Abstract
Background. The safety and immunogenicity of high-dose pandemic H1N1 (pH1N1) vaccination in perinatally human immunodeficiency virus type 1 (HIV-1)–infected children, adolescents, and young adults are unknown.
Methods. Two 30-μg doses of 2009 Novartis pH1N1 monovalent vaccine (Fluvirin) were administered 21–28 days apart to perinatally HIV-1–infected children, adolescents, and young adults. Antibodies were measured by hemagglutination inhibition (HAI) assay at baseline, 21–28 days after first vaccination, 7–13 days after the second vaccination, and 7 months after the first vaccination.
Results. Among the 155 participants, 54 were aged 4–8 years, 51 were aged 9–17 years, and 50 were aged 18–24 years. After 2 doses of Fluvirin, seroresponse (≥4-fold rise in HAI titers) was demonstrated in 79.6%, 84.8%, and 83% of participants in the aforementioned age groups, respectively, and seroprotection (HAI titers ≥40) was shown in 79.6%, 82.6%, and 85.1%, respectively. Of those lacking seroresponse (n = 43) or seroprotection (n = 37) after the first vaccination, 46.5% and 40.5% achieved seroresponse or seroprotection, respectively, after the second vaccination. Among participants who lacked seroprotection at entry, a “complete response” (both seroresponse and seroprotection) after first vaccination was associated with higher baseline log10 HAI titer and non-Hispanic ethnicity. No serious vaccine-related events occurred.
Conclusion. Two doses of double-strength pH1N1 vaccine are safe and immunogenic and may provide improved protection against influenza in perinatally HIV-1–infected children and youth.
Clinical Trials Registration. NCT00992836.
A novel swine-origin influenza A subtype H1N1 virus, designated 2009 H1N1 influenza A, was identified as the cause of pandemic febrile respiratory illnesses [1–4]. Although individuals of all ages were affected, the greatest increase in severe morbidity and mortality occurred in young children, pregnant women, and the morbidly obese [5]. In human immunodeficiency virus type 1 (HIV-1)–infected patients, influenza infection is more severe than that typical of age-matched uninfected people [6, 7]. HIV-1–nfected patients also may shed virus for greater periods of time, prolonging the need for isolation in the clinic or hospital [8]. Because of the increased severity of this pandemic in children and young adults, knowledge of the safety and immunogenicity of the pandemic influenza A (pH1N1) 2009 monovalent vaccine in this population is critically important.
Antibody responses to seasonal trivalent influenza vaccine (TIV) are blunted in HIV-1–infected children and adults who are not receiving antiretroviral therapy (ART) [9–11] but improved in patients who do not have progressive HIV-1 disease and/or are receiving combination ART (cART) [12–16]. Still, the immunological response is poorer compared to that of HIV-uninfected cohorts [15, 16]. Studies evaluating the effect of antigen dosage on the immune responses to TIV performed over the past 35 years demonstrate dose-related increases in serum and mucosal antibody responses [17–25]. Higher vaccine dosages are also associated with the development of higher levels of serum antibodies that recognize antigenically distinct drift variants [23] and can overcome suboptimal responses in immunologically impaired vaccinees, such as elderly patients [22, 24, 25]. However, higher dosages of hemagglutinin are also associated with more frequent adverse events.
We hypothesized that 2 doses of influenza vaccine would be necessary to achieve protection in perinatally HIV-1–infected children and youth who had no prior exposure to pH1N1. In addition, because of blunted response to TIV in HIV-1–infected persons, we investigated a 30-μg dose of antigen, double the 15-μg dose proposed for healthy children.
METHODS
Perinatally HIV-1–infected children, adolescents, and young adults, aged 4–24 years, were recruited from International Maternal Pediatric and Adolescent Clinical Trials (IMPAACT) group units in the United States and Puerto Rico. Participants were either receiving stable ART for at least 90 days prior to entry or no ART within 90 days prior to entry. Participants were excluded for platelet count ≤50 000/μL or absolute neutrophil count ≤500/μL within 30 days prior to study entry; known allergy to vaccine components; history of severe reactions after influenza vaccination; known pH1N1 infection or vaccination; receipt of a live vaccine within the prior 4 weeks or inactivated vaccine in the prior 2 weeks; receipt of immunoglobulin or other blood products within the prior 3 months; immunosuppressive condition other than HIV infection; personal or family history of Guillain-Barré syndrome (GBS); or onset of neurological disorder characterized by loss of strength or reflexes within the prior 6 months. Prior to the second vaccination, the participants were required to meet the same inclusion and exclusion criteria. Female participants of childbearing potential were required to have a negative pregnancy test within 72 hours before each vaccination.
Vaccine Administration
Participants received two 30-μg doses of 2009 Novartis influenza A (H1N1) monovalent vaccine (Fluvirin) separated by 21–28 days. Each 30-μg vaccine dose was administered in the deltoid or anterolateral thigh muscle as two 0.5-mL (15-μg) injections. Participants had assessment of vaccine safety using the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, version 1.0 (http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf). Participants were observed for at least 30 minutes after each vaccination and were contacted by telephone or other methods for reactogenicity assessments and safety monitoring on day 2 (±1 day) and day 10 (±3 days) after first vaccination and then again on day 2 (±1 day) after second vaccination; they were also seen on day 10 (±3 days) after second vaccination. Report of any symptom compatible with GBS (eg, weakness of legs, tingling of hands and/or feet, or difficulty walking) required a clinic visit within 24 hours of onset.
Immunogenicity Assessments
Immunogenicity was assessed by specific hemagglutination inhibition (HAI) titers in serum collected at baseline (the study entry visit), 21–28 days after first vaccination, 7–13 days after second vaccination (if received), and 7 months after first vaccination. Timing of the assessment following second vaccination was selected to coincide with the expected peak of an anamnestic response. Henceforth, these time points will be called baseline, after first vaccination, after second vaccination, and 7 months after first vaccination. The assay was adapted from previously described methods developed and validated for seasonal influenza viruses [12]. HAI titers ≥40 was defined as evidence of seroprotection [26]. Seroresponse was defined as having a ≥4-fold rise in HAI titers following vaccination as compared with baseline HAI. A complete responder was defined as a participant who achieved both seroprotection and seroresponse, regardless of baseline serology.
Statistical Analysis
The baseline characteristics and safety data for all participants enrolled in the study were summarized using descriptive measures. The HAI titers following the first vaccination were summarized for the eligible study participants who received at least 1 dose of vaccine; the titers following the second vaccination were summarized for the eligible study participants who received both doses of vaccine. Three study participants with pH1N1 infection during the study (after first vaccine) were excluded from all HAI analyses but not safety analysis. The serology analyses were stratified by 3 age groups: 4–8 years, 9–17 years, and 18–24 years.
HAI titers <10 were considered undetectable and were assigned a value of 5 for this analysis. The titers were summarized using geometric means and 95% confidence intervals (CIs) as well as medians. A Sign test was used to determine, within each age group, whether the number of participants showing increased titers from baseline to follow-up was greater than the number showing decreases. Rates of seroprotection (HAI titers ≥40) were computed, as well as fold changes from the titer values at baseline. Differences in the seroprotection and seroresponse rates after vaccination among the age groups as well as the differences in rates between those with detectable antibody at baseline (HAI titers ≥10) and those without were assessed using Fisher exact test. Among the study participants without seroprotection at baseline, the exact McNemar test of agreement was used to compare the rate of seroprotection after the second vaccination to the rate after the first vaccination. The persistence of seroprotective levels 7 months after vaccination was assessed, and the rates of participants with seroprotection at present up to 7 months after vaccination were computed.
Univariate logistic regression analysis was used to assess the association of demographic characteristics (age, sex, ethnicity [Hispanic vs other], race [black vs other]), use of cART at study entry, viral load [<400 copies/mL or ≥400 copies/mL], TIV vaccination prior to study entry, CD4 count and percentage, CD8 count and percentage, CD19 count and percentage, and log10 HAI titer at baseline) with serologic response following first and second vaccination. Combination ART was defined as a regimen containing at least 3 ART drugs from at least 2 drug classes. Data from all age groups were combined. For these analyses, participants with baseline HAI titers ≥40 were omitted to avoid a mixture of primary and secondary response to the pH1N1 antigen. Multivariable logistic regression modeling with backward selection was used to evaluate the association of the above factors on immunologic response, including all factors with P < .1 in univariate models as candidate predictors; the final model retained only those covariates with P < .05. The analyses were performed using SAS software, version 9.2 (SAS Institute), and the graphs were produced using the R software package.
RESULTS
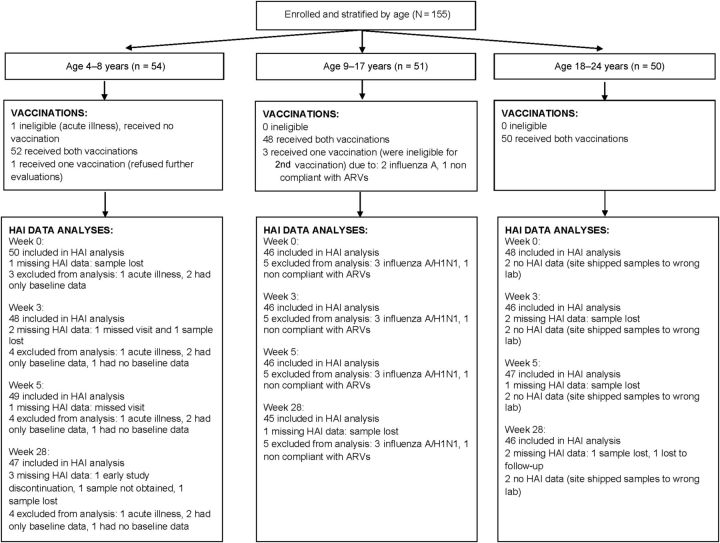
We enrolled 155 children, adolescents, and young adults from 37 sites, between 14 October 2009 and 12 November 2009; 54 participants were aged 4–8 years; 51 were aged 9–17 years; and 50 were aged 18–24 years. Among the 155 participants, 150 (97%) received both vaccinations, 4 (2%) received 1 vaccination, and 1 received no vaccination (Figure 1). Reasons for not receiving the second vaccination were documented pH1N1 infection after first vaccination (n = 2), refusing further follow-up (n = 1), and ineligibility due to nonadherence to ARV medications (n = 1). The participant who received no vaccinations became ineligible due to an acute illness after enrollment but prior to vaccination. Demographic characteristics, CD4 percentage, and viral load are displayed in Table 1.
Figure 1.
Patient flowchart after enrollment. Abbreviations: ARVs, antiretrovirals; HAI, hemagglutination inhibition titers.
Table 1.
Study Participant Baseline Characteristics
| Age Group |
|||
|---|---|---|---|
| 4–8 y, n = 54 (35%) | 9–17 y, n = 51 (33%) | 18–24 y, n = 50 (32%) | |
| Sex, male | 54% | 57% | 42% |
| Race | |||
| Black | 65% | 55% | 64% |
| White | 26% | 39% | 30% |
| Ethnicity | |||
| Hispanic | 33% | 39% | 24% |
| Age, y (median) | 6 | 14 | 19 |
| CD4 count, cells/μL, median (range) | 1161 (308–1921) | 642 (113–1488) | 589 (57–1031) |
| CD4%, median (range) | 37% (24–50) | 33% (11–47) | 30% (5–48) |
| Viral load, copies/mL, median (range) | 48 (40–19K) | 48 (40–80K) | 75 (40–81K) |
| Baseline ART | |||
| Combination ARTa | 50 | 45 | 42 |
| Otherb | 1 | 3 | 4 |
| None | 3 | 3 | 4 |
Abbreviation: ART, antiretroviral therapy.
a Combination ART is defined as a regimen containing at least 3 antiretroviral drugs from at least 2 drug classes.
b Includes regimens with nucleoside reverse transcriptase inhibitor only, combination protease inhibitor and nonnucleoside reverse transcriptase inhibitor, and other combinations of antiretroviral agents.
Safety
The vaccine was well tolerated; 12 grade 3 events were reported, including only 2 (both fever episodes) reported as possibly related to vaccine administration. Fever of 39.5°C was reported in 2 participants 3 and 7 days following first vaccination, respectively. The unrelated events included low neutrophil count (2), pH1N1 infection, tonsillitis, pharyngitis, sinusitis, dizziness, headache, neck pain, and nasal congestion (1 each). There were 10 grade 2 local and systemic events that were related to vaccine including injection site pain (3); tenderness (2); and itching, nausea, vomiting, headache, and leg pain (1 each). Seven of these events occurred in 3 participants 1–7 days following the first vaccination and 3 events occurred in 2 participants 1 day to 6 months following the second vaccination. Two participants had grade 2 herpes virus reactivation possibly related to vaccination, 1 with unilateral facial nerve palsy associated with an oral herpes simplex reactivation on day 4 after the second dose and 1 with dermatomal herpes zoster eruption 14 days after the first dose. There were no reported cases of GBS.
Influenza-like Illness
Seven study participants developed influenza-like illness during the course of the study. Six occurred 4–16 days following the first vaccination and 1 occurred 23 days after the second vaccination. Real-time reverse transcriptase polymerase chain reaction testing identified 3 as having influenza A: 2 confirmed pH1N1 influenza and 1 probable, based on local epidemiology. All influenza A infections occurred in the 9–17-year age group and were reported 6, 14, and 16 days following the first vaccination. Baseline HAI in these participants was 5, 10, and 160, respectively, and CD4 percentage was 16, 21 and 30, respectively.
Immunogenicity
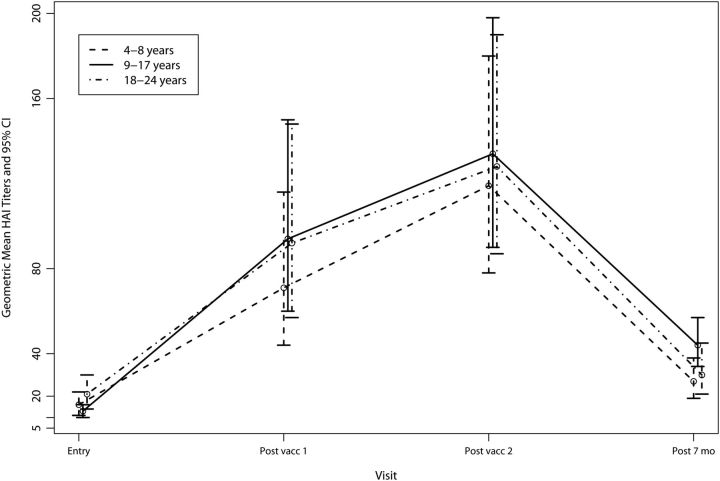
HAI titers were available for 140 participants following the first vaccination and for 142 participants following the second vaccination (Tables 2 and 3 and Figure 2). The median, range, geometric mean titers, and 95% CI HAI titers are presented in Table 2.
Table 2.
Summary of Hemagglutination Inhibition Titers
| Age | Time Point | Median (Range) | No. | GMT (95% CI) | P Valuea |
|---|---|---|---|---|---|
| All children | Baseline | 10 (5–1280) | 144 | 16 (13–19) | … |
| After first vaccination | 80 (5–1280) | 140 | 85 (65–111) | <.0001 | |
| After second vaccination | 160 (5–1280) | 142 | 127 (101–159) | <.0001 | |
| 7 mo after first vaccination | 40 (5–640) | 138 | 33 (27–40) | <.0001 | |
| 4–8 y | Baseline | 10 (5–1280) | 50 | 16 (11–22) | … |
| After first vaccination | 60 (5–1280) | 48 | 71 (44–116) | <.0001 | |
| After second vaccination | 160 (10–1280) | 49 | 119 (78–180) | <.0001 | |
| 7 mo after first vaccination | 20 (5–640) | 47 | 27 (19–38) | .0002 | |
| 9–17 y | Baseline | 10 (5–80) | 46 | 13 (10–16) | … |
| After first vaccination | 160 (5–1280) | 46 | 94 (60–150) | <.0001 | |
| After second vaccination | 160 (5–1280) | 46 | 134 (90–198) | <.0001 | |
| 7 mo after first vaccination | 40 (5–160) | 45 | 44 (34–57) | <.0001 | |
| 18–24 y | Baseline | 20 (5–320) | 48 | 21 (14–30) | … |
| After first vaccination | 120 (5–1280) | 46 | 92 (57–148) | <.0001 | |
| After second vaccination | 160 (10–1280) | 47 | 128 (87–190) | <.0001 | |
| 7 mo after first vaccination | 40 (5–320) | 46 | 30 (21–45) | .006 |
Abbreviations: CI, confidence interval; GMT, geometric mean titer.
a P value from Sign test to test whether the no. of participants showing increased titers from baseline to follow-up was greater than the no. showing decreases.
Table 3.
Hemagglutination Inhibition Assay Findings Among Participants
| Seroresponse (≥4-Fold Rise in HAI) |
Seroprotection (HAI Titers ≥40) |
|||||
|---|---|---|---|---|---|---|
| Age | After First Vaccination | After Second Vaccination | Baseline | After First Vaccination | After Second Vaccination | 7 mo After First Vaccination |
| All participants | ||||||
| 4–8 y | 34/48 (70.8%) | 39/49 (79.6%) | 13/50 (26%) | 31/48 (64.6%) | 39/49 (79.6%) | 19/47 (40.4%) |
| 9–17 y | 35/46 (76.1%) | 39/46 (84.8%) | 13/46 (28.3%) | 35/46 (76.1%) | 38/46 (82.6%) | 36/45 (80.0%) |
| 18–24 y | 27/46 (58.7%) | 39/47 (83%) | 21/48 (43.8%) | 35/46 (76.1%) | 40/47 (85.1%) | 24/46 (52.2%) |
| All | 96/140 (68.6%) | 117/142 (82.4%) | 47/144 (32.6%) | 101/140 (72.1%) | 117/142 (82.4%) | 79/138 (57.2%) |
| Participants with baseline HAI titers <40 | ||||||
| 4–8 y | 22/35 (62.9%) | 27/36 (75%) | 0/37 (0%) | 18/35 (51.4%) | 26/36 (72%) | 8/35 (22.9%) |
| 9–17 y | 23/33 (69.7%) | 27/33 (81.8%) | 0/33 (0%) | 22/33 (66.7%) | 25/33 (75.8%) | 23/32 (71.9%) |
| 18–24 y | 14/25 (56%) | 23/26 (88.5%) | 0/27 (0%) | 14/25 (56%) | 19/26 (73%) | 7/25 (28%) |
| All | 59/93 (63.4%) | 77/95 (81.1%) | 0/97 (0%) | 54/93 (58.1%) | 70/97 (73.7%) | 38/92 (41.3%) |
Abbreviation: HAI, hemagglutination inhibition.
Figure 2.
Geometric mean and 95% confidence interval (CI) hemagglutination inhibition (HAI) titers among vaccine recipients by age group.
Seroresponse
Overall, seroresponse occurred in 68.6% of participants following the first vaccination and increased to 82.4% after the second vaccination. There was a significantly higher rate of seroresponse after the second vaccination as compared to the first (P < .0001). Of those who lacked seroresponse after the first vaccination (n = 43), 46.5% achieved it after the second vaccination. Only 1 (1%) of those who had seroresponse after the first vaccination lost it after the second vaccination. Seroresponse rates were not related to age (P = .20 after first and P = .82 after second vaccination). Seroresponse was greater at all time points among participants with baseline HAI titers ≥10 (Table 4).
Table 4.
Proportion of Participants With Seroresponse, Seroprotection, and Complete Response by Baseline Hemagglutination Inhibition (HAI) Titers
| Baseline HAI Titers <10 | Baseline HAI Titers ≥10 | P Value | |
|---|---|---|---|
| Seroresponse | |||
| After first vaccination | 38.1% (16/42) | 86.7% (85/98) | <.0001 |
| After second vaccination | 63.6% (28/44) | 90.8% (89/98) | .0002 |
| 7 mo after first vaccination | 27.9% (12/43) | 70.5% (67/95) | <.0001 |
| Seroprotection | |||
| After first vaccination | 52.4% (22/42) | 75.5% (74/98) | .01 |
| After second vaccination | 79.5% (35/44) | 83.7% (82/98) | .63 |
| 7 mo after first vaccination | 51.2% (22/43) | 25.3% (24/95) | .004 |
| Complete response | |||
| After first vaccination | 38.1% (16/42) | 76.3% (74/97) | <.0001 |
| After second vaccination | 63.6% (28/44) | 84.5% (82/97) | .008 |
| 7 mo after first vaccination | 27.9% (12/43) | 25.5% (24/94) | .84 |
Abbreviation: HAI, hemagglutination inhibition.
Seroprotection
Seroprotection was demonstrated in 32.6% at baseline, increasing to 72.1% after the first vaccination and to 82.4% after the second. As with seroresponse, seroprotection was not related to age (P = .36 after first vaccination and P = .79 after second vaccination). In participants who were evaluated after both vaccinations and who were without seroprotection at baseline (n = 93), 59.3% and 73.6% achieved it after the first and second vaccinations, respectively. There was a significantly higher rate of seroprotection after the second vaccination as compared to the first (P = .002). Of those who lacked seroprotection after the first vaccination (n = 37), 40.5% achieved it after the second vaccination. Only 2 (3.7%) of those who had seroprotection after the first vaccination lost it after the second vaccination. Seroprotection was greater following first vaccination and 7 months after first vaccination among participants with baseline HAI titers ≥10 (Table 4).
Complete Response
Complete response (both seroresponse and seroprotection) was achieved in 61.7%, 73.9%, and 58.7% of participants in the 4–8-, 9–17-, and 18–24-year age groups after first vaccination, respectively. Among those who were not complete responders after 1 vaccination, 9 of 18, 4 of 12, and 6 of 18 were complete responders after the second vaccination in the 4–8-, 9–17-, and 18–24-year age groups, respectively. Only 1 of 29, 1 of 34, and 0 of 27 who were complete responders after the first vaccination were not complete responders after the second vaccination in the 4–8-, 9–17-, and 18–24-year age groups, respectively (P = .02, .38, and .03, respectively). Overall, 39.6% (19 of 48) of those who were not complete responders after first vaccination became complete responders after the second vaccination, whereas 2.2% (2 of 90) of those who were complete responders after first vaccination were no longer complete responders after second vaccination (P = .0002). Complete response was greater following first and second vaccinations among participants with baseline HAI titers ≥10 (Table 4).
Persistence
Seven months after the first vaccination, 57.2% of the participants demonstrated seroprotection. Seventy-three of the 113 (64.6%) participants with seroprotection after the second vaccination maintained HAI titers ≥40 seven months after first vaccination; the rates were 44.7% (17 of 38), 86.5% (32 of 37), and 63.2% (24 of 38) in the 4–8-, 9–17-, and 18–24-year age groups, respectively.
Correlates of Primary Response
Baseline factors associated with complete vaccine response (seroresponse and seroprotection) after first vaccination in univariate analysis among participants with baseline HAI titers <40 included baseline log10 HAI titers, Hispanic ethnicity, and receiving TIV prior to study vaccine (P < .1, Table 5). Only Hispanic ethnicity (adjusted odds ratio [AOR] = 0.3 [95% CI, .1–.9]; P = .03) and baseline log10 HAI (AOR = 68.7 for a 1 log10 increase [95% CI, 6.5–731.6]; P = .0005) were significant predictors of complete vaccine response in multivariable logistic regression analysis.
Table 5.
Baseline Factors and Relationship to Pandemic H1N1 Vaccine Response (Seroresponse and Seroprotection) After First and Second Vaccinations
| After First Vaccinationa |
After Second Vaccinationa |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | OR (95% CI) | P Valueb | AOR (95% CI) | P Valuec | OR (95% CI) | P Valueb | AOR (95% CI) | P Valuec |
| Male sex | 1.0 (.4–2.3) | .98 | 0.6 (.2–1.4) | .23 | ||||
| Hispanic ethnicity | 0.4 (.2–1.0) | .06 | 0.3 (.1–.9) | .03 | 1.1 (.4–3.0) | .80 | ||
| Black race | 0.8 (.4–2.0) | .67 | 0.8 (.3–2.2) | .71 | ||||
| Combination ART | 1.7 (.5–6.0) | .41 | 1.7 (.5–6.4) | .43 | ||||
| HIV RNA <400 copies/mL | 1.6 (.6–4.7) | .36 | 2.1 (.7–6.4) | .20 | ||||
| Preentry 2009 seasonal influenza vaccination | 0.3 (.1–.9) | .03 | 0.6 (.2–1.5) | .26 | ||||
| Age (y) | 1.0 (1.0–1.1) | .48 | 1.0 (.9–1.1) | .78 | ||||
| CD4 count | 1.0 (1.0–1.0) | .76 | 1.0 (1.0–1.0) | .11 | ||||
| CD4% | 1.0 (1.0–1.1) | .70 | 1.0 (1.0–1.1) | .15 | ||||
| CD4 count ≥200 cells/μL | 1.4 (.2–10.3) | .75 | 3.2 (.4–24.1) | .26 | ||||
| CD4 count ≥500 cells/μL | 1.5 (.6–4.1) | .43 | 2.8 (1.0–7.8) | .05 | ||||
| CD4% ≥15 | 1.3 (.2–10.0) | .77 | 1.9 (.3–12.4) | .48 | ||||
| CD8 count | 1.0 (1.0–1.0) | .52 | 1.0 (1.0–1.0) | .25 | ||||
| CD8% | 1.0 (1.0–1.0) | .83 | 1.0 (1.0–1.0) | .87 | ||||
| CD19 count | 1.0 (1.0–1.0) | .47 | 1.0 (1.0–1.0) | .98 | ||||
| CD19% | 1.0 (.9–1.1) | .61 | 1.0 (.9–1.0) | .30 | ||||
| Log10 baseline HAI titer | 34.9 (4.0–307.9) | .001 | 68.7 (6.5–731.6) | .0005 | 2.3 (.9–5.9) | .07 | 16.3 (1.3–201.2) | .03 |
Abbreviations: AOR, adjusted odds ratio; ART, antiretroviral therapy; CI, confidence interval; HAI, hemagglutination inhibition; HIV, human immunodeficiency virus; OR, odds ratio.
a Limited to participants with baseline HAI titers <40.
b P value from univariate regression analysis.
c P value from multivariable regression analysis.
Correlates of Secondary Response
Following second vaccination, log10 baseline HAI titer (odds ratio [OR] = 8.0 for a 1 log10 increase [95% CI, .8–76.4]; P = .07; Table 5) and CD4 count ≥500 cells/μL (OR = 2.8 [95% CI, 1.0–7.8]; P = .05) were associated with complete vaccine response (both seroprotection and seroresponse). However, in multivariable analysis, only log10 baseline HAI titers remained predictive (AOR = 16.3 for a 1 log10 increase [95% CI, 1.3–201.2]; P = .03).
DISCUSSION
In this study we demonstrated the safety and immunogenicity of 2 vaccinations with high-dose pH1N1 antigen in perinatally HIV-1–infected children and young adults. Although a substantial portion of the participants had seroprotective levels of antibody (HAI titers ≥40) at baseline, the rate increased to 82.4% after 2 vaccinations. Additionally, in those without seroprotection at baseline, the seroprotection was 59.3% and 73.6% after the first and second vaccinations, respectively. Of those participants with HAI titers <40 after the first vaccination, 40.5% achieved seroprotection (HAI titers ≥40) after the second vaccination. We also demonstrated an improved seroresponse after the second vaccination: from 68.6% of participants after the first vaccination to 82.4% after the second and in 46.5% of those who did not demonstrate seroresponse after first vaccination. In addition, the second vaccination resulted in significantly more complete responders (both seroresponse and seroprotection) than after 1 vaccination (P = .0002).
The levels of seroprotection after pH1N1 vaccination demonstrated in our population remain substantially lower than the seroprotection rates reported for HIV-uninfected children (85%–99%) in studies using a variety of inactivated vaccines, antigen doses, and vaccination schedules [27–35]. Similar to our findings in perinatally HIV-1–infected children and youth, 2 dose series yielded higher seroprotection rates in HIV-uninfected children [28, 31, 33, 34]. The current study regimen of 2 doses of 30 μg antigen per dose, when evaluated in healthy children, resulted in seroprotection rates ranging from 87.7% to 100%, depending on the population and vaccine [31, 33, 34].
The lower seroprotection rates found in our population compared with those in healthy children and youth receiving the pH1N1 vaccine are not surprising. Poor response to TIV in HIV-1–infected individuals has been previously demonstrated [9–16], though often associated with advanced disease states [12–15]. Of interest, the response of HIV-infected children (similar to our population) to a single dose of live, attenuated TIV was better than in the current study, demonstrating that 96%–100% of the participants achieved seroprotection for influenza A and 81%–88% for influenza B [36].
Seroprotection in HIV-infected adults following the recommended single dose of 15 μg antigen, unadjuvanted vaccine was achieved in only 54%–69% of participants [37–39]. Seroprotection rates reached 72.5%–88% after 1 dose [40–43] and 91%–97% following 2 doses of adjuvanted vaccine [38–40]. Experience with pH1N1 adjuvanted vaccines in HIV-infected children is limited to MF59 adjuvanted vaccine where seroprotection was achieved in 94%–100% and in all participants after 2 doses [43, 44].
Improved vaccine response in HIV-infected children with better immunologic status on cART has been demonstrated with hepatitis A and pneumococcal vaccines [45, 46]. However, we were unable to demonstrate an independent relationship between immune status at study entry and vaccine response, likely because our cohort was immunologically robust with a median CD4 count >500 cells/μL in the 2 older age groups and >1000 cells/μL in the youngest group. In addition, the median viral load was <100 copies/mL and only 6.4% participants were not receiving antiretroviral therapy. In contrast to our findings, studies of pH1N1 vaccination in HIV-infected adults demonstrated reduced response in those with lower baseline or nadir CD4 counts [39, 40, 43], longer duration of HIV infection [38, 40], or older age [38, 43]. There are differences in nadir CD4, duration of HIV, and age in perinatally infected children compared with adults because CD4 count is usually higher in young children and age correlates with duration of illness, thus possibly explaining the different findings.
Higher log-transformed baseline HAI titers were an independent predictor of improved primary and secondary complete response (both seroresponse and seroprotection). We do not know if higher log10 baseline HAI represented prior exposure to pH1N1 infection or cross-reaction with similar H1N1 antigens encountered in prior influenza vaccines or infections. We believe this demonstrated the benefit of antigenic boost and further supports the possible use of a 2-vaccine series for perinatally HIV-1–infected children and youth. Of interest, Hispanic ethnicity resulted in a lower rate of complete response following the first vaccination in the current study, which was not reported in other studies [27]. This relationship, however, was not seen following the second vaccination.
Consistent with data from prior studies and the Vaccine Adverse Event Reporting System [47], pH1N1 vaccination was safe in our population of HIV-1–infected children and youth, even at increased doses. Adverse events were few and mild in severity. No seizures were reported. Of interest are the 2 cases of herpes virus reactivation following vaccination, 1 with a concomitant facial nerve palsy, which has previously been reported as possibly related to seasonal influenza vaccine [48].
Our study had several limitations. Our baseline HAI titers were elevated; therefore, presumably, 32.6% of our participants were previously exposed to pH1N1 although there was no history of compatible symptoms. Our study did not initiate vaccination until the fall of 2009 and in most study sites; pH1N1 infection peaked in August 2009. This was a common finding in many other pH1N1 vaccine studies [27, 42, 43, 49]. Interestingly, Kok et al [50] assessed pre-pandemic serum samples from patients demonstrating seroprotection after the pandemic. Among the 34.2% of HIV-infected individuals demonstrating seroprotection following the pandemic, 12.8% had protective antibody levels prior to the pandemic, suggesting that cross-reacting antibodies may be present [50].
Additionally, we did not include groups of perinatally HIV-1–infected children and youth who received a single 15-μg dose or two 15-μg doses of pH1N1 vaccine. We did, however, conduct a companion study, P1089, that recruited perinatally HIV-1–infected children and youth, 6 months to 24 years of age, who were scheduled to receive 1 of the following commercially available pH1N1 vaccines: FluMist (MedImmune), Fluvirin (Novartis), or Fluzone (Sanofi Pasteur). In this nonrandomized evaluation, 93 participants in the present study who were 10 years to 24 years of age and received 2 high-dose vaccinations were compared to 50 P1089 participants who received the recommended 15-μg single dose.
Baseline demographics and seroprotection rates in P1088 (36.6%) and P1089 (42%) were similar (P = .52). No difference in seroprotection rates between studies was observed after the first vaccination (P1088, 76.9% and P1089, 75%; P = .80). The seroprotection rate after the second vaccination in P1088 (84%) was comparable to that following a single vaccination in P1089 (75%) (P = .22). Seroresponse rates were also similar following first vaccination (P1088, 68.1% and P1089, 62.5%; P = .50). However, participants in P1088 demonstrated greater seroresponse rates after the second vaccination compared with P1089 participants after the single standard-dose vaccination (84% vs 62.5%; P = .005).
In conclusion, we have demonstrated the safety and immunogenicity of 2 doses of 30-μg pH1N1 antigen in HIV-1–infected children and youth. A substantial proportion of children who failed to respond to the first vaccine dose achieved seroprotection and seroresponse after the second dose. In response to new influenza pandemics, a 2-increased-dose vaccine series may provide improved protection against this infection.
Notes
Acknowledgments. We thank the participating sites and site personnel: 60422: St Jude/Memphis IMPAACT Clinical Trials Unit [CTU] (Nehali Patel, MD; Sandra Boyd, RN, MSN, PNP; Tom Wride, MS; Aditya Gaur, MD); 60402: The Children's Hospital of Philadelphia International Maternal Pediatric Adolescent AIDS CTU (Steven D. Douglas, MD; Richard M. Rutstein, MD; Carol A. Vincent, CRNP, MSN; Margaret R. Duckett, RN, BSN); 60318: UCSD IMPAACT CTU (Rolando Viani, MD, MTP; Lisa Stangl, NP; Jeanne Manning, RN; Kimberly Norris, RN); 60336: Baylor College of Medicine CTU (Norma Cooper, MA, RN, BSN, ACRN; Mary Paul, MD, Kathleen Pitts, CPNP; Terry Raburn, RN); 60444: Bronx-Lebanon Hospital Family Center CTU (Seema Chittalae, MD; Mavis Dummitt, RN; Stefan Hagmann, MD; Murli Purswani, MD); 5031 San Juan City Hospital PR NICHD Clinical Research Site [CRS] (Midnela Acevedo-Flores, MD; Wanda I. Marrero, BSN, RN; Lizbeth Fabregas, BS, MS; Mario Paulino, MD); 5041: Children's Hospital of Michigan NICHD CRS (Chokechai Rongkavilit, MD; Ellen Moore, MD; Ulyssa Hancock, MSN, RN, PNP; Ayanna Walters, RN); 60325: Duke University Medical Center HIV/AIDS CTU (Joan Wilson, John Swetnam, Margaret Donnelly, Mary Jo Hassett); 60341: Columbia Collaborative—HIV/AIDS CTU; 2802 New Jersey Medical School CRS; 60466: UCLA–Los Angeles/Brazil AIDS Consortium CTU; 60349: University of Miami Pediatric Perinatal HIV/AIDS CTU (Gwendolyn B. Scott, MD; Charles D. Mitchell, MD; Patricia Bryant, RN, BSN; Claudia Florez, MD); 5013: Jacobi Medical Center Bronx NICHD CRS (Joanna Dobroszycki, MD; Marlene Burey, RN, MSHS, CPN; Raphaelle Auguste, RN, BSN; Karen Kassen, RN); 5017: Seattle Children's Hospital CRS (Ann J. Melvin, MD, MPH; Joycelyn Thomas, RN; Corry Venema-Weiss, ARNP; Lisa M Frenkel, MD); 5040: SUNY Stony Brook NICHD CRS (Denise Ferraro, FNP; Michele Kelly, NP; Erin Infanzon); 60323: WNE Maternal Pediatric Adolescent AIDS CTU; 60339: Children's Memorial Hospital–Chicago; 60446: University of Puerto Rico CTU (Carmen D. Zorrilla, MD; Irma Febo, MD; Vivian Tamayo-Agrait, MD; Ruth Santos-Otero, RN); 5015: Children's National Medical Center Washington DC NICHD CRS (Steven Zeichner, MD, PhD; Deidre Thompson, RN; Chrisa Thomas, BA); 5018: University of South Florida–Tampa NICHD CRS (Jorge Lujan-Zilberman, MD; Patricia Emmanuel, MD; Denise Casey, RN; Tammy Myers); 5009: Children's Hospital of Boston NICHD CRS (Charlotte Mao, MD, MPH; Catherine Kneut, RN, CPNP; Nancy Karthas, RN, CPN); 5012: New York University NY NICHD CRS (Sandra Deygoo, MS; Siham Akleh, RN; Aditya Kaul, MD; William Borkowsky, MD; CTSI grant: 1UL1RR029893); 5003: Metropolitan Hospital NICHD CRS; 5011: Boston Medical Center Pediatrics. HIV Program NICHD CRS (Ellen R. Cooper, MD; Debra McLaud, RN, Diana Clarke, Pharm D, CTSI grant: U54 RR025771).
Financial support. Overall support for the IMPAACT group was provided by the National Institute of Allergy and Infectious Diseases (NIAID) (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (AI068632). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported by the Statistical and Data Analysis Center at the Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases (cooperative agreement number 5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group and number 1 U01 AI068616 with the IMPAACT group). Support of the sites was provided by the NIAID and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 2.Peiris JS, Poon LL, Guan Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J Clin Virol. 2009;45:169–73. doi: 10.1016/j.jcv.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gatherer D. The 2009 H1N1 influenza outbreak in its historical context. J Clin Virol. 2009;45:174–8. doi: 10.1016/j.jcv.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global Alert and Response: Situation Updates (Pandemic) H1N1 2009. http://www.who.int/csr/disease/swineflu/updates/en/index.html. Accessed May 29, 2012. [Google Scholar]
- 5.Bautista E, Chotpitayasunondh T, Gao Z, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–19. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 6.Fine AD, Bridges CB, De Guzman AM, et al. Influenza A among patients with human immunodeficiency virus: an outbreak of infection at a residential facility in New York City. Clin Infect Dis. 2001;32:1784–91. doi: 10.1086/320747. [DOI] [PubMed] [Google Scholar]
- 7.Madhi SA, Ramasamy N, Bessellar TG, Saloojee H, Klugman KP. Lower respiratory tract infections associated with influenza A and B viruses in an area with a high prevalence of pediatric human immunodeficiency type 1 infection. Pediatr Infect Dis J. 2002;21:291–7. doi: 10.1097/00006454-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Mendoza Sanchez MC, Ruiz-Contreras J, Vivanco JL, et al. Respiratory virus infections in children with cancer or HIV infection. J Pediatr Hematol Oncol. 2006;28:154–9. doi: 10.1097/01.mph.0000210061.96075.8e. [DOI] [PubMed] [Google Scholar]
- 9.Kroon FP, van Dissel JT, de Jong JC, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS. 1994;8:469–76. doi: 10.1097/00002030-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Miotti PG, Nelson KE, Dallabetta GA, Farzadegan H, Margolick J, Clements ML. The influence of HIV infection on antibody responses to a two-dose regimen of influenza vaccine. JAMA. 1989;262:779–83. [PubMed] [Google Scholar]
- 11.King JC, Jr, Treanor J, Fast PE, et al. Comparison of the safety, vaccine virus shedding, and immunogenicity of influenza virus vaccine, trivalent, types A and B, live cold-adapted, administered to human immunodeficiency virus (HIV)-infected and non-HIV-infected adults. J Infect Dis. 2000;181:725–8. doi: 10.1086/315246. [DOI] [PubMed] [Google Scholar]
- 12.Levin MJ, Song LY, Fenton T, et al. Shedding of live vaccine virus, comparative safety, and influenza-specific antibody responses after administration of live attenuated and inactivated trivalent influenza vaccines to HIV-infected children. Vaccine. 2008;26:4210–7. doi: 10.1016/j.vaccine.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyall EG, Charlett A, Watkins P, Zambon M. Response to influenza virus vaccination in vertical HIV infection. Arch Dis Child. 1997;76:215–8. doi: 10.1136/adc.76.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson CR, Vavro CL, Valentine ME, et al. Effect of influenza immunization on immunologic and virologic characteristics of pediatric patients infected with human immunodeficiency virus. Pediatr Infect Dis J. 1997;16:200–4. doi: 10.1097/00006454-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Chadwick EG, Chang G, Decker MD, Yogev R, Dimichele D, Edwards KM. Serologic response to standard inactivated influenza vaccine in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1994;13:206–11. doi: 10.1097/00006454-199403000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Vigano A, Zuccotti GV, Pacei M, et al. Humoral and cellular response to influenza vaccine in HIV-infected children with full viroimmunologic response to antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;48:289–96. doi: 10.1097/QAI.0b013e3181632cda. [DOI] [PubMed] [Google Scholar]
- 17.Ennis FA, Mayner RE, Barry DW, et al. Correlation of laboratory studies with clinical responses to A/New Jersey influenza vaccines. J Infect Dis. 1977;136(Suppl):S397–406. doi: 10.1093/infdis/136.supplement_3.s397. [DOI] [PubMed] [Google Scholar]
- 18.Ruben FL, Jackson GG. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis. 1972;125:656–64. doi: 10.1093/infdis/125.6.656. [DOI] [PubMed] [Google Scholar]
- 19.Ruben FL, Potter CW, Stuart-Harris CH. Humoral and secretory antibody responses to immunization with low and high dosage split influenza virus vaccine. Arch Virol. 1975;47:157–66. doi: 10.1007/BF01320555. [DOI] [PubMed] [Google Scholar]
- 20.Palache AM, Beyer WE, Sprenger MJ, et al. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose-response study in elderly nursing-home residents and young volunteers. Vaccine. 1993;11:3–9. doi: 10.1016/0264-410x(93)90333-s. [DOI] [PubMed] [Google Scholar]
- 21.Keitel WA, Couch RB, Cate TR, et al. High doses of purified influenza A virus hemagglutinin significantly augment serum and nasal secretion antibody responses in healthy young adults. J Clin Microbiol. 1994;32:2468–73. doi: 10.1128/jcm.32.10.2468-2473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keitel WA, Cate TR, Atmar RL, et al. Increasing doses of purified influenza virus hemagglutinin and subvirion vaccines enhance antibody responses in the elderly. Clin Diagn Lab Immunol. 1996;3:507–10. doi: 10.1128/cdli.3.5.507-510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keitel WA, Atmar RL, Nino D, Cate TR, Couch RB. Increasing doses of an inactivated influenza A/H1N1 vaccine induce increasing levels of cross-reacting antibody to subsequent, antigenically different, variants. J Infect Dis. 2008;198:1016–8. doi: 10.1086/591465. [DOI] [PubMed] [Google Scholar]
- 24.Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med. 2006;166:1121–7. doi: 10.1001/archinte.166.10.1121. [DOI] [PubMed] [Google Scholar]
- 25.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200:172–80. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 26.Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol. 2010;10:18. doi: 10.1186/1471-2288-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arguedas A, Soley C, Lindert K. Responses to 2009 H1N1 vaccine in children 3 to 17 years of age. N Engl J Med. 2010;362:370–2. doi: 10.1056/NEJMc0909988. [DOI] [PubMed] [Google Scholar]
- 28.Plennevaux E, Blatter M, Cornish MJ, et al. Influenza A (H1N1) 2009 two-dose immunization of US children: an observer-blinded, randomized, placebo-controlled trial. Vaccine. 2011;29:1569–75. doi: 10.1016/j.vaccine.2010.12.116. [DOI] [PubMed] [Google Scholar]
- 29.Waddington CS, Walker WT, Oeser C, et al. Safety and immunogenicity of AS03B adjuvanted split virion versus non-adjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months-12 years: open label, randomised, parallel group, multicentre study. BMJ. 2010;340:c2649. doi: 10.1136/bmj.c2649. doi:10.1136/bmj.c2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plennevaux E, Sheldon E, Blatter M, Reeves-Hoché MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 2010;375:41–8. doi: 10.1016/S0140-6736(09)62026-2. [DOI] [PubMed] [Google Scholar]
- 31.Liang XF, Wang HQ, Wang JZ, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375:56–66. doi: 10.1016/S0140-6736(09)62003-1. [DOI] [PubMed] [Google Scholar]
- 32.Zhu FC, Wang H, Fang HH, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–23. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 33.Nolan T, McVernon J, Skeljo M, et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA. 2010;303:37–46. doi: 10.1001/jama.2009.1911. [DOI] [PubMed] [Google Scholar]
- 34.Lu CY, Shao PL, Chang LY, et al. Immunogenicity and safety of a monovalent vaccine for the 2009 pandemic influenza virus A (H1N1) in children and adolescents. Vaccine. 2010;28:5864–70. doi: 10.1016/j.vaccine.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 35.Oh CE, Lee J, Kang JH, et al. Safety and immunogenicity of an inactivated split-virus influenza A/H1N1 vaccine in healthy children from 6 months to <18 years of age: a prospective, open-label, multi-center trial. Vaccine. 2010;28:5857–63. doi: 10.1016/j.vaccine.2010.06.060. [DOI] [PubMed] [Google Scholar]
- 36.Weinberg A, Song LY, Walker R, et al. Anti-inflenza serum and mucosal antibody responses after administration of live, attenuated or inactivated influenza vaccines to HIV-infected children. J Acquir Immune Defic Syndr. 2010;55:189–96. doi: 10.1097/QAI.0b013e3181e46308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crum-Cianflone NF, Iverson E, Defang G, et al. Durability of antibody responses after receipt of the monovalent 2009 pandemic influenza A (H1N1) vaccine among HIV-infected and HIV-uninfected adults. Vaccine. 2011;29:3183–91. doi: 10.1016/j.vaccine.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crum-Cianflone NF, Eberly LE, Duplessis C, et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine in an immunocompromised population: a prospective study comparing HIV-infected adults with HIV-uninfected adults. Clin Infect Dis. 2011;52:138–46. doi: 10.1093/cid/ciq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tebas P, Frank I, Lewis M, et al. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS. 2010;24:2187–92. doi: 10.1097/QAD.0b013e32833c6d5c. [DOI] [PubMed] [Google Scholar]
- 40.Bickel M, von Hentig N, Wieters I, et al. Immune response after two doses of the novel split virion, adjuvanted pandemic H1N1 influenza A vaccine in HIV-1-infected patients. Clin Infect Dis. 2011;52:122–7. doi: 10.1093/cid/ciq003. [DOI] [PubMed] [Google Scholar]
- 41.Soonawala D, Rimmelzwaan GF, Gelinck LB, Visser LG, Kroon FP. Response to 2009 pandemic influenza A (H1N1) vaccine in HIV-infected patients and the influence of prior seasonal influenza vaccination. PLoS One. 2011;6:e16496. doi: 10.1371/journal.pone.0016496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manuel O, Pascual M, Hoschler K, et al. Humoral response to the influenza A H1N1/09 monovalent AS03-adjuvanted vaccine in immunocompromised patients. Clin Infect Dis. 2011;52:248–56. doi: 10.1093/cid/ciq104. [DOI] [PubMed] [Google Scholar]
- 43.Esposito S, Tagliaferri L, Daleno C, et al. Pandemic influenza A/H1N1 vaccine administered sequentially or simultaneously with seasonal influenza vaccine to HIV-infected children and adolescents. Vaccine. 2011;29:1677–82. doi: 10.1016/j.vaccine.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 44.Viganò A, Giacomet V, Pariani E, et al. Long-term immunogenicity after one and two doses of a monovalent MF59-adjuvanted A/H1N1 influenza virus vaccine coadministered with the seasonal 2009–2010 nonadjuvanted influenza virus vaccine in HIV-infected children, adolescents, and young adults in a randomized controlled trial. Clin Vaccine Immunol. 2011;18:1503–9. doi: 10.1128/CVI.05200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberg A, Gona P, Nachman SA, et al. Antibody responses to hepatitis A virus vaccine in HIV-infected children with evidence of immunologic reconstitution while receiving highly active antiretroviral therapy. J Infect Dis. 2006;193:302–11. doi: 10.1086/498979. [DOI] [PubMed] [Google Scholar]
- 46.Tarragó D, Casal J, Ruiz-Contreras J, et al. Assessment of antibody response elicited by a 7-valent pneumococcal conjugate vaccine in pediatric human immunodeficiency virus infection. Clin Diagn Lab Immunol. 2005;12:165–70. doi: 10.1128/CDLI.12.1.165-170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vellozzi C, Broder KR, Haber P, et al. Adverse events following influenza A (H1N1) 2009 monovalent vaccines reported to the Vaccine Adverse Event Reporting System, United States, October 1, 2009–January 31, 2010. Vaccine. 2010;28:7248–55. doi: 10.1016/j.vaccine.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 48.Zhou W, Pool V, DeStefano F, Iskander JK, Haber P, Chen RT the VAERS Working Group. A potential signal of Bell's palsy after parenteral inactivated influenza vaccines: reports to the Vaccine Adverse Event Reporting System (VAERS)–United States, 1991–2001. Pharmacoepidemiol Drug Saf. 2004;13:505–10. doi: 10.1002/pds.998. [DOI] [PubMed] [Google Scholar]
- 49.Greenberg ME, Lai MH, Hartel GF, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–13. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 50.Kok J, Tudo K, Blyth CC, Foo H, Hueston L, Dwyer DE. Pandemic (H1N1) 2009 influenza virus seroconversion rates in HIV-infected individuals. J Acquir Immune Defic Syndr. 2011;56:91–4. doi: 10.1097/QAI.0b013e318204a1c3. [DOI] [PubMed] [Google Scholar]