Abstract
Background. Brain histology and ophthalmoscopy suggest that approximately 25% of children with World Health Organization–defined cerebral malaria (CM) have a nonmalarial cause of death. Misclassification complicates clinical care, confounds studies of association, and may obfuscate successes in malaria control. Retinopathy predicts intracerebral parasite sequestration with >90% sensitivity and specificity, but detecting retinopathy requires well-trained personnel and expensive equipment.
Methods. We investigated the utility of plasma concentrations of parasite histidine-rich protein 2 (pHRP2), a Plasmodium-specific protein, as a predictor of intracerebral parasite sequestration at autopsy and of malaria retinopathy on clinical examination in patients with clinically defined CM.
Results. In 64 autopsy cases, 47 of whom had histological evidence of sequestration, the sensitivity and specificity of a plasma pHRP2 level of >1700 ng/mL were 98% and 94%, respectively, and the area under the receiver operating characteristic (AUROC) curve was 0.98. In a separate, prospectively studied group of 101 children with clinically defined CM, of whom 71 had retinopathy, the same pHRP2 cutoff predicted retinopathy-positivity with a sensitivity of 90% and specificity of 87% (AUROC, 0.90).
Conclusions. Elevated plasma pHRP2 concentrations can identify Malawian children with histologically confirmed or retinopathy-positive CM and is a more field-friendly approach to confirming the diagnosis than post mortem sampling or ophthalmoscopy.
(See the editorial commentary by John, on pages 307–8.)
Despite increasing malaria control measures, cerebral malaria (CM) remains a condition that affects millions of children, mostly in sub–Saharan Africa [1, 2]. The presentation is often dramatic, with profound coma and, frequently, convulsions. The clinical diagnosis, however, is not straightforward. Because asymptomatic parasitemia is common in malaria-endemic regions, parasitemic children in a coma are often initially assumed to have CM. Nonmalarial etiologies of coma are often considered only after a child has failed to respond to antimalarial treatment. The misdiagnosis of CM has implications for patient care, resource utilization, and research studies including estimates of clinical efficacy of antimalarial drugs, adjuvant therapies, and control measures.
The gold standard for the diagnosis of CM, the presence of intraerythrocytic parasites sequestered in cerebral microvessels, requires the examination of brain tissue collected after death [3]. The most useful surrogate for histologic or cytologic evidence of sequestration is malaria retinopathy [4–6]. Indirect ophthalmoscopy by a trained clinician has a sensitivity and specificity of 90% and 95%, respectively, when compared to autopsy [7]. Although exceedingly useful in a research setting, the cost of the equipment and the need for clinical training limit the utility of ophthalmoscopy in many clinical settings in malaria-endemic areas. A point-of-care, low-cost, low-technology assay that could distinguish children with retinopathy-positive CM from those with a nonmalarial cause of coma and a coincidental parasitemia would be very useful.
Parasite histidine-rich protein 2 (pHRP2) is a malaria parasite protein of unknown function. The protein has recently been shown to interact with host glycosaminoglycans and interfere with the coagulation cascade [8]. It is produced throughout the 48-hour life cycle of the malaria parasite. The presence of a signal sequence allows for secretion of the protein, although the majority of the protein remains intracellular until the mature schizont ruptures, releasing all of the intraerythrocytic contents [9].
A dichotomous measure of pHRP2 (presence or absence of the protein) is the basis of several rapid diagnostic tests for malaria. Quantitative assessments have shown a positive correlation between pHRP2 concentrations and disease severity [10, 11]. An observational study in Blantyre, Malawi, determined that pHRP2 concentrations are highest at the time of hospital admission and fall slowly once antimalarial treatment is initiated (authors’ unpublished data).
Other clinical parameters, such as increased peripheral parasitemia, increased lactate concentration, and decreased platelet count have been shown to be associated with increased disease severity in children with CM [12–16]. We evaluated the utility of a quantitative measure of plasma pHRP2 concentration to identify patients with histologically positive or retinopathy-positive CM and compared it with these other predictors of disease severity.
MATERIALS AND METHODS
Three studies were undertaken sequentially. All of the patients satisfied the standard clinical case definition of CM, and all had plasma concentrations of pHRP2 measured at the time of admission to the Paediatric Research Ward in Blantyre.
In the first study, an autopsy-based study of clinicopathological correlates of fatal malaria, we compared plasma pHRP2 concentrations of children with histopathological evidence of cerebral sequestration with that of a control population of parasitemic children who died of nonmalarial causes and who had no evidence of cerebral sequestration.
The second study was a retrospective case–control study comparing children with retinopathy-positive CM and children with retinopathy-negative CM. It was designed to identify a plasma concentration of pHRP2 that could best discriminate between these 2 patient populations.
The third study was a prospective cohort study designed to validate the cutoff concentration established during the retrospective case–control study.
Patients
Children aged 6 months–9 years who satisfied the clinical case definition of CM [17] were enrolled in the study after consent was obtained from a parent or guardian. Cerebral malaria was defined as a Blantyre Coma Score of ≤2, peripheral parasitemia with Plasmodium falciparum of any density, and no other discernible causes of coma (eg, hypoglycemia-associated coma reversed by glucose infusion; meningitis; or postictal state). Admission plasma samples were obtained from children enrolled in a large ongoing research study of CM centered at Queen Elizabeth Central Hospital in Blantyre, and laboratory values for peripheral parasitemia, hematocrit, lactate, glucose, and platelet count were determined as previously described [3]. Human immunodeficiency virus (HIV) status was determined with 2 rapid tests—Uni-Gold (Trinity Biotech) and Determine (Inverness Medical). In the event of discordant HIV results, a polymerase chain reaction was performed. A venous blood sample was obtained at the time of admission, anticoagulated in lithium heparin, and stored at −80°C until analysis. Retinopathy status was determined at the time of admission by a trained ophthalmologist using a combination of direct and indirect ophthalmoscopy.
Study 1: Autopsy Study
As previously described [3], cases coming to autopsy either showed microscopic evidence of CM (intracranial vessels contained sequestered parasites) or no microscopic evidence of CM (no sequestered parasites). Cases in the latter group were included in this study only if another cause of death was identified at autopsy. The autopsy cases were classified as autopsy-confirmed CM cause of death (ACCD) or nonmalarial causes of death (NMCD). All autopsy cases for which admission plasma samples were available were analyzed.
Study 2: Retrospective Case–Control Study
This study was designed on the basis of the data from study 1 and was powered to determine the ability of pHRP2 to detect the lower bound of the sensitivity and specificity values determined in the autopsy study with a 95% confidence interval (CI): 116 patients had retinopathy and 145 patients did not. The presence of retinopathy is a surrogate for intracerebral parasite sequestration in patients who met the standard clinical case definition of CM, and the absence of retinopathy suggests that the patient, albeit infected with P. falciparum, had a nonmalarial coma. Archived admission plasma samples were randomly chosen, and patients who had been involved in the autopsy study were excluded.
Study 3: Prospective Cohort Study
Every child admitted to the research ward at Queen Elizabeth Central Hospital during 2009 who fulfilled the clinical case definition for CM and had a full ophthalmologic examination was included in this study. The test characteristics were determined by comparing pHRP2 concentrations of retinopathy-positive CM patients with that of retinopathy-negative CM patients.
Laboratory Procedures
pHRP2 Determination
Frozen stocks of lithium heparin anticoagulated plasma were thawed. Plasma was diluted at a ratio of either 1:200 or 1:500 in phosphate-buffered saline (PBS). These samples, as well as a titration of a stock of recombinant pHRP2, were plated in duplicate (100 µL/well) onto a plate precoated with anti-pHRP2 antibody (Cellabs). The manufacturer's protocol was followed except that all incubations were carried out at 37°C in a humidified chamber instead of at room temperature. Briefly, a 1-hour sample incubation step was followed with extensive washing with PBS/0.1% Tween, after which 100 μL of conjugated antibody were plated and allowed to incubate for 1 hour. The conjugate was subsequently washed off and 100 µL of substrate were added for 15 minutes, during which color change was observed. This reaction was stopped with 50 µL of stop solution, and the plate was analyzed at an optical density (OD) of 450. A standard curve was generated from the recombinant protein, and pHRP2 concentrations in the samples were calculated using the standard curve. When the initial dilution of the sample was inappropriate, resulting in an OD reading outside the linear range of the standard curve, the samples were rediluted at either 1:1000 or 1:10. Samples that remained off the scale (low) despite redilution were assigned a value equivalent to the lowest possible detection value.
Parasite Production of pHRP2
To evaluate the possibility that the variability in pHRP2 concentration is a feature of different parasite strains, we compared pHRP2 levels produced by isolates from retinopathy-positive CM patients with isolates from retinopathy-negative CM patients. Cryopreserved parasite isolates from 13 patients (7 retinopathy-positive and 6 retinopathy-negative) with CM were thawed and cultured in Roswell Park Memorial Institute medium supplemented with 5% Albumax (Invtitrogen). After several rounds of culture, parasites were synchronized with 5% Sorbitol, and concentrations were adjusted to approximately 1% parasitemia at 5% hematocrit in fresh media. The absolute number of parasites placed into culture was calculated using a hemocytometer to calculate the total number of erythrocytes and by counting the number of parasites per 1000 erythrocytes on a thin smear. Parasites were then allowed to mature for 48 hours, at which point supernatant was collected, and the pHRP2 concentration was determined (using the methods described). The amount of pHRP2 produced per parasite was calculated by dividing the amount of pHRP2 produced by the absolute number of parasites initially placed into culture.
Statistical Analyses
Baseline characteristics between groups of interest were compared by χ2 or Fisher exact tests for categorical variables; continuous variables were compared by Student t tests or Mann–Whitney tests for continuous variables with nonnormal distribution.
To quantify the discriminative ability of pHRP2 levels to distinguish retinopathy-positive CM from retinopathy-negative CM, receiver operator characteristic (ROC) curves and the area under the ROC (AUROC) curve were calculated using SPSS software version 18.0 (IBM Corporation).
RESULTS
Autopsy Study
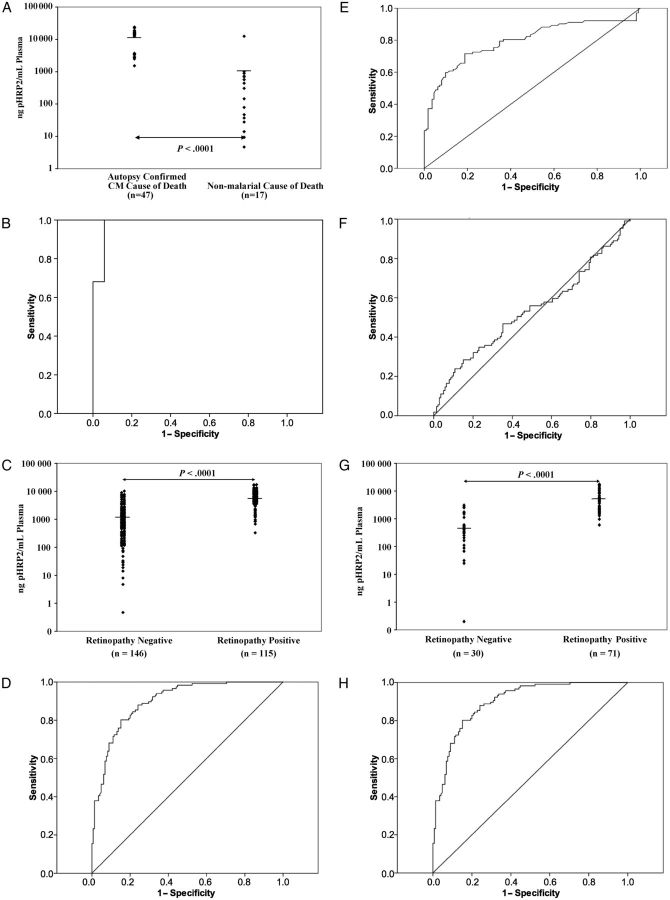
There were 47 cases of ACCD (parasites sequestered in intracerebral vessels) and 17 cases of NMCD (without intracerebral sequestration). Children with ACCD had statistically significant lower mean hematocrit concentrations, higher mean plasma lactate concentrations, and lower mean platelet counts than children with NMCD (Table 1). Plasma pHRP2 concentrations were substantially higher in children in the ACCD group than in children with NMCD (mean ± SD, 12 800 ± 7057 ng/mL vs 1028 ± 2970 ng/mL; P < .0001) (Table 1 and Figure 1A). The AUROC curve to discriminate between ACCD and NMCD was 0.98 (Figure 1B).
Table 1.
Characteristics of Study 1: Autopsy Study
| Characteristic | Autopsy-Confirmed Cerebral Malaria Cause of Death n = 47 | Nonmalarial Cause of Death n = 17 | P Value |
|---|---|---|---|
| Gender, % male | 48.9 | 70.6 | .16 |
| Age in months | 49.0 ± 37.1 | 35.0 ± 17.9 | .17 |
| Admission laboratory values | |||
| Parasitemia, parasites/μLa | 45 486 | 12 407 | .08 |
| Hematocrit, % | 21.4 ± 7.3 | 31.4 ± 9.1 | <.001 |
| Lactate, mmol/L | 10.1 ± 4.4 | 5.1 ± 2.9 | <.01 |
| pHRP2 concentration, ng/mL | 12 800 ± 7057 | 1028 ± 2970 | <.001 |
| Platelets, per μL | 77 710 ± 64 821 | 181 533 ± 109 501 | <.001 |
| White blood cell count, per μL | 16 120 ± 13 158 | 17 787 ± 11 304 | .67 |
| HIV positive, % | 32.6 | 6.3 | .05 |
| Time to death in hours | 19.4 ± 20.8 | 29.4 ± 36.5 | .18 |
Abbreviations: HIV, human immunodeficiency virus; pHRP2, parasite histidine-rich protein 2.
aGeometric mean.
Figure 1.
Parasite histidine-rich protein 2 (pHRP2) concentrations for study 1 (A), study 2 (C), and study 3 (G) and receiver operating characteristic (ROC) curves for pHRP2 for study 1 (area under curve [AUC], 0.98) (B); pHRP2 (AUC, 0.90) (D), platelets (AUC, 0.79) (E), and periperhal parisitemia (AUC, 0.53) (F) for study 2; and pHRP2 for study 3 (AUC, 0.96) (H). Each of the studies resulted in statistically significant differences in pHRP2 concentrations in the groups being compared.
Retrospective Case–Control Study
The patients with retinopathy-positive CM differed from those without retinopathy in several respects (Table 2). Those with retinopathy had statistically significant lower mean platelet counts and mean hematocrit concentrations than those without retinopathy. Admitting axillary temperatures were higher, on average, in the retinopathy-positive group, but the proportion of patients with a history of convulsions and the average depth of coma, as measured by the Blantyre Coma Score, were higher in the retinopathy-negative group.
Table 2.
Characteristics of Study 2: Retrospective Case Control Study
| Characteristic | Retinopathy Positive (n = 116) | Retinopathy Negative (n = 145) | P Value |
|---|---|---|---|
| Gender, % male | 54.3 | 57.3 | .71 |
| Age in months | 39.8 ± 19.8 | 41.9 ± 31.5 | .54 |
| Temperature on admission, °C | 38.7 ± 1.2 | 38.4 ± 1.3 | .04 |
| Blantyre Coma Score on admission | 1.5 ± 0.6 | 1.3 ± 0.7 | .02 |
| History of convulsions, % positive | 76.7 | 89.6 | <.01 |
| History of hypoglycemia,a % positive | 13.9 | 8.9 | .24 |
| Laboratory values on admission | |||
| Parasitemia, per μLb | 31 345 | 36 853 | .94 |
| Hematocrit | 18.1 ± 5.9 | 27.7 ± 6.3 | <.001 |
| Lactate, mmol/L | 7.4 ± 4.7 | 6.8 ± 4.4 | .39 |
| pHRP-2, ng/μL | 6362 ± 3862 | 1539 ± 2032 | <.001 |
| Platelets, per μL | 70 841 ± 52 117 | 186 419 ± 144 476 | <.001 |
| WBCs, per μL | 11 516 ± 6505 | 11 618 ± 6431 | .90 |
| HIV positive, % | 13.7 | 12.9 | .99 |
| Fever clearance time in hours | 46.0 ± 51.0 | 34.0 ± 42.3 | .06 |
| Coma resolution time in hours | 43.4 ± 31.1 | 36.8 ± 32.2 | .14 |
| Parasite clearance time in hours | 41.1 ± 17.5 | 38.9 ± 15.0 | .31 |
| Mortality rate, % | 12.8 | 11.1 | .70 |
Abbreviations: HIV, human immunodeficiency virus; pHRP2, parasite histidine-rich protein 2; WBCs, white blood cells.
a Whole blood glucose concentration ≤2.2 mmoL/L.
b Geometric mean.
The mean ± SD plasma pHRP2 concentration in children with retinopathy was 6362 ± 3862 ng/mL, compared with 1539 ± 2032 ng/mL in children without retinopathy (Table 2 and Figure 1C) (P < .001). The AUROC curve for distinguishing between the 2 conditions was 0.90 (Figure 1D). By comparison, the AUROC curve for platelet concentration and peripheral parasitemia were only 0.71 and 0.53 respectively (Figures 1E and 1F). A cutoff of 1700 ng/mL plasma was chosen using this ROC. This cutoff results in a sensitivity of 87% (95% CI, 80%–92%), a specificity of 73% (95% CI, 65%–80%), a positive predictive value of 72% (95% CI, 64%–79%), and a negative predictive value of 87% (95% CI, 81%–92%).
Prospective Cohort Study
Of 122 patients with clinically defined CM who were enrolled in 2009, 101 had both pHRP2 determination and eye examinations performed at admission. Seventy-one were retinopathy-positive, and 30 were retinopathy-negative. The mean admission values of hematocrit concentration and platelet count were lower in the retinopathy-positive group than in the retinopathy-negative group, and coma resolution times were shorter in the retinopathy-negative group than in the retinopathy-positive group (Table 3). The plasma lactate concentrations and admission parasitemias were not significantly different between the 2 groups. The mean pHRP2 concentration in the retinopathy-positive group was significantly higher than that in the retinopathy-negative group (6668 ± 5041 vs 810 ± 916 ng/mL, P < .001) (Table 3; Figure 1G), and the AUROC curve was 0.96 (Figure 1H). When the cutoff established in the retrospective case–control study (1700 ng/mL plasma) was used, the assay showed a sensitivity of 90% (95% CI, 81%–95%), a specificity of 87% (95% CI, 70%–95%), a positive predictive value of 94% (95% CI, 86%–98%), and a negative predictive value of 79% (95% CI, 62%–89%).
Table 3.
Characteristics of Study 3: Prospective Validation Group
| Characteristic | Retinopathy Positive (n = 71) | Retinopathy Negative (n = 30) | P Value |
|---|---|---|---|
| Gender, % male | 47.8 | 50 | 1.00 |
| Age in months | 45.3 ± 25.3 | 51.7 ± 25.3 | .25 |
| Temperature on admission, °C | 38.8 ± 1.3 | 39.0 ± 1.1 | .40 |
| Blantyre Coma Score on admission | 1.3 ± 0.6 | 1.3 ± 0.6 | .78 |
| History of convulsions, % positive | 85.7 | 93.5 | .33 |
| History of hypoglycemia,a % positive | 36.6 | 33.3 | .66 |
| Laboratory values on admission | |||
| Parasitemia, per μLb | 60 621 | 28 280 | .20 |
| Hematocrit | 19.5 ± 5.3 | 29.3 ± 6.5 | <.001 |
| Lactate, mmol/L | 7.7 ± 4.9 | 6.0 ± 3.7 | .09 |
| pHRP-2, ng/μL | 6668 ± 5041 | 810 ± 916 | <.001 |
| Platelets, per μL | 71 809 ± 61 558 | 171 923 ± 107 125 | <.001 |
| WBCs, per μL | 13 415 ± 10 519 | 13 712 ± 9 481 | .90 |
| HIV positive, % | 7.5 | 16.7 | .24 |
| Fever clearance time in hours | 44.2 ± 49.7 | 44.8 ± 37.1 | .96 |
| Coma resolution time in hours | 49.5 ± 37.7 | 32.1 ± 22.7 | .04 |
| Parasite clearance time in hours | 42.8 ± 14.2 | 37.9 ± 14.3 | .13 |
| Mortality rate, % | 14.3 | 19.4 | .56 |
Abbreviations: HIV, human immunodeficiency virus; pHRP2, parasite histidine-rich protein 2; WBCs, white blood cells.
a Whole blood glucose concentration ≤2.2 mmoL/L.
b Geometric mean.
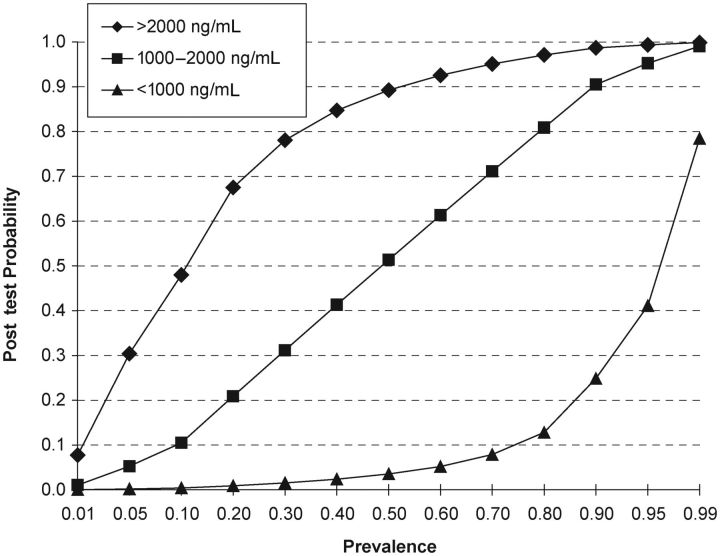
As shown in Table 4, this cutoff results in a likelihood ratio (LR) negative of 0.11 for cases with pHRP2 concentration ≤1700 ng/mL and an LR positive of 6.76 for cases with pHRP2 concentration >1700 ng/mL. Like sensitivity and specificity, the LR is independent of disease prevalence and is an indication of the accuracy of a test in classifying patients. In our population, in which the pretest probability is 70% (71 of 101 in the prospective cohort study), these values translate to a patient with pHRP2 less than the cutoff having a 21% posttest probability of having retinopathy-positive CM, and a patient with pHRP2 greater than the cutoff having a 94% posttest probability of having retinopathy-positive CM. To further improve the diagnostic discrimination, we generated interval LRs for 3 ranges of pHRP2 concentrations: low (<1000 ng/mL), medium (1000–2000 ng/mL), and high (>2000 ng/mL). Children with pHRP2 values of <1000 ng/mL have an LR of only 0.04 of having retinopathy-positive CM, leading to a posttest probability of only 8%, essentially ruling out the possibility. The LR increases to 8.31 in those with pHRP2 values >2000 ng/mL, allowing a clinician to be almost certain that the patient has retinopathy-positive CM (posttest probability of 95% in a population with a pretest prevalence of 70%). With this approach, for patients in the mid-range of 1000–2000 ng/mL, the LR is 1.06. These patients would be classified as indeterminate, and the test would not be useful for revising the pretest probability in this group. However, this group only included 14% of the patients in the prospective cohort study.
Table 4.
Diagnostic Characteristics of Parasite Histidine-Rich Protein Test
| Cutoff (ng/mL) | Retinopathy Positive | Retinopathy Negative | Likelihood Ratio |
|---|---|---|---|
| ≤1700 | 7 | 26 | 0.11 |
| >1700 | 64 | 4 | 6.76 |
| <1000 | 2 | 23 | 0.04 |
| 1000–2000 | 10 | 4 | 1.06 |
| >2000 | 59 | 3 | 8.31 |
Parasite Production of pHRP2
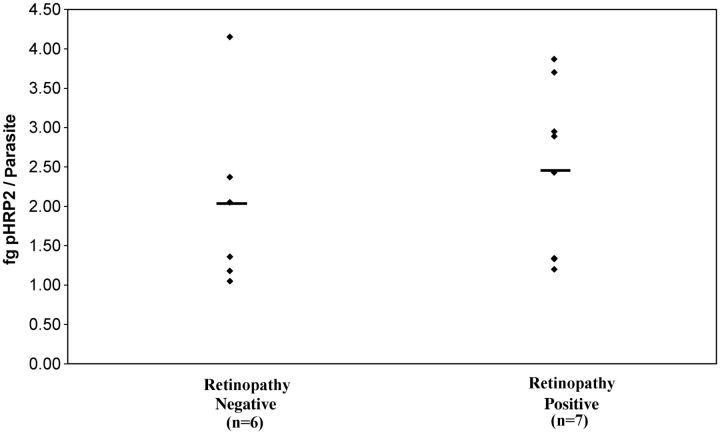
There was up to a 5-fold difference in pHRP2 production by isolates from different individuals, but the mean concentrations were not statistically significantly different between the 2 patient groups (mean, 2.03 fg/parasite for retinopathy-negative patients [n = 6] and 2.46 fg/parasite for retinopathy-positive patients [n = 7]) (Figure 2).
Figure 2.
Parasite histidine-rich protein 2 (pHRP2) concentrations for selected individual parasites in study 2. Although the individual production level varies widely among parasites, there is not a significant difference between parasites from patients with retinopathy-positive cerebral malaria and those with retinopathy-negative cerebral malaria.
DISCUSSION
Many questions remain regarding the diagnosis and pathogenesis of CM. Histological evidence of parasitized red cells sequestered in brain microvessels is the gold standard for diagnosis, but because this evidence can only be collected from the minority of patients who die, causality between sequestration and CM has not been definitively established. In an ongoing autopsy-based clinicopathological study of CM, all children who met the clinical case definition of CM either showed sequestration in the cerebral microvessels and had no other cause of death identified or had no sequestration and an alternative, nonmalarial cause of death [3], suggesting that children do not die of CM without the presence of parasite sequestration. The retina is an embryological outgrowth of the brain, and retinal blood vessels share a common ontogeny with vasculature in the brain. The retina can be examined for malaria retinopathy in patients who meet the standard clinical case definition of CM. Histological and angiographic studies suggest that there are direct correlates between the observable features of malaria retinopathy and the cerebral sequestration of parasitized red cells. Retinal vessels filled with parasitized erythrocytes appear orange or less red, presumably because the mature parasite has transformed some of the intraerythrocytic hemoglobin into hemozoin [18, 19]. Retinal whitening coincides with areas of nonperfusion demonstrated by retinal angiography [19]. The intensity of retinal hemorrhages observed antemortem correlates with the number of ring hemorrhages seen at autopsy in the white matter of the cerebral hemispheres and in the gray and white matter of the cerebellum [20]. If one assumes that cerebral sequestration is an essential feature of the pathogenesis of CM, then patients with retinopathy-positive CM can represent “cases,” and parasitemic patients with retinopathy-negative CM coma are likely to have a nonmalarial cause of coma and can be studied as controls.
Establishing retinopathy status requires training, practice, mydriatics, and 2 ophthalmoscopes (direct and indirect). These are unlikely to become the standard of care in many settings where diagnostic accuracy is important. In this study we have shown that elevated concentrations of plasma pHRP2 are closely associated with retinopathy, suggesting that this measure can be used as a surrogate for ophthalmoscopy in the diagnosis of retinopathy-positive CM.
The utility of a pHRP2-based assay would be to accurately identify malaria as the etiology of coma in parasitemic patients. The assay performs very well in distinguishing between malarial coma in patients with retinopathy-positive CM and coma that is likely due to a cause other than malaria in a patient who is infected with P. falciparum. When a cutoff plasma pHRP2 concentration of 1700 ng/mL was employed and the test was evaluated over the course of an entire malaria transmission season in Blantyre, the sensitivity was 90%, the specificity was 87%, the positive predictive value was 94%, and the negative predictive value was 79%. The AUROC curve for the test, 0.96, is superior to that of many diagnostic tests currently in widespread clinical use, including the use of troponin T to measure for acute myocardial infarction [21].
Choosing a pHRP2 cutoff is influenced by the consequences of false negatives and false positives, the underlying disease prevalence, and the planned use of the test. Clinicians might prefer to minimize false negatives (ie, increase sensitivity) because these cases would be receiving unnecessary additional diagnostic tests and, in some situations, presumptive treatment for nonmalarial coma etiologies. Individuals conducting interventional clinical trials for CM might want to minimize false positives (ie, increase specificity) because these subjects would be unlikely to respond to the intervention and would diminish the power of a study. Malaria control program managers would be interested in minimizing the number of false negatives because, in an era of dwindling disease, false negatives would generate underestimates of the incidence of CM.
The prevalence of the disease in the test population has a major impact on the performance of any diagnostic test. In the prospective cohort study, a population of comatose parasitemic children, the prevalence of retinopathy-positive CM was 70%, and the test functioned well to discriminate retinopathy-positive CM from retinopathy-negative CM. However, in a different population in which the prevalence of retinopathy-positive CM was only 10%, a patient with a pHRP2 concentration >2000 ng/mL would have only a 48% posttest probability of having retinopathy-positive CM. Figure 3 shows the posttest probabilities for each of the 3 ranges of pHRP2 over the full spectrum of disease prevalence.
Figure 3.
Posttest probability for the 3 possible assay results (low: <1000 ng/mL; medium: 1000–2000 ng/mL; and high: >2000 ng/mL) for a wide range of disease prevalence rates. Calculations are based on data from study 3.
These assays were performed using plasma in a 96-well enzyme-linked immunosorbent assay plate, a format that is not amenable to rapid diagnostic testing of whole blood in resource-limited settings. The development of a more user-friendly technique (eg, a lateral flow quantitative dipstick assay) would allow for this test to be performed rapidly at the bedside in conjunction with other important point-of-care assessments (eg, hemoglobin, glucose, and lactate concentrations).
There have recently been several reports regarding variable levels of pHRP2 expression among different P. falciparum isolates, including reports of the complete lack of the pHRP2 gene and protein in some parasite lines [22]. This is not a common phenotype in our patient population. Of the 426 samples evaluated for this study, only 5 were below the detection level of the assay. Of these 5, 3 also had extremely low peripheral parasitemias, suggesting that the low pHRP2 levels merely reflected the paucity of parasites, leaving 2 samples that could possibly have the pHRP2− genotype. When we evaluated the levels of pHRP2 produced by different clinical isolates, we found a variance of up to 5-fold, but we did not find that parasites from retinopathy-positive cases produced significantly more pHRP2 than did parasites from retinopathy-negative cases (Figure 2). Genetic differences in pHRP2 production levels are therefore unlikely to account for the highly significant differences in plasma pHRP2 levels seen between these groups. Given the long half-life of this protein in the circulation [23–25], our findings suggest that pHRP2 concentrations may reflect both duration and intensity of infection, including the sequestered mass of mature parasites, which are rarely identified in the usual diagnostic smears of peripheral blood.
The biologic significance of increased pHRP2 levels remains unclear. pHRP2 is unique to P. falciparum, the most pathogenic of the Plasmodium species, suggesting that the protein may have a role in disease pathogenesis. Owing to its abundance, it was one of the first Plasmodium genes to be cloned and has been extensively studied [26]. Proposed functions include binding zinc, binding heme, forming hemozoin, immunosuppression, and perturbations of the coagulation cascade [8, 27–30]. Although all of these are plausible pathogenetic mechanisms, none have been verified. The recent description of naturally occurring P. falciparum parasites lacking the pHRP2 gene suggests that gene modification techniques may help to elucidate potential roles of the protein both in parasite biology and in pathogenesis of human malaria infections [22].
Patients with retinopathy-positive CM and retinopathy-negative CM differed significantly with respect to several clinical parameters (Table 2). These features are useful discriminators on the population level, but they are not helpful for identifying and treating individual patients. In contrast, with the appropriate cutoff, the pHRP2 concentrations generate an LR that can help to establish the etiology of coma in a parasitemic individual.
The diagnosis of CM remains a challenge in endemic areas where the disease is most prevalent. Accurate recognition of this clinical syndrome is needed for clinical care, for research purposes, and to evaluate the impact of malaria control efforts. Our findings suggest that the use of a quantitative, pHRP2-based bedside diagnostic assay would greatly aid in identifying patients with retinopathy-positive CM.
Notes
Acknowledgments. The authors wish to thank David Sullivan for providing recombinant pHRP2. Laboratory space and support provided by the Malawi-Liverpool-Wellcome Trust is gratefully acknowledged. Authors also wish to thank Jimmy Vareta for excellent technical support, Clemency Borgstein for enzyme-linked immunosorbent assay assistance, and the patients’ families for allowing their children to participate in these studies.
Financial support. This work was funded by grants from the National Insitutes of Health (R01 AI34969 to T. E. T.; K23AI079402 to K. B. S.). M. E. M. was supported by a programme grant from The Wellcome Trust.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hay SI, Okiro EA, Gething PW, et al. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 2010;7:e1000290. doi: 10.1371/journal.pmed.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe AK, Rowe SY, Snow RW, et al. The burden of malaria mortality among African children in the year 2000. Int J Epidemiol. 2006;35:691–704. doi: 10.1093/ije/dyl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor T, Fu W, Carr R, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–5. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 4.Beare NA, Southern C, Chalira C, Taylor TE, Molyneux ME, Harding SP. Prognostic significance and course of retinopathy in children with severe malaria. Arch Ophthalmol. 2004;122:1141–7. doi: 10.1001/archopht.122.8.1141. [DOI] [PubMed] [Google Scholar]
- 5.Lewallen S, Taylor TE, Molyneux ME, Wills BA, Courtright P. Ocular fundus findings in Malawian children with cerebral malaria. Ophthalmology. 1993;100:857–61. doi: 10.1016/s0161-6420(93)31563-0. [DOI] [PubMed] [Google Scholar]
- 6.Lewallen S, Bronzan RN, Beare NA, Harding SP, Molyneux ME, Taylor TE. Using malarial retinopathy to improve the classification of children with cerebral malaria. Trans R Soc Trop Med Hyg. 2008;102:1089–94. doi: 10.1016/j.trstmh.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. 2006;75:790–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Ndonwi M, Burlingame OO, Miller AS, Tollefsen DM, Broze GJ, Goldberg DE. Inhibition of antithrombin by Plasmodium falciparum histidine-rich protein II. Blood. 2011;117:6347–54. doi: 10.1182/blood-2010-12-326876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desakorn V, Dondorp AM, Silamut K, et al. Stage-dependent production and release of histidine-rich protein 2 by Plasmodium falciparum. Trans R Soc Trop Med Hyg. 2005;99:517–24. doi: 10.1016/j.trstmh.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Desakorn V, Silamut K, Angus B, et al. Semi-quantitative measurement of Plasmodium falciparum antigen PfHRP2 in blood and plasma. Trans R Soc Trop Med Hyg. 1997;91:479–83. doi: 10.1016/s0035-9203(97)90292-3. [DOI] [PubMed] [Google Scholar]
- 11.Dondorp AM, Desakorn V, Pongtavornpinyo W, et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2005;2:e204. doi: 10.1371/journal.pmed.0020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gérardin P, Rogier C, Ka AS, Jouvencel P, Brousse V, Imbert P. Prognostic value of thrombocytopenia in African children with falciparum malaria. Am J Trop Med Hyg. 2002;66:686–91. doi: 10.4269/ajtmh.2002.66.686. [DOI] [PubMed] [Google Scholar]
- 13.Gravenor MB, van Hensbroek MB, Kwiatkowski D. Estimating sequestered parasite population dynamics in cerebral malaria. Proc Natl Acad Sci USA. 1998;95:7620–4. doi: 10.1073/pnas.95.13.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishna S, Waller DW, ter Kuile F, et al. Lactic acidosis and hypoglycaemia in children with severe malaria: pathophysiological and prognostic significance. Trans R Soc Trop Med Hyg. 1994;88:67–73. doi: 10.1016/0035-9203(94)90504-5. [DOI] [PubMed] [Google Scholar]
- 15.Ladhani S, Lowe B, Cole AO, Kowuondo K, Newton CR. Changes in white blood cells and platelets in children with falciparum malaria: relationship to disease outcome. Br J Haematol. 2002;119:839–47. doi: 10.1046/j.1365-2141.2002.03904.x. [DOI] [PubMed] [Google Scholar]
- 16.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–59. [PubMed] [Google Scholar]
- 17.Severe falciparum malaria. World Health Organization, communicable diseases cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- 18.Beare NA, Harding SP, Taylor TE, Lewallen S, Molyneux ME. Perfusion abnormalities in children with cerebral malaria and malarial retinopathy. J Infect Dis. 2009;199:263–71. doi: 10.1086/595735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White VA, Lewallen S, Beare NA, Molyneux ME, Taylor TE. Retinal pathology of pediatric cerebral malaria in Malawi. PLoS One. 2009;4:e4317. doi: 10.1371/journal.pone.0004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White VA, Lewallen S, Beare N, Kayira K, Carr RA, Taylor TE. Correlation of retinal haemorrhages with brain haemorrhages in children dying of cerebral malaria in Malawi. Trans R Soc Trop Med Hyg. 2001;95:618–21. doi: 10.1016/s0035-9203(01)90097-5. [DOI] [PubMed] [Google Scholar]
- 21.Johnson PA, Goldman L, Sacks DB, et al. Cardiac troponin T as a marker for myocardial ischemia in patients seen at the emergency department for acute chest pain. Am Heart J. 1999;137:1137–44. doi: 10.1016/s0002-8703(99)70374-1. [DOI] [PubMed] [Google Scholar]
- 22.Gamboa D, Ho MF, Bendezu J, et al. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoS One. 2010;5:e8091. doi: 10.1371/journal.pone.0008091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biswas S, Tomar D, Rao DN. Investigation of the kinetics of histidine-rich protein 2 and of the antibody responses to this antigen, in a group of malaria patients from India. Ann Trop Med Parasitol. 2005;99:553–62. doi: 10.1179/136485905X51463. [DOI] [PubMed] [Google Scholar]
- 24.Iqbal J, Siddique A, Jameel M, Hira PR. Persistent histidine-rich protein 2, parasite lactate dehydrogenase, and panmalarial antigen reactivity after clearance of Plasmodium falciparum monoinfection. J Clin Microbiol. 2004;42:4237–41. doi: 10.1128/JCM.42.9.4237-4241.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyabayinze DJ, Tibenderana JK, Odong GW, Rwakimari JB, Counihan H. Operational accuracy and comparative persistent antigenicity of HRP2 rapid diagnostic tests for Plasmodium falciparum malaria in a hyperendemic region of Uganda. Malar J. 2008;7:221. doi: 10.1186/1475-2875-7-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wellems TE, Howard RJ. Homologous genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. Proc Natl Acad Sci USA. 1986;83:6065–9. doi: 10.1073/pnas.83.16.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panton LJ, McPhie P, Maloy WL, Wellems TE, Taylor DW, Howard RJ. Purification and partial characterization of an unusual protein of Plasmodium falciparum: histidine-rich protein II. Mol Biochem Parasitol. 1989;35:149–60. doi: 10.1016/0166-6851(89)90117-5. [DOI] [PubMed] [Google Scholar]
- 28.Schneider EL, Marletta MA. Heme binding to the histidine-rich protein II from Plasmodium falciparum. Biochemistry. 2005;44:979–86. doi: 10.1021/bi048570p. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan DJ, Jr, Gluzman IY, Goldberg DE. Plasmodium hemozoin formation mediated by histidine-rich proteins. Science. 1996;271:219–22. doi: 10.1126/science.271.5246.219. [DOI] [PubMed] [Google Scholar]
- 30.Das P, Grewal JS, Chauhan VS. Interaction of Plasmodium falciparum histidine-rich protein II with human lymphocytes leads to suppression of proliferation, IFN-gamma release, and CD69 expression. Parasitol Res. 2006;100:39–50. doi: 10.1007/s00436-006-0228-6. [DOI] [PubMed] [Google Scholar]