Abstract
Background. Few studies have assessed genital human papillomavirus (HPV) concordance and factors associated with concordance among asymptomatic heterosexual couples.
Methods. Genotyping for HPV was conducted with male and female sex partners aged 18–70 years from Tampa, Florida. Eligibility included no history of HPV-associated disease. Type-specific positive concordance (partners with ≥1 genotype in common) and negative concordance (neither partner had HPV) were assessed for 88 couples. Factors associated with concordance were assessed with Fisher exact tests and tests for trend.
Results. Couples reported engaging in sexual intercourse for a median of 1.7 years (range, 0.1–49 years), and 75% reported being in the same monogamous relationship for the past 6 months. Almost 1 in 4 couples had type-specific positive concordance, and 35% had negative concordance for all types tested, for a total concordance of 59%. Concordance was not associated with monogamy. Type-specific positive concordance was associated with an increasing difference in partners’ lifetime number of sex partners and inversely associated with an increasing difference in age. Negative concordance was inversely associated with both the couple's sum of lifetime number of sex partners and the difference in the partners’ lifetime number of sex partners.
Conclusions. Genital HPV concordance was common. Viral infectiousness and number of sex partners may help explain concordance among heterosexual partners.
Research over the past several decades has definitively demonstrated that human papillomavirus (HPV) is the necessary cause of cervical cancer, the primary cause of anal canal cancer, and an important etiologic agent in cancer of the oropharynx in both men and women, vaginal and vulvar cancer in women, and penile cancer in men [1].
Since HPV is a sexually transmitted infection (STI), a number of studies have reported concordance of HPV infection among sex partners, particularly heterosexual partners. In a recent meta-analysis of 30 studies reporting concordance, approximately 1 in 4 couples exhibited type-specific positive concordance [2]; however, concordance estimates obtained by these studies may be an overestimation of couple concordance because of the inclusion of participants with HPV-associated disease or preexisting HPV infection [2].
Type-specific positive concordance varies greatly, from a high of 41% to a low of 4%, among cross-sectional or case-control studies enrolling asymptomatic partners in heterosexual relationships [3–9]) and likely depends on study population, sampling methods, and HPV DNA detection techniques. For example, Burchell et al observed a concordance of 41% among 263 young couples in Montreal, Canada [3], while Parada et al reported a type-specific positive concordance of only 4% among 504 primarily rural couples recruited in Mexico [8].
In addition, there is little understanding of the factors associated with concordance among heterosexual couples, particularly among healthy sex partners with no history of HPV-related disease. To our knowledge, only 1 study has assessed factors associated with type-specific positive concordance among couples with no HPV-associated disease and who were not seeking testing or care for STIs [3]. Among monogamous and newly formed heterosexual couples, Burchell et al reported that recent vaginal sex was associated with type-specific infection in both partners. The same study also observed increased type-specific positive concordance among couples who had engaged in vaginal sex with each other for 5–6 months, compared with couples with a sexual relationship of shorter duration. Examination of the factors associated with type-specific positive concordance in healthy heterosexual couples is essential to increase our understanding of HPV acquisition and transmission dynamics.
The objective of the current analysis was to characterize HPV infection concordance among heterosexual partners with no history of HPV-associated disease and to determine factors associated with HPV concordance and its corollary, discordance, among these couples.
MATERIALS AND METHODS
Study Design and Enrollment
Between 2005 and 2009, men were recruited from a large university and the general community in the Tampa, Florida, area for enrollment in the 48-month longitudinal HPV in Men (HIM) Study, whose methods have been described in detail previously [10]. Criteria for enrollment of men in the HIM Study included an age of 18–70 years, no prior diagnosis of an HPV-associated cancer or genital warts, and no current STI diagnosis, including HIV infection.
A total of 1258 HIM Study male recruits with clinic visits from November 2006 to May 2010 were asked whether they had a steady female partner, and, if so, whether they would invite their partner to join the couples’ study. A total of 560 men agreed to invite their partners, with 222 female partners responding affirmatively. Of these, 165 of 222 (74%) met inclusion criteria for the study; however, 27 did not attend the first clinical visit, and 1 woman dropped out after successfully meeting the inclusion criteria. Women were excluded if they reported an abnormal Papanicolaou (Pap) test finding in the prior 6 months, a hysterectomy, pregnancy, or enrollment in an HPV vaccine trial; however, they were not excluded if they chose to receive an HPV vaccine after enrollment. Thus, a total of 137 female sex partners of HIM Study participants were enrolled in the current study.
While partners in couples were required to be each other's current primary sex partners, they were not required to acknowledge sexual intercourse in the recent past. Each partner independently consented to the current study's protocol, which was approved by the Institutional Review Board of the University of South Florida. Participants received a nominal incentive for study involvement.
Procedure
Couples were instructed to not have sex for 48 hours before a clinic visit, to avoid detection of superficial HPV deposited by partners. Women were asked to not douche for 24 hours before an appointment. Couples were encouraged to complete clinical visits with ≤14 days separating each partner's visit.
The clinical protocol for both partners was similar and included a physical examination and the collection of biological and behavioral data in private. Behavioral data were collected with a computer-assisted self-interview which elicited information about participant demographic characteristics, substance use, and sexual behaviors with primary and nonprimary partners. Females were also questioned about Pap cytology screening and pregnancy history.
A clinician collected blood and urine specimens and examined the participant, including an inspection of the skin and external genitalia. By use of a saline-wetted cotton swab, warts and/or lesions, if present, were sampled. For women, the cervix, vulva/labia (including perineum), and anal canal were sampled. These specimens were collected by swabbing from the clitoral prepuce down to the posterior fourchette (including collection between the folds of the labia minora and majora). Then, by use of a separate swab, cells were collected from between the anal os and the anal canal dentate line. The anal canal was not visualized. To sample the endocervix/ectocervix, the cervix was visualized and then a swab moistened with normal saline was introduced into the cervical os, rotated 1–2 times, and brushed back and forth across the ectocervix. Swabs from the cervix, vulva, and anal canal were kept separate with each swab placed into standard transport medium. The cervix was then swabbed to assess cytological status. To sample the men, the clinician used a saline-wetted swab to sweep 360° around the coronal sulcus, glans penis, and, if present, the retracted prepuce. A second swab was used to sample the entire surface of the penile shaft, while a third was used to sample the scrotum. These 3 swabs were combined and placed into standard transport media. Specimens from both partners were stored at −80°C until polymerase chain reaction (PCR) analyses and genotyping were conducted.
HPV Testing
Samples were analyzed for HPV DNA as described previously [11]. Briefly, DNA was extracted using the QIAamp Media MDx Kit (Qiagen). The PCR consensus primer system (PGMY 09/11) was used to amplify a fragment of the HPV L1 gene [12]. HPV genotyping was conducted on all samples, regardless of HPV PCR findings, using DNA probes labeled with biotin to detect the following 36 HPV types: 6, 11, 16, 18, 26, 31, 33, 34, 35, 39, 40, 42, 44, 45, 51–54, 56, 58, 59, 61, 62, 66–73, 81–84, and 89 [13]. Accuracy and potential contamination were assessed using nontemplate negative controls and CaSki DNA positive controls. For 121 of 137 enrolled couples (88.3%), both the female and male were either β-globin or HPV-genotype positive.
Statistical Analyses
The concordance analysis is based on the couples’ enrollment data. For men, a genital sample contained exfoliated cells from the coronal sulcus, glans penis (and, if present, a retracted prepuce), the entire surface of the penile shaft, and the scrotum. For women, a genital sample contained exfoliated cells from the cervix and vulva.
Prevalence estimates and exact 95% confidence intervals (CIs) were derived for HPV species, genotypes, and groups of genotypes. A specimen was considered positive for the group “any HPV DNA” if it was HPV positive by PCR or positive for ≥1 of 36 HPV genotypes. A specimen was considered positive for the group “any genotype” if it was positive for ≥1 of 36 HPV genotypes. Specimens were labeled as “any oncogenic” if ≥1 of 13 oncogenic HPV types were detected (ie, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 [14]), regardless of the presence of other genotypes. Similarly, specimens were labeled as “any nononcogenic” if any of the remaining 23 HPV types in the linear array were detected, regardless of the presence of oncogenic types. In contrast, a specimen was labeled “only oncogenic” if it contained only oncogenic HPV types with no nononcogenic coinfection. A specimen was labeled “only nononcogenic” if it contained only nononcogenic HPV types with no oncogenic coinfection. Specimens were also labeled according to species [15].
For HPV genotypes with the highest prevalence (ie, ≥5.0%), expected and observed concordance was assessed where the expected concordance was calculated as the product of the type-specific prevalence at genital sites in women (cervical and vulvar) and men (penile and scrotal). The proportion of men and women who had partners with corresponding type-specific infection was also assessed.
The proportion of couples who were concordant and discordant was calculated. A couple was classified as having “type-specific positive concordance” if the man and woman had ≥1 HPV genotype in common. A couple was classified as having “negative concordance” if both the man and woman were negative for all 36 genotypes. Finally, if the man and woman were discordant for ≥1 HPV genotype, regardless of the presence of concordant genotypes, the couple was classified as having “any discordance.”
Sociodemographic characteristics and behaviors reported by each partner were compared and/or averaged to create variables for the couple's combined characteristics. For example, each partner's reported number of sex partners in the prior 6 months was used to create a variable with the values “monogamous” (both partners report sex only with each other) and “nonmonogamous” (either partner reports ≥2 sex partners). Associations between couple characteristics and concordance/discordance were assessed with Fisher exact tests and Cochran-Armitage tests for trend. Because each couple characteristic was assessed with 3 different types of concordance, P values for concordance outcomes were adjusted for multiple comparisons, using a bootstrap method with 200 000 resamples [16]. Data were analyzed using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
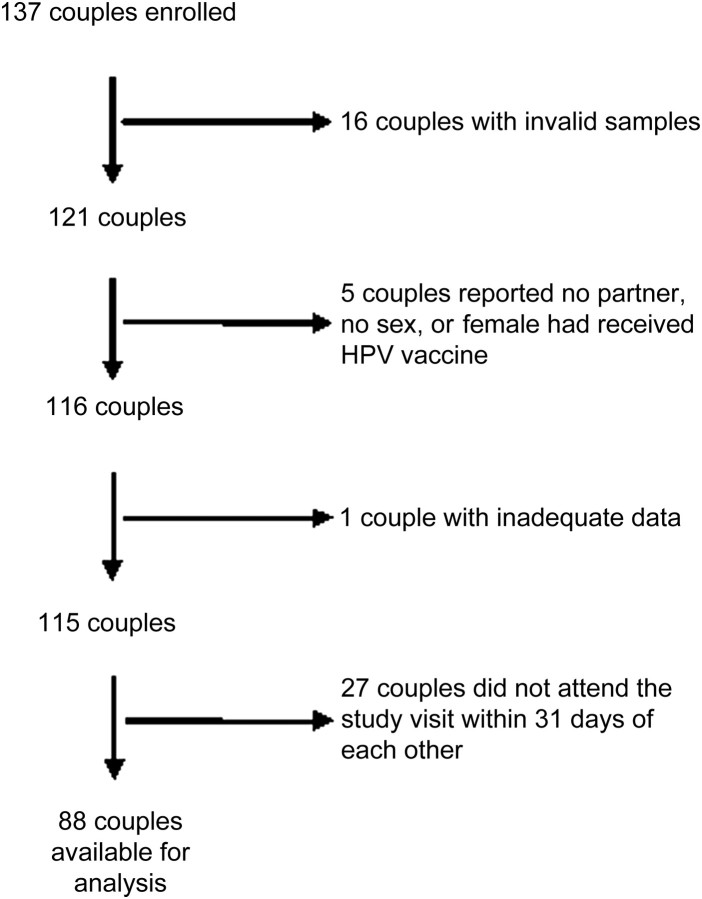
Of 121 couples who provided valid biological samples, 6 reported either no sex with their partner, inadequate data on the computer-assisted self-interview, or HPV vaccine receipt by the woman prior to the study. These couples were removed from further analysis. An additional 27 couples were excluded from analysis because the partners did not appear for their clinical appointments within 31 days of each other, leaving a total of 88 couples available for analysis (Figure 1).
Figure 1.
Numbers of couples enrolled, attrition, and couples available for analysis in a study of genital human papillomavirus (HPV) concordance, 2006–2010.
Sociodemographic and behavioral characteristics of the men and women were generally similar. The median age of men and women was 24.5 and 24.0 years, respectively, while 69.3% of men and 72.7% of women were white; however, the median lifetime number of sex partners of the opposite sex was 8 among men and 5.5 among women (Table 1). Couples reported engaging in sexual intercourse for a median of 1.7 years (range, 0.1–49 years). The median time between partners’ study visits was 9 days (range, 0–30 days). A total of 76 of 88 women (86.4%) had normal cervical cytology findings, 9 of 88 (10.2%) had atypical cells of undetermined significance, and 3 of 88 (3.4%) had low-grade squamous intraepithelial lesions (data not shown).
Table 1.
Selected Characteristics of Men and Women in Heterosexual Couples in a Study of Genital Human Papillomavirus (HPV) Concordance, 2006–2010
| Variable | Men (n = 88) | Women (n = 88) |
|---|---|---|
| Age | ||
| 18–30 y | 55 (62.5) | 55 (62.5) |
| 31–44 y | 17 (19.3) | 17 (19.3) |
| 45–70 y | 16 (18.2) | 16 (18.2) |
| Refuse | Not applicable | Not applicable |
| Median y, (range) | 24.5 (18–70) | 24.0 (18–70) |
| Race | ||
| White | 61 (69.3) | 64 (72.7) |
| Black | 15 (17.1) | 7 (8.0) |
| Mixed/other | 12 (13.6) | 17 (19.3) |
| Ethnicity | ||
| Hispanic | 19 (21.6) | 16 (18.2) |
| Non-Hispanic | 69 (78.4) | 71 (80.7) |
| Refuse | 0 | 1 (1.1) |
| Education, years | ||
| <12 | 4 (4.6) | 1 (1.1) |
| 12 | 14 (15.9) | 9 (10.2) |
| 13–16 | 67 (76.1) | 75 (85.2) |
| ≥17 | 3 (3.4) | 3 (3.4) |
| Marital status | ||
| Single, never married | 49 (55.7) | 39 (44.3) |
| Married | 23 (26.1) | 23 (26.1) |
| Cohabitating | 7 (8.0) | 17 (19.3) |
| Divorced/separated/widowed | 9 (10.2) | 9 (10.2) |
| Woman received HPV vaccine | ||
| Yes | Not applicable | 0 |
| No | Not applicable | 80 (90.9) |
| Refuse, missing | Not applicable | 8 (9.1) |
| Man has prepuce (clinician record) | ||
| Yes | 20 (22.7) | Not applicable |
| No | 70 (77.3) | Not applicable |
| Lifetime no. of sex partners of opposite sex | ||
| 1–2 | 13 (14.8) | 17 (19.3) |
| 3–9 | 29 (33.0) | 41 (46.6) |
| ≥10 | 40 (45.5) | 28 (31.8) |
| Refuse | 6 (6.8) | 2 (2.3) |
| Median no. (range) | 8 (1–200) | 5.5 (1–40) |
| No. of sex partners of opposite sex past 6 mo | ||
| 0 | 11 (12.5) | 1 (1.1) |
| 1 | 64 (72.7) | 74 (84.1) |
| ≥2 | 9 (10.2) | 12 (13.6) |
| Refuse | 4 (4.6) | 1 (1.1) |
| Median no. (range) | 1 (0–30) | 1 (0–8) |
| Frequency of condom use for vaginal sex with primary partner in past 6 mo | ||
| Always | 5 (5.7) | 11 (12.5) |
| Sometimes | 31 (35.2) | 27 (30.7) |
| Never | 44 (50.0) | 44 (50.0) |
| No recent vaginal sex | 1 (1.1) | 0 (.0) |
| Refuse | 7 (8.0) | 6 (6.8) |
Data are no. (%) of subjects, unless otherwise indicated.
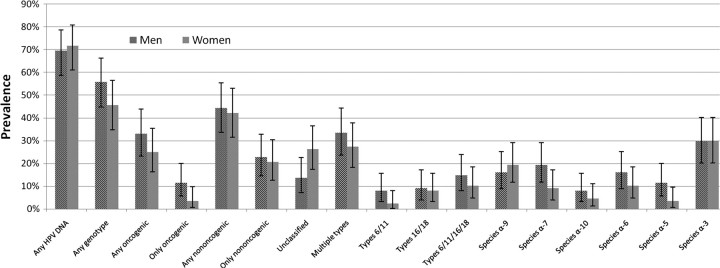
Overall, the prevalence of genital HPV was comparable among men and women, with a prevalence for any genotype of 55.7% (95% CI, 44.7%–66.3%) and 45.5% (95% CI, 34.8%–56.4%), respectively (Figure 2). We detected either HPV-16 or HPV-18 in 16.3% of couples and HPV-6,-11,-16, or -18 in 23.3% of couples (data not shown).
Figure 2.
Genital human papillomavirus (HPV) prevalence in 88 couples, by sex, 2006–2010. Genital prevalence is derived from penile and scrotal specimens in men and from cervical and vulvar specimens in women. HPV DNA was detected with the Roche linear array for 36 genotypes. Species α-9 genotypes observed in either men or women were HPV-16, -31, -33, -35, -52, -58, and -67. Species α-7 genotypes observed were HPV-18, -39, -45, -59, -68, and -70. Species α-10 genotypes observed were HPV-6, and -44. Species α-6 genotypes observed were HPV-53, -56, and -66. Species α-5 genotypes observed were HPV-51 and -82. Species α-3 genotypes observed were HPV-61, -62, -72, -81, -83, -84, and -89.
HPV Concordance
A total of 31 couples had negative concordance (Table 2). Among 8 couples, we detected no HPV in the man, while the woman was positive for ≥1 genotype. Among 17 couples, we detected no HPV in the woman, while the man was positive for ≥1 genotype. Partners were concordant for all genotypes in 2 couples.
Table 2.
Genital Human Papillomavirus (HPV) Genotypes in Couples, by Partner HPV Status, 2006–2010
| HPV Genotype(s) |
||
|---|---|---|
| Couple | Men | Women |
| Man−/woman− (n = 31) | ||
| 1–31 | 0 | 0 |
| Man−/woman+ (n = 8) | ||
| 32 | 0 | 16 |
| 33 | 0 | 16, 54, 66 |
| 34 | 0 | 35, 62 |
| 35–36 | 0 | 54 |
| 37 | 0 | 44,a 62, 72 |
| 38 | 0 | 62 |
| 39 | 0 | 62, 83, 89a |
| Man+/woman− (n = 17) | ||
| 40–41 | 6 | 0 |
| 42 | 6, 16, 44a | 0 |
| 43 | 16, 18, 45, 51, 59 | 0 |
| 44 | 39, 58, 67, 68, 84 | 0 |
| 45 | 45, 51, 59 | 0 |
| 46 | 51 | 0 |
| 47 | 54 | 0 |
| 48 | 56 | 0 |
| 49–50 | 59 | 0 |
| 51 | 61 | 0 |
| 52 | 62, 70, 81 | 0 |
| 53 | 73 | 0 |
| 54–56 | 84 | 0 |
| Man+/woman+ | ||
| Full concordance (n = 2) | ||
| 57 | 61, 68, 82a | 61, 68, 82a |
| 58 | 89a | 89a |
| Partial concordance (n = 19) | ||
| 59 | 6, 83 | 6, 44, 62, 71, 83 |
| 60 | 16 | 16, 52, 66 |
| 61 | 16, 39, 54, 84, 89a | 16, 39, 54, 89a |
| 62 | 16, 51, 89a | 89a |
| 63 | 16, 59, 84, 89a | 16, 59, 73 |
| 64 | 18, 31, 66 | 31, 83 |
| 65 | 31, 51, 52, 58 | 31, 52, 62 |
| 66 | 31, 68 | 31 |
| 67 | 31, 81 | 31, 66, 67, 70 |
| 68 | 39, 52, 58 | 52, 58, 62 |
| 69 | 45, 54, 59, 66, 68, 83 | 45, 54, 44, 61, 66, 70, 89a |
| 70 | 45, 70 | 70 |
| 71 | 51, 62, 66, 82a | 62, 82a |
| 72 | 53, 68 | 53 |
| 73 | 54, 59, 66, 89a | 16, 42, 59, 66, 89a |
| 74 | 56, 84 | 56, 66, 84 |
| 75 | 59, 62, 66, 68, 89a | 62 |
| 76 | 62 | 16, 62 |
| 77 | 81 | 56, 81 |
| No concordance (n = 11) | ||
| 78 | 6 | 31, 61 |
| 79 | 6, 16, 31, 44,a 83 | 72 |
| 80 | 6, 35, 52 | 40 |
| 81 | 51, 53 | 52 |
| 82 | 51, 53, 61 | 58, 72 |
| 83 | 53 | 54, 82a |
| 84 | 53, 62 | 83 |
| 85 | 53, 84 | 59, 89a |
| 86 | 62, 82a | 54, 84 |
| 87 | 66 | 6, 33, 52, 71, 84 |
| 88 | 72 | 66 |
The men's genital specimen combines penile and scrotal sites, and the women's genital specimen combines cervical and vulvar sites.
Abbreviations: −, HPV-genotype negative; +, HPV-genotype positive.
a HPV-55 is considered a subtype of HPV-44, IS39 is considered a subtype of HPV-82, and HPV-89 was previously known as CP6108.
For the most common genotypes (ie, prevalence ≥5.0%), observed concordance between genital specimens of men and women was higher than expected. For example, while it was expected that 0.6% of couples would be concordant for HPV-16 by chance, we observed 3.4% concordance for HPV-16 (Table 3). Among men and women with HPV-16, 42.9% (3 of 7) of their partners were concordant. Among men and women with HPV-6, 14.3% (1 of 7) and 50.0% (1 of 2) of their partners, respectively, were concordant (Table 3).
Table 3.
Human Papillomavirus (HPV) Genotype Concordance of Genital Specimens Between Men and Women, 2006–2010
| Men, No. (%) |
Women, No. (%) |
Concordance, % |
||||
|---|---|---|---|---|---|---|
| HPV Type | Genital Prevalence | Partner Has Same Type | Genital Prevalence | Partner Has Same Type | Expecteda | Observed |
| Oncogenic | ||||||
| 16 | 7 (8.0) | 3 (42.9) | 7 (8.0) | 3 (42.9) | 0.6 | 3.4 |
| 31 | 5 (5.7) | 4 (80.0) | 5 (5.7) | 4 (80.0) | 0.3 | 4.6 |
| 51 | 8 (9.1) | 0 | 0 | … | 0 | 0 |
| 59 | 8 (9.1) | 2 (25.0) | 3 (3.4) | 2 (66.7) | 0.3 | 2.3 |
| 68 | 6 (6.8) | 1 (16.7) | 1 (1.1) | 1 (100.0) | 0.1 | 1.1 |
| Nononcogenic | ||||||
| 6 | 7 (8.0) | 1 (14.3) | 2 (2.3) | 1 (50.0) | 0.2 | 1.1 |
| 53 | 6 (6.8) | 1 (16.7) | 1 (1.1) | 1 (100.0) | 0.1 | 1.1 |
| 54 | 4 (4.6) | 2 (50.0) | 7 (8.0) | 2 (28.6) | 0.4 | 2.3 |
| 62 | 6 (6.8) | 3 (50.0) | 10 (11.4) | 3 (30.0) | 0.8 | 3.4 |
| 66 | 6 (6.8) | 2 (33.3) | 7 (8.0) | 2 (28.6) | 0.5 | 2.3 |
| 84 | 8 (9.1) | 1 (12.5) | 3 (3.4) | 1 (33.3) | 0.3 | 1.1 |
| 89b | 6 (6.8) | 4 (66.7) | 7 (8.0) | 4 (57.1) | 0.5 | 4.6 |
Only genotypes with ≥5% prevalence among either male or female genital specimens are shown. The men's genital specimen combines penile and scrotal sites, and the women's genital specimen combines cervical and vulvar sites.
a Equal to the product of the prevalence in men and prevalence in women.
b Previously known as CP6108.
Factors Associated With Concordance
Type-specific positive concordance was observed in 23.9% of couples (21 of 88), while 35.2% had negative concordance (Table 4), for a total of 59.1% exhibiting either kind of concordance. Genotype discordance for ≥1 HPV genotype was observed in 62.5% of couples. Type-specific positive concordance was higher among couples with more similar ages (P = .04). Neither concordance nor discordance was associated with difference in races, ethnicities, or education or with disagreement between partners about marital status. We were more likely to observe type-specific positive concordance among couples who reported sexual intercourse for <1 year (41.4%), compared with couples who reported sexual intercourse for ≥ 1 year (14.6%) (P = .04).
Table 4.
Concordance and Discordance for Any Human Papillomavirus (HPV) Type, by Selected Characteristics of Couples, 2006–2010
| Variablea | Couples, No. (%) | Type-Specific Positive Concordance, %b | Negative Concordance, %c | Any Discordance, %d |
|---|---|---|---|---|
| All | 88 (100.0) | 23.9 | 35.2 | 62.5 |
| Age difference between partners | ||||
| ≤1 y | 46 (52.3) | 30.4 | 39.1 | 56.5 |
| 2–4 y | 23 (26.1) | 30.4 | 34.8 | 65.2 |
| ≥5 y | 19 (21.6) | 0 | 26.3 | 73.7 |
| Pe | .04 | .59 | .36 | |
| Partners’ races | ||||
| Different | 18 (20.5) | 33.3 | 27.8 | 72.2 |
| Same | 70 (79.6) | 21.4 | 37.1 | 60.0 |
| P | .51 | .79 | .62 | |
| Partners’ ethnicities | ||||
| Different | 17 (19.3) | 17.7 | 29.4 | 70.6 |
| Same | 70 (79.6) | 25.7 | 35.7 | 61.4 |
| Either partner refused | 1 (1.1) | 0 | 100.0 | 0 |
| P | .87f | .93 | .80 | |
| Partners’ education level | ||||
| Different | 27 (30.7) | 29.6 | 29.6 | 66.7 |
| Same | 61 (69.3) | 21.3 | 37.7 | 60.7 |
| P | .60 | .83 | .86 | |
| Marital status | ||||
| Disagree | 24 (27.3) | 29.2 | 33.3 | 62.5 |
| Agree | 64 (72.7) | 21.9 | 35.9 | 62.5 |
| P | .75 | 1.00 | 1.00 | |
| Length of relationship with partner | ||||
| <1 y | 29 (33.0) | 41.4 | 27.6 | 65.5 |
| ≥1 y | 41 (46.6) | 14.6 | 34.2 | 65.9 |
| Either partner refused | 18 (20.5) | 16.7 | 50.0 | 50.0 |
| P | .04 | .83 | 1.00 | |
| Monogamy | ||||
| Both partners report monogamy | 66 (75.0) | 19.7 | 34.9 | 63.6 |
| Either partner reports nonmonogamy | 17 (19.3) | 41.2 | 23.5 | 70.6 |
| Either partner refused | 5 (5.7) | 20.0 | 80.0 | 20.0 |
| P | .18 | .75 | .93 | |
| Man has prepuce (clinician record) | ||||
| Yes | 20 (22.7) | 20.0 | 50.0 | 50.0 |
| No | 68 (77.3) | 25.0 | 30.9 | 66.2 |
| P | .90 | .27 | .32 | |
| Sum of lifetime no. of sex partners of opposite sex | ||||
| 2 | 2 (2.3) | 0 | 100.0 | 0 |
| 3–9 | 21 (23.9) | 14.3 | 71.4 | 28.6 |
| 10–19 | 25 (28.4) | 24.0 | 20.0 | 72.0 |
| ≥20 | 32 (36.4) | 34.4 | 9.4 | 90.6 |
| Either partner refused | 8 (9.1) | 12.5 | 75.0 | 25.0 |
| Pe | .13 | < .0001 | < .0001 | |
| Difference in lifetime no. of sex partners of opposite sex | ||||
| 0–2 | 24 (27.3) | 12.5 | 58.3 | 41.7 |
| 3–4 | 15 (17.1) | 13.3 | 40.0 | 60.0 |
| 5–9 | 16 (18.2) | 31.3 | 18.8 | 68.8 |
| ≥10 | 25 (28.4) | 40.0 | 8.0 | 92.0 |
| Either partner refused | 8 (9.1) | 12.5 | 75.0 | 25.0 |
| Pe | .03 | .0001 | .0003 | |
| Days since last vaginal sex | ||||
| 1–2 | 23 (26.1) | 34.8 | 13.0 | 82.6 |
| 3–7 | 39 (44.3) | 20.5 | 41.0 | 56.4 |
| >7 | 18 (20.5) | 16.7 | 38.9 | 61.1 |
| Either partner refused | 8 (9.1) | 25.0 | 62.5 | 37.5 |
| Pe | .32 | .15 | .24 | |
| Frequency of condom use for vaginal sex with primary partner in past 6 mo | ||||
| Condoms used at least sometimesg | 30 (34.1) | 20.0 | 40.0 | 56.7 |
| Condoms never used or partners disagreeh | 47 (53.4) | 29.8 | 27.7 | 70.2 |
| Either partner refused | 11 (12.5) | 9.1 | 54.6 | 45.5 |
| P | .60 | .49 | .50 |
a P values are calculated by the Fisher exact test, unless otherwise noted
b Couple had ≥1 HPV genotype in common.
c Couple was negative for all 36 genotypes.
d Couple was discordant for ≥1 HPV genotype, regardless of the presence of concordant genotypes. Thus, a couple may appear in both the “Type-Specific Positive Concordance” column and the “Any Discordance” column.
e By the Cochrane-Armitage test for trend.
f Missing and refused data were not included in statistical testing.
g Each partner reported using condoms either sometimes or all of the time.
h Includes partners who agreed that condoms were never used, in addition to partners who disagreed with their partner's report that condoms were never used.
Overall type-specific positive concordance was 41.2% (7 of 17) among nonmonogamous couples and 19.7% (13 of 66) among monogamous couples (P = .18), while negative concordance was 23.5% among nonmonogamous couples and 34.9% among monogamous couples (P = .75).
When the lifetime number of sex partners for each partner in a couple was summed, there was a linear trend toward negative HPV concordance among couples who reported the lowest number of sex partners (P < .0001). Likewise, couples in which the man and woman had the least difference in reported lifetime number of partners (eg, a difference of 0–2 partners vs ≥10 partners) were more likely to have negative concordance (P = .0001) and less likely to have type-specific positive concordance (P = .03).
Couples who reported vaginal sex in the prior 2 days had higher type-specific positive concordance, compared with couples who reported no vaginal sex in the past week (34.8% vs 16.7%); however, findings from the test for trend were not statistically significant (P = .32).
DISCUSSION
To our knowledge, this is the first study to assess a range of sociobehavioral factors associated with concordance among a group of heterosexual couples with diverse ages and no history of HPV-associated disease. HPV concordance (both type-specific positive concordance and negative concordance) was common, while the proportion of couples sharing specific genotypes was greater than would have been expected by chance.
We observed higher type-specific positive concordance (41.4%) among couples whose relationship was <1-year old, compared with couples who had been together longer. Burchell et al reported a very similar proportion of type-specific positive concordance (41%) among couples whose sexual relationship was ≤6 months old [3]. Sexually experienced men and women who form new couples may have recently had sex with other people and therefore had recent access to a pool of HPV genotypes. Given that HPV is easily transmitted [17], these new couples are then likely to share their genital microbiota, resulting in concordance for ≥1 genotype.
Our observation that an increased difference in age between men and women was inversely associated with type-specific positive concordance may be related to host immunity. That is, if one partner is seronegative and the other is seropositive and has increased ability to clear an HPV-genotype that has been transmitted between partners, it would decrease our chances to observe concordance in the couple. For example, a younger partner, more likely be HPV naive than an older partner, might have increased time to clearance and, in turn, might have decreased the likelihood of our observing concordance in the couple. The lag time between detection of incident HPV infection and seroconversion in women is generally 8–18 months, as observed in a small number of natural history studies of serum HPV antibodies [18–21]; thus, even partners close in age may have different abilities to clear an HPV genotype that enters the relationship.
There was also an expected linear trend toward negative HPV concordance among couples with a smaller lifetime number of sex partners. A lower number of partners among both partners would tend to decrease the number of genotypes in a couple, which, in turn would increase the probability of negative concordance. While not statistically significant, we also observed increased type-specific positive concordance among couples with the highest lifetime number of sex partners. Kero et al also observed increased type-specific positive concordance among couples in which the woman (sampled during the third trimester of pregnancy) acknowledged a significantly higher lifetime number of sex partners than similar women in discordant couples [22]. As mentioned above, men and women with a higher number of out-of-relationship sex partners had access to novel pools of HPV genotypes, which then may be efficiently shared within the couple.
We observed no statistically significant differences in concordance between monogamous and nonmonogamous couples. This finding is counterintuitive in that some might predict that greater nonmonogamy would lead to less type-specific positive concordance among couples. But as previously mentioned, the infectious nature of HPV may quickly result in the establishment of a concordant couple soon after out-of-relationship sex, which would lead to greater type-specific concordance in the context of nonmonogamy. Subsequently, differential HPV clearance rates would lead to type-specific discordance among the partners. Our definition of monogamy in this cross-sectional study is limited to 6 months of prior sexual behavior; thus, this definition would classify partners as monogamous even if there was out-of-relationship sex as little as 7–12 months prior to study enrollment. Incorporation of data from follow-up visits in a longitudinal study will not only allow use of a more long-term definition of monogamy, but also will allow estimation of the time of acquisition and clearance of infections in each partner. Our data also cannot distinguish between serial monogamy and concurrent relationships. It is possible that these 2 forms of sexual coupling could lead to different patterns of HPV concordance outcomes.
We also observed neither concordance nor discordance in association with couples whose partners had different races, ethnicities, or education or disagreed about marital status. To our knowledge, there have been no such comparisons in the literature; however, in our prior paper examining factors associated with prevalent genital HPV among men, race and marital status were associated with genital HPV [23]. Thus, we felt it prudent to examine several sociodemographic variables within the context of the composition of the dyad. In addition to the cross-sectional nature of the current study, statistical power may have limited our ability to detect some associations, including those related to nonmonogamy and concordance.
It is worth noting that the concordance/discordance outcome of interest affects interpretation. For example, a difference of ≥10 lifetime number of sex partners between male and female partners was associated not only with type-specific positive concordance but also with any discordance. This dual result is possible because an increased number of partners likely increases the pool of genotypes in a relationship, which increases the likelihood of observing not only concordant but also discordant genotypes.
Information bias, such as that stemming from the self-reported nature of the behavioral data, is also possible. A total of 11 men and 1 woman reported 0 sex partners in the prior 6 months. Recall bias may have led to these differential reports.
Among couples in which at least 1 partner had detectable HPV infection, Burchell et al observed an association between type-specific HPV infection and young couples who reported vaginal sex 1–2 days before their study visit. Likewise, we observed a higher proportion of couples with type-specific positive concordance if they reported vaginal sex in the prior 1–2 days; however, the linear trend was not statistically significant (P = .32). While couples were instructed to not have sex for the prior 48 hours, 6 couples who reported vaginal sex within that period were still retained in the study. HPV detection among these persons may reflect viral deposition rather than true infection.
In 17 couples, we observed the woman to be HPV free, while the man harbored ≥1 genotype. Conversely, the reverse was true in only 8 couples; that is, the man was HPV free while the woman harbored ≥1 types. Such discordance might be related to increased nonmonogamous sexual behavior by the man, but we found no association between monogamy and discordance; indeed, approximately the same percentage of men and women acknowledged sex with ≥2 persons in the prior 6 months. An alternative explanation points to a stronger immune response in the woman and, thus, increased probability of HPV clearance as compared to the man. Higher HPV antibody seroprevalence in women has been observed in a number of reports [24, 25]. On the other hand, if we assume that the frequency of male positive/female-negative couples would be equal to the frequency of female-positive/male-negative couples, a McNemar exact test cannot exclude the possibility that the finding of 8 of 25 couples who were male-negative/female-positive was due to chance (P = .11).
In summary, we found type-specific and negative HPV concordance to be common features of heterosexual couples and that this finding may be explained by lifetime number of sex partners or, possibly, by duration of the couple's relationship and by age differences between the partners. Given the cross-sectional nature and sample size of our study, these findings should be interpreted as preliminary observations subject to subsequent investigations, especially longitudinal studies.
Notes
Acknowledgments. We offer special thanks to the men and women who provided personal information and biological samples for the study. We also thank the HIM Study Team in Tampa, including Kathy Eyring, Nadia Lambermont, Kayoko Kennedy, Kimberly Isaacs, Andrea Leto, Abidemi Ajidahun, and Bradley Sirak.
Disclaimer. Publication and report contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCI/NIH or GlaxoSmithKline.
Financial support. This work was supported by the NCI, NIH (3R03CA134204-02S1 to A. G. N.), the NIH (RO1 CA098803 01-A1 to A. R. G.), and GlaxoSmithKline (EPI-HPV-036 BOD US CRT to A. R. G.).
Potential conflicts of interest. A. G. N. has previously received research support from Merck. A. R. G. receives research support from GlaxoSmithKline and Merck and is on the speaker's bureau of Merck. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolescent Health. 2010;46:S20–6. doi: 10.1016/j.jadohealth.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Reiter PL, Pendergraft WF, 3rd, Brewer NT. Meta-analysis of human papillomavirus infection concordance. Cancer Epidemiol Biomarkers Prev. 2010;19:2916–31. doi: 10.1158/1055-9965.EPI-10-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burchell AN, Tellier PP, Hanley J, Coutlee F, Franco EL. Human papillomavirus infections among couples in new sexual relationships. Epidemiology. 2010;21:31–7. doi: 10.1097/EDE.0b013e3181c1e70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franceschi S, Castellsague X, Dal Maso L, et al. Prevalence and determinants of human papillomavirus genital infection in men. Br J Cancer. 2002;86:705–11. doi: 10.1038/sj.bjc.6600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta A, Arora R, Gupta S, et al. Human papillomavirus DNA in urine samples of women with or without cervical cancer and their male partners compared with simultaneously collected cervical/penile smear or biopsy specimens. J Clin Virol. 2006;37:190–4. doi: 10.1016/j.jcv.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Kyo S, Inoue M, Koyama M, Fujita M, Tanizawa O, Hakura A. Detection of high-risk human papillomavirus in the cervix and semen of sex partners. J Infect Dis. 1994;170:682–5. doi: 10.1093/infdis/170.3.682. [DOI] [PubMed] [Google Scholar]
- 7.Mbulawa ZZA, Marais DJ, Johnson LF, Boulle A, Coetzee D, Williamson A-L. Influence of human immunodeficiency virus and CD4 count on the prevalence of human papillomavirus in heterosexual couples. J Gen Virol. 2010;91:3023–31. doi: 10.1099/vir.0.020669-0. [DOI] [PubMed] [Google Scholar]
- 8.Parada R, Morales R, Giuliano AR, Cruz A, Castellsague X, Lazcano-Ponce E. Prevalence, concordance and determinants of human papillomavirus infection among heterosexual partners in a rural region in central Mexico. BMC Infect Dis. 2010;10:223. doi: 10.1186/1471-2334-10-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abalos AT, Harris RB, Nyitray AG, et al. Human papillomavirus type distribution among heterosexual couples. J Low Genit Tract Dis. 2012;16:10–5. doi: 10.1097/LGT.0b013e31822a8404. [DOI] [PubMed] [Google Scholar]
- 10.Giuliano AR, Lee JH, Fulp W, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377:932–40. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The Human Papillomavirus Infection in Men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:2036–43. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens—part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 15.de Villiers E-M, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Westfall PH, Young SS. P value adjustments for multiple tests in multivariate binomial models. J Am Stat Assoc. 1989;84:780–6. [Google Scholar]
- 17.Burchell AN, Richardson H, Mahmud SM, et al. Modeling the sexual transmissibility of human papillomavirus infection using stochastic computer simulation and empirical data from a cohort study of young women in Montreal, Canada. Am J Epidemiol. 2006;163:534–43. doi: 10.1093/aje/kwj077. [DOI] [PubMed] [Google Scholar]
- 18.Andersson-Ellstrom A, Dillner J, Hagmar B, et al. Comparison of development of serum antibodies to HPV16 and HPV33 and acquisition of cervical HPV DNA among sexually experienced and virginal young girls: a longitudinal cohort study. Sex Transm Dis. 1996;23:234–8. doi: 10.1097/00007435-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Carter JJ, Koutsky LA, Hughes JP, et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181:1911–9. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 20.Ho GY, Studentsov YY, Bierman R, Burk RD. Natural history of human papillomavirus type 16 virus-like particle antibodies in young women. Cancer Epidemiol Biomarkers Prev. 2004;13:110–6. doi: 10.1158/1055-9965.epi-03-0191. [DOI] [PubMed] [Google Scholar]
- 21.Carter JJ, Koutsky LA, Wipf GC, et al. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J Infect Dis. 1996;174:927–36. doi: 10.1093/infdis/174.5.927. [DOI] [PubMed] [Google Scholar]
- 22.Kero K, Rautava J, Syrjanen K, Grenman S, Syrjanen S. Human papillomavirus genotypes in male genitalia and their concordance among pregnant spouses participating in the Finnish Family HPV study. J Sex Med. 2011;8:2522–31. doi: 10.1111/j.1743-6109.2011.02378.x. [DOI] [PubMed] [Google Scholar]
- 23.Giuliano AR, Lazcano E, Villa LL, et al. Circumcision and sexual behavior: factors independently associated with human papillomavirus detection among men in the HIM study. Int J Cancer. 2009;124:1251–7. doi: 10.1002/ijc.24097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003-2004. J Infect Dis. 2009;200:1059–67. doi: 10.1086/604729. [DOI] [PubMed] [Google Scholar]
- 25.Kreimer AR, Alberg AJ, Viscidi R, Gillison ML. Gender differences in sexual biomarkers and behaviors associated with human papillomavirus-16,-18, and-33 seroprevalence. Sex Transm Dis. 2004;31:247–56. doi: 10.1097/01.olq.0000118425.49522.2c. [DOI] [PubMed] [Google Scholar]