Abstract
Background. Chlamydia trachomatis (CT), Mycoplasma genitalium (MG), and Trichomonas vaginalis (TV) are sexually transmitted infections (STIs) associated with nongonococcal urethritis (NGU). We assessed their predictors and persistence after treatment.
Methods. We analyzed data from an NGU treatment trial among symptomatic heterosexual men aged 16–45 years from STI clinics. Nucleic acid amplification tests detected CT, MG, and TV at baseline and at 1 and 4 weeks after therapy. Associations between variables and STI detection were investigated.
Results. Among 293 participants, 44% had CT, 31% had MG, and 13% had TV at baseline. In multivariate analysis, CT infection was associated with young age and STI contact. Young age was also associated with MG, and having ≥1 new partner was negatively associated with TV. We detected persistent CT in 12% and MG in 44% of participants at 4 weeks after therapy, which were associated with signs and symptoms of NGU. Persistent CT was detected in 23% of participants after azithromycin treatment vs 5% after doxycycline treatment (P = .011); persistent MG was detected in 68% of participants after doxycycline vs 33% after azithromycin (P = .001). All but 1 TV infection cleared after tinidazole.
Conclusions. Persistent CT and MG after treatment of NGU are common, and were associated with clinical findings and drug regimen.
Nongonococcal urethritis (NGU) is a frequent diagnosis worldwide among adolescent and adult men presenting with urethral discharge or dysuria. There are no recent estimates of the prevalence of NGU in the United States; however, data from the 1970s indicate that 19%–78% of urethritis cases among men in sexually transmitted infection (STI) clinics and 85% of urethritis cases on college campuses in the United States were due to NGU [1, 2]. Chlamydia trachomatis (CT), Mycoplasma genitalium (MG), and Trichomonas vaginalis (TV) are the most recognized pathogens contributing to NGU. However, recent studies have demonstrated the contribution of Ureaplasma urealyticum, herpes simplex virus, and adenovirus to the etiology of NGU, owing in part to advancements in nucleic acid amplification tests (NAATs) for detection [3–5]. Empiric therapy is directed primarily at chlamydial infection, yet the clinical manifestations of these pathogens are usually indistinguishable and may require different treatment considerations. Persistent NGU can occur in a significant proportion of men after treatment; whether this is due to a slow resolution of the primary inflammatory response or due to persistent pathogens causing infection is uncertain.
Treatment recommendations for NGU include azithromycin or doxycycline therapy [6]. A randomized controlled trial conducted by Schwebke et al [7] found that microbiological cure differed between CT and MG, with CT having the highest clearance rate (94.8%) after doxycycline therapy, and MG having the highest clearance rate (66.7%) after treatment with azithromycin. This finding raises concern about the different response rates of each pathogen to recommended therapy and their contribution to persistent NGU. Although a test-of-cure for NGU (ie, repeat testing at 3–4 weeks after therapy) is not recommended unless symptoms persist or reinfection is suspected, the proportion of cases with persistent infection after treatment should be further delineated.
We conducted an analysis of the data collected by Schwebke et al [7] to ascertain characteristics associated with CT, MG, and TV infection at baseline and persistence after therapy in symptomatic heterosexual men with NGU. In the absence of point-of-care tests for these organisms in men, recognition of factors that predict specific infections at baseline could direct optimal therapy and management of sexual partners. We also assessed factors associated with microbiological persistence of CT or MG after standard treatment with azithromycin or doxycycline, and their correlation with persistent NGU.
METHODS
Study Design and Intervention
The multicenter, randomized controlled trial was conducted from November 2006 to April 2009. Symptomatic heterosexual men aged 16–45 years with NGU were recruited from 4 STI clinics (Birmingham, Alabama; New Orleans, Louisiana; Durham, North Carolina; and Baltimore, Maryland). The master protocol was approved by the University of Alabama at Birmingham Institutional Review Board (IRB) for Human Subjects and by IRBs serving each study site.
The primary study objectives, eligibility criteria, enrollment, and follow-up procedures were previously described [7]. Participants were randomized to 1 of 4 arms with standard treatment regimens of azithromycin or doxycycline, with or without tinidazole [7], and asked to return to the clinics for 2 follow-up visits at 1 week (visit 2) and 3–4 weeks (visit 3) after completion of therapy. Participants were counseled regarding sexual abstinence or condom use during the study and were instructed to refer their sexual partners to the clinic as contacts to NGU.
At enrollment and each follow-up visit, urethral Gram stains were conducted using standard clinic procedures to quantify ≥5 polymorphonuclear neutrophils (PMNs) per 3–5 oil immersion high-power fields (HPFs), with 3 of the 4 clinics also quantifying 5–15 PMNs per HPF or >15 PMNs per HPF. A urine specimen was obtained for detection of prevalent CT, MG, and TV infections. CT testing was performed according to the manufacturer's instructions using a licensed transcription-mediated amplification (TMA) assay (APTIMA COMBO 2, Gen-Probe Inc). TV polymerase chain reaction (PCR) testing was performed centrally at the University of Alabama at Birmingham using specific primers TV3 and TV7 for PCR amplification as previously described [5]. Detection of MG was performed centrally at Louisiana State University using a TMA-hybridization protection MG analyte specific reagent assay (Gen-Probe Inc).
Definitions
NGU was defined as new-onset urethral discharge or dysuria accompanied by a urethral smear with ≥5 PMNs per HPF and without evidence of Neisseria gonorrhoeae infection determined by the TMA assay. Persistent NGU was defined as continued clinical signs and/or symptoms of NGU after treatment. Microbiological persistence of CT, MG, or TV was defined as a positive TMA test for these pathogens during the last evaluable follow-up among participants who were positive at baseline, irrespective of clinical findings.
In our study, clinical failures after treatment were defined similar to previous clinical trials [8], based on persistent symptoms and ≥5 PMNs per HPF on the urethral smear or persistent urethral discharge on exam at visit 2, and based on ≥5 PMNs per HPF on urethral smear or persistent urethral discharge regardless of symptoms at visit 3. Clinical failures received standard treatment for persistent NGU and were discontinued from further study follow-up.
Data Analysis
Associations between demographic (age, marital status), behavioral (age at first sexual encounter, number of sexual partners in the past 3 months, new partner in the past 30 days, number of oral or vaginal sexual encounters in the past 30 days, days since last sexual encounter, condom use during last encounter, urination after sex), and clinical variables (reason for clinic visit, symptoms, duration of symptoms, physical exam findings, circumcision status, and urethral Gram stain results) with presence of organisms or no detectable pathogens at baseline were initially investigated with χ2 tests. Factors that were significantly associated at the 0.10 level were entered into a multivariate logistic model and those whose significance level remained at <0.10 were retained. Adjusted prevalence odds ratios (AORs) were estimated with 95% confidence intervals (CIs) to determine independent associations with CT, MG, and TV infections or having none of these pathogens at baseline. Associations between clinical outcomes at visits 2 and 3 and microbiological detection of prevalent STIs were examined with χ2 tests. The associations between variables and persistent infection based on the last evaluable test result for each participant after treatment were examined using exact χ2 or Mantel-Haenszel tests. Only participants with the organism detected at baseline and who returned for at least 1 of the follow-up visits were included in these analyses. Statistical significance was assessed at the 0.05 level.
RESULTS
Three hundred five male participants were enrolled in the study, and we included 293 with symptomatic NGU after excluding 12 who did not meet eligibility criteria (4 had gonorrhea, 3 had NGU in the past 30 days, 3 were asymptomatic, and 2 were excluded for other exclusion criteria). The majority (98%) of participants were African-American, with a median age of 24 years (range, 17–45). Among those enrolled, 245 (84%) returned for visit 2 (median of 1 week after treatment). Of the 260 patients not treated as clinical failures at visit 2, 169 (65%) returned for visit 3 (median of 4 weeks from treatment).
Persistence of STIs Detected by NAATs After Treatment
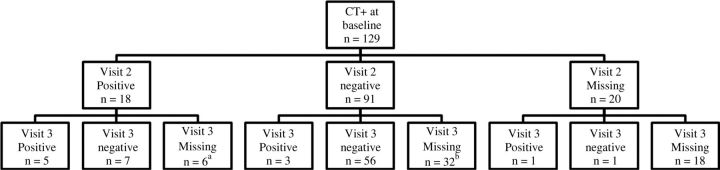
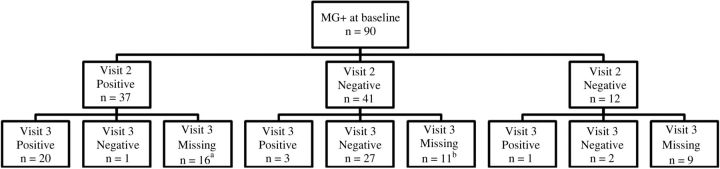
At the baseline visit, 44.0% (129/293) of our male participants had CT infection, 31% (90/232) had MG, 13% (39/293) had TV, and 28% (82/292) had none of the 3 pathogens detected. Among men identified with CT at baseline who were treated with either azithromycin or doxycycline, 17% (18/109) had a positive CT result at visit 2 after treatment and 12% (9/73) at visit 3. Among 12 men with CT at visit 2 who returned at visit 3, 5 still had persistent CT (Figure 1). Three of the CT infections at visit 3 were noted in men who were negative at visit 2, possibly due to reinfection or relapse. Among men with MG at baseline treated with either azithromycin or doxycycline, 47% (37/78) had a positive result at visit 2, and 44% (24/54) at visit 3 (Figure 2). Among the 37 men with MG at visit 2 who returned at visit 3, 20 had persistent MG. For TV, only 14% (2/14) of men with baseline infection had persistence after treatment with tinidazole at visit 2, 1 of whom cleared at visit 3 and the other did not return for follow-up.
Figure 1.
Distribution of Chlamydia trachomatis (CT) test results after treatment (visits 2 and 3) for men with positive results at baseline. aThree of the 6 participants who missed visit 3 were excluded from further study follow-up because of clinical failure at visit 2. bNineteen of the 32 participants who missed visit 3 were excluded from further study follow-up owing to clinical failure at visit 2.
Figure 2.
Distribution of Mycoplasma genitalium (MG) test results after treatment (visits 2 and 3) for men with positive results at baseline. aEleven of the 16 participants who missed visit 3 were excluded from further study follow-up because of clinical failure at visit 2. bFour of the 11 participants who missed visit 3 were excluded from further study follow-up owing to clinical failure at visit 2.
Associations Between Clinical Outcomes and Microbiological Data
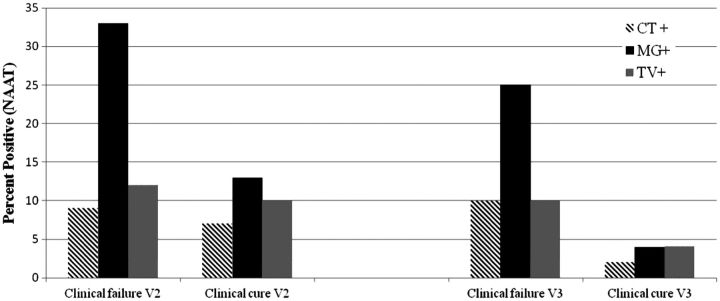
Following any treatment regimen, 13% (33/245) of the male participants who were reassessed at visit 2 met the criteria for clinical failure. However, 55% of these men with clinical failure had none of the 3 pathogens detected upon retesting at visit 2; prevalent CT infection was detected in 9%, MG in 33%, and TV in 12% (Figure 3). At visit 3, 47% (80/169) of the men were identified with clinical failure, of which 10% had prevalent CT, 25% had MG, 10% had TV (Figure 3), and 56% had no detectable pathogens. CT was associated with clinical failure at visit 3 (P = .048), whereas MG was strongly correlated with clinical failure at both visit 2 (P = .003) and visit 3 (P < .001).
Figure 3.
Microbiological detection of prevalent Chlamydia trachomatis (CT), Mycoplasma genitalium (MG), and Trichomonas vaginalis (TV) in participants identified with clinical failure or clinical cure at visit 2 (V2) and visit 3 (V3) after treatment. Abbreviation: NAAT, nucleic acid amplification test.
Predictors of Infection at Baseline in Univariate Analysis
Increasing age was negatively associated with the risk of CT or MG infection. Conversely, there was a positive association of increasing age with having none of the 3 pathogens at baseline and TV infection, but the latter was not statistically significant (Table 1). Among behavioral factors, reported sex in exchange for drugs/money or with a prostitute was negatively associated with CT and positively associated with having none of the 3 pathogens detected at baseline. Reporting ≥1 new sexual partner in the past 30 days was associated with a reduced risk of having trichomoniasis compared to no new partners, while ≥2 sexual partners in the past 3 months was negatively associated with having no pathogens at baseline. Among reasons for the clinic visit, reported sexual contact with an STI was strongly associated with CT infection (Table 1). Having >15 PMNs per HPF vs 5–15 PMNs per HPF on urethral Gram stain was also associated with an increased risk of CT infection. Other variables including marital status, age at first sexual encounter, days since last sexual encounter, number of oral or vaginal sex in the past 30 days, condom use during last encounter, urination after sex, type or duration of urethral symptoms, and circumcision status were not significantly associated with CT, MG, or TV infections.
Table 1.
Univariate Analysis of Baseline Chlamydia trachomatis, Mycoplasma genitalium, and Trichomonas vaginalis Infections According to Demographic, Behavioral, and Clinical Characteristics in Symptomatic Men With Nongonococcal Urethritis
| No.a |
Chlamydia trachomatis |
Mycoplasma genitalium |
Trichomonas vaginalis |
No Pathogen |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline Variable | No.(Row %) | OR (95% CI) | No. (Row %) | OR (95% CI) | No. (Row %) | OR (95% CI) | No. (Row %) | OR (95% CI) | |
| Demographics | |||||||||
| Age, mean (SD) | |||||||||
| With specified STI | … | 24.9 (5.8) | 0.72 (.60–.87)* | 25.2 (5.9) | 0.80 (.66–.98)* | 28.1 (6.0) | 1.20 (.95–1.52) | 28.6 (7.8) | 1.36 (1.13–1.63)* |
| Without specified STI | … | 27.7 (7.2) | 27.1 (7.0) | 26.3 (6.8) | 25.7 (6.6) | ||||
| Sexual partners | |||||||||
| No. in past 3 mo | |||||||||
| <2 | 80 | 29 (36) | 1 | 22 (28) | 1 | 9 (11) | 1 | 31 (39) | 1 |
| ≥2 | 211 | 99 (47) | 1.55 (.91–2.64) | 68 (32) | 1.26 (.71–2.23) | 29 (14) | 1.26 (.57–2.79) | 51 (24) | 0.51 (.29–.88)* |
| New partner in past 30 d | |||||||||
| 0 | 12 | 2 (17) | 1 | 3 (25) | 1 | 5 (42) | 1 | 5 (42) | 1 |
| ≥1 | 279 | 126 (45) | 4.12 (.89–19.13) | 87 (31) | 1.37 (.36–5.17) | 33 (12) | 0.19 (.06–.63)* | 77 (28) | 0.54 (.17–1.74) |
| Ever had sex in exchange for drugs/money or with prostitute | |||||||||
| No | 264 | 123 (47) | 1 | 85 (32) | 1 | 32 (12) | 1 | 68 (26) | 1 |
| Yes | 28 | 5 (18) | 0.25 (.09–.68)* | 5 (18) | 0.46 (.17–1.24) | 7 (25) | 2.42 (.95–6.14) | 14 (50) | 2.87 (1.3–6.32)* |
| Sexual encounter | |||||||||
| No. of vaginal sex encounters in past 30 d | |||||||||
| ≤4 | 146 | 66 (45) | 1 | 43 (29) | 1 | 21 (14) | 1 | 42 (29) | 1 |
| >4 | 144 | 62 (43) | 0.92 (.58–1.46) | 46 (32) | 1.14 (.69–1.87) | 17 (12) | 0.80 (.40–1.58) | 40 (28) | 0.96 (.58–1.6) |
| Condom use during last encounter | |||||||||
| No | 180 | 73 (41) | 1 | 50 (28) | 1 | 25 (14) | 1 | 56 (31) | 1 |
| Yes | 112 | 55 (49) | 1.41 (.88–2.27) | 40 (36) | 1.46 (.88–2.43) | 14 (13) | 0.89 (.44–1.79) | 26 (23) | 0.68 (.39–1.16) |
| Reason for visit (not mutually exclusive) | |||||||||
| Symptoms | |||||||||
| Yes | 220 | 90 (41) | 0.59 (.34–1.00)* | 69 (31) | 1.09 (.61–1.95) | 32 (15) | 1.58 (.67–3.75) | 63 (29) | 1.18 (.64–2.17) |
| No | 72 | 39 (54) | 1 | 21 (30) | 1 | 7 (10) | 1 | 18 (25) | 1 |
| Contact with an STI | |||||||||
| Yes | 49 | 34 (69) | 3.53 (1.83–6.83)* | 12 (24) | 0.68 (.34–1.38) | 6 (12) | 0.89 (.35–2.25) | 10 (20) | 0.62 (.29–1.3) |
| No | 243 | 95 (39) | 1 | 78 (32) | 1 | 33 (14) | 1 | 71 (29) | 1 |
| Symptoms (not mutually exclusive) | |||||||||
| Drip or discharge | |||||||||
| Yes | 179 | 82 (46) | 1.21 (.75–1.94) | 59 (33) | 1.30 (.78–2.18) | 23 (13) | 0.90 (.45–1.79) | 44 (25) | 0.64 (.38–1.08) |
| No | 114 | 47 (41) | 1 | 31 (27) | 1 | 16 (14) | 1 | 38 (34) | 1 |
| Burning on urination | |||||||||
| Yes | 131 | 57 (44) | 0.96 (.61–1.53) | 40 (31) | 0.98 (.59–1.61) | 22 (17) | 1.72 (.87–3.40) | 31 (24) | 0.67 (.4–1.13) |
| No | 162 | 72 (44) | 1 | 50 (31) | 1 | 17 (10) | 1 | 51 (32) | 1 |
| Physical exam findings | |||||||||
| Discharge amount | |||||||||
| None | 24 | 9 (38) | 1 | 8 (33) | 1 | 2 (8) | 1 | 8 (33) | 1 |
| Scant | 141 | 52 (37) | 0.97 (.40–2.38)b | 45 (32) | 0.95 (.38–2.38) | 18 (13) | 1.61 (.35–7.43) | 45 (32) | 0.95 (.38–2.38) |
| Moderate | 117 | 63 (54) | 1.94 (.79–4.80)b | 33 (28) | 0.79 (.31–2.01) | 16 (14) | 1.74 (.37–8.13) | 18 (24) | 0.63 (.24–1.63) |
| Large | 11 | 5 (45) | 1.39 (.33–5.90)b | 4 (36) | 1.14 (.26–5.09) | 3 (27) | 4.13 (.58–29.39) | 1 (9) | 0.2 (.02–1.85) |
| Circumcised | |||||||||
| No | 53 | 24 (45) | 1 | 12 (23) | 1 | 8 (15) | 1 | 16 (30) | 1 |
| Yes | 240 | 105 (44) | 0.94 (.52–1.71) | 78 (33) | 1.65 (.82–3.32) | 31 (13) | 0.83 (.36–1.94) | 66 (28) | 0.88 (.46–1.69) |
| Urethral Gram stainc | |||||||||
| 5–15 PMNs/HPF | 176 | 64 (36) | 1 | 51 (29) | 1 | 18 (10) | 1 | 63 (35) | 1 |
| >15 PMNs/HPF | 80 | 43 (54) | 2.03 (1.19–3.48)* | 28 (35) | 1.31 (.75–2.30) | 15 (19) | 2.03 (.96–4.26) | 14 (18) | 0.39 (.2–.74)* |
Abbreviations: CI, confidence interval; OR, odds ratio; PMNs/ HPF, polymorphonuclear leukocytes per high-power field; STI, sexually transmitted infection.
a One participant did not have results for Mycoplasma genitalium testing.
b The overall test for discharge amount was significant for C. trachomatis. The OR based on the 2 largest groups was significant with OR = 2.00 (95% CI, 1.21–3.29) for moderate discharge with scant discharge as the reference category.
c Excludes 37 participants from 1 site who had urethral Gram stains reported only as ≥5 PMNs per HPF.
*P < .05.
Predictors of Infection at Baseline by Multivariate Analysis
After adjusting for other variables in the model, every 5-year increase in age was significantly associated with a reduction in the risk of CT (AOR = 0.74, 95% CI, .60–.91) (Table 2). Age was the only variable retained in the multivariate model for MG, and every 5-year age increase was also associated with a 20% reduction in the risk of MG. Conversely, every 5-year increase in age remained positively associated with having no pathogens at baseline (AOR = 1.27, 95% CI, 1.04–1.55).
Table 2.
Multivariate Analysis of Characteristics Associated With Chlamydia trachomatis, Mycoplasma genitalium, Trichomonas vaginalis, and Absence of Pathogen at Baseline in Symptomatic Men With Nongonococcal Urethritis
| Baseline Variable | Chlamydia trachomatis (n = 290), AOR (95% CI) | Mycoplasma genitalium (n = 291), AOR (95% CI) | Trichomonas vaginalis (n = 291), AOR (95% CI) | No Pathogen (n = 289), AOR (95% CI) |
|---|---|---|---|---|
| Age | ||||
| Per 5 y | 0.74 (.60–.91)* | 0.80 (.66–.98)* | … | 1.27 (1.04–1.55)* |
| Partners in last 3 mo | ||||
| ≥2 vs 0–1 | 1.81 (1.00–3.30) | … | … | 0.52 (.29–.92)* |
| New partners in last 30 d | ||||
| ≥1 vs 0 | 4.42 (.86–22.74) | … | 0.20 (.06–.66)* | … |
| Ever had sex for drugs/money or with prostitute | ||||
| Yes vs no | 0.38 (.13–1.10) | … | 2.39 (.92–6.22) | 2.29 (.97–5.42) |
| Visit for contact with an STI | ||||
| Yes vs no | 3.92 (1.93–7.98)* | … | … | … |
| Discharge amounta | ||||
| Scant vs none | 0.87 (.33–2.30) | … | … | … |
| Moderate vs none | 2.00 (.75–5.33) | … | … | … |
| Large vs none | 1.09 (.23–5.17) | … | … | … |
| Reported drip or discharge | ||||
| Yes vs no | … | … | … | 0.62 (.36–1.07) |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; STI, sexually transmitted infection.
a The overall test for discharge amount was significant for Chlamydia trachomatis (P = .027). The odds ratio based on the 2 largest groups was significant with AOR = 2.30 (1.33–3.97) for moderate discharge with scant discharge as the reference category.
* P < .05.
Regarding behavioral factors, men who had a reported contact with an STI had a nearly 4-fold increased risk of CT infection (AOR = 3.92, 95% CI, 1.93–7.98) compared to those without a reported contact. Men who reported at least 1 new sexual partner in the past 30 days had a significant reduction in the risk of TV infection (AOR = 0.20, 95% CI, .06–.66) compared to those who reported no new sexual partners. A significant negative association remained between having ≥2 sexual partners in the last 3 months and having no pathogens at baseline.
When urethral Gram stain results from 3 of the sites that quantified ≥5 PMNs per HPF were added to the multivariate model, the AOR was 2.01 (95% CI, 1.09–3.72) for >15 PMNs per HPF for CT and 2.31 (95% CI, 1.05, 5.09) for >15 PMNs per HPF for TV compared with 5–15 PMNs per HPF. Conversely, having >15 PMNs per HPF on urethral Gram stain was associated with a reduced likelihood of having no pathogen (AOR = 0.39, 95% CI, .19–.78) compared with 5–15 PMNs per HPF.
Factors Associated With Persistent CT or MG Detection at Follow-up
There were no apparent associations between persistent CT or MG and risk factors for possible reinfection including reported sex since the last visit or lack of condom use. However, among men with CT infection at baseline, there was a higher proportion of persistent CT detected by NAATs in those who had a visible discharge on follow-up examination compared to those without a visible discharge (30% vs 10%, P = .028). Men with >15 PMNs per HPF on urethral Gram stain also had a higher proportion of persistent CT compared to men with a lower number of PMNs per HPF (36% vs 21%, P = .001). A higher proportion of men treated with azithromycin had persistent CT compared to those treated with doxycycline (23% vs 5%, respectively; P = .011).
Among men with MG infection at baseline, there was a higher proportion of persistent MG detected in participants who reported symptoms of discharge (81% vs 38%, P = .001) or burning on urination (76% vs 43%, P = .016) at follow-up compared to those without these symptoms. Similar to CT, the presence of urethral discharge (P < .001), ≥5 PMNs per HPF on the urethral Gram stain at follow-up (P = .001), and the initial treatment regimen were also associated with persistent MG. However, a higher proportion of men with MG at baseline who were treated with doxycycline had persistent MG compared to those treated with azithromycin (68% vs 33%, respectively; P = .001).
DISCUSSION
Overall, we detected persistent CT in 12% and MG in 44% by NAATs at 4 weeks after therapy. Persistent CT and MG at 4 weeks after therapy were both associated with signs of clinical failure. Persistent CT or MG were proportionally higher in men with a urethral discharge on examination or high number of PMNs per HPF on urethral Gram stain at follow-up compared to men without a discharge or <5 PMNs per HPF. However, the proportion of persistent CT or MG differed based on the initial therapy, with the highest proportion of persistent CT detected among men treated with azithromycin and persistent MG among men treated with doxycycline.
CT infection at baseline among symptomatic men with NGU was independently associated with young age and reported contact to an STI. We found that young age was a predictor of MG infection at baseline, similar to other investigators [5, 9], while having at least 1 new sexual partner in the past 2 weeks was the only factor associated with a reduced likelihood of TV infection. Young age has been identified as a predictor of CT and MG infections in women [10–12], so our findings are expected given the likelihood of age matching among sexual partners and higher rates of partner change in the young population [13]. In comparison, TV infection has been associated with older age in both men [14, 15] and women [16]. We found that increasing age was associated with an increased likelihood of having none of the 3 pathogens or TV identified at baseline; however, the latter was not statistically significant, likely owing to the small number of TV infections in our study.
The decreased odds of TV infection among men who reported at least 1 new sexual partner in the past 30 days was surprising, given that there were no other associations with direct or indirect measures of STI risk. A study evaluating partnership status and condom use among women with TV infections found that the most likely source of women's exposure to TV was their regular partners owing to the frequency of sex and lack of condom use [17]. Future studies involving TV infections in men are needed to substantiate this finding and its clinical relevance.
Our finding that a proportion of men had persistent CT and MG detected by NAATs at 4 weeks after recommended NGU therapy is concerning. Determining whether the persistence of these organism is due to reinfection or treatment failure is important, given that 13% of men diagnosed with chlamydia are noted to have repeat infections [8]. Although we did not monitor treatment of sexual partners in our study, we found no significant associations between persistent CT or MG and risk factors for possible reinfection including reported sex since the last visit or frequency of condom use. Furthermore, only 2% and 3% of male participants with CT and MG at baseline, respectively, had negative results at visit 2 and positive results at visit 3 consistent with reinfection. In comparison, 4% and 22% of men with CT and MG at baseline, respectively, had persistent detection at both visits 2 and 3, suggestive of treatment failure.
Persistent CT has already been noted in >5% of women after azithromycin, raising the question of whether a longer duration of treatment is needed, especially among those with higher bacterial burdens [18, 19]. A few clinical isolates of CT with mutations in the 23S ribosomal RNA gene associated with resistance to macrolides have been reported [20]; however, antimicrobial resistance in CT has not been well investigated [21]. MG has also been reported to have high treatment failures following both azithromycin and doxycycline therapy [22, 23]. A study conducted among men with NGU who were treated with doxycycline detected MG in 41% of their participants with recurrent or persistent NGU [24].
Both persistent CT and MG in our study was associated with the presence of a urethral discharge on examination and elevated number of PMNs per HPF on the urethral Gram stain at the follow-up visit. The presence of a visible discharge and inflammatory cells may be indicative of an ongoing inflammatory process from a persistent infection or persistent antigens or endotoxins from the initial infection. Whether the ongoing inflammation noted in some men after NGU therapy is due to a higher inoculum of CT/MG or the infecting serovar (CT) is unknown, and warrants future study. The cutoff point for separating men with urethritis from those without urethritis has been established at 4 PMNs per HPF [25], but the value of distinguishing between 5–15 vs >15 PMNs per HPF requires further consideration.
The possibility remains that persistent CT or MG after NGU therapy may represent shedding of nonviable organisms detected by NAATs, which is a plausible explanation for the detection of CT, MG, and TV in some participants considered to have achieved cure at 1 week after therapy (Figures 1 and 2). The time for CT clearance after azithromycin treatment has been predicted to be between 16 and 18 days [26, 27]; however, the duration of NAAT positivity for MG after treatment has not been reliably examined. We did not use cultures to assess for viable organisms nor quantitative PCR to determine the copy number of organisms associated with persistence after therapy. Therefore, further correlations between in vitro studies and microbiological persistence of CT assessed by culture and quantitative PCR would be important.
Limitations in our study that should be acknowledged include our enrollment of participants from STI clinics that are expected to have a higher prevalence of CT, MG, or TV than other male populations. We did not investigate other organisms associated with NGU including U. urealyticum and viruses, which could have been present in some of our participants who had none of the other pathogens detected. Our inclusion of only symptomatic men with possibly higher organism burdens of CT, MG, or TV may have overestimated the proportion of persistent infections, which may be lower in asymptomatic men. We also defined persistent infections based partly on a positive NAAT at 1 week after therapy if the participant did not return thereafter, potentially resulting in its overestimation since DNA from killed organisms may have been detected in these cases. However, we identified only 3 such men in the CT group and 3 in the MG group.
Our results demonstrate that clinical failures among men treated for NGU with recommended therapies are common and that the majority are attributable to MG. Overall, persistent detection of CT and MG via NAATs after treatment is associated with clinical correlates of infection, although the significance of persistent pathogens in men who are no longer symptomatic is unknown. Unfortunately, the absence of commercially available tests for MG detection precludes the diagnosis of MG infections in clinical practice. Considering that MG had the highest prevalence relative to the other STIs at baseline and that a high proportion of our male participants had persistent MG after doxycycline, the preferred current treatment regimen for NGU appears to be azithromycin despite the possibility of CT treatment failures. Therefore, future randomized clinical trials exploring the contribution of other pathogens, potentially higher doses, or longer treatment durations of azithromycin or other drug regimens (ie, moxifloxacin) are vital to determining the optimal therapy and management of persistent NGU.
Notes
Acknowledgments. We gratefully acknowledge Peter Wolff, MHA, for his significant management contributions on behalf of the Sexually Transmitted Infections Clinical Trials Group Executive Committee; Linda McNeil, for assisting with study coordination, and the other data management staff at FHI 360; the University of Alabama at Birmingham research staff at the Jefferson County Health Department; Louisiana State University research staff at the Delgado Center for Personal Health; the University of North Carolina at Chapel Hill research staff at the Durham County Health Department; and the Johns Hopkins University research staff at the Baltimore City Health Department. Mission Pharmacal (San Antonio, Texas) kindly provided tinidazole for the study.
Financial support. This work was supported by the Division of Microbiology and Infectious Diseases (DMID) of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (DMID contract number HHSN266200400073c), through the collaboration of the Sexually Transmitted Infections Clinical Trials Group.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Weisner PJ. Selected aspects of the epidemiology of nongonococcal urethritis. In: Hobson D, Holmes KK, editors. Nongonococcal urethritis and related infections. Washington, DC: American Society for Microbiology; 1977. p. 9. [Google Scholar]
- 2.McChesney JA, Zedd A, King H, Russell CM, Hendley JO. Acute urethritis in male college students. JAMA. 1973;226:37–9. [PubMed] [Google Scholar]
- 3.Bradshaw CS, Tabrizi SN, Read TR, et al. Etiologies of nongonococcal urethritis: bacteria, viruses, and the association with orogenital exposure. J Infect Dis. 2006;193:336–45. doi: 10.1086/499434. [DOI] [PubMed] [Google Scholar]
- 4.Martin DH. Nongonococcal urethritis: new views through the prism of modern molecular microbiology. Curr Infect Dis Rep. 2008;10:128–31. doi: 10.1007/s11908-008-0023-x. [DOI] [PubMed] [Google Scholar]
- 5.Wetmore CM, Manhart LE, Lowens MS, et al. Demographic, behavioral, and clinical characteristics of men with nongonococcal urethritis differ by etiology: a case-comparison study. Sex Transm Dis. 2011;38:180–6. doi: 10.1097/OLQ.0b013e3182040de9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. MMWR Morb Mort Wkly Rep. 2010;59:41–4. [Google Scholar]
- 7.Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: emphasizing emerging pathogens—a randomized clinical trial. Clin Infect Dis. 2011;52:163–70. doi: 10.1093/cid/ciq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamm WE, Hicks CB, Martin DH, et al. Azithromycin for empirical treatment of the nongonococcal urethritis syndrome in men. A randomized double-blind study. JAMA. 1995;274:545–9. [PubMed] [Google Scholar]
- 9.Mena L, Wang X, Mroczkowski TF, Martin DH. Mycoplasma genitalium infections in asymptomatic men and men with urethritis attending a sexually transmitted diseases clinic in New Orleans. Clin Infect Dis. 2002;35:1167–73. doi: 10.1086/343829. [DOI] [PubMed] [Google Scholar]
- 10.La Montagne DS, Patrick LE, Fine DN, Marrazzo JM Region X Infertility Prevention Project. Re-evaluating selective screening criteria for chlamydial infection among women in the U S Pacific Northwest. Sex Transm Dis. 2004;31:283–9. doi: 10.1097/01.olq.0000124613.85111.6b. [DOI] [PubMed] [Google Scholar]
- 11.Hilger TM, Smith EM, Ault K. Predictors of Chlamydia trachomatis infection among women attending rural Midwest family planning clinics. Infect Dis Obstet Gynecol. 2001;9:3–8. doi: 10.1155/S1064744901000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Short VL, Totten PA, Ness RB, et al. The demographic, sexual health and behavioural correlates of Mycoplasma genitalium infection among women with clinically suspected pelvic inflammatory disease. Sex Transm Infect. 2010;86:29–31. doi: 10.1136/sti.2009.037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris M, Goodreau S, Moody J. Sexual networks, concurrency, and STD/HIV. In: Holmes K, Sparling PF, Stamm WE, et al., editors. Sexually transmitted diseases. 4th ed. New York: McGraw-Hill; 2008. pp. 109–25. [Google Scholar]
- 14.Joyner JL, Douglas JM, Jr, Ragsdale S, Foster M, Judson FN. Comparative prevalence of infection with Trichomonas vaginalis among men attending a sexually transmitted diseases clinic. Sex Transm Dis. 2000;27:236–40. doi: 10.1097/00007435-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Varela JA, Otero L, García MJ, et al. Trends in the prevalence of pathogens causing urethritis in Asturias, Spain, 1989–2000. Sex Transm Dis. 2003;30:280–3. doi: 10.1097/00007435-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis. 2007;45:1319–26. doi: 10.1086/522532. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenstein B, Desmond RA, Schwebke JR. Partnership concurrency status and condom use among women diagnosed with Trichomonas vaginalis. Womens Health Issues. 2008;18:369–74. doi: 10.1016/j.whi.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golden MR, Whittington WL, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676–85. doi: 10.1056/NEJMoa041681. [DOI] [PubMed] [Google Scholar]
- 19.Horner P. The case for further treatment studies of uncomplicated genital Chlamydia trachomatis infection. Sex Trans Infect. 2006;82:340–3. doi: 10.1136/sti.2005.019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misyurina OY, Chipitsyna EV, Finashutina YP, et al. Mutations in a 23S rRNA gene of Chlamydia trachomatis associated with resistance to macrolides. Antimicrob Agents Chemother. 2004;48:1347–9. doi: 10.1128/AAC.48.4.1347-1349.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SA, Papp JR, Stamm WE, Peeling RW, Martin DH, Holmes KK. Evaluation of antimicrobial resistance and treatment failures for Chlamydia trachomatis: a meeting report. J Infect Dis. 2005;191:917–23. doi: 10.1086/428290. [DOI] [PubMed] [Google Scholar]
- 22.Mena LA, Mroczkowski TF, Nsuami M, et al. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649–54. doi: 10.1086/599033. [DOI] [PubMed] [Google Scholar]
- 23.Bradshaw CS, Jensen JS, Tabrizi SN, et al. Azithromycin failure in Mycoplasma genitalium urethritis. Emerg Infect Dis. 2006;12:1149–52. doi: 10.3201/eid1207.051558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wikstrom A, Jensen JS. Mycoplasma genitalium: a common cause of persistent urethritis among men treated with doxycycline. Sex Transm Infect. 2006;82:276–9. doi: 10.1136/sti.2005.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arya OP, Mallinson H, Andrews BE, Sillis M. Diagnosis of urethritis: role of polymorphonuclear leukocyte counts in gram-stained urethral smears. Sex Transm Dis. 1984;11:10–7. [PubMed] [Google Scholar]
- 26.Workowski KA, Lampe MF, Wong KG, Watts MB, Stamm WE. Long-term eradication of Chlamydia trachomatis genital infection after antimicrobial therapy. Evidence against persistent infection. JAMA. 1993;270:2071–5. [PubMed] [Google Scholar]
- 27.Renault CA, Israelski DM, Levy V, Fujikawa BK, Kellogg TA, Klausner JD. Time to clearance of Chlamydia trachomatis ribosomal RNA in women treated for chlamydial infection. Sex Health. 2011;8:69–73. doi: 10.1071/SH10030. [DOI] [PubMed] [Google Scholar]