Abstract
Background. The understanding of dengue virus (DENV) transmission dynamics and the clinical spectrum of infection are critical to informing surveillance and control measures. Geographic cluster studies can elucidate these features in greater detail than cohort studies alone.
Methods. A 4-year longitudinal cohort and geographic cluster study was undertaken in rural Thailand. Cohort children underwent pre-/postseason serology and active school absence–based surveillance to detect inapparent and symptomatic dengue. Cluster investigations were triggered by cohort dengue and non-dengue febrile illnesses (positive and negative clusters, respectively).
Results. The annual cohort incidence of symptomatic dengue ranged from 1.3% to 4.4%. DENV-4 predominated in the first 2 years, DENV-1 in the second 2 years. The inapparent-to-symptomatic infection ratio ranged from 1.1:1 to 2.9:1. Positive clusters had a 16.0% infection rate, negative clusters 1.1%. Of 119 infections in positive clusters, 59.7% were febrile, 20.2% were afebrile with other symptoms, and 20.2% were asymptomatic. Of 16 febrile children detected during cluster investigations who continued to attend school, 9 had detectable viremia.
Conclusions. Dengue transmission risk was high near viremic children in both high- and low-incidence years. Inapparent infections in the cohort overestimated the rate of asymptomatic infections. Ambulatory children with mild febrile viremic infections could represent an important component of dengue transmission.
Dengue virus (DENV) causes more human morbidity and mortality than any other arboviral disease. Each year, an estimated 3.6 billion people are at risk, 36 million develop dengue fever (DF), 2.1 million develop severe dengue hemorrhagic fever (DHF)/dengue shock syndrome, and 21 000 die [1]. Much of the available epidemiologic and clinical data on dengue are based on passive reporting. An improved understanding of both the patterns of DENV transmission and clinical spectrum of infection would improve public health surveillance and inform mitigation strategies. Prospective cohort studies in Thailand [2–6], Indonesia [7, 8], Nicaragua [9], Vietnam [10], and Peru [11, 12] are addressing knowledge gaps by providing a more comprehensive picture of the spectrum of DENV infection from clinically inapparent infection to severe disease. These increasingly detailed datasets have revealed marked spatial and temporal heterogeneity in the intensity of DENV transmission and the ratio of inapparent-to-symptomatic infections [13]. To further explore the range of DENV infection outcomes, especially with regard to inapparent infections that may contribute to transmission dynamics, we and others have initiated studies incorporating geographic cluster designs [6, 14, 15] in which symptomatic DENV infections trigger sampling of people who live in close proximity to an individual with a documented DENV infection. Geographic cluster studies can elucidate transmission dynamics on a finer spatial and temporal scale than is possible with longitudinal cohort studies, thus allowing increasingly detailed analysis of the factors that affect transmission dynamics.
We previously published the findings from the first 2 years of a 4-year combined longitudinal cohort and geographic cluster study in Kamphaeng Phet, Thailand [6]. Those data indicated that DENV transmission in rural locations occurs in a remarkably focal manner and suggested that environmental factors may influence transmission risk. In the current report, we present a more detailed analysis of the full 4-year prospective study, which includes a transition in the predominant circulating DENV serotype. Our results indicate that many DENV infections that were considered clinically inapparent in the longitudinal cohort were, in fact, viremic and therefore potentially contributed to DENV transmission.
METHODS
Ethics Statement
The study protocol and informed consents were approved by the institutional review boards of the Thai Ministry of Public Health, Walter Reed Army Institute of Research, University of Massachusetts Medical School, University of California, Davis, and San Diego State University.
Study Location and Population
Our study methodology was previously described [6]. In brief, the study was conducted at 11 primary schools in Muang district, Kamphaeng Phet province, in north-central Thailand. Students attending the 11 schools came from 32 villages consisting of >8445 houses. Demographics of house residents and house spatial coordinates were geocoded into a geographic information system database (MapInfo [2000] version 6.0; MapInfo Corporation).
Prospective Longitudinal Cohort and Geographic Cluster Investigations
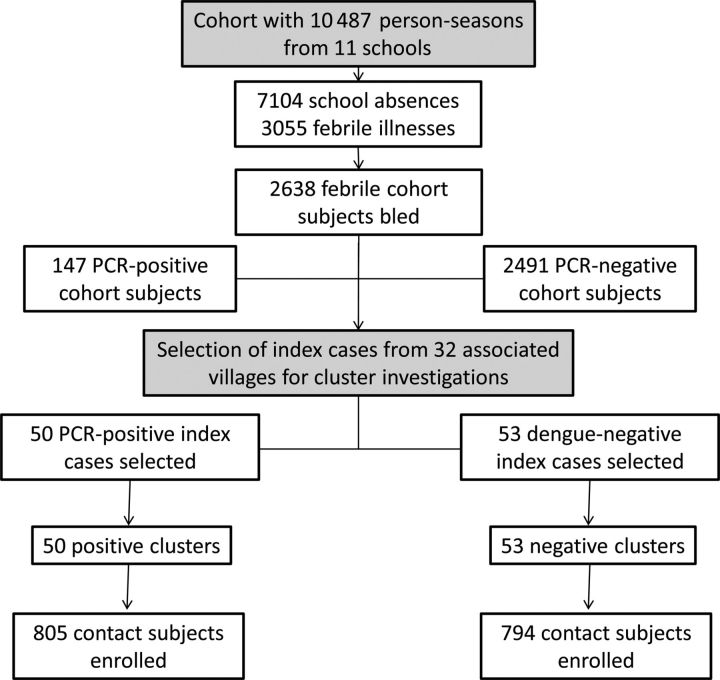
From 2004 to 2007, a dynamic prospective longitudinal cohort of approximately 2000 primary school children in kindergarten through grade 6 was followed by active school absence–based surveillance during June to November [5, 6]. An acute blood sample was drawn from cohort subjects who reported a fever in the previous 7 days or who had a measured temperature of ≥38°C. A convalescent blood sample was drawn 14 days later along with an evaluation of symptoms. Acute blood samples underwent dengue testing, including semi-nested reverse-transcriptase polymerase chain reaction (PCR) for detection of DENV RNA [16, 17]. Cohort subjects who were dengue PCR positive from an acute blood sample drawn within 3 days of illness onset served as an “index” case for a positive cluster investigation around the subject's house. Cohort subjects who were dengue PCR negative served as an “index” case for a negative cluster investigation (Figure 1). When possible, paired positive and negative clusters were carried out in the same village, within 5 days of each other.
Figure 1.
Study design overview. Abbreviation: PCR, polymerase chain reaction.
In each cluster, 10–25 children aged 6 months to 15 years living within a 100-meter radius of the index case were enrolled regardless of symptomatology. These contact subjects were evaluated at days 0, 5, 10, and 15 with temperature measurement and symptom questionnaire concerning the previous 5 days. Blood samples were collected on days 0 and 15.
Paired acute and convalescent blood samples from febrile cohort subjects were tested using an in-house dengue/Japanese encephalitis immunoglobulin M (IgM)/immunoglobulin G (IgG) capture enzyme immunoassay (EIA) [18]. Paired day 0 and day 15 blood samples from cluster contact subjects were tested by both dengue PCR and IgM/IgG EIA. Virus isolation was attempted in C6/36 cells from selected cohort and cluster dengue PCR-positive samples [16].
Each year, cohort subjects underwent scheduled phlebotomy in May and January, before and after the peak dengue transmission season. Paired pre- and postseason blood samples were tested by hemagglutination inhibition (HAI) assay [19] for all 4 DENV serotypes and Japanese encephalitis. Samples that had a 4-fold rise in HAI titers were retested by serotype-specific plaque reduction neutralization testing (PRNT) for DENV and Japanese encephalitis to confirm DENV seroconversion [20].
Study Definitions
Subjects were considered to have “acute” DENV infection if their acute and/or convalescent blood samples (or day 0 and/or day 15 blood samples in cluster contacts) were dengue IgM positive; or, if IgM was negative, IgG was positive with rising titer. Subjects had “recent” DENV infection if IgM was negative but IgG was positive with declining titer. These were further categorized as “primary” infections if the IgM to IgG ratio was ≥1.8 and “secondary” infection if the ratio was <1.8 [18, 21].
Within the cohort, subjects with DENV infection were clinically characterized as having “symptomatic” infection, “inapparent” infection, or “unclassified” infection. Symptomatic DENV infection was defined as any febrile illness (reported or measured fever) associated with a positive dengue EIA from the acute and/or convalescent blood sample. These symptomatic infections were further classified as hospitalized DF if a hospitalized subject met World Health Organization (WHO) 1997 criteria for DF, and as DHF if he/she met WHO 1997 guidelines for DHF [22]. “Inapparent” DENV infection was defined by paired pre- and postseason blood samples that showed a rise in dengue HAI and PRNT titers but was not associated with a symptomatic DENV infection between May and January. DENV infections in the cohort were defined as “unclassified” if paired pre- and postseason blood samples showed a rise in dengue HAI and PRNT titers and the subject had a febrile illness between May and January but did not have acute/convalescent blood samples collected at the time of the febrile illness.
Within the clusters, contact subjects with DENV infections were clinically characterized as being febrile, afebrile with other symptoms, or asymptomatic (ie, no detectable symptoms). Contact subjects who were dengue PCR positive on day 15 but did not have serological confirmation owing to lack of follow-up after day 15 were considered as having DENV infection. They were not, however, included in the analysis of clinical characteristics because no follow-up data were available.
Entomological Studies
On day 1 of each cluster investigation, adult Aedes aegypti mosquitoes were collected using backpack aspirators from inside and within the immediate vicinity of each house within a cluster. Immature A. aegypti (larvae and pupae) were collected from development sites (eg, containers with water) using the shape, use, and material method [23]. After mosquito collections were complete, a pyrethrin mixture insecticide spray (BP-300: Pyronyl oil concentrate OR-3610A, Prentiss Inc) was applied by ultralow volume aerosol inside and around each house to kill adult mosquitoes [24]. Temephos was applied to artificial water holding containers to kill immature mosquitoes. On day 7, the Thai Ministry of Public Health (MOPH) sprayed deltamethrin or permethrin 10% in and around each house in a cluster according to the standard procedures of the MOPH.
Statistical Analyses
Data were analyzed using IBM SPSS for Windows, version 19, and Stata Statistical Software, version 10. Demographic, environmental, and entomological parameters were analyzed at the cluster and house levels. Positive and negative clusters were compared using independent t test weighted by the number of houses per cluster. Student t test or analysis of variance was used to determine differences in continuous variables. The χ2 test was used for proportions.
RESULTS
Characteristics of the Prospective Cohort Population
The prospective cohort ranged in size from 2060 to 2088 subjects at the start of each surveillance season with ages ranging from 4 to 15 years and a male-to-female ratio of 1.1:1. A total of 7103 school absences were evaluated during the 4-year study, 3055 (43%) of which were associated with reported or measured fever; 2638 subjects (86.4%) underwent acute and convalescent blood collection (Figure 1; Table 1).
Table 1.
Cohort Description and Dengue Virus Infections
| Year |
|||||
|---|---|---|---|---|---|
| Description | 2004 | 2005 | 2006 | 2007 | Total |
| Cohort size (at start/end of surveillance season) | 2078/2023 | 2088/2021 | 2086/2039 | 2060/2007 | … |
| Median age, y (range) | 9.0 (4–15) | 9.0 (4–15) | 9.0 (4–15) | 10.0 (4–15) | 9.0 (4–15) |
| Sex | |||||
| Female (%) | 998 (48) | 1005 (48) | 994 (48) | 964 (47) | 5011 (48) |
| Male (%) | 1080 (52) | 1083 (52) | 1092 (52) | 1096 (53) | 5476 (52) |
| School absences (no. of episodes) | 1747 | 1737 | 1837 | 1782 | 7103 |
| Fever history (no. of episodes) | 663 | 764 | 871 | 757 | 3055 |
| Phlebotomized (% of febrile illnesses) | 504 (76) | 715 (94) | 779 (89) | 640 (85) | 2638 (86) |
| Dengue EIA positive (% of phlebotomized cases) | 33 (6.5) | 27 (3.8) | 90 (11.6) | 39 (6.1) | 189 (7.2) |
| Serological category | |||||
| Acute primary (% of dengue EIA-positive) | 6 (18.2) | 2 (7.4) | 5 (5.6) | 0 (0) | 13 (6.9) |
| Acute secondary (% of dengue EIA-positive) | 27 (81.8) | 24 (88.9) | 80 (88.9) | 38 (97.4) | 169 (89.4) |
| Recent (% of dengue EIA-positive) | 0 (0) | 1 (3.7) | 5 (5.6) | 1 (2.6) | 7 (3.7) |
| Seasonal incidence by EIA (%) | 1.6 | 1.3 | 4.4 | 1.9 | 2.3 |
| Dengue PCR positive (% of dengue EIA-positive) | 28 (85) | 20 (74) | 68 (76) | 31 (79) | 147 (78) |
| Serotype | |||||
| DENV-1 | 0 | 2 | 46 | 21 | 69 |
| DENV-2 | 9 | 2 | 1 | 8 | 20 |
| DENV-3 | 3 | 1 | 0 | 0 | 4 |
| DENV-4 | 16 | 15 | 21 | 2 | 54 |
| All symptomatic DENV infections | 33 | 27 | 90 | 39 | 189 |
| Symptomatic category | |||||
| Outpatient symptomatic DENV infections | 27 | 22 | 67 | 33 | 149 |
| Hospitalized dengue fever | 3 | 3 | 19 | 6 | 31 |
| DHF | 3 | 2 | 4 | 0 | 9 |
| Inapparent dengue | 81 | 77 | 103 | 85 | 346 |
| Unclassified dengue | 6 | 2 | 8 | 4 | 20 |
| Inapparent-to-symptomatic ratio | 2.5:1 | 2.9:1 | 1.1:1 | 2.2:1 | 1.8:1 |
| Combined inapparent, symptomatic and unclassified DENV infections | 120 | 106 | 201 | 128 | 554 |
| Combined inapparent, symptomatic and unclassified dengue seasonal incidence (%) | 5.9 | 5.2 | 9.9 | 6.4 | 6.8 |
All data are no. or no. (%) unless otherwise specified.
Abbreviations: DENV, dengue virus; DHF, dengue hemorrhagic fever; EIA, enzyme immunoassay.
Occurrence and Clinical Spectrum of DENV Infection in the Cohort Population
There were 189 EIA-confirmed symptomatic DENV infections in the cohort (Table 1). For the entire study, 7.2% of all febrile illnesses were dengue EIA positive, ranging from 3.8% in 2005 to 11.6% in 2006. The average incidence of symptomatic DENV infection was 2.3% per season, ranging from 1.3% in 2005 to 4.4% in 2006. On average, 0.5% of the cohort per season was hospitalized with laboratory-confirmed dengue. Hospitalized dengue accounted for 21.2% of symptomatic DENV infections and 7.2% of all DENV infections (inapparent, symptomatic, and unclassified). Thirteen of the 189 symptomatic infections (6.9%) were due to acute primary infections, 169 (89.4%) to acute secondary infections, and 7 (3.7%) to recent infections (Table 1).
There were 346 inapparent DENV infections and 20 clinically unclassified DENV infections in the cohort, resulting in an average total dengue incidence of 6.9% per season, ranging from 5.2% in 2005 to 9.9% in 2006. The inapparent-to-symptomatic infection ratio was 1.8:1 for the entire study, ranging from 1.1:1 in 2006 to 2.9:1 in 2005 (Table 1).
Of the 189 EIA-positive symptomatic DENV infections, 147 (77.8%) were dengue PCR positive. All 4 DENV serotypes were recovered. DENV-1 (46.9%) and DENV-4 (36.7%) were most commonly identified. DENV-4 was predominant during the first 2 years; DENV-1 was predominant in the last 2 years (Table 1).
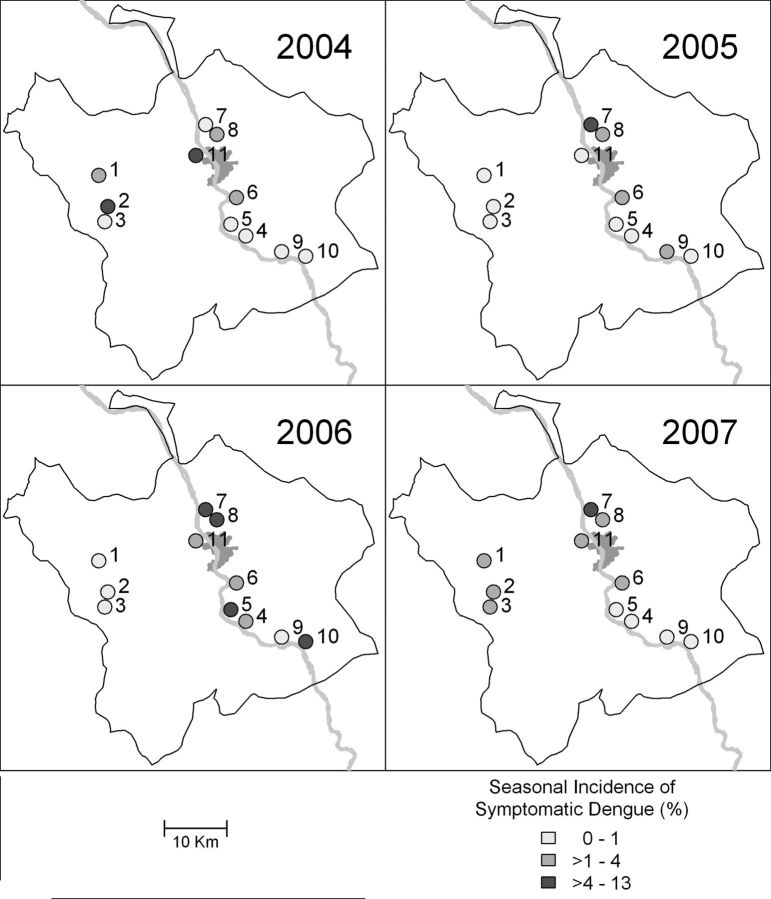
There was wide diversity in dengue incidence at different schools and in different years of the study, even though some schools were in close proximity (Figure 2).
Figure 2.
Geographic and temporal diversity of symptomatic dengue virus infections in prospective cohort. The boundary shows the Muang district of Kamphaeng Phet; the gray area indicates the urbanized portion of the study area; the Ping River is included for reference.
DENV Transmission Within Geographic Clusters
A total of 103 cluster investigations were initiated during the study: 50 positive clusters with 805 contact subjects and 53 negative clusters with 794 contact subjects (Table 2). One hundred twenty-nine (16.0%) contacts had DENV infection in positive clusters; only 9 (1.1%) had infection in negative clusters (P < .0001). Of the 129 DENV infections in positive clusters, 119 had EIA results consistent with DENV infection; the other 10 infections (in 8 different clusters) had only a positive dengue PCR on day 15 (Table 2). Of the 119 EIA-positive infections, 22 (18.5%) had acute primary infections, 82 (68.9%) acute secondary infections, and 15 (12.6%) recent infections. Eighteen positive clusters could be spatially and temporally matched closely with a corresponding negative cluster. When only these 18 closely matched pairs were considered, the DENV infection rate was 19.5% (65 of 333 contacts) in positive clusters compared with 0.8% (2 of 259 contacts) in negative clusters (P < .0001).
Table 2.
Cluster Description and Dengue Virus Infections
| Year |
|||||
|---|---|---|---|---|---|
| Description | 2004 | 2005 | 2006 | 2007 | Total |
| Positive Clusters | |||||
| Clusters (no.) | 8 | 4 | 22 | 16 | 50 |
| Index serotype | |||||
| DENV-1 | 0 | 2 | 12 | 12 | 26 |
| DENV-2 | 1 | 1 | 1 | 3 | 6 |
| DENV-3 | 0 | 1 | 0 | 0 | 1 |
| DENV-4 | 7 | 0 | 9 | 1 | 17 |
| Contact subjects | 151 | 66 | 339 | 249 | 805 |
| Median age, y (range) | 9.0 (1–15) | 9.0 (1–15) | 9.0 (1–15) | 10.0 (1–15) | 9.0 (1–15) |
| Sex | |||||
| Male (%) | 73 (48) | 35 (53) | 175 (52) | 126 (51) | 409 (51) |
| Female (%) | 78 (52) | 31 (47) | 164 (48) | 123 (49) | 396 (49) |
| Dengue EIA-positive contacts | 18 | 7 | 55 | 39 | 119 |
| Serological category | |||||
| Acute primary (%) | 3 (16.7) | 3 (42.9) | 11 (20.0) | 5 (12.8) | 22 (18.5) |
| Acute secondary (%) | 14 (77.8) | 4 (57.1) | 38 (69.1) | 26 (66.7) | 82 (68.9) |
| Recent (%) | 1 (5.6) | 0 (0) | 6 (10.9) | 8 (20.5) | 15 (12.6) |
| Dengue PCR-positive contacts at day 0 | 4 | 2 | 20 | 14 | 40 |
| Dengue PCR-positive contacts at day 15 | 2 | 0 | 7 | 1 | 10 |
| Infection rate by EIA and/or PCR (%) | 13.2 | 10.6 | 18.3 | 16.1 | 16.0 |
| Negative Clusters | |||||
| Clusters (no.) | 15 | 7 | 17 | 14 | 53 |
| Contact subjects | 235 | 104 | 233 | 222 | 794 |
| Median age, y (range) | 9.0 (1–15) | 9.0 (1–15) | 9.0 (1–15) | 9.0 (1–15) | 9.0 (1–15) |
| Sex | |||||
| Male (%) | 122 (52) | 53 (51) | 121 (52) | 121 (55) | 417 (53) |
| Female (%) | 113 (48) | 51 (49) | 112 (48) | 101 (45) | 377 (47) |
| Dengue EIA-positive contacts | 0 | 0 | 6 | 1 | 7 |
| Serological category | |||||
| Acute primary (%) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 1 (14.3) |
| Acute secondary (%) | 0 (0) | 0 (0) | 5 (83.3) | 1 (100) | 6 (85.7) |
| Recent (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dengue PCR-positive contacts at day 0 | 0 | 0 | 3 | 0 | 3 |
| Dengue PCR-positive contacts at day 15 | 0 | 0 | 2 | 0 | 2 |
| Infection rate by EIA and/or PCR (%) | 0 | 0 | 3.4 | 0.5 | 1.1 |
All data are no. or no. (%) unless otherwise specified.
Abbreviations: DENV, dengue virus; EIA, enzyme immunoassay; PCR, polymerase chain reaction.
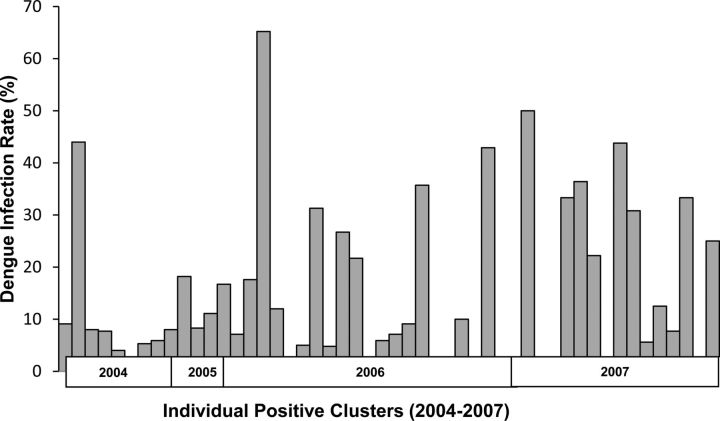
The overall proportion of positive clusters in which DENV infection was detected in contact subjects was 38 of 50 (76%). The proportion of negative clusters with DENV infection was 6 of 53 (11%). The DENV infection rate among positive clusters was highly variable (Figure 3). Demographic, environmental, and entomological factors were not significantly different between positive and negative clusters (Table 3). Similarly, there were no significant differences in measured demographic, environmental, or mosquito density parameters between houses with DENV infections versus those without DENV infections in positive clusters (data not shown).
Figure 3.
Range of dengue virus (DENV) infection rates among contact subjects in individual positive clusters.
Table 3.
Positive vs Negative Clusters: Comparison of Selected Demographic, Environmental, and Entomological Parameters
| Variable | Mean (SD) in 50 Positive Clusters | Mean (SD) in 53 Negative Clusters | Difference, P Value |
|---|---|---|---|
| People per cluster | 84.3 (31.2) | 79.1 (40.0) | .47 |
| Children per cluster | 24.5 (9.3) | 22.9 (11.1) | .44 |
| Houses per cluster | 22.9 (8.07) | 21.7 (11.5) | .55 |
| People per house | 3.71 (0.58) | 3.76 (0.73) | .72 |
| Proportion of houses with piped water | 0.60 (0.39) | 0.70 (0.36) | .22 |
| Aedes aegypti pupae per person | 1.41 (1.5) | 1.49 (1.6) | .78 |
| A. aegypti adults per person | 1.06 (0.78) | 0.93 (0.68) | .38 |
| Proportion of houses recently fumigated | 0.20 (0.37) | 0.17 (0.33) | .63 |
| Key containers | 118.24 (50.2) | 115.57 (55.1) | .80 |
| Total containers | 292.82 (116.15) | 294.17 (131.45) | .97 |
| Proportion of containers with temephos | 0.142 (0.05) | 0.136 (0.07) | .61 |
Clinical Spectrum and Virological Features of DENV Infection in Geographic Clusters
Of the 119 dengue EIA-positive contact subjects in positive clusters, 71 (59.7%) were febrile, 24 (20.2%) were afebrile with other symptoms, and 24 (20.2%) were asymptomatic. The ratio of afebrile to febrile infections in positive clusters was 0.7:1 for the entire study, ranging from 0.4:1 to 1.2:1 depending on year (Table 4). When the presence or absence of any symptom was used as the distinguishing criterion, the ratio of asymptomatic infection to infection with any symptom was lower, with a narrow range of 0.2:1–0.3:1 depending on the year (although it is possible that some of these symptoms, especially among the afebrile contacts, may have been coincidental and not truly due to DENV infection).
Table 4.
Clinical Spectrum of Dengue Enzyme Immunoassay–Positive Infections in Positive Clusters
| Year |
|||||
|---|---|---|---|---|---|
| Clinical Description of DENV Infections in Positive Clusters | 2004 | 2005 | 2006 | 2007 | Total |
| All EIA-positive DENV infections | 18 | 7 | 55 | 39 | 119 |
| Hospitalized DF or DHF | 1 | 0 | 1 | 2 | 4 |
| EIA-positive cases with and without fever history, no. | |||||
| Primary | |||||
| No fever history | 3 | 2 | 4 | 0 | 9 |
| Fever history | 0 | 1 | 7 | 5 | 13 |
| Ratio (afebrile:febrile) | 3:0 | 2.0:1 | 0.6:1 | 0:1 | 0.7:1 |
| Secondary | |||||
| No fever history | 7 | 0 | 20 | 12 | 39 |
| Fever history | 8 | 4 | 24 | 22 | 58 |
| Ratio (afebrile:febrile) | 0.9:1 | 0:4 | 0.8:1 | 0.5:1 | 0.7:1 |
| Total | |||||
| No fever history | 10 | 2 | 24 | 12 | 48 |
| Fever history | 8 | 5 | 31 | 27 | 71 |
| Ratio (afebrile:febrile) | 1.2:1 | 0.4:1 | 0.8:1 | 0.4:1 | 0.7:1 |
| EIA-positive cases with and without any symptoms, no. | |||||
| Primary | |||||
| No symptoms | 1 | 1 | 2 | 0 | 4 |
| Any symptom | 2 | 2 | 9 | 5 | 18 |
| Ratio (asymptomatic:any symptom) | 0.5:1 | 0.5:1 | 0.2:1 | 0:5 | 0.2:1 |
| Secondary | |||||
| No symptoms | 2 | 0 | 10 | 8 | 20 |
| Any symptom | 13 | 4 | 34 | 26 | 77 |
| Ratio (asymptomatic:any symptom) | 0.2:1 | 0:4 | 0.3:1 | 0.3:1 | 0.3:1 |
| Total | |||||
| No symptoms | 3 | 1 | 12 | 8 | 24 |
| Any symptom | 15 | 6 | 43 | 31 | 95 |
| Ratio (asymptomatic:any symptom) | 0.2:1 | 0.2:1 | 0.3:1 | 0.3:1 | 0.3:1 |
Abbreviations: DENV, dengue virus; DF, dengue fever; DHF, dengue hemorrhagic fever; EIA, enzyme immunoassay.
Thirty-one dengue EIA- and/or PCR-positive cluster contacts were also cohort subjects simultaneously involved in active surveillance at the time of the cluster investigation. Only 8 of these 31 were detected as symptomatic DENV infections by cohort surveillance. Of the remaining 23 infections, 16 were classified as “inapparent” dengue in the cohort based on pre- and postseason HAI. Seven were not detected as either inapparent or symptomatic dengue in the cohort. Of the 16 subjects with “inapparent” infections, 12 reported fever during the cluster investigation but had not missed school based on cohort surveillance; 3 were afebrile with other symptoms; 1 was asymptomatic. Of the 7 subjects with infections not detected in the cohort, 4 were febrile and 3 were afebrile with other symptoms.
Dengue viremia was detected by PCR in 34 of the 71 febrile dengue EIA-positive contact subjects in positive clusters. Dengue viremia was detected by PCR and culture in 9 of the 16 febrile DENV-infected children in the clusters (12 HAI positive, 4 HAI negative) who did not miss school (4 DENV-1, 5 DENV-4). Dengue viremia was detected by PCR and culture in only 2 of 24 infected children in clusters who were afebrile with other symptoms, and in only 1 of 24 asymptomatic infected children. Viremia in the 2 children who were afebrile with other symptoms and 1 asymptomatic child was due to DENV-4 in a single cluster. Not all viremias would have been detected given the 15-day interval between blood collections in the clusters.
DISCUSSION
By linking a prospective longitudinal cohort with geographic clusters, we obtained information about DENV transmission and the clinical spectrum of infection that has not been available from other studies. The previously reported focal nature of DENV transmission risk within 100 meters of an “index” viremic infection [6] was confirmed in the full 4-year study. Although clinically inapparent DENV infection rates have been estimated in other studies [25], this is the first study to demonstrate the low rate of infections having no detectable symptoms. Our results support the potential role of children with mildly symptomatic viremic infections in DENV transmission. These mild infections would otherwise have been characterized as clinically inapparent in school absence–based cohort studies.
Our findings are subject to several limitations. First, our study population included only children. We did not address the role of adults in DENV transmission. Second, movement patterns of cohort and cluster contact subjects and the risk of DENV infection related to the various places they visited were not determined. Describing the epidemiologic impact of adults and appropriately measuring human movement patterns would help better define the spatial and temporal scale for dengue surveillance and control.
DENV-4 was the most commonly detected serotype during the first 2 years of the study. This serotype was uncommon during the earlier cohort study reported by Endy et al [5]. The predominant serotype changed to DENV-1 in 2006, the same year when dengue incidence was highest and the inapparent-to-symptomatic infection ratio was lowest. Endy et al similarly detected a negative association between dengue incidence and inapparent-to-symptomatic infection ratio for individual schools [13]. A 4-year cohort study of children in Nicaragua [9] reported a shift in predominant serotype from DENV-1 to DENV-2 coincident with the highest dengue incidence and a decrease in inapparent-to-symptomatic infection ratio from the previous year. Introduction of a new predominant serotype into a relatively susceptible population may lead to an overall increase in incidence in concert with a lower proportion of inapparent infections. One possible explanation is that the susceptible population may have preexisting cross-reactive, nonprotective immunity to the newly introduced serotype, thus promoting immune enhancement leading to a lower inapparent-to-symptomatic infection ratio.
The 2006 season, when dengue incidence in the cohort population was highest, was the only year in which a significant number of DENV infections were detected in negative clusters. Eight DENV infections were detected in 5 different negative clusters in 2006 (infection rate, 3.4%), compared with only 1 infection detected in negative clusters during the other 3 years combined (Table 2). Although the DENV infection rate in positive clusters was also highest in 2006 (18.3%), the infection rate in positive clusters was also high during the other 3 years (range, 10.6%–16.1%), indicating that focal clustering of DENV transmission occurs in both high- and low-incidence years. In high-incidence years, however, additional transmission risk is dispersed over a larger geographic area, likely reflecting the more dispersed, larger number of immunologically susceptible hosts. These kinds of heterogeneities in patterns of transmission will be important to take into account when predicting virus transmission patterns, for example, during vaccine trials.
Positive and negative clusters were not distinguishable by any of the demographic, environmental, or entomological factors that were evaluated. This suggests that mosquito densities were high so that entomological thresholds for DENV transmission were exceeded and that, in this setting, preexisting immunity, at both the individual and population levels, played a more important role than mosquito population density in determining transmission patterns. To test this hypothesis directly would require collection of pre-illness blood samples from cluster contacts. Mammen et al reported from the first 2 years of our study that piped water and A. aegypti pupae per person were higher in positive than negative clusters [6]. Those relationships were not statistically significant when all 4 years of the study were taken into account. Relevant risk factors during lower-incidence years (ie, first 2 study years) may have been overshadowed by other determinants of transmission, such as host immunity, when the force of infection was higher (ie, second 2 study years).
The overall ratio of inapparent-to-symptomatic infection in the cohort was, based on the definition of inapparent infection that we used, an overestimate. Of the 16 cluster contacts with DENV infection who could be simultaneously characterized as having “inapparent” infection in the cohort, 12 did, in fact, report fever in the cluster investigation. Asymptomatic infections accounted for only 20% of all DENV infections in positive clusters. As suggested by other investigators [11], the different surveillance and sampling methods used in the 2 components of our study most likely contributed to these differences. Cohort subjects were evaluated for acute illness only when they were ill enough to be absent from school. Cluster contacts were evaluated whether or not they felt ill, which resulted in detection of milder DENV infections. The design of vaccine trials will benefit by accounting for these kinds of differences in surveillance sensitivities.
Nine of 16 febrile children with DENV infections in the clusters who did not miss school had detectable viremia by PCR and culture, suggesting that these individuals were potentially infectious to mosquitoes and could participate in the spread of DENV. These 16 children with mild febrile infections were more likely to be viremic when sampled than the 48 children with DENV infections in the clusters who were afebrile with other symptoms or were asymptomatic. If human movement plays an important role in DENV spread to geographically disparate locations [26–28], individuals with mild febrile viremic infections could contribute disproportionately to virus spread given their proven mobility as demonstrated by continued school attendance. In contrast, individuals with symptomatic viremic infections who miss school could potentially play a lesser role in transmission if they are homebound and geographically restricted for part of their illness. Investigators in other cluster studies have reported occasional viremia in asymptomatic individuals [14, 15]. Blood transfusion studies have also revealed viremia in subclinical blood donors [29, 30]. Our study detected a larger group of mildly symptomatic viremic individuals who may be more important contributors than previously recognized to overall patterns of DENV transmission.
Our study design, which linked geographic clusters to a longitudinal cohort, allowed fine-scale spatial and temporal study of DENV transmission. Results from this approach are relevant to improved strategies for the prevention and management of dengue. The focal and heterogeneous nature of DENV transmission may warrant the use of more intensive and targeted interventions guided by local surveillance within the context of broad-scale dengue management efforts. More thorough investigation is warranted, particularly on the role of inapparent/mild DENV infections in promoting virus spread and the challenges that human movement presents for the assessment and implementation of dengue control efforts.
Notes
Acknowledgments. The authors acknowledge the contributions of Dr Chonticha Klungthong; Dr Butsaya Thaisomboonsuk; Ms Chaleaw Saengchan; Mr Udom Kijchalao; and other clinical, laboratory, and entomological personnel of the Armed Forces Research Institute of Medical Sciences. The collaboration has benefited from the consultative support of Dr Frank Ennis. We thank Dr Kamchai Rungsimanphaiboon for his support of the field laboratory; Dr Siripen Kalayanarooj for clinical grading of hospitalized cases; and the political, educational, medical, and community workers and leaders in Kamphaeng Phet, Thailand, for their support. We are especially grateful to the children and parents involved in this study for their enthusiastic participation. This research benefited from discussions with working group members in the Research and Policy for Infectious Disease Dynamics program of the Science and Technology Directorate, US Department of Homeland Security; and the Fogarty International Center, US National Institutes of Health.
Disclaimer. The views expressed in this article are those of the authors and do not represent the official policy or position of the US Department of the Army, US Department of Defense, or US government.
Financial support. This work was supported by the US National Institutes of Health (grant numbers P01 AI34533 and R01 GM083224); the US Military Infectious Diseases Research Program (grant number S0016-04-AF); and the Bill & Melinda Gates Foundation Global Health Program (grant number OPP52250).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Pediatric Dengue Vaccine Initiative, International Vaccine Institute. Global burden of dengue. Available at: http://www.pdvi.org/about_dengue/GBD.asp. Accessed 22 May 2011. [Google Scholar]
- 2.Sangkawibha N, Rojanasuphot S, Ahandrik S, et al. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984;120:653–69. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 3.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–80. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 4.Endy TP, Chunsuttiwat S, Nisalak A, et al. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 5.Endy TP, Nisalak A, Chunsuttiwat S, et al. Spatial and temporal circulation of dengue virus serotypes: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:52–9. doi: 10.1093/aje/kwf006. [DOI] [PubMed] [Google Scholar]
- 6.Mammen MP, Pimgate C, Koenraadt CJ, et al. Spatial and temporal clustering of dengue virus transmission in Thai villages. PLoS Med. 2008;5:e205. doi: 10.1371/journal.pmed.0050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham RR, Juffrie M, Tan R, et al. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia I. studies in 1995–1996. Am J Trop Med Hyg. 1999;61:412–9. doi: 10.4269/ajtmh.1999.61.412. [DOI] [PubMed] [Google Scholar]
- 8.Porter KR, Beckett CG, Kosasih H, et al. Epidemiology of dengue and dengue hemorrhagic fever in a cohort of adults living in Bandung, West Java, Indonesia. Am J Trop Med Hyg. 2005;72:60–6. [PubMed] [Google Scholar]
- 9.Balmaseda A, Standish K, Mercado JC, et al. Trends in patterns of dengue transmission over 4 years in a pediatric cohort study in Nicaragua. J Infect Dis. 2010;201:5–14. doi: 10.1086/648592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tien NT, Luxemburger C, Toan NT, et al. A prospective cohort study of dengue infection in school children in Long Xuyen, Viet Nam. Trans R Soc Trop Med Hyg. 2010;104:592–600. doi: 10.1016/j.trstmh.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Rocha C, Morrison AC, Forshey BM, et al. Comparison of two active surveillance programs for the detection of clinical dengue cases in Iquitos, Peru. Am J Trop Med Hyg. 2009;80:656–60. [PubMed] [Google Scholar]
- 12.Morrison AC, Minnick SL, Rocha C, et al. Epidemiology of dengue virus in Iquitos, Peru 1999 to 2005: interepidemic and epidemic patterns of transmission. PLoS Negl Trop Dis. 2010;4:e670. doi: 10.1371/journal.pntd.0000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endy TP, Anderson KB, Nisalak A, et al. Determinants of inapparent and symptomatic dengue infection in a prospective study of primary school children in Kamphaeng Phet, Thailand. PLoS Negl Trop Dis. 2011;5:e975. doi: 10.1371/journal.pntd.0000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckett CG, Kosasih H, Faisal I, et al. Early detection of dengue infections using cluster sampling around index cases. Am J Trop Med Hyg. 2005;72:777–82. [PubMed] [Google Scholar]
- 15.Reyes M, Mercado JC, Standish K, et al. Index cluster study of dengue virus infection in Nicaragua. Am J Trop Med Hyg. 2010;83:683–9. doi: 10.4269/ajtmh.2010.10-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klungthong C, Gibbons RV, Thaisomboonsuk B, et al. Dengue viral detection using whole blood for RT-PCR and viral isolation. J Clin Microbiol. 2007;45:2480–5. doi: 10.1128/JCM.00305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–51. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Innis BL, Nisalak A, Nimmannitya S, et al. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg. 1989;40:418–27. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 19.Clarke DH, Casals J. Techniques for hemagglutination and hemagglutination inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–73. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 20.Russell PK, Nisalak A, Sukhavachana P, Vivona S. A plaque reduction test for dengue virus neutralization antibodies. J Immunol. 1967;99:285–90. [PubMed] [Google Scholar]
- 21.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue in the early febrile phase: viremia and antibody responses. J Infect Dis. 1997;176:322–30. doi: 10.1086/514048. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. 2nd ed. Geneva, Switerland: World Health Organization; 1997. [Google Scholar]
- 23.Koenraadt CJ, Jones JW, Sithiprasasna R, Scott TW. Standardizing container classification for immature Aedes aegypti surveillance in Kamphaeng Phet, Thailand. J Med Entomol. 2007;44:938–44. doi: 10.1603/0022-2585(2007)44[938:sccfia]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Koenraadt CJ, Aldstadt J, Kijchalao U, Kengluecha A, Jones JW, Scott TW. Spatial and temporal patterns in the recovery of Aedes aegypti (Diptera: Culicidae) populations after insecticide treatment. J Med Entomol. 2007;44:65–71. doi: 10.1603/0022-2585(2007)44[65:satpit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Endy TP, Yoon IK, Mammen MP. Prospective cohort studies of dengue viral transmission and severity of disease. Curr Top Microbiol Immunol. 2010;338:1–13. doi: 10.1007/978-3-642-02215-9_1. [DOI] [PubMed] [Google Scholar]
- 26.Kuno G. Review of the factors modulating dengue transmission. Epidemiol Rev. 1995;17:321–35. doi: 10.1093/oxfordjournals.epirev.a036196. [DOI] [PubMed] [Google Scholar]
- 27.Stoddard ST, Morrison AC, Vazquez-Prokopec GM, et al. The role of human movement in the transmission of vector-borne pathogens. PLoS Negl Trop Dis. 2009;3:e481. doi: 10.1371/journal.pntd.0000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrington LC, Scott TW, Lerdthusnee K, et al. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg. 2005;72:209–20. [PubMed] [Google Scholar]
- 29.Mohammed H, Linnen JM, Munoz-Jordan JL, et al. Dengue virus in blood donations, Puerto Rico, 2005. Transfusion. 2008;48:1348–54. doi: 10.1111/j.1537-2995.2008.01771.x. [DOI] [PubMed] [Google Scholar]
- 30.Linnen JM, Vinelli E, Sabino EC, et al. Dengue viremia in blood donors from Honduras, Brazil, and Australia. Transfusion. 2008;48:1355–62. doi: 10.1111/j.1537-2995.2008.01772.x. [DOI] [PubMed] [Google Scholar]