Abstract
Background. Diagnosis and treatment of sexually transmitted infections (STIs) is a public health priority, particularly in regions where the incidence of human immunodeficiency virus (HIV) infection is high. In most developing countries, STIs are managed syndromically. We assessed the adequacy of syndromic diagnosis of STIs, compared with laboratory diagnosis of STIs, and evaluated the association between STI diagnosis and the risk of HIV acquisition in a cohort of high-risk women.
Methods. HIV-uninfected high-risk women (n = 242) were followed for 24 months. Symptoms of STIs were recorded, and laboratory diagnosis of common STI pathogens was conducted every 6 months. Forty-two cytokines were measured by Luminex in cervicovaginal lavage specimens at enrollment. Human immunodeficiency virus type 1 (HIV-1) infection was evaluated monthly.
Results. Only 12.3% of women (25 of 204) who had a laboratory-diagnosed, discharge-causing STI had clinically evident discharge. Vaginal discharge was thus a poor predictor of laboratory-diagnosed STIs (sensitivity, 12.3%; specificity, 93.8%). Cervicovaginal cytokine concentrations did not differ between women with asymptomatic STIs and those with symptomatic STIs and were elevated in women with asymptomatic STIs, compared with women with no STIs or bacterial vaginosis. Although laboratory-diagnosed STIs were associated with increased risk of HIV infection (hazard ratio, 3.3 [95% confidence interval, 1.5–7.2)], clinical symptoms were not.
Conclusions. Syndromic STI diagnosis dependent on vaginal discharge was poorly predictive of laboratory-diagnosed STI. Laboratory-diagnosed STIs were associated with increased susceptibility to HIV acquisition, while vaginal discharge was not.
(See the editorial commentary by Cohen, on pages 1–2.)
Sexually transmitted infections (STIs) impose a major health burden, particularly in developing countries such as South Africa, where the prevalence of human immunodeficiency virus type 1 (HIV-1) infection is high [1, 2]. In South Africa, most new HIV infections are sexually transmitted, and women are at higher risk of infection than men [3]. As STIs are associated with increased susceptibility to HIV infection, they have likely played a central role in facilitating the spread of HIV [4–11]. STI management is thus a key issue in preventing HIV infection in countries where both HIV infection and other STIs are prevalent.
Developing countries such as South Africa manage most STIs syndromically, on the basis of signs and symptoms [12]. Etiological diagnosis is expensive and time-consuming and requires laboratory equipment and suitably trained staff, whereas syndromic management is easily implemented and inexpensive, with immediate treatment available [12, 13]. However, because the STI syndromes used for diagnosis are nonspecific and because a large proportion of STIs are asymptomatic, undertreatment, and sometimes overtreatment, are pervasive [14–18]. In South Africa, asymptomatic infections occur in almost 50% of STI-infected women who do not seek care and therefore remain untreated [19]. Untreated STIs are associated with significant direct sequelae, including pelvic inflammatory disease (PID), ectopic pregnancy, and tubal factor infertility [20]. On the other hand, a significant number of women with conditions such as vaginal discharge resulting from a derangement of normal vaginal flora may be treated for STIs that they do not have. The costs associated with overtreatment include the financial burden of supplying medicines, the potential development of antimicrobial resistance, and the social cost of misdiagnosis.
Several studies have investigated the influence of different strategies for STI management on the risk of HIV infection. Population-wide treatment of bacterial infections or therapy for herpes simplex virus 2 (HSV-2) was found to be ineffective at reducing HIV infection [21–25]. Two of 3 syndromic management interventions found no difference in HIV acquisition [26–29], suggesting that asymptomatic infections may play a role. In addition to causing visible clinical symptoms, certain STIs may be associated with subclinical manifestations, including elevated genital tract inflammatory cytokine responses [14, 30]. Although a direct association between genital tract inflammatory cytokines and susceptibility to HIV infection has not yet been demonstrated, previous studies have suggested that inflammatory cytokines may directly upregulate HIV replication in the genital tract by activating nuclear factor κB and by recruiting and activating various immune cells which act as targets for HIV infection [31–34]. Additionally, proinflammatory cytokines such as tumor necrosis factor α (TNF-α) may facilitate HIV infection by disrupting tight junctions between epithelial cells, reducing the integrity of this barrier [35–37].
The aim of this study was to establish the prevalence and incidence of STIs in a longitudinal cohort of HIV-uninfected women with multiple sexual partners in South Africa. The adequacy of syndromic management was evaluated by comparing clinical signs and symptoms to laboratory diagnosis of STIs. Inflammatory cytokine concentrations in cervicovaginal lavage (CVL) specimens were measured to determine whether women who had asymptomatic laboratory-diagnosed STIs also had subclinical inflammation that may facilitate HIV infection. Last, associations between STIs, cytokine concentrations, and HIV acquisition were investigated.
MATERIALS AND METHODS
Study Design
A total of 775 high-risk women were screened for HIV infection, and 245 HIV-uninfected women were enrolled into a prospective observational cohort study, the CAPRISA 002 Acute HIV Infection study, which was conducted in Durban, South Africa [38]. Participants attended the clinic monthly for risk-reduction counseling, condom provision, and HIV testing. STIs were assessed at enrollment and every 6 months thereafter. Acute HIV infection was diagnosed by detection of HIV RNA in the absence of HIV antibodies or on the basis of a reactive HIV antibody test within 5 months of a previously known negative antibody result. Laboratory diagnosis of STIs was conducted at enrollment and every 6 months thereafter. Ethics approval for the study was obtained from the University of KwaZulu-Natal and the University of Cape Town. Written informed consent was obtained from all participants.
Laboratory Diagnosis of STIs
A gynecological examination, including a speculum examination using an unlubricated sterile bivalve speculum, was performed at enrollment and every 6 months thereafter to visualize any cervical changes, and appropriate samples were collected from any suspicious lesions. Two vulvovaginal swab specimens were collected from the posterior fornices and lateral vaginal walls at each examination. Blood specimens were collected for serological testing for Treponema pallidum and HSV-2. All specimens were transported to the diagnostic laboratory (Medical Microbiology Laboratory, University of KwaZulu-Natal) within 1 hour of collection for same-day processing. One vulvovaginal swab specimen was rolled onto a glass slide for Gram staining for diagnosis of bacterial vaginosis, using Nugents criteria. The second swab specimen was used to extract DNA for detection of Neisseria gonorrhoeae and Chlamydia trachomatis, using the BDProbe Tec ET polymerase chain reaction (PCR) assay (Becton Dickinson Microbiology Systems, United States), and for detection of Trichomonas vaginalis, Mycoplasma genitalium, and HSV-2, using an in-house PCR assay [39].
Genital ulcers were diagnosed as previously described, on the basis of PCR testing for T. pallidum, Haemophilus ducreyi, C. trachomatis (lymphogranuloma venereum types), and HSV-2 [40]. An impression slide of ulcers for microscopy was collected for the diagnosis of Calymmatobacterium granulomatis, if suspected clinically. HerpeSelect-1 and HerpeSelect-2 enzyme immunoassays were used to confirm genital herpes diagnosis and to identify asymptomatic carriers. Syphilis screening was done using the Becton Dickinson Macro-Vue RPR (rapid plasma reagin [RPR]) card test, and positive reactions were confirmed by the T. pallidum hemagglutination test (Omega ImmuTrep TPHA test). The RPR test was done on serum samples that were undiluted or diluted at ratio of 1:8.
Collection of CVL Specimens and Cytokine Measurements
CVL samples for cytokine measurements were collected from study participants at enrollment into the study, as previously described [41]. Sterile normal saline (10 mL) was used to repeatedly bathe the cervix. The fluid was allowed to pool in the posterior fornix, where it was then aspirated into a plastic bulb pipette. Samples were centrifuged, and the supernatant was stored at −80°C. CVL samples were not collected from menstruating participants, for whom sampling was postponed to the following week. CVL samples for cytokine analysis were available for a subset of 227 of 242 women who were included in this study, of whom 66 had ≥1 STI (excluding bacterial vaginosis and HSV-2 serology findings). Prior to cytokine measurements, CVL samples were prefiltered by centrifugation using 0.2 μm cellulose acetate filters (Sigma, United States). Concentrations of epidermal growth factor, eotaxin/CCL11, fibroblast growth factor 2, fms-like tyrosine kinase 3 ligand (Flt-3L), fractalkine/CX3CL1, granulocyte colony-stimulating factor (CSF), granulocyte macrophage CSF, growth related oncogene family (CXCL1-CXCL3), interferon α (IFN-α), interferon γ (IFN-γ), interleukin 1α (IL-1α), interleukin 1β (IL-1β), interleukin 1 receptor antagonist, interleukin 2 (IL-2), interleukin 3, interleukin 4, interleukin 5, interleukin 6 (IL-6), interleukin 7, interleukin 8 (IL-8)/CXCL8, interleukin 9, interleukin 10, interleukin 12p40, interleukin 12p70 (IL-12p70), interleukin 13 (IL-13), interleukin 15, interleukin 17 (IL-17), IFN-γ–induced protein 10/CXCL10, monocyte chemotactic protein 1/CCL2, monocyte chemotactic protein 3/CCL7, macrophage-derived chemokine/CCL22, macrophage inflammatory protein 1α/CCL3, macrophage inflammatory protein 1β/CCL4, platelet-derived growth factor AA, platelet-derived growth factor AB/BB, RANTES/CCL5, soluble CD40 ligand (sCD40L), soluble IL-2 receptor α, transforming growth factor α, tumor necrosis factor α (TNF-α), tumor necrosis factor β (TNF-β), and vascular endothelial growth factor (VEGF) were measured using Human Cytokine LINCOplex kits (LINCO Research, MO), according to the manufacturer's protocol. The sensitivity of these kits ranged from 0.01 and 18.3 pg/mL for each of the 42 cytokines measured. Data were collected using a Bio-Plex Suspension Array Reader (Bio-Rad Laboratories), and a 5-parameter logistic regression formula was used to calculate sample concentrations from the standard curves. Data were analyzed using BIO-plex manager software (version 4). Cytokine levels that were below the lower limit of detection of the assay were reported as the midpoint between the lowest concentration measured and zero.
Statistical Methods
Data was analyzed using SAS, version 9.2 (SAS Institute, Cary, NC), GraphPad Prism version 5 (GraphPad Software, San Diego, CA), and STATA (StataCorp, TX). Demographic and baseline behavioral characteristics are described overall and by STI and genital symptom profile at enrollment. The Fisher exact test and the Kruskal–Wallis test were used to compare characteristics between the groups. STI incidence was calculated during follow-up for participants who were STI negative at baseline or for whom resolution of a previous infection allowed detection of a newly acquired STI. Sensitivity, specificity and positive and negative predictive values were calculated for STI diagnosis by discharge and genital ulcers, using laboratory results as the true diagnosis. A Mann–Whitney U test was used to compare cytokine concentrations at enrollment in unmatched women with asymptomatic and symptomatic STIs and women with none of the assessed STIs. Hierarchical clustering was used to cluster women according to the relatedness of their cytokine expression profiles (Qlucore Omics Explorer). Principal component analysis (PCA) was used to simplify the data set and generate new variables (component estimates), which are representative of the unique and common variance of each of the included cytokines. P values were adjusted using a false discovery rate step-down procedure in order to reduce false-positive results when multiple comparisons were made [42]. The hazard ratios (HRs) for HIV infection were calculated using proportional hazards regression models with STIs as time-varying covariates. A multivariate proportional hazard model was also fitted, with adjustment for all laboratory-diagnosed STIs, clinical symptoms, and demographic and behavioral factors. Only STI infections diagnosed prior to the estimated date of HIV infection were included in the models predicting HIV infection. Demographic and behavioral factors associated with HIV risk will, however, be explored in more detail in a separate analysis. Participants lost to follow-up were censored at the last contact visit, while all other participants were censored at the time of the last clinic visit.
RESULTS
High Prevalence and Incidence of STIs in this Cohort of High Risk Women
A total of 245 high-risk HIV-uninfected women were enrolled into this study and followed for up to 24 months. Of these, 242 were included in the STI analysis, as 3 participants did not have preinfection data available. At enrollment, 57.0% of women reported having multiple “stable” partners, while 35.7% had 1 stable partner or were married. However, most women (95.0%) reported having had >1 casual sex partner within the last 3 months, and 78.8% were self-identified female sex workers (Table 1) [38]. The prevalence of laboratory-diagnosed STIs at enrollment was high: 20.3% had T. vaginalis, 5.4% had N. gonorrhoeae, 4.2% had C. trachomatis, 1.2% had M. genitalium, and 3.7% were HSV-2 positive on PCR. Of these, 25.3% of women received a diagnosis one of 1 STI, and 5.8% received a diagnosis of ≥2 STIs. HSV-2 antibodies were detected in 86.0% of the women, while the prevalence of bacterial vaginosis was 52.7% (Table 2). During the study period, the incidence of any STI (excluding bacterial vaginosis and HSV-2 serology findings) was 26.7 cases per 100 person-years, and the incidences of T. vaginalis, N. gonorrhoeae, C. trachomatis, M. genitalium, and T. pallidum were 14.7, 3.3, 4.8, 3.8, and 2.1 cases per 100 person-years, respectively.
Table 1.
Demographic and Behavioral Characteristics of Study Participants
| Laboratory-Diagnosed STI |
|||||
|---|---|---|---|---|---|
| Characteristic | Total (n = 245) | No STI, BV, or Symptoms (n = 83) | Asymptomatic (n = 65) | Symptomatic (n = 12) | P |
| Education | |||||
| Primary school | 60 (24.5) | 18 (21.7) | 14 (21.5) | 2 (16.7) | .7827 |
| Secondary school to grade 10 | 84 (34.3) | 29 (34.9) | 28 (43.1) | 6 (50.0) | |
| Secondary school grade 11 and higher | 101 (41.2) | 36 (43.4) | 23 (35.4) | 4 (33.3) | |
| Marital status | |||||
| Single | 18 (7.4) | 9 (11.0) | 6 (9.2) | 0 (0.0) | .1336 |
| Stable partner/married | 87 (35.7) | 32 (39.0) | 22 (33.9) | 1 (8.3) | |
| Many partners | 139 (57.0) | 41 (50.0) | 37 (56.9) | 11 (91.7) | |
| No. of casual sex partners in last 3 mo | |||||
| 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .0475 |
| 1 | 12 (5.0) | 5 (6.2) | 2 (3.1) | 0 (0.0) | |
| 2–5 | 205 (85.4) | 73 (90.1) | 51 (79.7) | 10 (83.3) | |
| >5 | 23 (9.6) | 3 (3.7) | 11 (17.2) | 2 (16.7) | |
| No. of steady sexual partners in last 3 mo | |||||
| 0 | 8 (3.3) | 1 (1.2) | 5 (7.8) | 0 (0.0) | .0717 |
| 1 | 157 (65.2) | 59 (72.0) | 35 (54.7) | 10 (83.3) | |
| 2–5 | 70 (29.1) | 20 (24.4) | 22 (34.4) | 1 (8.3) | |
| >5 | 6 (2.5) | 2 (2.4) | 2 (3.1) | 1 (8.3) | |
| No. who reported condom use at last sex act | 144 (58.8) | 54 (65.1) | 36 (55.4) | 8 (66.7) | .4390 |
| Mean age in years (SD) | 34.2 (10.5) | 34.6 (9.7) | 34.2 (11.5) | 31.6 (8.0) | .5974 |
| Mean age of sexual debut in years (SD) | 17.0 (2.4) | 17.1 (2.4) | 16.4 (1.9) | 16.4 (2.6) | .2830 |
Abbreviations: BV, bacterial vaginosis; STI, sexually transmitted infection.
Table 2.
Prevalence and Incidence of Laboratory-Diagnosed Sexually Transmitted Infections (STIs) and Clinical Symptoms
| Prevalence, % (Proportion) |
Incidence, Cases/100 py (95% CI) | |||
|---|---|---|---|---|
| Variable | Enrollment (n = 242) | 6 mo (n = 260) | 12 mo (n = 189) | |
| Laboratory diagnosis | ||||
| HSV-2 serology | 86.0 (208/242) | 90.1 (183/203) | 91.0 (171/188) | 26.0 (11.2–51.2) |
| HSV-2 PCR | 3.7 (9/241) | 1.5 (3/205) | 2.2 (4/185) | 4.1 (2.2–6.9) |
| T. pallidum | 2.9 (7/242) | 3.0 (6/202) | 1.6 (3/184) | 2.1 (.8–4.3) |
| BV | 52.7 (127/241) | 46.8 (96/205) | 42.9 (79/184) | 50.5 (40.9–61.6) |
| T. vaginalis | 20.3 (49/241) | 13.2 (27/205) | 17.3 (32/185) | 14.7 (10.7–19.7) |
| N. gonorrhoeae | 5.4 (13/240) | 2.0 (4/205) | 2.2 (4/184) | 3.3 (1.6–5.8) |
| C. trachomatis | 4.2 (10/240) | 4.4 (9/205) | 3.3 (6/184) | 4.8 (2.7–7.7) |
| M. genitalium | 1.2 (3/241) | 1.5 (3/205) | 3.8 (7/185) | 3.8 (2.0–6.5) |
| Any STI (excluding BV and HSV-2 serology) | 31.3 (75/240) | 21.7 (44/203) | 27.5 (50/182) | 26.7 (20.8–33.7) |
| 1 | 25.3 (61/241) | 17.6 (36/205) | 24.3 (45/185) | … |
| >1 | 5.8 (14/241) | 3.9 (8/205) | 2.7 (5/185) | … |
| Clinical diagnosis | ||||
| Vaginal discharge | 15.3 (37/242) | 1.5 (3/203) | 4.8 (9/189) | 14.2 (10.6–18.5) |
Abbreviations: BV, bacterial vaginosis; CI, confidence interval; C. trachomatis, Chlamydia trachomatis; HSV-2, herpes simplex virus 2; Mycoplasma genitalium, M. genitalium; N. gonorrhoeae, Neisseria gonorrhoeae; PCR, polymerase chain reaction; py, person-years; T. pallidum, Treponema pallidum; T. vaginalis, Trichomonas vaginalis.
Most Women With Laboratory-Diagnosed STIs Were Asymptomatic
Occurrence of genital ulcer disease and abnormal vaginal discharge was compared to laboratory diagnosis of STIs. Only 3 participants presented with genital ulcers during the follow-up period, and none presented at enrollment. Each of these women tested positive for HSV-2 IgG antibodies prior to the ulcer episode. However, only one of these women tested positive for HSV-2 by PCR after receiving an etiological diagnosis. The other 2 women were negative for all of the ulcer-causing organisms assessed (T. pallidum, H. ducreyi, C. trachomatis, HSV-2, and C. granulomatis).
At enrollment, 15.3% of women presented with vaginal discharge, while only 1.5% and 4.8% reported vaginal discharge at their 6- and 12-month study visits, respectively (Table 2). Contrary to this, at enrollment 27.5% of women tested positive for a vaginal discharge–associated STI (T. vaginalis, N. gonorrhoeae, M. genitalium, or C. trachomatis), with 18.5% and 23.9% testing positive at their 6- and 12-month visits, respectively.
Overall, only 34.3% of vaginal discharge incidents were accompanied by a positive laboratory test for ≥1 discharge-associated STI (Table 3). Meanwhile, a total of 87.7% laboratory-diagnosed STIs (discharge associated) had no accompanying clinical symptoms. Assuming laboratory testing of the above STIs to be the reference standard, the presence of discharge for screening had a sensitivity of 12.3%, a specificity of 93.8%, a positive predictive value of 34.3%, and a negative predictive value of 80.2%. Therefore, only 12.3% of women with a confirmed laboratory-diagnosed STI would have been appropriately treated using vaginal discharge as an indicator for syndromic treatment.
Table 3.
Sensitivity and Specificity of Discharge and Other Symptoms of Sexually Transmitted Infections (STIs) in Detecting STIs
| Laboratory Diagnosis |
|||||||
|---|---|---|---|---|---|---|---|
| Variable | Clinical Symptoms | + | − | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
| Any discharge-causing STI (including BV) | + | 55 | 18 | 10.5 | 96.0 | 75.3 | 48.1 |
| − | 468 | 433 | |||||
| Any discharge-causing STI (excluding BV) | + | 25 | 48 | 12.3 | 93.8 | 34.3 | 80.2 |
| − | 179 | 723 | |||||
| BV | + | 44 | 29 | 10.0 | 94.4 | 59.5 | 55.9 |
| − | 398 | 504 | |||||
| T. vaginalis | + | 20 | 54 | 13.8 | 93.5 | 27.0 | 86.2 |
| − | 125 | 778 | |||||
| N. gonorrhoeae | + | 5 | 68 | 17.9 | 92.8 | 6.9 | 97.5 |
| − | 23 | 879 | |||||
| C. trachomatis | + | 5 | 68 | 13.9 | 92.8 | 6.9 | 96.6 |
| − | 31 | 871 | |||||
| M. genitalium | + | 2 | 72 | 10.5 | 92.5 | 2.7 | 98.1 |
| − | 17 | 886 | |||||
| HSV-2 (by PCR) | + | 2 | 72 | 8.7 | 92.5 | 2.7 | 97.7 |
| − | 21 | 882 | |||||
Abbreviations: BV, bacterial vaginosis; C. trachomatis, Chlamydia trachomatis; HSV-2, herpes simplex virus 2; Mycoplasma genitalium, M. genitalium; N. gonorrhoeae, Neisseria gonorrhoeae; NPV, negative predictive value; PCR, polymerase chain reaction; PPV, positive predictive value; T. vaginalis, Trichomonas vaginalis.
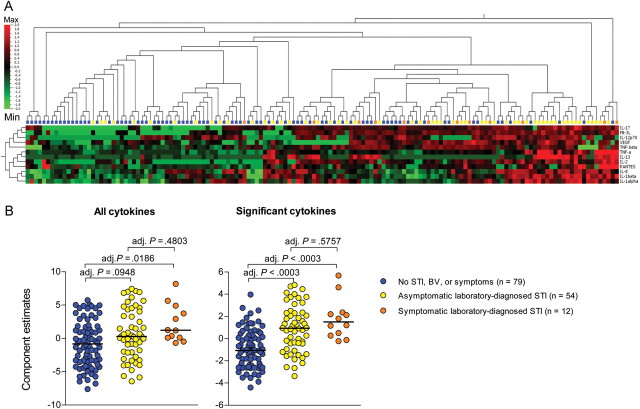
Asymptomatic Laboratory-Diagnosed STIs Were Associated With Elevated Genital Tract Inflammatory Cytokine Concentrations
The concentrations of 42 cytokines were measured in CVL samples from study participants in order to determine whether women who had asymptomatic STIs also had subclinical genital inflammation. Cytokine concentrations in CVL specimens were compared in (1) women who tested negative for all assessed STIs and bacterial vaginosis, (2) women who had vaginal discharge and tested positive for ≥1 STI (excluding bacterial vaginosis and HSV-2 serology findings), and (3) women who had no clinical symptoms but tested positive for ≥1 STI (excluding bacterial vaginosis and HSV-2 serology findings; Figure 1A). The concentrations of IL-1α, IL-1β, IL-12p70, TNF-α, TNF-β, RANTES, Flt-3L, VEGF, IL-2, and IL-17 were significantly elevated in CVL specimens from women who had vaginal discharge and a laboratory-diagnosed STI, compared with women who had no STI or bacterial vaginosis, after adjustment for multiple comparisons (adjusted P values: .0043, .0016, .0016, .0152, .007, .0064, .0039, .014, .012, and .0294, respectively). Levels of 7 of these cytokines (IL-1α, IL-1β, IL-12p70, TNF-α, VEGF, IL-2, and IL-17), as well as levels of IL-8 and IL-13, were elevated in women who had ≥1 active STI but no clinical symptoms (adjusted P values: < .0016, < .0042, .012, .0027, .0429, .0115, .0204, .0084, and .0462, respectively). Cytokine concentrations did not differ significantly between women who had asymptomatic STIs and those with symptomatic STIs. It was found that women who had an STI, either symptomatic or asymptomatic, clustered separately from women who did not have an STI (Figure 1A). PCA was used to further reduce the complexity of the data set by defining new variables that were representative of either (1) the unique and common variance of each of the 42 cytokines assessed or (2) the variance of only those 12 cytokines that had significantly elevated levels in CVL from women who had an STI (Figure 1B). It was found that, although the groups of women were not clearly differentiated using the cluster of 42 cytokines, the estimates of a component including only the 12 significant cytokines were significantly higher in women who had either a symptomatic STI or an asymptomatic infection, compared with women who did not have an STI. Therefore, women who had asymptomatic STIs had subclinical inflammation that may increase their susceptibility to HIV infection.
Figure 1.
Cervicovaginal (CVL) cytokine profiles of women who did not have a sexually transmitted infection (STI), compared with women who had asymptomatic or symptomatic infections. A, Cytokine concentrations were measured in CVL samples that were available for 227 of 242 participants in this study. Women who did not have an STI, bacterial vaginosis (BV), or vaginal discharge (blue dots/blocks), women who had an asymptomatic STI (yellow), and women who had a symptomatic STI (orange) were clustered according to their genital cytokine concentrations. Only the cytokines that differed significantly between these groups after adjustment for multiple comparisons were included in this analysis. Abbreviations: Max, maximum standardized cytokine concentration measured; Min, minimum standardized cytokine concentration. B, Principal component analysis was used to group either (1) all cytokines or (2) cytokines that differed significantly between the groups into single components and generate estimates representative of each component. P values were adjusted for multiple comparisons, using a false discovery rate step-down procedure in order to reduce false-positive results when multiple comparisons were made. Adjusted (adj.) P values < .05 were considered statistically significant.
Asymptomatic Laboratory-Diagnosed STIs Were Associated With Increased Risk of HIV Infection
Of the enrolled 245 HIV-seronegative participants, 28 became HIV infected, yielding an HIV incidence of 7.2 cases per 100 women-years (95% confidence interval [CI], 4.5–9.8) [38]. As 3 of these 28 did not have preinfection data, they were excluded from analysis. Among the STIs tested, N. gonorrhoeae, C. trachomatis, and M. genitalium were associated with HIV acquisition (Table 4). After control for demographic and behavioral factors, clinical symptoms, and other STIs, only N. gonorrhoeae remained significant (adjusted HR, 4.62 [95% CI, 1.34–15.93]). The presence of any STI (excluding bacterial vaginosis and HSV-2 serology findings) was also significantly associated with HIV seroconversion (HR, 3.27 [95% CI, 1.49–7.21]). Additionally, the number of STIs was predictive of HIV infection, with women who had 1 STI having a 3-fold increased risk of acquiring HIV (HR, 2.93 [95% CI, 1.26–6.82]), compared with women with no STI, and those who had ≥2 concurrent STIs had a 6-fold increased risk of HIV infection (HR, 6.15 [95% CI, 1.72–22.03]). HSV-2 seropositivity, bacterial vaginosis, and vaginal discharge were not found to increase the risk of HIV acquisition. Genital ulcers were also not associated with increased risk of HIV infection, although the prevalence of ulcers was low in this cohort (n = 3).
Table 4.
Sexually Transmitted Infections (STIs) and Risk of Human Immunodeficiency Virus (HIV) Type 1 Infection
| Unadjusted Analysis |
Adjusted Analysis |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P | HR (95% CI) | P |
| Symptom | ||||
| Genital ulcers | 0 | … | … | … |
| Discharge | 1.04 (.24–4.58) | .9594 | 0.59 (.12–3.00) | .5283 |
| Laboratory diagnosis | ||||
| BV | 2.04 (.90–4.63) | .0892 | 1.69 (.71–4.06) | .2375 |
| HSV-2 serology | 1.30 (.31–5.52) | .7234 | 2.12 (.43–10.50) | .3558 |
| HSV-2 (by PCR) | 1.96 (.26–14.59) | .5108 | 1.42 (.18–11.44) | .7426 |
| T. pallidum | 0a | … | … | … |
| T. vaginalis | 1.78 (.71–4.50) | .2203 | 1.74 (.62–4.92) | .2935 |
| N. gonorrhoeae | 7.74 (2.82–21.24) | <.0001 | 4.62 (1.34–15.93) | .0154 |
| C. trachomatis | 3.99 (1.19–13.39) | .0250 | 0.90 (.18–4.63) | .9006 |
| M. genitalium | 4.49 (1.04–19.44) | .0446 | 4.08 (.83–20.19) | .0846 |
| Any STI (excluding HSV-2 and BV) | 3.27 (1.49–7.21) | .0033 | … | … |
| No. of concurrent STIs (excluding HSV-2 and BV) | ||||
| 1 | 2.93 (1.26–6.82) | .0124 | … | … |
| ≥2 | 6.15 (1.72–22.03) | .0053 | … | … |
The multivariate model adjusted for all laboratory-diagnosed STIs and clinical symptoms, as well as for demographic and behavioral factors (data not shown).
Abbreviations: BV, bacterial vaginosis; C. trachomatis, Chlamydia trachomatis; HR, hazard ratio; HSV-2, herpes simplex virus 2; Mycoplasma genitalium, M. genitalium; N. gonorrhoeae, Neisseria gonorrhoeae; PCR, polymerase chain reaction; T. pallidum, Treponema pallidum; T. vaginalis, Trichomonas vaginalis.
a Participants who tested positive for T. pallidum remained HIV uninfected, and a HR could not be calculated.
Despite the long period between cytokine measurements in CVL specimens (at enrollment) and HIV infection (median, 302 days prior to infection [range, 14–686 days]), several inflammatory cytokines were associated with risk of HIV infection, before adjustment for multiple comparisons. Elevated concentrations of IL-1β, IL-6, IL-8, and sCD40L were associated with greater risk of HIV infection (HR [95% CI], 1.25 [1.05–1.50], 1.28 [1.07–1.54], 1.39 [1.04–1.87], and 1.45 [1.02–2.07], respectively, per 1 log10 pg/mL increase in cytokine concentration). Of these cytokines, IL-1β and IL-8 were elevated in women who had asymptomatic STIs, relative to women who had no STIs.
DISCUSSION
The prevalence of STIs is generally very high in sub-Saharan Africa [1, 2], and these infections are associated with increased susceptibility to HIV acquisition and secondary transmission [4–11]. There is conflicting evidence regarding the use of syndromic management of STIs for reducing HIV incidence, and it is thought that this approach may underestimate STI prevalence, as many infections are asymptomatic [26–29, 43]. The prevalence and incidence of laboratory-diagnosed STIs in this cohort were high; however, only 12.3% of women who tested positive for ≥1 STI had visible clinical symptoms. As a result, 87.7% of STIs in this cohort of high-risk women would have been left untreated in a syndromic management setting. The presence of any laboratory-diagnosed STI was associated with a 3-fold increased risk of HIV infection, and this association was independent of clinical symptoms. The STI most significantly associated with HIV infection was N. gonorrhoeae, increasing the risk of HIV infection by almost 5-fold. No associations were found between abnormal discharge or genital ulcers and susceptibility to HIV-infection, although only a small proportion of women presented with ulcers in this study. Furthermore, HSV-1 was not tested for in this study but may be considered in future testing algorithms, given the increasing prevalence of this organism [44]. The prevalence and incidence of STIs were much higher than the values of approximately 50% reported in most studies. This might reflect a higher frequency of reinfection due to the higher number of partners in this cohort of mainly commercial sex workers.
Genital tract inflammatory cytokine concentrations were similar in women who had symptomatic and asymptomatic laboratory-diagnosed STIs and were elevated in these women, relative to women who had no STIs, bacterial vaginosis, or symptoms. Previous studies have suggested that elevated genital tract inflammatory cytokine concentrations may facilitate HIV transmission by directly upregulating HIV replication, by recruiting and activating immune cell targets for HIV infection, and by disrupting tight junctions between epithelial cells [31–37]. Although confounded by the long interval between CVL cytokine measurements and time of HIV infection and by the fact that women were treated for STIs in the period between enrollment and the time of HIV infection, we found that higher concentrations of inflammatory cytokines (IL-1β, IL-6, IL-8, and sCD40L) in CVL specimens were associated with increased risk of HIV infection, albeit weakly. This supports our finding that women who had no clinical symptoms but had subclinical STIs were at increased risk of HIV acquisition, with N. gonorrhoeae remaining associated after adjustment for behavioral factors. Although this suggests that underlying genital tract inflammation may be a biological mechanism for HIV transmission in these women, it is also possible that the relationship between elevated inflammation and risk of HIV infection is indirect. Elevated cytokine concentrations were found to be associated with STIs, which may be markers of high-risk sexual activity and thus increased risk of HIV infection due to behavior.
The finding that clinically evident and syndromically managed STIs declined during follow-up while the prevalence of laboratory-diagnosed STI remained relatively high confirms that syndromic management does not address subclinical STI infections, which may contribute to genital inflammation and risk of HIV infection. This has important implications not only for HIV prevention strategies, but also for complications that are associated with untreated inflammatory STIs, including pelvic inflammatory diseases, ectopic pregnancy, and infertility [20, 30].
These data create a compelling argument for readdressing the STI management strategy in high-risk populations in which negotiating condom use is a challenge for women. Healthcare systems should include regular screening for STIs by means of laboratory testing in these groups rather than relying on symptoms only. The increased diagnostic power of PCR technology and the related potential to impact the risk of HIV acquisition could outweigh its high costs. This strategy would lead to treatment of greater numbers of STIs, reducing prevalence and incidence. Another diagnostic approach in underresourced areas would be to explore point-of-care STI testing as an alternative to laboratory testing. Some of these point-of-care tests have proven to be sensitive diagnostic tools in trials and would offer significant benefit in both rural and urban poor-resourced settings that do not have good access to a larger STI sentinel screening laboratory [45, 46].
Findings from this study and those from studies of other high-risk cohorts in South Africa [13] suggest that the current symptom-driven syndromic management system is untenable for high-risk populations and underscores the need for a paradigm shift in diagnosing STIs. Only when more effective STI treatment is achieved are we likely to see STI management playing a role in HIV prevention.
Notes
Acknowledgments. We thank the following people for their contribution to this work: Ms Fazana Karim, for her assistance with the microbiological diagnosis; the Centre for the AIDS Programme of Research in South Africa (CAPRISA) Acute Infection (CAPRISA 002) Study Team; and the participants of the CAPRISA 002 study, without whom this work would not have been possible.
Financial support. This work was supported by grants from the Comprehensive International Program of Research on AIDS of the Division of AIDS, National Institute of Allergy and Infectious Disease, National Institutes of Health, US Department of Health and Human Services (grant5U19 AI051794) and the National Research Foundation, South Africa (grant UID 67385).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Johnson LF, Dorrington RE, Bradshaw D, Coetzee DJ. The effect of syndromic management interventions on the prevalence of sexually transmitted infections in South Africa. Sex Reprod Healthc. 2011;2:13–20. doi: 10.1016/j.srhc.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS. Geneva: Joint United Nations Programme on HIV/AIDS; 2010. UNAIDS report on the global AIDS Epidemic 2010. Available at: http://www.unaids.org/globalreport/ Accessed 11 February 2011. [Google Scholar]
- 3.Rehle T, Shisana O, Pillay V, Zuma K, Puren A, Parker W. National HIV incidence measures—new insights into the South African epidemic. S Afr Med J. 2007;97:194–9. [PubMed] [Google Scholar]
- 4.Stamm WE, Handsfield HH, Rompalo AM, Ashley RL, Roberts PL, Corey L. The association between genital ulcer disease and acquisition of HIV infection in homosexual men. JAMA. 1988;260:1429–33. [PubMed] [Google Scholar]
- 5.Plummer FA, Simonsen JN, Cameron DW, et al. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1991;163:233–9. doi: 10.1093/infdis/163.2.233. [DOI] [PubMed] [Google Scholar]
- 6.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 7.Laga M, Manoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868–73. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MS. Sexually transmitted diseases enhance HIV transmission: no longer a hypothesis. Lancet. 1998;351((Suppl 3):5–7. doi: 10.1016/s0140-6736(98)90002-2. [DOI] [PubMed] [Google Scholar]
- 10.McClelland RS, Wang CC, Mandaliya K, et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS. 2001;15:105–10. doi: 10.1097/00002030-200101050-00015. [DOI] [PubMed] [Google Scholar]
- 11.Ramjee G, Williams B, Gouws E, Van Dyck E, De Deken B, Karim SA. The impact of incident and prevalent herpes simplex virus-2 infection on the incidence of HIV-1 infection among commercial sex workers in South Africa. JAIDS. 2005;39:333–9. doi: 10.1097/01.qai.0000144445.44518.ea. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Guidelines for the management of sexually transmitted infections. Available at http://www.who.int/hiv/pub/sti/en/STIGuidelines2003.pdf . Accessed 14 March 2011.
- 13.Moodley P, Sturm AW. Management of vaginal discharge syndrome: how effective is our strategy? Int J Antimicrob Agents. 2004;24(Suppl 1):S4–7. doi: 10.1016/j.ijantimicag.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Bogaerts J, Ahmed J, Akhter N, Begum N, Van Ranst M, Verhaegen J. Sexually transmitted infections in a basic healthcare clinic in Dhaka, Bangladesh: syndromic management for cervicitis is not justified. Sex Transm Infect. 1999;75:437–8. doi: 10.1136/sti.75.6.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettifor A, Walsh J, Wilkins V, Raghunathan P. How effective is syndromic management of STDs? A review of current studies. Sex Transm Dis. 2000;27:371–85. doi: 10.1097/00007435-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Behets FM, Miller WC, Cohen MS. Syndromic treatment of gonococcal and chlamydial infections in women seeking primary care for the genital discharge syndrome: decision-making. Bull World Health Organ. 2001;79:1070–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Desai VK, Kosambiya JK, Thakor HG, Umrigar DD, Khandwala BR, Bhuyan KK. Prevalence of sexually transmitted infections and performance of STI syndromes against aetiological diagnosis, in female sex workers of red light area in Surat, India. Sex Transm Infect. 2003;79:111–5. doi: 10.1136/sti.79.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pepin J, Deslandes S, Khonde N, et al. Low prevalence of cervical infections in women with vaginal discharge in west Africa: implications for syndromic management. Sex Transm Infect. 2004;80:230–5. doi: 10.1136/sti.2003.007534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson D, Abdool Karim SS, Harrison A, et al. Unrecognized sexually transmitted infections in rural South African women: a hidden epidemic. Bull World Health Organ. 1999;77:22–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Moodley P, Sturm AW. Sexually transmitted infections, adverse pregnancy outcome and neonatal infection. Semin Neonatol. 2000;5:255–69. doi: 10.1053/siny.2000.0026. [DOI] [PubMed] [Google Scholar]
- 21.Wawer MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group. Lancet. 1999;353:525–35. doi: 10.1016/s0140-6736(98)06439-3. [DOI] [PubMed] [Google Scholar]
- 22.Kaul R, Kimani J, Nagelkerke NJ, et al. Monthly antibiotic chemoprophylaxis and incidence of sexually transmitted infections and HIV-1 infection in Kenyan sex workers: a randomized controlled trial. JAMA. 2004;291:2555–62. doi: 10.1001/jama.291.21.2555. [DOI] [PubMed] [Google Scholar]
- 23.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 24.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:2109–19. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. New Engl J Med. 2008;358:1560–1571. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grosskurth H, Mosha F, Todd J, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet. 1995;346:530–6. doi: 10.1016/s0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]
- 27.Grosskurth H, Gray R, Hayes R, Mabey D, Wawer M. Control of sexually transmitted diseases for HIV-1 prevention: understanding the implications of the Mwanza and Rakai trials. Lancet. 2000;355:1981–7. doi: 10.1016/S0140-6736(00)02336-9. [DOI] [PubMed] [Google Scholar]
- 28.Kamali A, Quigley M, Nakiyingi J, et al. Syndromic management of sexually-transmitted infections and behaviour change interventions on transmission of HIV-1 in rural Uganda: a community randomised trial. Lancet. 2003;361:645–52. doi: 10.1016/s0140-6736(03)12598-6. [DOI] [PubMed] [Google Scholar]
- 29.Gregson S, Adamson S, Papaya S, et al. Impact and process evaluation of integrated community and clinic-based HIV-1 control: a cluster-randomised trial in eastern Zimbabwe. PLoS Med. 2007;4:e102. doi: 10.1371/journal.pmed.0040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiesenfeld HC, Hillier SL, Krohn MA, et al. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet Gynecol. 2002;100:456–63. doi: 10.1016/s0029-7844(02)02118-x. [DOI] [PubMed] [Google Scholar]
- 31.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989;86:2336–40. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swingler S, Mann A, Jacque J, et al. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat Med. 1999;5:997–1003. doi: 10.1038/12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–8. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nkwanyana NN, Gumbi PP, Roberts L, et al. Impact of human immunodeficiency virus 1 infection and inflammation on the composition and yield of cervical mononuclear cells in the female genital tract. Immunology. 2009;128:e746–57. doi: 10.1111/j.1365-2567.2009.03077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724–7. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz H, Epple HJ, Fromm M, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha (TNF-a) impairs barrier function in epithelial monolayers of HT-29/B6 cells. Gastroenterol. 1995;108:A322. [Google Scholar]
- 37.Nazli A, Chan O, Dobson-Belaire WN, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Loggerenberg F, Mlisana K, Williamson C, et al. Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PLoS One. 2008;3:e1954. doi: 10.1371/journal.pone.0001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sturm PD, Moodley P, Khan N, et al. Aetiology of male urethritis in patients recruited from a population with a high HIV prevalence. Int J Antimicrob Agents. 2004;24(Suppl 1):S8–14. doi: 10.1016/j.ijantimicag.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Moodley P, Sturm PD, Vanmali T, Wilkinson D, Connolly C, Sturm AW. Association between HIV-1 infection, the etiology of genital ulcer disease, and response to syndromic management. Sex Transm Dis. 2003;30:241–5. doi: 10.1097/00007435-200303000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Bebell LM, Passmore JA, Williamson C, et al. Relationship between levels of inflammatory cytokines in the genital tract and CD4+ cell counts in women with acute HIV-1 infection. J Infect Dis. 2008;198:710–4. doi: 10.1086/590503. [DOI] [PubMed] [Google Scholar]
- 42.Columb MO, Sagadai S. Multiple comparisons. Curr Anaesth Crit Care. 2006;17:233–6. [Google Scholar]
- 43.White RG, Moodley P, McGrath N, et al. Low effectiveness of syndromic treatment services for curable sexually transmitted infections in rural South Africa. Sex Transm Infect. 2008;84:528–34. doi: 10.1136/sti.2008.032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis. 2003;30:797–800. doi: 10.1097/01.OLQ.0000092387.58746.C7. [DOI] [PubMed] [Google Scholar]
- 45.Mania-Pramanik J, Kerkar SC, Mehta PB, Potdar S, Salvi VS. Use of vaginal pH in diagnosis of infections and its association with reproductive manifestations. J Clin Lab Anal. 2008;22:375–9. doi: 10.1002/jcla.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madhivanan P, Krupp K, Hardin J, Karat C, Klausner JD, Reingold AL. Simple and inexpensive point-of-care tests improve diagnosis of vaginal infections in resource constrained settings. Trop Med Int Health. 2009;14:703–8. doi: 10.1111/j.1365-3156.2009.02274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]