE results were recorded for both ears following pure-tone threshold determination. The 2f1–f2 cubic difference frequency distortion product was measured in the field in a quiet test environment using the GSI-60 DPOAE system (Grason-Stadler, Inc., Eden Prairie, MN, USA). To elicit the 2f1–f2 distortion product, primary tone pairs (f1 and f2) were presented via a non-invasive probe tip placed at the entrance to the ear canal, and a miniature microphone housed in the probe tip was used to record the responses. The f1 primary tone was presented at 65 dB sound pressure level (SPL) (L1), and the f2 primary tone was presented at 55 dB SPL (L2). The L1/L2 (65/55 dB SPL) ratio was maintained within 1-dB in the ear canal of each participant, and the f2/f1 ratio was held constant at 1.2. DPOAE data were obtained for both ears of most participants in a three-octave range encompassing the frequency region of 1000–2000, 2000–4000, and 4000–8000 Hz, and plotted as a function of the f2 frequency, since the response of the cochlear sensory cells corresponds more closely with the f2 tonotopic location. Unlike a controlled clinical environment, in field investigations, there are inherent uncontrollable variables and constrains, such as participants’ availability, work and school schedules, and time limitations. Because of some of these constraints, replicate DPOAE data could not be obtained on 7 (13%) of the 53 participants. Therefore, the mean of the replicate DPOAE recordings obtained on 87% of the participants and the single DPOAE recordings obtained on 13% of participants were analyzed for the following f2 frequencies: 1187, 1500, 1906, 2406, 3031, 3812, 4812, 6031, and 7625 Hz. A sampling rate of 16,000 Hz and three data points per octave were used for DPOAE analysis. A DPOAE was accepted as a response if the amplitude of the emission was 6 dB or greater than the average noise floor recorded from the ear canal.
Statistical Analysis
Means, standard deviations, medians, and ranges were computed for PbB level, pure-tone threshold, DPOAE amplitude, DPOAE noise floor, and DPOAE signal-to-noise ratio. Since many of the variables had skewed distributions, nonparametric statistical tests, as well as parametric tests, were used for data analysis. The Mann-Whitney U test and the unpaired t-test were used to analyze differences in DPOAE amplitude between the Pb-exposed children and a non-Pb-exposed reference group. The associations of PbB level with pure-tone threshold, and PbB level with DPOAE variables (amplitude, and signal-to-noise ratio) were probed with the Spearman correlation coefficient. An a priori alpha level of ≤ 0.05 was accepted as indicative of statistical significance.
RESULTS
Blood lead levels
The PbB levels for the 53 children ranged from 4.2 to 94.3 µg/dl with a mean PbB level of 37.7 µg/dl (SD: 25.7; median: 36.4 [CDC Classification III]). The data distribution indicated that 11 of the children had PbB levels of less than 10 µg/dl (CDC I or action line). Of the remaining 42 children, 10 had PbB levels in the CDC II (10–19.9 µg/dl), 8 in the CDC III (20–44.9 µg/dl), 20 in the CDC IV (45–69.9 µg/dl), and 4 had PbB levels that placed them in the CDC V Classification (≥ 70 µg/dl), which calls for emergency medical attention.
Pure-Tone Thresholds
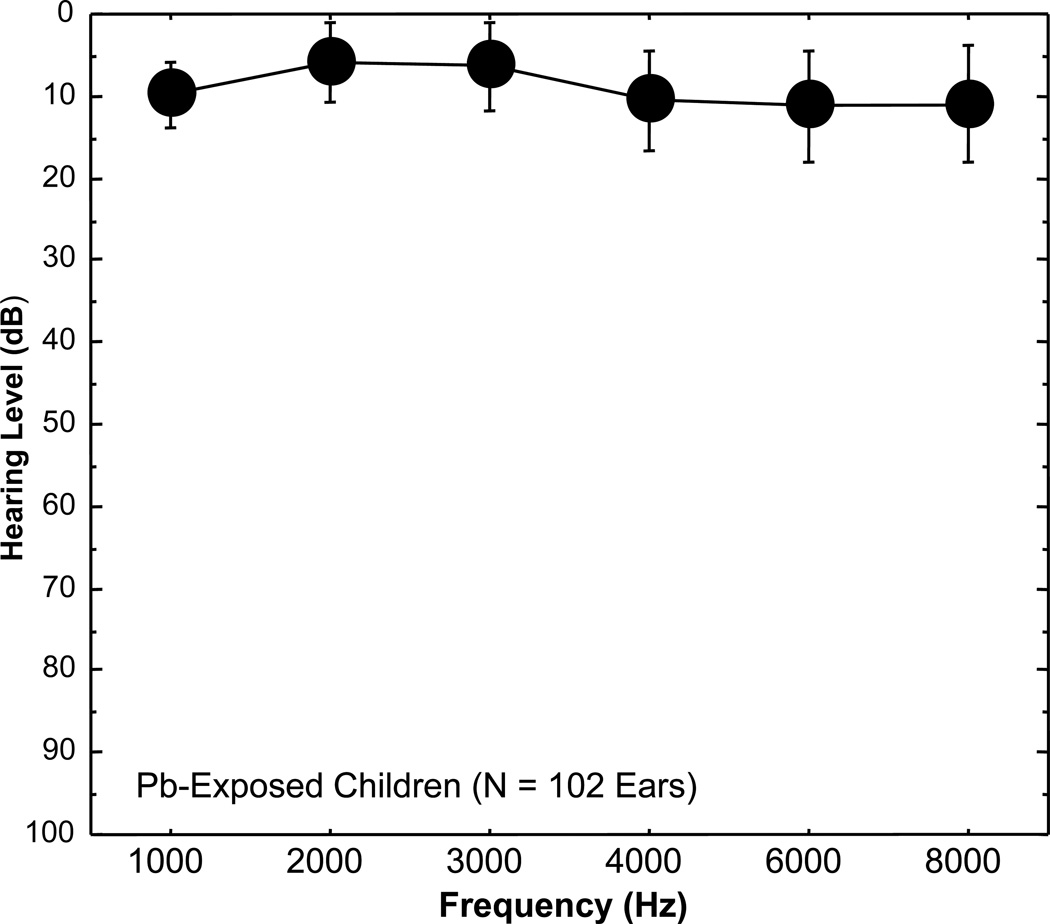
Mean hearing thresholds (dB HL) for 102 ears of the 53 Pb-exposed children are illustrated in a traditional audiogram format in Figure 1 for 1000, 2000, 3000, 4000, 6000 and 8000 Hz. The results indicated normal hearing (HLs ≤ 20 dB) for the children despite their high PbB levels and chronic environmental Pb exposure. Mean HLs for the children ranged from 5.9 dB (SD: 4.8) at 2000 Hz to 11.2 dB (SD: 6.8) at 6000 Hz. To determine if auditory sensitivity correlated with Pb exposure, the relation of pure-tone threshold to PbB level for the 102 ears at each test frequency was probed with the Spearman rho correlation analysis. As shown in Table 1, the resulting analysis revealed no significant associations between PbB level and hearing threshold for any of the pure-tone test frequencies.
Figure 1.
Mean hearing levels (HL) with standard deviation bars are plotted as a function of frequency (1000, 2000, 3000, 4000, 6000 or 8000 Hz) using a traditional audiogram format for 102 ears of 53 lead (Pb) exposed children residing in rural Pb-glazing areas in the Andes Mountains of Ecuador. Note that on the traditional audiogram, zero is plotted at the top of the graph. The audiogram shows that the Pb-exposed children’s mean hearing thresholds are well within the normal range (≤ 20 dB HL).
Table 1.
Spearman rho correlation coefficients for blood lead (PbB) level and hearing level (HL) [pure-tone threshold] for 102 ears of Pb-exposed Andean children for the frequencies 1000, 2000, 3000, 4000, 6000 and 8000 Hz. The values for rho are corrected for ties, and the p-values are tied p-values. NS = non-significant
| Comparison Variables | rho | P | Significance |
|---|---|---|---|
| PbB (µg/dL), HL at 1000 Hz | −0.170 | 0.088 | NS |
| PbB (µg/dL), HL at 2000 Hz | −0.073 | 0.462 | NS |
| PbB (µg/dL), HL at 3000 Hz | 0.002 | 0.982 | NS |
| PbB (µg/dL), HL at 4000 Hz | −0.005 | 0.959 | NS |
| PbB (µg/dL), HL at 6000 Hz | −0.060 | 0.546 | NS |
| PbB (µg/dL), HL at 8000 Hz | −0.176 | 0.079 | NS |
Distortion Product Otoacoustic Emissions
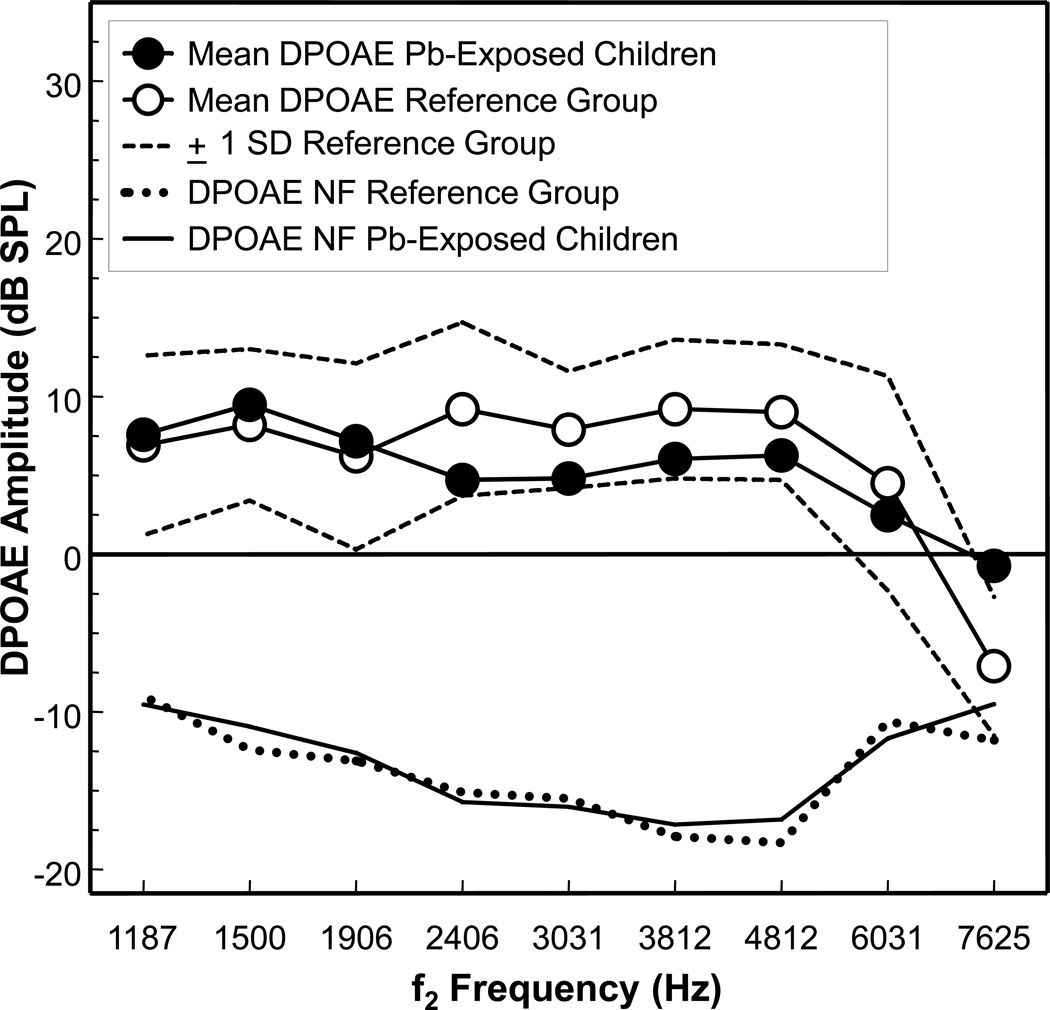
Mean DPOAE amplitude as a function of f2 frequency for the 102 ears of the Pb-exposed children are shown in Figure 2. The results for the Pb-exposed children are compared to 33 ears of a non-Pb-exposed DPOAE reference group. The prevalence of DPOAE meeting the 6-dB or greater S/N ratio acceptance criterion for the Pb-exposed children ranged from 94% to 100% for f2 frequencies from 1187 to 4812 Hz. For f2 frequencies of 6031 and 7625 Hz, the prevalence was 78% and 85%, respectively. These prevalence rates are similar to the DPOAE data of the reference group, which also ranged from 94–100% for f2 frequency from 1187 to 4812 Hz, and 91% for f2 frequencies 6031 and 7625 Hz. The mean DPOAE amplitude for the Pb-exposed children ranged from a low of 2.5 dB SPL at f2 6031 Hz to a high of 9.5 dB SPL at f2 1500 Hz. The mean DPOAE amplitude for the reference group ranged from a low of 4.5 dB SPL at f2 6031 Hz to a high of 9.2 dB SPL at f2 2406 and 3812 Hz. The mean DPOAE amplitude levels of −0.7 and −7.1 dB SPL at f2 7625 Hz for the Pb-exposed and reference groups, respectively, are rather low and thus, may not reliably represent true DPOAE, especially for the reference group. Using the Mann-Whitney U test and the unpaired t-test for statistical analyses, mean DPOAE amplitude for the Pb-exposed children were found to be significantly different from the reference group at f2 frequencies of 2406 Hz (tied Z = −3.58, tied p = 0.0003; t = −3.56, p = 0.0005), 3031 Hz (tied Z = −2.69, tied p = 0.007; t = −2.60, p = 0.010), 3812 Hz (tied Z = −2.72, tied p = 0.006; t = −2.53, p = 0.012), and 4812 Hz (tied Z = −2.53, tied p = 0.012; t = −2.19, p = 0.030). The statistically significant differences notwithstanding, further inspection of Figure 3 reveals that the DPOAE amplitudes for the Pb-exposed children were never unequivocally outside of the normal ± 1 SD range of the reference group. There were no significant differences in DPOAE amplitudes between the Pb-exposed children and the reference group at f2 frequencies of 1187, 1500, 1906, and 6031 Hz. Because of the small number of ears (7) for the reference group at f2 7625, a statistical comparison was not made at this frequency.
Figure 2.
Mean distortion product otoacoustic emissions (DPOAE) amplitude (dB SPL) for 102 ears of 53 lead (Pb)-exposed children living in rural Pb-glazing villages in the Andes Mountains of Ecuador. Mean DPOAE amplitude data for the Pb-exposed children are compared to mean DPOAE data of 33 ears of a reference group of non-Pb exposed children and young adults. The broken lines in the upper portion of the graph represent the ± 1 standard deviation range for the reference group. Mean DPOAE noise floors (NF) are shown as solid and dotted lines at the bottom of the graph for the Pb-exposed and reference groups, respectively. The N at 7625 Hz consists of 45 ears of the Pb-exposed group and 7 ears of the reference group. Mean DPOAE amplitude for the Pb-exposed children were significantly different from the reference group at f2 frequencies of 2406 Hz (p = 0.0003), 3031 Hz (p = 0.007), 3812 Hz (p = 0.006), and 4812 Hz (p = 0.012). See text for details of the statistical analyses.
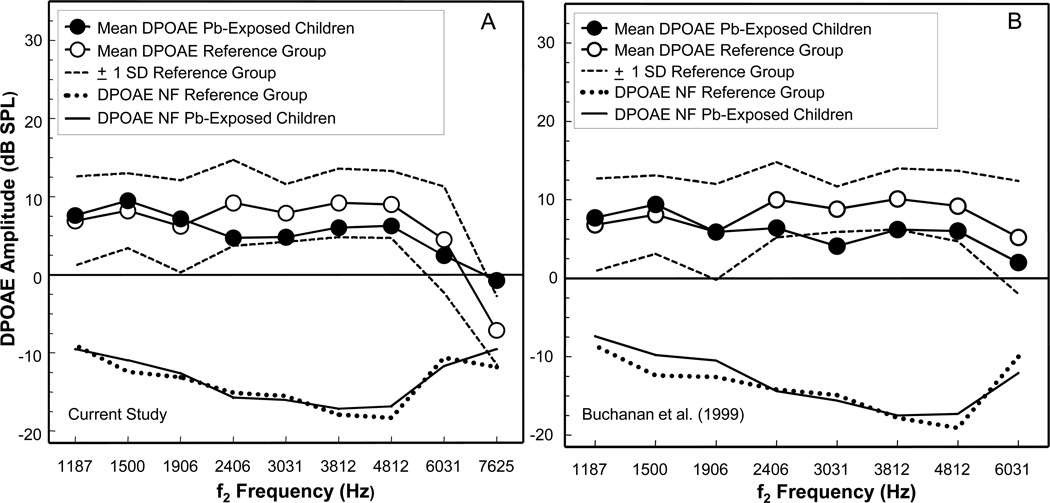
Figure 3.
Mean distortion product otoacoustic emissions (DPOAE) amplitude (dB SPL) data obtained in the current study (A) for 102 ears of 53 lead (Pb)-exposed children residing in rural Pb-glazing villages in the Ecuadorian Andes are compared to DPOAE results obtained in a previous study (B) on a different group of 28 ears of 14 Pb-exposed children residing in the same study area. For both graphs, mean DPOAE amplitude for the Pb-exposed children are compared to mean DPOAE amplitude of a reference group (N = 33 and 26 for graph A and B, respectively) of non-Pb exposed children and young adults. The broken lines in the upper portion of the graphs represent the ± 1 standard deviation range for the reference group. Mean DPOAE noise floors (NF) are shown as solid and dotted lines at the bottom of each graph for the Pb-exposed and reference group, respectively. Note that graph A includes the f2 frequency of 7625 Hz, which is not included in graph B. Figure 2B is adapted from Buchanan et al. (1999).
The association between PbB level and DPOAE amplitude for the Pb-exposed children was analyzed using the Spearman rho correlation coefficient. The results of this analysis are presented in Table 2, which shows no significant associations between PbB level and DPOAE amplitude at any of the f2 frequencies tested for the Pb-exposed children. Figure 3A, 3B presents a comparison of results obtained in the current study (A) to those obtained in a previous investigation (B) by the authors on a smaller group of Pb-exposed children from the same Pb-glazing area (Buchanan et al., 1999). As can be seen from Figure 3, the otoacoustic emissions data obtained in the current investigation on a larger group of children are remarkably similar to those obtained in the earlier field study (B).
Table 2.
Spearman rho correlation coefficients for blood lead (PbB) level and distortion product otoacoustic emissions (DPOAE) amplitude at the f2 frequencies 1187, 1500, 1906, 2406, 3031, 3812, 4812, 6031 and 7625 Hz for Pb-exposed Andean Children. N = 102 ears, except at 7625 Hz where N = 45. The values for rho are corrected for ties, and the p-values are tied p-values. NS = non-significant.
| Comparison Variables | rho | P | Significance |
|---|---|---|---|
| PbB (µg/dL), DPOAE at f2 1187 Hz | 0.104 | 0.295 | NS |
| PbB (µg/dL), DPOAE at f2 1500 Hz | 0.025 | 0.803 | NS |
| PbB (µg/dL), DPOAE at f2 1906 Hz | 0.036 | 0.715 | NS |
| PbB (µg/dL), DPOAE at f2 2406 Hz | −0.114 | 0.251 | NS |
| PbB (µg/dL), DPOAE at f2 3031 Hz | −0.117 | 0.239 | NS |
| PbB (µg/dL), DPOAE at f2 3812 Hz | −0.140 | 0.161 | NS |
| PbB (µg/dL), DPOAE at f2 4812 Hz | −0.147 | 0.139 | NS |
| PbB (µg/dL), DPOAE at f2 6031 Hz | −0.072 | 0.467 | NS |
| PbB (µg/dL), DPOAE at f2 7625 Hz | −0.232 | 0.123 | NS |
DPOAE responses were also examined by analyzing the DPOAE signal-to-noise (S/N) ratio, which is calculated as the DPOAE amplitude level in dB SPL minus the noise floor level in dB SPL. The noise floor level refers to the noise in the enclosed ear canal that is generated by the participant’s breathing and movement, environmental noise, test microphone noise, or blood flow noise. As seen in Figure 2, the DPOAE amplitude for the Pb-exposed children are well above the noise floor of the ear canal, with mean S/N ratios ranging from 8.8 dB at f2 7625 Hz to 23.0 dB at f2 3812 Hz. These levels were similar to those of the reference group, which ranged from 4.6 dB at f2 7625 Hz to 27.2 dB at f2 4812 Hz. To ascertain if the S/N ratio was associated with PbB level, Spearman rho correlation analyses were performed. Similar to the analysis for DPOAE amplitude, the Spearman correlation coefficients did not show any statistically significant relations between PbB level and DPOAE S/N ratio at any of the f2 frequencies (see Table 3).
Table 3.
Spearman rho correlation coefficients for blood lead (PbB) level and distortion product otoacoustic emissions signal-to-noise ratio (S/N) at the f2 frequencies 1187, 1500, 1906, 2406, 3031, 3812, 4812, 6031 and 7625 Hz for Pb-exposed Andean children. N = 102 ears, except at 7625 Hz where N = 45. The values for rho are corrected for ties, and the p-values are tied p-values. NS = non-significant.
| Comparison Variables | rho | P | Significance |
|---|---|---|---|
| PbB (µg/dL), S/N at f2 1187 Hz | 0.168 | 0.091 | NS |
| PbB (µg/dL), S/N at f2 1500 Hz | 0.135 | 0.174 | NS |
| PbB (µg/dL), S/N at f2 1906 Hz | 0.128 | 0.197 | NS |
| PbB (µg/dL), S/N at f2 2406 Hz | −0.018 | 0.854 | NS |
| PbB (µg/dL), S/N at f2 3031 Hz | −0.112 | 0.259 | NS |
| PbB (µg/dL), S/N at f2 3812 Hz | −0.140 | 0.159 | NS |
| PbB (µg/dL), S/N at f2 4812 Hz | −0.153 | 0.125 | NS |
| PbB (µg/dL), S/N at f2 6031 Hz | −0.072 | 0.467 | NS |
| PbB (µg/dL), S/N at f2 7625 Hz | −0.211 | 0.162 | NS |
DISCUSSION
The current study, which used DPOAE to investigate the outer hair cell system of the inner ear in Andean children with chronic Pb poisoning from Pb-contaminated living environments, found no unequivocal evidence to support the notion that Pb exposure is associated with sensory or cochlear hearing impairment. The results are consistent with other investigations conducted by the authors on different groups of Pb-exposed children from the same study area, using otoacoustic emissions, pure-tone thresholds and auditory brainstem responses as outcome measures (Counter et al., 1997a; 1997b; Buchanan et al., 1999; Counter, 2002). While the Pb-exposed children in the current study showed DPOAE amplitudes that were within the normal ±1 SD range of the reference group, their DPOAE amplitudes were significantly reduced relative to the reference group at the f2 frequencies of 2406, 3031, 3812, and 4812 Hz. Since DPOAE amplitude was not correlated with PbB level in the Pb-exposed children, the amplitude difference in DPOAE between the Pb-exposed children and the reference group is presumed to be related to other variables not evaluated in this study. The most likely explanation is that the reference group had significantly better hearing thresholds than the Pb-exposed children for the 1000–6000 Hz pure-tone test frequency range. There was no significant difference in pure-tone thresholds between the groups at 8000 Hz. The difference in pure-tone thresholds between the Pb-exposed children and the reference group is not surprising, since the reference group was chosen on the basis of having normal hearing (pure-tone thresholds ≤ 20 dB HL), whereas, the Pb-exposed children were excluded from the study only if there were indications of ear canal or middle ear pathology or family history of deafness. As a result, some of the Pb-exposed children included in the study had HL of 25 and 30 dB.
Of the previous studies that showed an association between PbB level and auditory sensory function, the ones that are most relevant to the current investigation are the 4 studies that found a relation between pediatric Pb exposure and sensory hearing dysfunction (Schwartz & Otto, 1987, 1991; Osman et al., 1999; Lin et al., 2008). An analysis of PbB and hearing threshold data collected during the second U.S. National Health and Nutrition Examination Survey (NHANES II, 1976–1980) (Schwartz & Otto, 1987) and the Hispanic Health and Nutrition Examination Survey (HHANES, 1982–1984) (Schwartz & Otto, 1991) for 4,519 and 3,262 children and adolescents, respectively, indicated that hearing thresholds were significantly elevated by Pb exposure. Despite the fact that median hearing thresholds for all test frequencies were ≤ 10 dB HL in both surveys, it was concluded that Pb exposure (even below 10 µg/dl) was associated with “subtle” or “minor” hearing loss (Schwartz & Otto, 1987, 1991). For example, the HNANES results showed a 2-dB increase in hearing threshold for increments in PbB levels from 7–18 µg/dl (Schwartz & Otto, 1991). Considering the NHANES and HHANES results (Schwartz & Otto, 1987, 1991), one would have expected the children in the current study and in our previous studies, who were both chronically Pb-exposed and high PbB levels, to have clinically significant sensory hearing impairment, since the effects of ototoxic agents are dose-related. As previously indicated, the children in the current study had normal mean hearing thresholds with no statistically significant threshold-PbB or DPOAE-PbB associations. The NHANES and HHANES (Schwartz & Otto, 1987, 1991) results are somewhat difficult to interpret because audiometer nonlinearities at low intensity levels necessitated that all HLs less than 0 dB be grouped together at 0 dB HL, or required a constant to be added to the threshold. It is unclear how this may have affected the hearing threshold-PbB relation. The NHANES and HHANES (Schwartz & Otto, 1987, 1991) data may be examples of large sample sizes producing significant, but small 1–2 dB effects that would have no clinical, biological or medical significance, and would not be detected in the clinic since pure-tone audiometric hearing thresholds are obtained in 5-dB steps. Unfortunately, at the time the NHANES (1976–1980) and HHANES (1982–1984) survey data were collected, otoacoustic emissions were either not discovered or were not in general clinical use for assessment of inner ear sensory impairment.
Osman et al. (1999) using pure-tone thresholds as an outcome measure, also reported that hearing thresholds increased significantly with increasing PbB levels at all test frequencies for 155 children (aged 4–14 years) with low PbB concentrations (median PbB: 7.2 µg/dl) living in Katowice, Poland. Most of the children in the Katowice study (Osman et al., 1999) had low PbB levels (approximately 69% of the children had PbB levels less than 10 µg/dl, 61% had PbB levels less than 5 µg/dl, and 88% of children had PbB levels ≤ 15 µg/dl), indicating a rather restricted exposure range. It was concluded that auditory function in the Katowice children was impaired even at PbB levels below 10 µg/dl, despite the fact that the children had normal mean hearing thresholds ranging from 10.3 dB HL at 1000 Hz to 15.1 dB HL at 6000 Hz. Furthermore, 43 of the 155 children in the Katowice study showed evidence of middle ear pathology, which can adversely affect pure-tone thresholds. Interestingly, in the Katowice study, the highest regression coefficient for pure-tone threshold and PbB level occurred at 500 Hz. This is inconsistent with ototoxic damage, considering that ototoxins usually damage the basal end of the cochlea partition (the high frequency place) more prominently than its apical end (the low frequency place), at least initially. Otoacoustic emissions, which would have been a more direct measure of the effects of Pb on the sensory status of the inner ear, were not recorded in the Katowice investigation.
Aside from the current study and Buchanan et al. (1999), only one other study, Lin et al. (2008), used otoacoustic emissions to investigate sensory hearing impairment in Pb-exposed children. Unfortunately, only the English abstract of the Lin et al. (2008) study is available (the full text is published in an inaccessible Chinese journal). According to the abstract, results obtained on 256 children (aged 6–7 years) living near a Pb and zinc mining area in China revealed a significantly negative correlation between PbB level and DPOAE S/N ratio. The PbB distribution or mean PbB level was not specified in the abstract. Because no mention is made in the abstract of the Lin et al. (2008) study of an association between PbB level and DPOAE amplitude, or PbB level and pure-tone threshold, it may be assumed that no significant relations were found among these variables. Since the DPOAE S/N ratio is an indirect reflection of the DPOAE amplitude, it is difficult to understand why the S/N ratio would be significantly correlated with PbB level and the DPOAE amplitude would not.
There are several nonhuman primate experimental studies that are relevant to the issue of whether Pb exposure produces sensory hearing impairment. Lasky et al. (1995) investigated DPOAE in monkeys (Macaca mulatta) exposed to Pb early in life and found that only 2 of 11 Pb-exposed monkeys had abnormal DPOAE (1 of the 2 monkeys was later discovered to have a conductive hearing loss [Lasky et al., 2001a]). In follow-up investigations, using DPOAE and other physiologic auditory measures with monkeys, no evidence was found for Pb effects on middle ear, auditory sensory or auditory neural functioning (Lasky et al., 2001a, 2001b). In a recent investigation by Laughlin, et al. (2009), mean behavioral pure-tone thresholds for 5 monkeys (Macaca mulatta) exposed to Pb during early development were observed to be elevated compared to 5 control monkeys for frequencies from 125–8000 Hz, but this difference was statistically significant only at 250 Hz. As previously indicated, if one assumes that Pb is an ototoxin, significant effects would be expected at the higher frequencies more so than lower frequencies. It is difficult to draw any firm conclusions from the Laughlin, et al. (2009) study because of the small sample size, and because the Pb-exposed monkeys were reported to be more difficult to test and less engaged in the auditory threshold task than controls. The less than optimal task performance behaviors by the Pb-exposed monkey may have contributed to suprathreshold responses unrelated to Pb exposure. Another study (Rice, 1997) found 3 of 6 monkeys (Macaca fascicularis) with chronic (lifetime) Pb exposure to have quantitatively elevated pure-tone thresholds relative to non-Pb-exposed control monkeys, but the threshold configuration was not consistent with that typical of ototoxicity. Other animal studies also found no significant Pb-related effects on the cochlea. Experimental studies using guinea pigs exposed to Pb, or in vitro experiments on dissociated guinea pig outer hair cells bathed in Pb acetate found no apparent morphological changes in the cochlea, no marked effects on the endocochlear potential or the cochlear microphonic response, and no significant effects on outer hair cell electromotility (the process leading to the generation of otoacoustic emissions) (Gozdzik-Zolnierkiewicz & Moszynski, 1969; Yamamura, et al., 1989; Tuncel, et al., 2002; Liang et al., 2004). Because the cochlear microphonic response (or receptor potential) is generated primarily by the outer hair cells of the inner ear, ototoxic effects should be manifested in both a diminished cochlear microphonic response and abnormal DPOAE, although DPOAE may be more hair cell place-specific than the cochlear microphonic.
Recent studies relate delta-aminolevulinic acid dehydratase (ALAD) gene polymorphisms to Pb poisoning susceptibility, indicating that ALAD2 genotype carriers may be less susceptible to the neurotoxic effects of Pb (Gao et al., 2010). ALAD gene polymorphisms may not explain the lack of association between auditory sensory functioning and Pb exposure found in the Andean children in the current study, since children from the same study area showed no apparent genetic protection against Pb-related adverse neurocognitive effects (Counter et al., 2008). Individuals living in high-altitude environments have an elevation in oxygen-carrying capacity of the blood because of increased hemoglobin concentration. However, there is no indication from the current study that this physiological adaptation to high-altitude living made the Andean children less susceptible to Pb-related auditory sensory impairment. Studying the effects of hemoglobin concentration of otoacoustic emissions in these children may yield informative data in this regard.
In conclusion, the current study further explored the issue of Pb-induced sensory hearing impairment by eliciting distortion product otoacoustic emissions, physiologic responses emanating from the sensory outer hair cells of the cochlea. The results showed no significant evidence of a Pb-exposure mediated effect on the electromotility function of the outer hair cells of the cochlea, and suggests that Pb uptake in the inner ear is not great enough to reduce significantly the amplitude of otoacoustic emissions. These findings also confirm the results of our previous auditory studies, and are in agreement with some experimental animal studies.
This study was part of an on-going medical and educational program related to the prevention of Pb exposure for inhabitants of rural communities in the Andes Mountains of Ecuador. The results of the current study were presented to the parents of the children who participated in the study. The parents of the children who participated in this study were counseled concerning their children’s Pb exposure levels, and were referred to local health officials for medical treatment where appropriate. It is noteworthy that since data in the current study were collected, the PbB levels of some of the children in this study have been reduced through preventative measures and chelation therapy.
Acknowledgments
The authors thank the Administration of Universidad San Francisco de Quito and the Fundacíon Capacitar of Ecuador for support of this project. We are grateful to Harvard University Biological Laboratories, the David Rockefeller Center of Latin American Studies at Harvard University, the University of Massachusetts Medical School/Shriver Center, and Dr. Jeremy Bloxham of Harvard University for support. We are thankful to Anthony B. Jacobs for excellent technical assistance, and to the Boston Children’s Hospital Department of Laboratory Medicine, and the Channing Trace Metals Laboratory of the Harvard School of Public Health for laboratory support.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- Alvarenga KF, Jacob LCB, Martins CHF, Costa OA, Coube CZV, Marques JM. Emissões otoacústicas – produto de distorção em indivíduos expostos ao chumbo e ao ruído. [Distortion product otoacoustic emissions in workers exposed to lead and noise.] Rev. Bras. Otorrinolaringol. 2003;69:681–689. (in Portuguese). [Google Scholar]
- American Academy of Pediatrics Committee on Environmental Health. Lead exposure in children: Prevention, detection and management. Pediatrics. 2005;116:1036–1046. doi: 10.1542/peds.2005-1947. [DOI] [PubMed] [Google Scholar]
- Anderson AC, Pueschel SM, Linakis JG. Pathophysiology of lead poisoning. In: Pueschel SM, Linakis JG, Anderson AC, editors. Lead Poisoning in Childhood. Baltimore, MD: Paul H. Brookes Publishing Co. Inc.; 1996. pp. 75–96. [Google Scholar]
- Baloh RW, Spivey GH, Brown CP, Morgan D, Campion DS, Browdy BL, Valentine JL, Gonick HC, Massey FJ, Jr, Culver BD. Subclinical effects of chronic increased lead absorption a prospective study. II. Results of baseline neurologic testing. J. Occup. Med. 1979:490–496. [PubMed] [Google Scholar]
- Bellinger D. Learning and behavioral sequelae of lead poisoning. In: Pueschel SM, Linakis JG, Anderson AC, editors. Lead Poisoning in Childhood. Baltimore, MD: Paul H. Brookes Publishing Co. Inc.; 1996. pp. 97–115. [Google Scholar]
- Brownell WE. Outer hair cell electromotility and otoacoustic emissions. Ear Hear. 1990;11:82–92. doi: 10.1097/00003446-199004000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan LH, Counter SA, Ortega F, Laurell G. Distortion product otoacoustic emissions in Andean children and adults with chronic lead intoxication. Acta Otolaryngol. 1999;119:652–658. doi: 10.1080/00016489950180586. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Atlanta: U.S. Government Printing Office; 1991. Preventing lead poisoning in young children: A statement by the Centers for Disease Control. [Google Scholar]
- Chuang HY, Kuo CH, Chiu YW, Ho CK, Chen CJ, Wu TN. A case-control study on the relationship of hearing function and blood concentrations of lead, manganese, arsenic, and selenium. Sci. Total Environ. 2007;387:79–85. doi: 10.1016/j.scitotenv.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Cole C, Winsler A. Protecting children from exposure to lead: Old problem, new data, and new policy needs. Soc. Policy Rep. 2010;24:1–23. [Google Scholar]
- Counter SA. Brainstem neural conduction biomarkers in lead-exposed children of Andean lead-glaze workers. J. Occup. Environ. Med. 2002;44:855–864. doi: 10.1097/00043764-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH. Neuro-ototoxicity in Andean adults with chronic lead and noise exposure. J. Occup. Environ. Med. 2002;44:30–38. doi: 10.1097/00043764-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH, Laurell G, Ortega F. Field screening of blood lead levels in remote Andean villages. Neurotoxicology. 1998;19:871–878. [PubMed] [Google Scholar]
- Counter SA, Buchanan LH, Ortega F. Neurocognitive screening of lead-exposed Andean adolescents and young adults. J. Toxicol. Environ. Health, A. 2009a;72:625–632. doi: 10.1080/15287390902769410. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH, Ortega F. Neurophysiologic and neurocognitive case profiles of Andean patients with chronic environmental lead poisoning. J. Toxicol. Environ. Health, A. 2009b;72:1150–1159. doi: 10.1080/15287390903091772. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH, Ortega F. Zinc protoporphyrin levels, blood lead levels and neurocognitive deficits in Andean children with chronic lead exposure. Clin. Biochem. 2008;40:787–792. doi: 10.1016/j.clinbiochem.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH, Ortega F, Rifai N. Blood lead and hemoglobin levels in Andean children with chronic lead intoxication. Neurotoxicology. 2000;21:301–308. [PubMed] [Google Scholar]
- Counter SA, Buchanan LH, Ortega F, Laurell G. Normal auditory brainstem and cochlear function in extreme pediatric plumbism. J. Neurol. Sci. 1997a;152:85–92. doi: 10.1016/s0022-510x(97)00149-4. [DOI] [PubMed] [Google Scholar]
- Counter SA, Vahter M, Laurell G, Buchanan LH, Ortega F, Skerfving S. High lead exposure and auditory sensory-neural function in Andean children. Environ. Health Persp. 1997b;105:522–526. doi: 10.1289/ehp.97105522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich KN, Succop PA, Berger OG, Keith RW. Lead exposure and the central auditory processing abilities and cognitive development of urban children: The Cincinnati lead study cohort at age 5 years. Neurotoxicol. Teratol. 1992;14:51–56. doi: 10.1016/0892-0362(92)90028-9. [DOI] [PubMed] [Google Scholar]
- Farahat TM, Abdel-Rasoul GM, El-Assy AR, Kandil SH, Kabil MK. Hearing thresholds of workers in a printing facility. Environ. Res. 1997;73:189–192. doi: 10.1006/enrs.1997.3700. [DOI] [PubMed] [Google Scholar]
- Forst LS, Freels S, Persky V. Occupational lead exposure and hearing loss. J. Occup. Environ. Med. 1997;39:658–660. doi: 10.1097/00043764-199707000-00011. [DOI] [PubMed] [Google Scholar]
- Gao A, Lu XT, Li QY, Tian L. Effect of the delta-aminolevulinic acid dehydratase gene polymorphism on renal and neurobehavioral function in workers exposed to lead in China. Sci. Total Environ. 2010;408:4052–4055. doi: 10.1016/j.scitotenv.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Gozdzik-Zolnierkiewicz T, Moszynski B. VIII nerve in experimental lead poisoning. Acta Otolaryngol. 1969;68:85–89. doi: 10.3109/00016486909121546. [DOI] [PubMed] [Google Scholar]
- Holdstein Y, Pratt H, Goldsher M, Rosen G, Shenhav R, Linn S, Mor A, Barkai A. Auditory brainstem evoked potentials in asymptomatic lead-exposed subjects. J Laryngol. Otol. 1986;100:1031–1036. doi: 10.1017/s0022215100100519. [DOI] [PubMed] [Google Scholar]
- Hwang YH, Chiang HY, Yen-Jean MC, Wang JD. The association between low levels of lead in blood and occupational noise-induced hearing loss in steel workers. Sci. Total Environ. 2009;408:43–49. doi: 10.1016/j.scitotenv.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Stimulated otoacoustic emissions from within the human auditory system. J. Acoust. Soc. Am. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- Lasky RE, Luck ML, Laughlin NK. The effects of succimer chelation therapy on auditory function in rhesus monkeys. Neurotoxicol. Teratol. 2001a;23:651–658. doi: 10.1016/s0892-0362(01)00176-3. [DOI] [PubMed] [Google Scholar]
- Lasky RE, Luck ML, Torre P, Laughlin NK. The effects of early lead exposure on auditory function in rhesus monkeys. Neurotoxicol. Teratol. 2001b;23:634–649. doi: 10.1016/s0892-0362(01)00175-1. [DOI] [PubMed] [Google Scholar]
- Lasky RE, Maier MM, Snodgrass EB, Hecox KE, Laughlin NK. The effects of lead on otoacoustic emissions and auditory evoked potentials in monkeys. Neurotoxicol. Teratol. 1995;17:633–644. doi: 10.1016/0892-0362(95)02006-3. [DOI] [PubMed] [Google Scholar]
- Laughlin NK, Luck ML, Lasky RE. Early lead exposure effects on an auditory threshold task in the Rhesus monkey (Macaca mulatta) Dev. Psychobiol. 2009;51:289–300. doi: 10.1002/dev.20364. [DOI] [PubMed] [Google Scholar]
- Liang GH, Järlebarka L, Ulfendahla M, Bianb JT, Moore EJ. Lead (Pb2+) modulation of potassium currents of guinea pig outer hair cells. Neurotoxicol. Teratol. 2004;26:253–260. doi: 10.1016/j.ntt.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Lidsky TI, Schneider JS. Lead neurotoxicity in children: Basic mechanisms and clinical correlates. Brain. 2003;126:5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- Lin J, Liu R, Chen Q. Relationship between otoacoustic emissions and blood-lead levels in school children. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi [J Otorhinolaryngol Head Neck Surg] 2008;22:446–448. [abstract]. [PubMed] [Google Scholar]
- Lonsbury-Martin BL, Martin GK. Evoked otoacoustic emissions as objective screeners for ototoxicity. Semin. Hear. 2001;22:377–391. [Google Scholar]
- Lonsbury-Martin BL, Whitehead ML, Martin GK. Clinical applications of otoacoustic emissions. J. Speech Hear. Res. 1991;34:964–981. doi: 10.1044/jshr.3405.964. [DOI] [PubMed] [Google Scholar]
- Markowitz M. Lead Poisoning. Pediatr. Rev. 2000;21:327–335. doi: 10.1542/pir.21-10-327. [DOI] [PubMed] [Google Scholar]
- Martins CHF, Vassoler TMF, Bergonse GFR, Alvarenga KF, Costa OA. Emissões otoacústicas e potencial evocado auditivo de tronco encefálico em trabalhadores expostos a ruído e ao chumbo. [Otoacustic emissions and brainstem evoked auditory response in workers exposed to noise and lead.] Acta ORL/Técnicas Otorrinolaringol. 2007;25:293–298. (in Portuguese). [Google Scholar]
- Mulheran M, Degg C. Comparison of distortion product OAE generation between a patient group requiring frequent gentamicin therapy and control subjects. Br. J. Audiol. 1997;31:5–9. doi: 10.3109/03005364000000004. [DOI] [PubMed] [Google Scholar]
- Olympio KPK, Gonçalves C, Günther WMR, Bechara EJH. Neurotoxicity and aggressiveness triggered by low-level lead in children: A review. Pan Am. J. Public Health. 2009;26:266–275. doi: 10.1590/s1020-49892009000900011. [DOI] [PubMed] [Google Scholar]
- Osman K, Pawlas K, Schütz A, Gazdzik M, Sokal JA, Vahter M. Lead exposure and hearing effects in children in Katowice, Poland. Environ. Res. 1999;80:1–8. doi: 10.1006/enrs.1998.3886. [DOI] [PubMed] [Google Scholar]
- Otto D, Robinson G, Bauman S, Schroeder S, Mushak P, Kleinbaum D, Boone L. 5-year follow-up study of children with low-to-moderate lead absorption: Electrophysiological evaluation. Environ. Res. 1985;38:168–186. doi: 10.1016/0013-9351(85)90082-9. [DOI] [PubMed] [Google Scholar]
- Park SK, Elmarsafawy S, Mukherjee B, Spiro A, III, Vokonas PS, Huiling N, Weisskopf MG, Schwartz J, Hu H. Cumulative lead exposure and age-related hearing loss: The VA normative aging study. Hear. Res. 2010;269:48–55. doi: 10.1016/j.heares.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DC. Effects of lifetime lead exposure in monkeys on detection of pure tones. Fundam. Appl. Toxicol. 1997;36:112–118. doi: 10.1006/faat.1996.2268. [DOI] [PubMed] [Google Scholar]
- Rosin A. The long-term consequences of exposure to lead. Israel Med. Assoc. J. 2009;11:689–694. [PubMed] [Google Scholar]
- Schwartz J, Otto D. Blood lead, hearing thresholds and neurobehavioral development in children and youth. Arch. Environ. Health. 1987;42:153–160. doi: 10.1080/00039896.1987.9935814. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Otto D. Lead and minor hearing impairment. Arch. Environ. Health. 1991;46:300–305. doi: 10.1080/00039896.1991.9934391. [DOI] [PubMed] [Google Scholar]
- Stavroulaki P, Apostolopoulos N, Segas J, Tsakanikos M, Adamopoulos G. Evoked otoacoustic emissions—An approach for monitoring cisplatin induced ototoxicity in children. Int. J. Pediatr. Otorhinolaryngol. 2001;59:47–57. doi: 10.1016/s0165-5876(01)00455-4. [DOI] [PubMed] [Google Scholar]
- Stavroulaki P, Vossinakis IC, Dinopoulou D, Doudounakis S, Adamopoulos G, Apostolopoulos N. Otoacoustic emissions for monitoring aminoglycoside-induced ototoxicity in children with cystic fibrosis. Arch. Otolaryngol. Head Neck Surg. 2002;128:150–155. doi: 10.1001/archotol.128.2.150. [DOI] [PubMed] [Google Scholar]
- Trautwein P, Hofstetter P, Wang J, Salvi R, Nostrant A. Selective inner hair cell loss does not alter distortion product otoacoustic emissions. Hear. Res. 1996;96:71–82. doi: 10.1016/0378-5955(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Tuncel U, Clerici WJ, Jones RO. Deferential ototoxicities induced by lead acetate and tetraethyl lead. Hear. Res. 2002;166:113–123. doi: 10.1016/s0378-5955(02)00303-9. [DOI] [PubMed] [Google Scholar]
- Vahter M, Counter SA, Laurell G, Buchanan LH, Ortega F, Schütz A, Skerfving S. Extensive lead exposure in children living in an area with production of lead-glazed tiles in the Ecuadorian Andes. Int. Arch. Occup. Environ. Health. 1997;70:282–286. doi: 10.1007/s004200050220. [DOI] [PubMed] [Google Scholar]
- Wu TN, Shen CY, Lai JS, Goo CF, Ko KN, Chi HY, Chang PY, Liou SH. Effects of lead and noise exposures on hearing ability. Arch. Environ. Health. 2000;55:109–114. doi: 10.1080/00039890009603396. [DOI] [PubMed] [Google Scholar]
- Yamamura K, Terayama K, Yamamoto N, Kohyama A, Kishi R. Effects of acute lead acetate exposure on adult guinea pigs: Electrophysiological study of the inner ear. Fundam. Appl. Toxicol. 1989;13:509–515. doi: 10.1016/0272-0590(89)90287-x. [DOI] [PubMed] [Google Scholar]