Abstract
Background. Understanding the earliest central nervous system (CNS) events during human immunodeficiency virus (HIV) infection is crucial to knowledge of neuropathogenesis, but these have not previously been described in humans.
Methods. Twenty individuals who had acute HIV infection (Fiebig stages I-IV), with average 15 days after exposure, underwent clinical neurological, cerebrospinal fluid (CSF), magnetic resonance imaging, and magnetic resonance spectroscopy (MRS) characterization.
Results. HIV RNA was detected in the CSF from 15 of 18 subjects as early as 8 days after estimated HIV transmission. Undetectable CSF levels of HIV (in 3 of 18) was noted during Fiebig stages I, II, and III, with plasma HIV RNA levels of 285 651, 2321, and 81 978 copies/mL, respectively. On average, the CSF HIV RNA level was 2.42 log10 copies/mL lower than that in plasma. There were no cases in which the CSF HIV RNA level exceeded that in plasma. Headache was common during the acute retroviral syndrome (in 11 of 20 subjects), but no other neurological signs or symptoms were seen. Intrathecal immune activation was identified in some subjects with elevated CSF neopterin, monocyte chemotactic protein/CCL2, and interferon γ–induced protein 10/CXCL-10 levels. Brain inflammation was suggested by MRS.
Conclusions. CSF HIV RNA was detectable in humans as early as 8 days after exposure. CNS inflammation was apparent by CSF analysis and MRS in some individuals during acute HIV infection.
Prior to widespread availability of combination antiretroviral therapy (cART), the most severe form of human immunodeficiency virus type 1 (HIV) encephalopathy, previously termed “AIDS dementia complex,” occurred almost exclusively in late disease, often when circulating CD4+ lymphocyte counts were <100 cells/mm3. In the current era of widespread cART availability, the estimated prevalence of severe impairment, now termed “HIV-associated dementia” (HAD), is about 2%, down from a reported 15% in the pre-cART era [1, 2]. However, the frequency of milder impairment detected through neuropsychological testing remained unchanged after the introduction of cART, occurring in about one-half of all community-dwelling persons living with HIV and having a measurable impact on daily function [1, 3].
A clear understanding of mechanisms underlying central nervous system (CNS) injury despite cART has remained elusive. Cognitive impairment now occurs even within the normal range CD4+ lymphocyte counts, although an association between impairment and the lowest ever CD4+ lymphocyte count (ie, nadir CD4+ lymphocyte count) has been consistently reported [4]. Elevated plasma HIV RNA level remains associated with cognitive impairment. However, more information is needed to inform mechanisms of injury for the majority of individuals with impaired testing despite adequate adherence and viral suppression. Attention has turned to the inability of cART to eradicate reservoirs of HIV thought to be critical to CNS impairment, particularly the reservoir in cerebrospinal fluid (CSF) as a proxy for the brain and the reservoir in circulating monocytes, each linked to HIV neuropathogenesis [5, 6]. Mechanistically, peripheral monocyte infection may be crucial to establishing persistent CNS infection [7, 8]. Both reservoirs may be established very early during infection.
Animal models of acute simian immunodeficiency virus (SIV) and limited human data captured during primary HIV infection suggest that the events of very early infection may negatively impact long-term cognition [9]. Based on anecdotal reports, HIV is able to cross the blood brain barrier early during acute infection, but the precise timing and variability of CNS infection, predictors of this timing, and degree of CNS inflammation or injury are poorly understood in humans, owing to a lack of data captured during this very early period prior to seroconversion [10, 11]. A clearer understanding of the earliest host immunological response and degree of reservoir burden would inform our understanding of early CNS injury and may provide insight into long-term HIV neuropathogenesis.
In this study, we describe these earliest CNS events in 20 HIV-infected subjects evaluated before Western Blot evidence of HIV infection and before substantial host antibody response was detected (acute HIV infection, defined as Fiebig stages I-IV disease) [12]. We found HIV RNA in CSF as early as 8 days after exposure and detected CNS inflammation through analysis of CSF and by magnetic resonance spectroscopy (MRS).
METHODS
Subject Selection
Thai subjects seeking HIV voluntary counseling and testing services at the Anonymous Clinic of the Thai Red Cross AIDS Research Center and men who have sex with men (MSM) enrolled in a study at the Silom Community Clinic in Bangkok, Thailand, had specimens screened in real time for acute HIV infection, using a hierarchical algorithm for testing of pooled specimens [12, 13]. All individuals who provided contact information were notified if their test results were found to be consistent with acute HIV infection. For the purpose of this report, we selected the first 20 subjects with Fiebig stage I-IV disease who agreed to CNS characterization on the basis of a more comprehensive parent protocol focused on peripheral immunology and virology of acute HIV (RV 254 of the Division of Retrovirology, Walter Reed Army Institute of Research; ClinicalTrials.gov identifier NCT00796146). One subject with marked CSF lymphocytic pleocytosis and a positive serum syphilis test was excluded because of presumed neurosyphilis and was replaced by the next enrollee.
We compared MRS data for these 20 subjects to those of Thais with chronic HIV infection who were enrolled in 1 of 2 concurrent chronic HIV studies (ClinicalTrials.gov identifiers NCT00782808 and NCT00777426), selecting all males and the first 5 females enrolled to obtain 17 cases in an effort to match for sex to the acutely HIV-infected subjects. These chronically infected subjects had advanced HIV infection that met criteria for initiation of treatment on the basis of Thai Ministry of Public Health guidelines and were evaluated just prior to initiating cART for the first time [14]. All were cognitively normal at the time of MRS, having undergone neuropsychological testing, symptom assessment, neurological examination, and subsequent consensus diagnostic classification by trained clinicians, as previously described [15]. Seven HIV-negative healthy community dwelling Thais also underwent MRS.
Clinical Characterization
All subjects underwent comprehensive medical and social evaluations by HIV clinicians to determine HIV risk factors and timing of estimated exposure. When a range of exposures was given (for 7 of 20 individuals), the mean date of exposures was recorded. An HIV clinician determined all symptoms occurring since the date of estimated exposure, and a neurologist completed a comprehensive neurological examination to evaluate cognitive status and neurological signs. Fiebig stages were defined according to standard criteria [12].
Laboratory Measurements
Blood CD4+ and CD8+ lymphocyte subsets were measured using standard flow cytometry. The plasma HIV RNA level was measured by the Roche Amplicor HIV-1 Monitor Test v1.5 (Roche Diagnostics, Branchburg, NJ; detection range, 400–750 000 copies/mL) in specimens that had not been frozen; if the HIV RNA load was >750 000 copies/mL, specimens were diluted and remeasured to obtain precise values. The CSF HIV RNA level was measured in specimens that had been frozen for <6 months, using a modification of the Roche Amplicor HIV-1 Monitor Test v1.5; RNA was extracted from ≤200 µL of CSF by using the Boom silica extraction procedure (NucliSens Basic Isolation Reagents and Lysis buffer; bioMerieux, Durham, NC). The purified RNA specimens were amplified according to the manufacturer's approved procedure, allowing a lower limit of detection of 50 copies/mL. HIV subtypes were determined in plasma by a multiregion hybridization assay for subtypes B, C, and CRF 01_AE (MHAbce), as previously described [16]. Clinical laboratory testing included serum Venereal Disease Research Laboratory testing for syphilis. The CSF level of monocyte chemotactic protein (MCP-1/CCL2) was measured using a multiplex assay on a Quansys platform. CSF levels of neopterin and interferon γ–induced protein (IP-10/CXCL10) were measured using a standard enzyme-linked immunosorbent assay developed by GenWay Biotech (San Diego, CA) and Life Technologies (formerly Invitrogen; Grand Island, NY), respectively.
Magnetic Resonance Imaging (MRI)/MRS
The same technician performed MRI/MRS prior to lumbar puncture, using a 1.5T General Electric whole-body clinical magnetic resonance scanner (software version 12x) with an 8-channel phased-array head coil for signal reception and body coil for transmission. High resolution, multislice, axial T1-weighted spoiled gradients-echo images were acquired and used for 8-cm3 voxels placed in the middle frontal gray matter, left frontal white matter, occipital grey matter at the middle posterior cingulate gyrus, and basal ganglia, using the automated Proton Brain Exam (PROBE-P) with an echo time of 35 milliseconds, a repetition time of 1.5 seconds, and a protocol that minimized partial-volume effects [17]. We acquired T2-weighted decay spectra of the fully relaxed unsuppressed water free-induction decays, with a repetition time of 10 seconds and 9 different echo times (30, 35, 45, 65, 85, 120, 200, 500, and 1500 milliseconds). Quality assurance was performed by scanning a gradient echo MRS phantom after each examination. Images were electronically stored, anonymously transferred, and processed by a single investigator (N. S.), using a time domain fitting routine LCModel for spectral quantification (available at: http://s-provencher.com/pages/lcmodel.shtml). The metabolite concentrations were determined for myoinositol, N-acetylaspartate, choline, and creatine. Relative levels of major metabolites were standardized to total creatine.
Ethical Approval and Statistical Analyses
All subjects signed consent forms approved by human subject review boards at Chulalongkorn University (Bangkok, Thailand), the Walter Reed Army Medical Center (Rockville, MD), and the University of California at San Francisco. Descriptive statistics were analyzed using common nonparametric tests or t tests on log10-transformed HIV RNA in SAS v9.2 (SAS Institute, Cary, NC). We used PROC GLM to include age in the models for MRS analyses.
RESULTS
Clinical Composition
Screening of 18 242 specimens between April 2009 and September 2010 identified 32 subjects with acute HIV infection consistent with Fiebig stages I-IV. Of the 32, 25 (78%) agreed to participation in the parent study. Three of these subjects were excluded because of progression beyond Fiebig stage IV prior to enrollment. One subject was excluded from the CNS study because of neurosyphilis, and 1 subject who agreed to participate was excluded because of incomplete participation. Two subjects retained for the analyses were positive for hepatitis B virus surface antigen, and all were negative for hepatitis C virus antibody. Our final sample of 20 subjects with acute HIV infection were evaluated during Fiebig stages I (n = 3), II (n = 4), III (n = 11), and IV (n = 2) and had a median estimated duration since exposure of 14.5 days (Table 1). Eighteen underwent lumbar puncture (2 declined because of fear about potential adverse events), and 17 underwent MRS (1 was claustrophobic and 2 had metallic dental braces). Lumbar puncture was completed a median of 2 days after enrollment. Individuals tended to be young MSM, and no subjects reported injection drug use. The median blood CD4+ lymphocyte count was 384 cells/mm3 (range, 218–740 cells/mm3). HIV subtypes were CRF_01AE (n = 13), B (n = 1) and six cases were indeterminate by MHAbce. Of the 15 subjects whose samples could be amplified, plasma HIV tropism analysis, using the Trofile Assay (Monogram Biosciences), revealed all samples to be R5 tropic, except for 1, which was dually R5/X4 tropic.
Table 1.
Demographic and Clinical Characteristics and Self-Reported History of Risk Factors for 20 Study Subjects With Acute Human Immunodeficiency Virus Infection
| Characteristic | Value |
|---|---|
| Male sex | 90% |
| Age, y, mean (range) | 31 (21–46) |
| Education, y, mean (range) | 17 (6–23) |
| Risk factor | |
| Male-male sex | 85 |
| Injection drug use | 0 |
| Heterosexual sex | 15 |
| CD4+ cell count, cells/mm3, median (range) | 384 (218–740) |
| Infection duration, d, median (range)a | 14.5 (4–31) |
Data are no. (%) of subjects, unless otherwise indicated.
a Interval between estimated time of exposure and enrollment. If a range of dates was provided, the mean was used.
Neurological Laboratory and Clinical Characterization
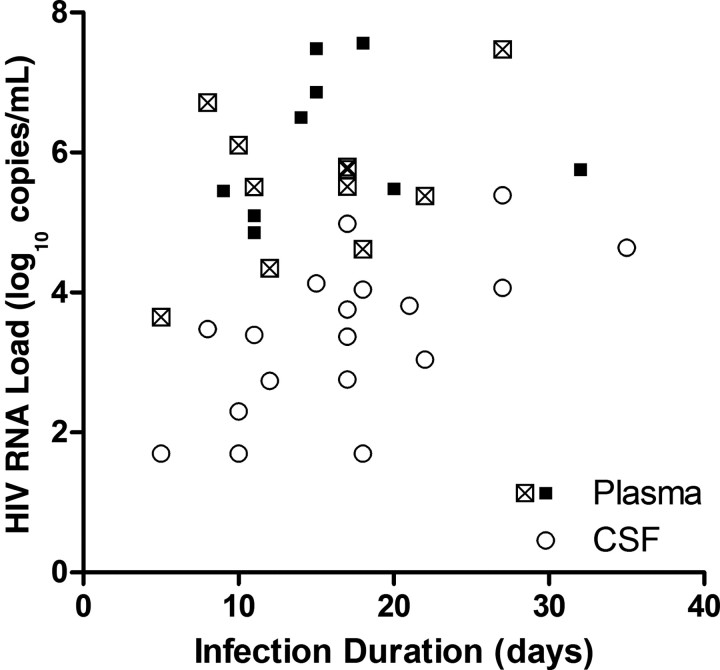
CSF sampling occurred a median of 17 days (range, 5–35 days) after estimated exposure and identified HIV RNA in CSF as early as 8 days. Only 1 subject was sampled prior to 8 days, and CSF HIV RNA was not detected (Fiebig stage I; 4 days after estimated exposure; plasma HIV RNA load, 2321 copies/mL). There were 2 additional cases with undetectable HIV RNA in CSF, with one at Fiebig stage II (10 days; plasma HIV RNA load, 285 651 copies/mL) and the other at Fiebig stage III (18 days; plasma HIV RNA load, 81 978 copies/mL).
Eleven subjects underwent lumbar puncture on the same day that plasma HIV RNA specimens were obtained. No CSF specimen had an HIV RNA level that was greater than the level measured in plasma. Overall, mean CSF HIV RNA level was lower than mean plasma HIV RNA level (3.38 vs 5.53 log10 copies/mL, respectively; P < .001). With addition of cases in which plasma HIV RNA specimens were not obtained on the same day as lumbar puncture and use of entry plasma HIV RNA values for these additional cases (median, 2 days prior [range, 0–3 days prior]), there were still no cases in which the CSF HIV RNA load was greater than that in plasma (Figure 1). The mean CSF HIV RNA load was 2.42 log10 copies/mL lower than that measured in plasma (3.38 vs 5.80 log10 copies/mL; P < .001). The CSF HIV RNA level correlated to that in plasma (r2 = .37; P = .007), and we found similar CSF HIV RNA levels in Fiebig stages I/II (without plasma antibodies to HIV, 2.78 log10 copies) and Fiebig stages III/IV (with antibodies, 3.62 log10 copies; P = .15).
Figure 1.
Plasma (squares) and cerebrospinal fluid (CSF; circles) human immunodeficiency virus (HIV) RNA levels, by infection duration. Cases with plasma HIV RNA level measured on the same day as lumbar puncture are represented by open squares, and cases with plasma HIV RNA measured at enrollment are represented by solid squares (median of 2 days before lumbar puncture).
About one-half of subjects (11 of 20) experienced new headache in the period prior to study enrollment but after the estimated date of HIV exposure. No other new clinical neurological symptoms were reported. There were no differences in mean HIV RNA levels between cases with and cases without headache (plasma level, 6.02 vs 5.42 log10 copies/mL, respectively [P = .19]; CSF level, 3.61 vs 3.11 log10 copies/mL, respectively [P = .374]). All subjects were normal on the basis of detailed neurological examination, except for one individual, who had decreased pin prick sensation at the right hand to the level of the wrist, known to be present prior to exposure.
CNS Inflammatory Findings
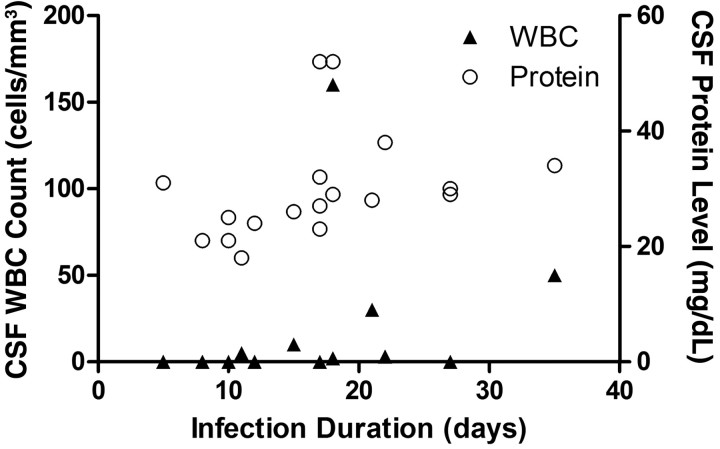
The mean CSF white blood cell (WBC) count was 14 cells/µL (median, 0 cells/µL [range, 0–160 cells/µL]). Values were above the normal range of 5 cells/µL in 4 subjects (22%), and 2 subjects had CSF protein levels above the normal range (15–45 mg/dL) (Figure 2). Consequently, 5 of 18 subjects (28%) had evidence of CSF inflammation or blood brain barrier disruption, using common testing parameters. We did not identify differences in CSF protein levels or WBC counts between patients in Fiebig stages I/II and those in Fiebig stages III/IV. The CSF neopterin level in many individuals was elevated, compared with published parameters reported for US, HIV-negative control subjects <45 years old (Figure 3; S. S., unpublished data from a previously published study [18]). Fewer cases of elevated MCP-1/CCL2 and IP-10/CXCL-10 levels were noted. CSF neopterin levels were correlated with plasma (r2 = .35; P = .009) and CSF (r2 = .36; P = .010) log10 HIV RNA loads. We identified a trend for CSF neopterin level correlating with duration of infection (P = .051; r2 = .22), but no difference was found when comparing CSF neopterin levels between patients in Fiebig stages I/II and those among patients in Fiebig stages III/IV.
Figure 2.
Cerebrospinal fluid (CSF) protein levels (open circles) and white blood cell (WBC) counts (solid triangles) in individuals with acute human immunodeficiency virus infection, by infection duration.
Figure 3.
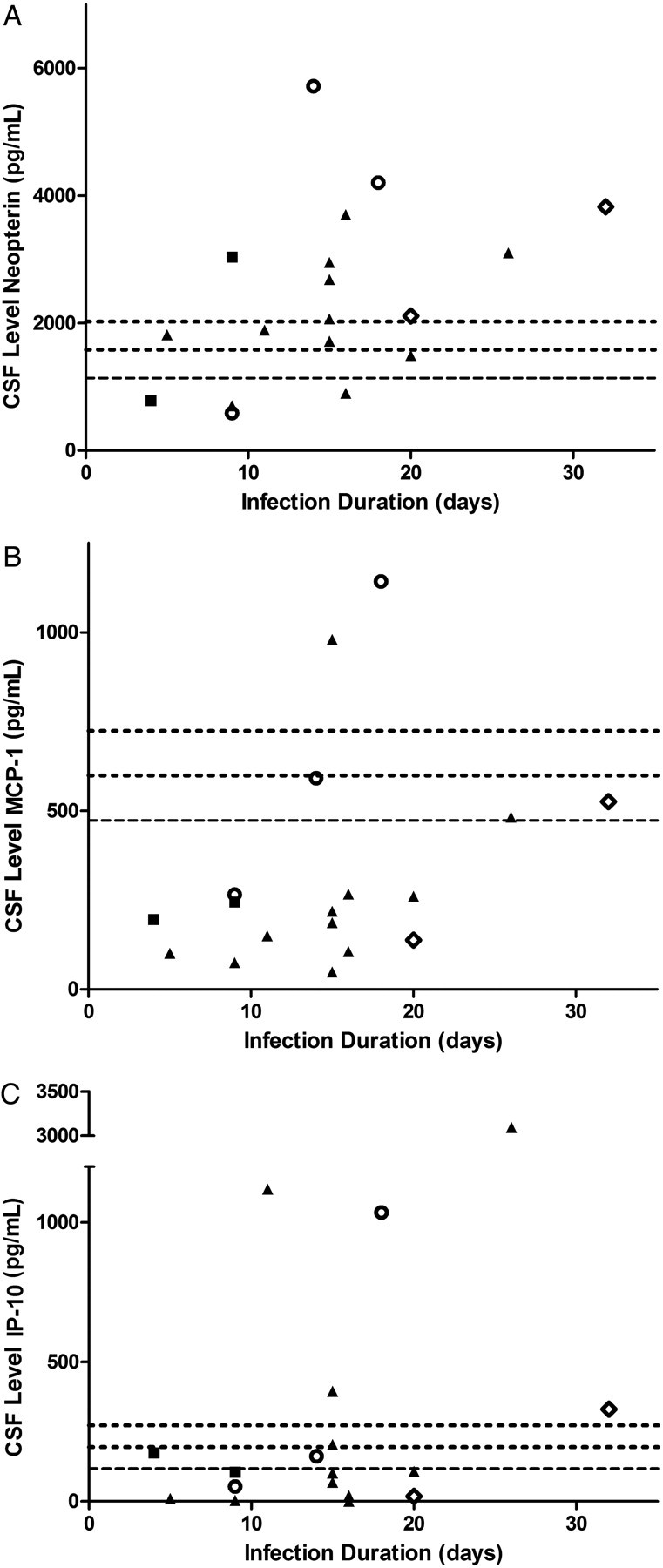
Cerebrospinal fluid (CSF) inflammatory markers during acute human immunodeficiency virus (HIV) infection. CSF neopterin (A), monocyte chemotactic protein (MCP-1/CCL2; B), and interferon γ–induced protein 10 (IP-10/CXCL-10; C) levels, demonstrating cases with elevated levels, compared with published normative data among US, HIV-negative controls <45 years old. Long dashes indicate the mean value for HIV-negative controls, with shorter dashes denoting 1 and 2 SDs above the mean value. Fiebig stages are indicated by solid squares (for stage I), open circles (for stage II), solid triangles (for stage III, and open diamonds (for stage IV).
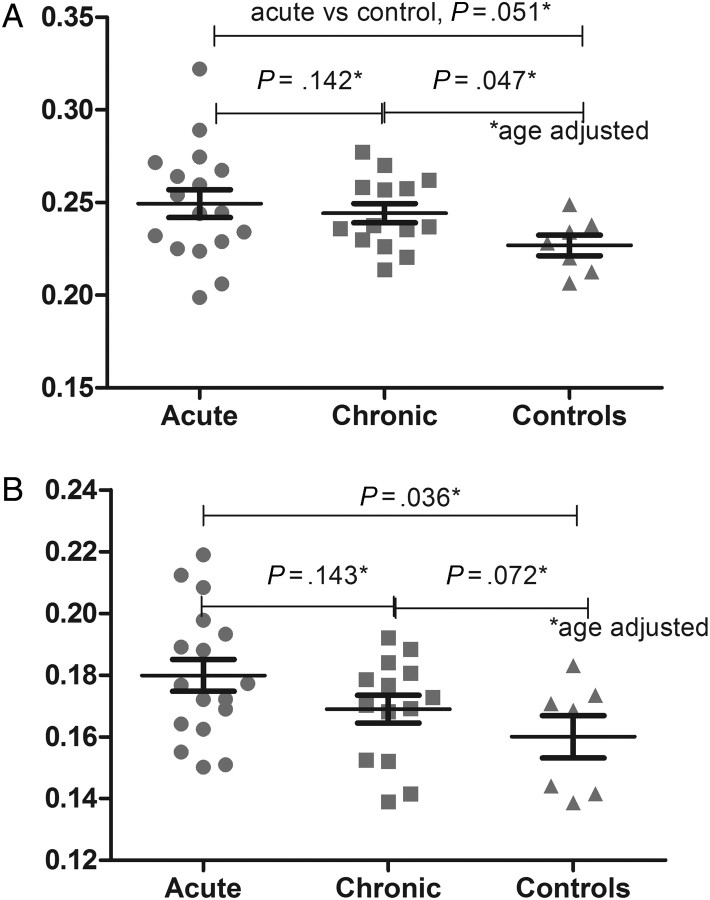
We identified no structural or T2-weighted signal abnormalities on MRI. Our Thai subjects with chronic HIV infection were similar to acute cases in sex and age (data not shown). HIV-negative Thai controls differed from the subjects with acute infection with regard to male sex (43% and 90%, respectively; P = .024) but were of similar age. The median CD4+ lymphocyte count among chronic HIV-infected subjects was 239 cells/mm3 (range, 19–426 cells/mm3), which differed from that among acute HIV-infected subjects (median, 384 cells/mm3 [range, 218–740 cells/mm3]; P < .001). After adjustment for age, we identified elevated choline/creatine levels in occipital grey matter at the middle posterior cingulate gyrus (P = .036) and a trend for elevated choline/creatine levels in basal ganglia among subjects with acute HIV infection, compared with control subjects (P = .051; Figure 4). We noted an associations between higher CSF neopterin and elevated choline/creatine levels in occipital grey matter at the middle posterior cingulate gyrus (r2 = .27; P = .048), as well as elevated myoinositol/creatine levels in left frontal white matter (r2 = .051; P = .051).
Figure 4.
Magnetic resonance spectroscopy for choline/creatine in basal ganglia (A) and occipital grey matter (B) in individuals with acute human immunodeficiency virus (HIV) infection, compared with individuals with chronic HIV infection and HIV-negative Thai controls.
DISCUSSION
This work identifies early HIV invasion of the CNS, as evidenced by HIV RNA detection in CSF as early as 8 days after estimated HIV exposure and during the earliest stage (Fiebig I) of infection, in a cohort of subjects identified through pooled nucleic acid screening. Our findings are consistent with those from animal models despite the models’ use of a more neurovirulent design with neurotropic strains of simian immunodeficiency virus, controlled intravenous injection, and CD8+ lymphocyte–depleted macaques [9, 19]. We further demonstrate that early viral invasion of the CNS occurs in humans after mucosal (vs intravenous) exposure, when there is expected to be attendant selection of transmitted/founder viruses rather than inoculation of all viral strains [20]. Although most of our cases involved clade CRF 01_AE, 1 case had confirmed clade B virus and low level of detectable HIV RNA (200 copies/mL) in CSF. In this case (Fiebig stage I), the concurrent plasma HIV RNA load was much higher, at 5.4 log10 copies/mL. It remains possible that clade differences impact neurovirulence during acute infection, since clade CRF 01_AE differs from other clades in the expression of tissue necrosis factor in a manner that could support altered neurovirulence [21]. Although most subjects expressed confidence in identifying a single exposure episode, estimating the duration since exposure may be subject to recall bias and other inaccuracies. However, congruent Fiebig staging based on laboratory data accompanied historical recall in our study, strengthening the confidence of such estimates. Our inability to detect CSF HIV RNA in 3 subjects demonstrates some variability in the timing of HIV penetration into the CNS during this vulnerable period and may point to unique viral and host factors that influence the early establishment of viremia in the CNS. This phenomenon occurred as late as Fiebig stage III, when the plasma HIV RNA load was 5.46 log10 copies/mL.
We identified the HIV RNA level in CSF to be, on average, 2.4 log10 copies/mL lower than that in plasma. This is a greater difference than that reported during chronic HIV infection (approximately 1 log10 copies/mL lower than that in plasma) [22]. In our participant with the highest HIV RNA level in plasma (7.56 log10 copies/mL), the corresponding CSF HIV RNA level was >4 log10 copies/mL lower (3.37 log10 copies/mL). Poor monocyte infection by transmitted/founder acute HIV strains, leading to decreased intramonocyte trafficking of virus to the brain, or a relatively preserved blood brain barrier in this early stage of disease are 2 possible explanations for this finding [20]. Both suggest a potential opportunity in which early intervention may protect against later CNS dysfunction. In all cases, CSF HIV RNA levels were lower than those measured in plasma. In contrast, there are case reports of patients with CSF HIV RNA levels that exceed those in plasma among chronic HIV-infected patients receiving cART and presenting with neurological syndromes [23]. We caution interpretation of this finding, because of our small sample size. The variable levels of CSF HIV RNA observed in this study may suggest a dynamic host systemic-CNS relationship that may alter the degree of HIV infection in the CNS during the earliest phase of infection. Our data suggest that plasma HIV RNA level is likely the largest determinant of that in CSF.
We identified evidence for CNS inflammation by standard clinical parameters of cellular pleocytosis and elevated CSF protein in some cases. Reports identify lymphocytic pleocytosis with HIV encephalopathy among acutely HIV-infected persons, and neurological complications are known to occur during HIV seroconversion [24]. Headache was commonly noted in this cohort, and this is consistent with other series of symptomatic patients enrolled in HIV seroconversion studies [25]. Our data strengthen these other reports since our findings were less subject to referral biases. Our reported headache rate may slightly overestimate the true frequency because 1 subject had chronic recurring migraines and 1 had a recent history of sinus headaches. However, the character of pain in these individuals was not typical of their previous headaches. We did not identify a relationship between plasma or CSF HIV RNA levels and headache.
Early neuroinvasion was characterized by measurable markers of CSF inflammation (eg, neopterin level) and by brain parenchymal inflammation as detected by MRS. Group comparisons for each marker demonstrated only modest statistical significance; however, the distribution of values in both MRS and CSF analyses demonstrated notable cases with measured values beyond expected ranges. Optimally, CSF specimens from local Thai controls would be used for interpretation of our CSF markers. By use of a conservative cut point of 2 SDs above the mean from our sample individuals from the US, we still identified cases with elevated levels. The variability in levels of inflammatory markers did not appear to be explained by the range of infection durations in our study, raising the possibility that other unique viral or host factors influence the early CNS response to infection. The individual differences provide an opportunity for future investigations aimed at defining factors associated with a lesser CNS inflammatory response during acute HIV infection.
Soluble markers of immune activation may be expressed in CSF of patients with HIV infection and may correlate with cognitive impairment [26]. MCP-1/CCL2 is produced by activated macrophages, microglia, and astrocytes, whereas neopterin is tightly linked to activated macrophages [27, 28]. Considered with IP-10, these markers of intrathecal immune activation are supportive of a monocyte-driven inflammatory profile that would be anticipated on the basis of accepted theories of CNS HIV invasion [29]. Measurements of CSF markers as surrogates for the brain have inherent limitations because these may not reflect activity in tissue. The combination of MRS and CSF sampling in this study strengthens our findings. Disruptions in MRS metabolites are reported in HAD and thought to indicate effects on both neuronal and glial cell populations [30]. These changes occur most prominently in subcortical structures, including the frontal white matter, basal ganglia, and thalamus. In animal models of acute SIV, MRS changes include reduction in N-acetylaspartate/creatine levels, with early elevation of choline/creatine levels in both frontal grey matter and subcortical structures [31]. Similarly, our data suggest early elevation of choline/creatine levels, which is thought to represent cellular immune activation or infiltration. We, however, did not identify lower N-acetylaspartate/creatine levels, a neuronal metabolite that is presumed to represent neuronal injury. These data need to be interpreted with caution because of limitations related to sample size and multiple comparisons.
Our data provide added insight into the earliest CNS events in HIV infection; yet, many questions remain. Since HIV infection is not universally associated with long-term CNS consequences, there is a potential to uncover factors that are present in acute HIV infection and may be associated with preserved CNS function during the chronic stage of HIV infection. We identify important variability in levels of CSF HIV RNA and inflammatory markers that may inform these paradigms. It is important to determine whether early intervention may spare some long-term CNS consequence. The advent of less complex regimens and safer antiretroviral toxicity profiles makes early intervention a possibility when HIV is identified; but current international recommendations remain vague on treatment recommendation during acute HIV infection, and, importantly, equipoise remains among experts [32].
Our future work will comprehensively characterize the systemic virological and immunological factors and their relationship to CNS viral dynamics, immune response, and brain injury. We will also determine how early treatment impacts these relationships and long-term outcomes. Because circulating monocytes have been tightly associated with late-stage HAD and are likely influenced during early in disease, future work will also focus on factors that impact the magnitude of this reservoir as it relates to long-term CNS consequences of infection with HIV.
Notes
Acknowledgments. We thank our study participants and staff from the Thai Red Cross AIDS Research Centre and the Silom Community Clinic in Bangkok for their valuable contributions to this study. SEARCH is a research collaboration between the Thai Red Cross AIDS Research Centre (TRCARC), the University of Hawaii (UH), and the Department of Retrovirology, US Army Medical Component, Armed Forces Research Institute of Medical Sciences (USAMC-AFRIMS).
The RV254/SEARCH 010 Study Group includes the following individuals: from SEARCH/TRCARC/HIV-NAT, Praphan Phanuphak, Nittaya Phanuphak, James Fletcher, Nipat Teeratakulpisarn, Nitiya Chomchey, Somprartthana Rattanamanee, Pairoa Praihirunkit, Sasiwimol Ubolyam, and Suteeraporn Pinyakorn; from Chulalongkorn University, Rungsun Rerknimitr, Wiriyaporn Ridtitid, and Mantana Pothisri; from AFRIMS, Alexandra Schuetz, Rapee Trichavaroj, Vatcharain Assawadarachai, Yuwadee Phuangngern, Wiriya Rutvisuttinunt, Nantana Tantibul, Panadda Sawangsinth, Bessara Nuntapinit, Siriwat Akapirat, Wanwarang Khobchit, Sakuna Suksawad, Ajchariyarat Sangdara, Kultida Poltavee, Hathairat Savadsuk, Suwittra Chaemchuen, Surat Jongrakthaitae, Chayada Sajiaweerawan, Nipattra Tragonlugsana, Putida Saetun, Joseph Chiu, Robert Paris, and Viseth Ngauy; from the Thailand Ministry of Public Health–US Centers for Disease Control and Prevention Collaboration, Phunlerd Phyaraj, Supaporn Chaikummao, Anchalee Varangruat, Pikun Luechai, Jaray Thongtojay, Anuwat Sriporn, and Wipas Wimonsate; from UCSF, Stephanie Chiao, Edgar Busovaca, and Lauren Wendelken; from UH, Cecilia Shikuma; from the Military HIV Research Program, Jeff Currier, Sodsai Tovanabutra, Merlin Robb, Bonnie Slike, Sheila Peel, Ying Liu, and Silvia Ratto-Kim; from the US National Institutes of Allergy and Infectious Diseases (NIAID), Irini Sereti and Jessica Hodge; from the US National Cancer Institute: Frank Maldarelli, Mary Kearney, and Ann Wiggins; from SAIC-Frederic: Jacob Estes, Robin Dewar, and Adam Rupert; from VGTI-Florida, Rafick Sekaly, Nicolas Chomont, and Claire Vandergeeten; and from Monogram Biosciences, Laura Napolitano, Molly Martell, Yolanda Lie, and the R&D and PDO groups (for technical assistance).
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health, the US Army, the US Department of Defense, or the US Centers for Disease Control and Prevention.
Financial support. This work was supported by the National Institute of Mental Health (NIH-R21 MH086341). The Military HIV Research Program, Walter Reed Army Institute of Research (Rockville, MD), funded the main acute HIV study. This work was supported by a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine and the US Department of Defense. This work was also supported in part by the Intramural research programs of the NIAID and of the Vaccine and Gene Therapy Institute. Antiretroviral therapy was supported by Gilead (Truvada, Atripla), Merck (Sustiva, Isentress), and Pfizer (Selzentry). Monogram Biosciences supported the Trofile test.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–52. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 3.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–31. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 4.Tozzi V, Balestra P, Lorenzini P, et al. Prevalence and risk factors for human immunodeficiency virus-associated neurocognitive impairment, 1996 to 2002: results from an urban observational cohort. J Neurovirol. 2005;11:265–73. doi: 10.1080/13550280590952790. [DOI] [PubMed] [Google Scholar]
- 5.Ellis RJ, Moore DJ, Childers ME, et al. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol. 2002;59:923–8. doi: 10.1001/archneur.59.6.923. [DOI] [PubMed] [Google Scholar]
- 6.Shiramizu B, Gartner S, Williams A, et al. Circulating proviral HIV DNA and HIV-associated dementia. AIDS. 2005;19:45–52. doi: 10.1097/00002030-200501030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burdo TH, Soulas C, Orzechowski K, et al. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Tang XP, McArthur JC, Scott J, Gartner S. Analysis of human immunodeficiency virus type 1 gp160 sequences from a patient with HIV dementia: evidence for monocyte trafficking into brain. J Neurovirol. 2000;6(Suppl 1):S70–81. [PubMed] [Google Scholar]
- 9.Fuller RA, Westmoreland SV, Ratai E, et al. A prospective longitudinal in vivo 1H MR spectroscopy study of the SIV/macaque model of neuroAIDS. BMC Neurosci. 2004;5:10. doi: 10.1186/1471-2202-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Resnick L, Berger JR, Shapshak P, Tourtellotte WW. Early penetration of the blood-brain-barrier by HIV. Neurology. 1988;38:9–14. doi: 10.1212/wnl.38.1.9. [DOI] [PubMed] [Google Scholar]
- 11.Davis LE, Hjelle BL, Miller VE, et al. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–9. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 12.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. Aids. 2003;17:1871–9. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 13.Ananworanich J, Phanuphak N, de Souza M, et al. Incidence and characterization of acute HIV-1 infection in a high-risk Thai population. J Acquir Immune Defic Syndr. 2008;49:151–5. doi: 10.1097/QAI.0b013e318183a96d. [DOI] [PubMed] [Google Scholar]
- 14.Somnuek S, Techasathit W, Utaipiboon C, et al. Practice Guideline: Thai national guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents. Asian Biomedicine. 2010;4:515–28. [Google Scholar]
- 15.Valcour VG, Sithinamsuwan P, Nidhinandana S, et al. Neuropsychological abnormalities in patients with dementia in CRF 01_AE HIV-1 infection. Neurology. 2007;68:525–7. doi: 10.1212/01.wnl.0000253196.78193.c7. [DOI] [PubMed] [Google Scholar]
- 16.Arroyo MA, Phanuphak N, Krasaesub S, et al. HIV type 1 molecular epidemiology among high-risk clients attending the Thai Red Cross Anonymous Clinic in Bangkok, Thailand. AIDS Res Hum Retroviruses. 2010;26:5–12. doi: 10.1089/aid.2009.0150. [DOI] [PubMed] [Google Scholar]
- 17.Webb PG, Sailasuta N, Kohler SJ, Raidy T, Moats RA, Hurd RE. Automated single-voxel proton MRS: technical development and multisite verification. Magn Reson Med. 1994;31:365–73. doi: 10.1002/mrm.1910310404. [DOI] [PubMed] [Google Scholar]
- 18.Spudich S, Gisslen M, Hagberg L, et al. Central nervous system immune activation characterizes primary HIV-1 infection even in subjects with minimal cerebrospinal fluid viral burden. J Infect Dis. 2011;204:753–60. doi: 10.1093/infdis/jir387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith MO, Heyes MP, Lackner AA. Early intrathecal events in rhesus macaques (Macaca mulatta) infected with pathogenic or nonpathogenic molecular clones of simian immunodeficiency virus. Lab Invest. 1995;72:547–58. [PubMed] [Google Scholar]
- 20.Salazar-Gonzalez JF, Salazar MG, Keele BF, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206:1273–89. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranjbar S, Rajsbaum R, Goldfeld AE. Transactivator of transcription from HIV type 1 subtype E selectively inhibits TNF gene expression via interference with chromatin remodeling of the TNF locus. J Immunol. 2006;176:4182–90. doi: 10.4049/jimmunol.176.7.4182. [DOI] [PubMed] [Google Scholar]
- 22.Spudich SS, Nilsson AC, Lollo ND, et al. Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment. BMC Infect Dis. 2005;5:98. doi: 10.1186/1471-2334-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50:773–8. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 24.Denning DW. The neurological features of acute HIV infection. Biomed Pharmacother. 1988;42:11–4. [PubMed] [Google Scholar]
- 25.Vanhems P, Allard R, Cooper DA, et al. Acute human immunodeficiency virus type 1 disease as a mononucleosis-like illness: is the diagnosis too restrictive? Clin Infect Dis. 1997;24:965–70. doi: 10.1093/clinids/24.5.965. [DOI] [PubMed] [Google Scholar]
- 26.Cinque P, Vago L, Mengozzi M, et al. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. Aids. 1998;12:1327–32. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Hagberg L, Cinque P, Gisslen M, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuchs D, Spira TJ, Hausen A, et al. Neopterin as a predictive marker for disease progression in human immunodeficiency virus type 1 infection. Clin Chem. 1989;35:1746–9. [PubMed] [Google Scholar]
- 29.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 30.Salvan AM, Vion-Dury J, Confort-Gouny S, Nicoli F, Lamoureux S, Cozzone PJ. Brain proton magnetic resonance spectroscopy in HIV-related encephalopathy: identification of evolving metabolic patterns in relation to dementia and therapy. AIDS Res Hum Retroviruses. 1997;13:1055–66. doi: 10.1089/aid.1997.13.1055. [DOI] [PubMed] [Google Scholar]
- 31.Greco JB, Westmoreland SV, Ratai EM, et al. In vivo 1H MRS of brain injury and repair during acute SIV infection in the macaque model of neuroAIDS. Magn Reson Med. 2004;51:1108–14. doi: 10.1002/mrm.20073. [DOI] [PubMed] [Google Scholar]
- 32.Bell SK, Little SJ, Rosenberg ES. Clinical management of acute HIV infection: best practice remains unknown. J Infect Dis. 2010;202(Suppl 2):S278–88. doi: 10.1086/655655. [DOI] [PMC free article] [PubMed] [Google Scholar]