Summary
This dual-site study sought to identify the appropriate role for TCM (acupuncture and herbs) in conjunction with a validated psychosocial self-care intervention (SC) for treating chronic TMD-associated pain. Participants with RDC-TMD-confirmed TMD (n=168) entered a stepped-care protocol that began with a basic TMD class. At weeks 2 and 10, patients receiving SC whose worst facial pain was above predetermined levels were reallocated by minimization to SC or TCM with experienced practitioners. Characteristic facial pain (CFP: mean of worst pain, average pain when having pain, current pain; each VAS 0-10) was the primary outcome. Social activity interference (VAS 0-10) was a secondary outcome. Patients were monitored for safety.
TCM provided significantly greater short-term (8-week) relief than SC (CFP reduction difference, −0.60 [SDE 0.26], p=0.020), and greater reduction in interference with social activities (−0.81 [SDE 0.33], p=0.016). In two of five treatment trajectory groups, more than 2/3 of participants demonstrated clinically meaningful responses (> 30% improvement) in pain interference over 16 weeks. This study provides evidence that TMD patients referred for TCM in a community-based model will receive safe treatment that is likely to provide some short-term pain relief and improved quality of life. Similar designs may also apply to evaluations of other kinds of chronic pain. (ClinicalTrials.gov number NCT00856167)
PERSPECTIVE
This short-term comparative effectiveness study of chronic facial pain suggests that Traditional Chinese Medicine is safe and frequently efficacious alone or subsequent to standard psychosocial interventions. TCM is widely available throughout North America and may provide clinicians and patients with a reasonable addition or alternative to other forms of therapy.
INTRODUCTION
Temporomandibular Disorders (TMD) include a cluster of related conditions affecting the hard and soft structures involved in movement of the mandible. Chronic pain radiating from the temporomandibular joint/masticatory muscles affects >10% of adults at any one time, and one-third of adults will experience TMD symptoms over their lifespan [50; 53]. TMD is commonly characterized by comorbidities, including headache, back pain, widespread pain and fibromyalgia, and psychosocial challenges including depression, anxiety, and multiple nonspecific physical symptoms [7; 19]. Impairment in daily activities, excess reliance on health care [14; 37;40; 55] and dependence on narcotic analgesics [11] are documented.
Pain relief is the primary therapeutic treatment objective for patients and clinicians.[15; 25; 33; 52]. Strategies include various chronic pain medications; intra-oral occlusal devices; physiotherapy; various surgeries; and arthroscopy. Psychosocial interventions have also been evaluated, with some successes [21][16–18; 22; 28]. Recent short-term trials have assessed the effectiveness of physically-based treatments [6; 30; 41], with many TMD patients seeking repeated courses of treatment over several years. Yet over a five-year period, TMD signs and symptoms are, for a significant minority, not adequately addressed [40]. No overarching evidence-based rationale for selecting among TMD treatments has emerged [50]. There is relatively frequent use of complementary and alternative medicine (CAM) in conjunction with conventional biomedicine by TMD patients [13; 42]. Patients appear to be seeking a more comprehensive and longer-lasting approach to the management of their syndrome.
Traditional Chinese Medicine (TCM) views disease as a constitutional imbalance, and has the potential to provide a whole system strategy for managing TMD. TCM encompasses acupuncture, herbal therapy, massage (Tuina) and breathing and relaxation exercises (Qigong and Tai Chi), yet acupuncture as monotherapy has been the predominant TCM-based clinical trial approach for TMD. The RCT literature to date is encouraging for acupuncture treatment of TMD [31]. Of four reasonably high quality sham-controlled studies, three reported significant acupuncture-related short-term reduction in facial pain [47–49], while the fourth reported statistically significant pain reduction but no significant between-group difference [24]. Three earlier trials had found acupuncture to be at least as effective as usual care (mainly occlusal splint therapy), but none of the 3 trials reported blinded treatment assessor(s) [29; 34; 43].
In our previous phase 2, randomized pilot RCT, TCM (individualized acupuncture and herbal therapy) provided short-term reduction in TMD pain and decrease in disability beyond that achieved by specialty dental care [45]. TCM was provided over a relatively short time period -- 20 visits in 12–16 weeks. In the 3–6 month follow-up, gains achieved during treatment declined, similar to other TMD treatments. These results informed the present trial where we shifted our focus from “cure” to “rehabilitation” management. The design was based on a real-world pain clinic within a comprehensive health plan.
METHODS
Overview of study design and rationale
This project was designed as a comparative effectiveness (CER) study. According to the IOM report, comparative effectiveness research has 6 key features: Informing a specific clinical decision from the patient perspective or a health policy decision from the population perspective, comparing at least two alternative interventions, each with the potential to be “best practice”, describing results at the population and subgroup levels, measuring outcomes – both benefits and harms - that are important to patients, employing methods and data sources appropriate for the decision of interest, conducted in settings that are similar to those in which the intervention will be used in practice.[27] Here we compare the effectiveness of whole system TCM to self-care management (SC) in a manner consistent with an integrative TMD specialty clinic stepped-care strategy. This sequential allocation design is similar to an approach that is being used in behavioral medicine/addiction research [32]. The community-based TCM practitioners are free to tailor treatment and, alongside the patients, to select timing of the visits to reflect real-world clinical practice. The pain and functional outcomes reflect those of importance to patients. The question we evaluate here is whether there is a benefit to providing TCM treatment, either before or after a standard self-care intervention.
Participants who passed a phone screen were recruited, consented, and began a 4-step eligibility process that included (1) a baseline questionnaire, (2) the Research Diagnostic Criteria for Temporomandibular Disorders (RDC-TMD) clinical examination by a project dentist (http://www.rdc-tmdinternational.org/TMDAssessmentDiagnosis/RDCTMD.aspx), (3) a standardized TCM diagnostic interview by the project TCM diagnostician, and (4) a 2-hour educational session, modeled on the educational session provided within the Kaiser Permanente Northwest TMD clinic for all new TMD patients. Those potential participants who remained interested and eligible after the educational session (the final step) were entered into the study and continued to the “week 2” telephone interview data collection. Based on the week 2 data, those with worst facial pain above a pre-determined level (see below and Figure 1) were dynamically allocated by the statistician to the psychosocial intervention (called here Self-Care or SC and described below) or whole system TCM [45] for Period 1. We have used S and T to denote these allocated groups. Those with pain below the cut-point automatically went to SC, and are denoted as group s. Project managers notified participants of their treatment assignments and facilitated the scheduling of appropriate appointments. After data collection in week 10, those who received SC (groups S and s) who had substantial pain beyond a 2nd cut-point were again dynamically allocated by the project statistician to TCM (now group ST or sT) or continued SC (groups SS or sS) for Period 2 using the same process. Participants who were automatically given SC in the first period (s) could continue in SC if they fell at or below the cut-point (group ss); they are the only group not included in the main analyses because there was not an allocation that would provide a basis for comparison. All participants ever allocated to TCM treatment remained in that arm (group Tt). (These design elements can be visualized in Figure 1, described below.) Final data collection for this report occurred in week 18. Eight weeks was chosen as the interval between allocations to represent a reasonable trial period for SC and to evaluate an allocation flow representative of actual clinic practice. Here, the first allocation reflects sending patients with substantial pain to TCM early. The second allocation reflects sending patients to TCM subsequent to a reasonable trial of usual care, as represented by the Self-Care intervention. The design anticipated minimal heterogeneity between these two allocations. The study permitted up to 20 TCM visits distributed, at practitioner and participant discretion, over a period of up to one year. Here we report the short-term two period treatment evaluation. The longer-term observational study will be reported elsewhere.
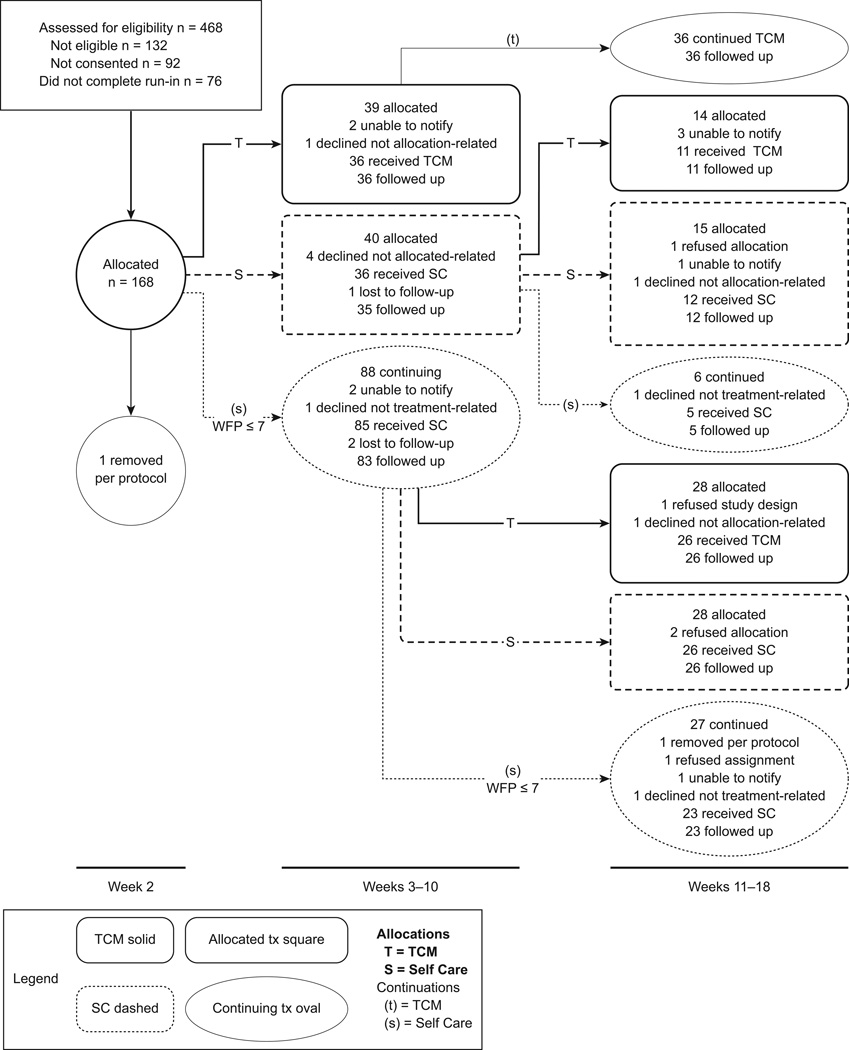
Figure 1.
The CONSORT diagram shows the progression of study participants through the first two allocation periods of the study, the short-term component reported here. It illustrates the study design and worst facial pain (WFP) cut-points, and provides the number of participants providing outcome data, beginning with the first allocation. In each group, the number of participants beginning the intervention in that phase is shown as the top number, and the number of evaluable participants at the end of that phase is shown in the bottom number. Details of the reasons for participant losses in each group are shown. Details for the losses in the recruitment process are provided in the text.
Study setting and recruitment
The study took place in Tucson, Arizona, and Portland, Oregon. Screening for TMD, TCM diagnosis, TCM treatment and SC intervention activities were all based in community practice settings. Participants at both sites were recruited through newspaper advertisements, and email list-serves of various types. In Tucson, there was also an active community outreach component to enhance the recruitment of minorities. At both sites, the response to newspaper advertisements was strong, reflecting a high interest in complementary and alternative medicine (CAM) as well as the relative lack of insurance coverage for TMD (considered a dental condition) and for CAM. Individuals responding to outreach in either city contacted our call center in Tucson using a toll-free number. The call center staff had all the information available for both sites, and provided all screening and scheduling to all participants. Potential participants completed an initial phone screen asking about TMD symptoms. Those who were ages 18–70 and reported worst facial pain at 5 or above were eligible to continue to the in-person local screening and consenting interview. At the screening interview, participants consented for the study, and completed the baseline questionnaire. They were sent for the clinical RDC-TMD examination by a study dentist, and for a TCM diagnostic interview by a study TCM evaluation practitioner.
Participant eligibility
Inclusion criteria were age 18–70, worst facial pain ≥ 5, research diagnosis of TMD [36], presence of one of ten TCM diagnoses (chosen to account for 90% of participants in prior study [45]), and completion of the run-in (TMD class) process. Exclusion criteria, evaluated at the consent interview or RDC-TMD clinical evaluation, included: (1) serious pathology of the temporomandibular joint, e.g., infection, rheumatoid arthritis, fracture; presence of cancer or acute infection of the teeth, ears, eyes, nose, or throat, as well as individuals undergoing active orthodontic treatment; (2) serious psychiatric conditions; (3) surgical implants for treatment of TMD; (4) bleeding disorders; (5) other life-threatening conditions, e.g. cancer, uncontrolled severe hypertension; (6) severe joint/disk displacement; (7) use of full dentures; (8) use of medications for which study herbs are contraindicated; (9) current pregnancy or plans to become pregnant during active treatment. Women were queried about pregnancy at every assessment point during the study and at every TCM visit. (This is usual practice in TCM as pattern differentiations, altered in pregnancy, might necessitate use of acupuncture points and/or herbs not included in the study protocol.)
All participants who met the RDC-TMD case-definition criteria and had an eligible TCM diagnosis participated in a 2-hour class at the local study site. The class, developed for this project by SD, covered the nature of TMD, its patterns of progression/non-progression, precipitating and relieving factors, and suggestions to help with jaw relaxation. If still interested, participants were moved to “enrolled” status, which led to the week 2 data collection.
Study dentists were trained and calibrated to meet research criteria by one of the investigators (SD) and were re-calibrated mid-way through the study. Study TCM diagnosticians along with the study TCM practitioners were calibrated in TCM diagnoses by another investigator (SM) [39] and recalibrated mid-way through the study as described below in greater detail. The RDC-TMD dentists and TCM diagnosticians, one each per city, remained with the study throughout its duration.
TCM Intervention Protocol
The protocol, designed to provide the best individualized TCM care within the confines of a research study, could include acupuncture, moxibustion, Chinese herbs, massage (Tuina), and lifestyle and nutrition counseling. Participants had a total of 20 acupuncture visits and 20 weeks of herbs available within a one-year period from the first treatment visit. The TCM practitioner and the participant were encouraged to schedule 6–10 visits in the initial eight-week period and to partition the remainder of visits in an individually appropriate manner. As TMD has a recurrent nature, this schedule was intended to permit treatment of flare-ups when they occurred. The initial TCM diagnostic interview by the treating TCM practitioner followed the same protocol as that used in the screening TCM diagnostic interview mentioned above and fully described in [39]. The process included a detailed history, assessment of wrist pulse taking, examination of the tongue, and concluding diagnosis, with data collected on standard forms. This diagnosis, which benefited from inter-practitioner calibration [39], guided the selection of acupuncture points, herbal formulas, and lifestyle recommendations.
Acupuncture treatments included a core set of points congruent with those identified in the meta-analysis of facial pain treatment with acupuncture [46] supplemented by diagnosis-specific points as well as points, where needed, for headaches, insomnia, stomach upset, or depression (see Table 1). The total number of needles per participant visit was limited to 20.
Table 1.
Herb and Acupuncture protocols for 2 most frequent TCM differentiations: examples are for participants presenting similar symptoms, including insomnia and headache.
| Base acupuncture points | ST7 and/or ST6, GB20 and/or GB21, taiyang, LI4, LV3 |
|---|---|
| Liver Qi Constraint | |
| Additional Points for diagnosis | GB41, GB40, GB34, LV14, LV13 |
| Base Herbal Formula | Xiao yao san, or Chai hu shu gan tang |
| Adjustments to Formula | Increase dose of bai zhu and fu ling, add suan zao ren [Increase Rhizoma Atractylodis Macrocephalae and Poria, add Semen Zizyphi Spinosae] |
| Qi and Blood Stagnation | |
| Additional Points for diagnosis | Local and distal Ah Shi |
| Base Herbal Formula | Tong qiao huo xue tang |
| Adjustments to Formula | Add dan shen and shi chang pu [Add Radix Salviae Miltiorrhizae and Radix Rehmanniae preparata] |
The herbal protocol was developed as an investigational new drug (IND) application to the FDA. For each of 12 TCM diagnoses, base herbal formulas were specified. Each formula was modifiable with any of the 65 herbs that composed the formulary (within recommended ranges) and practitioners had guidelines of traditional ranges of herbs comprising each formula as outlined in standard texts [9; 12]. As part of the protocol per FDA and NCCAM, practitioners documented the exact formulation prepared on each occasion, and participants were asked to keep a log of their herbal ingestion.
Accordingly, the FDA aspects of the protocol were focused exclusively on safety. All herbs, supplied in 5:1 granule form, were purchased from Mayway Corporation (Oakland, CA). They were GMP and ATG certified, which included testing for heavy metals, and microbial content. Granules were prepared by spraying dried herbal concentrates onto either maltose or microcrystalline cellulose, a non-allergenic carrier, to form a fine powder. Samples were retained from each lot for potential examination in the case of problems arising. Per FDA requirements for IND approval of the herbs, participants had laboratory tests for liver function (AST, ALT, total bilirubin,) renal function (creatinine, BUN,) coagulation (INR), blood count (ABC) and urinalysis performed at the time of assignment to the TCM protocol, and at 6 weeks and 1 year following start of TCM. Study medical directors at each site reviewed all laboratory tests with any out of range values and provided guidance to the PI and participants, when any remediation or treatment was necessary.
Table 1 demonstrates the flexibility of the protocol by showing the interventions for an example participant with either Liver Qi Constraint, or Qi and Blood Stagnation due to injury, and experiencing both insomnia and headache.
TCM Practitioner Qualifications and Training
The 8 TCM practitioners who provided treatments (4 in each community) had a minimum of five years’ experience with acupuncture and herbs. The two diagnosing practitioners each had more than 10 years experience and were faculty members at collaborating traditional Chinese medicine schools. Three practitioners were trained in China, and the rest had training from accredited Master’s level programs in the US. In order to increase the likelihood that similar diagnoses were made and therefore similar treatments were provided, the TCM practitioners from both sites met in Tucson in project year 1 to review the protocol and make consensus adjustments, and to practice diagnosing and giving per protocol treatments [39]. The protocol was originally developed by a consensus process among faculty at the Oregon College of Oriental Medicine in Portland as part of the prior study. Practitioners met in person or by conference call every three months with SM to review the protocol and discuss any unusual circumstances that were encountered. Practitioners were not aware of the specifics of the study design, nor were they aware of any details of participant assessments prior to beginning treatments. Participants were assigned to practitioners to maximize convenience for participants and to achieve balanced distributions of caseloads over time. Because of the geographic distributions of participants and practitioners, both goals were achieved. Assignment was not based on any other participant characteristics.
Self-care (SC) Intervention Protocol
TMD introductory class
The intervention for all participants began with a 2-hour self-care class; completing the class was considered part of the run-in phase. The inclusion of this initial educational component (self-care class) for all participants was based on our prior study finding that many TMD patients did not understand that their condition was chronic and non-progressive and were anxious about their long-term prognosis. The class, based on an existing TMD class, provided basic information on TMD and provided a few self-care strategies aimed toward relaxation of the mandibular muscles.
Self-care interventions
The two self-care interventions were designed to match the TCM interventions in time and attention during the specified study intervals. The time and attention matching in the first two 8 week periods was based on the following assumptions: (1) there would be a TCM intake (1-hour) and 7 TCM treatment sessions at 0.5 hours each (3.5 hours) in the first 8 weeks for a total of 4.5 hours; (2) there would be 2 in-person self-care education/training sessions (1.5 hours each) and 3 phone call follow-ups (0.5 hours each) in each self-care arm for a total of 4.5 hours.
Period 1
The period 1 SC protocol developed for this study was modified from the tailored self-care intervention cited earlier [16] by SD and associates who oversaw all aspects of Phase I SC, described below, including manual development, training, and quality assurance on the intervention. [The manuals are available from SD.] The Period 1 SC protocol was oriented primarily to TMD patients who, independent of pain level, were not psychosocially disabled. Self-care included the following elements: (1) Education about the bio-psycho-social model of TMD, chronic pain, the multi-faceted aspects of TMD etiology, management methods, and the rationale for self-management; (2) Guided reading with structured feedback, using participant-completed forms to explore the participant’s understanding of and identification with major themes, such as rationale for breathing and relaxation methods, TMD knowledge, communicating with health care providers, emotions, and bodily changes; (3) Relaxation and stress management training, including training in abdominal breathing, general muscle relaxation methods, and relaxation of head, neck, and masticatory muscles (the 2-hour class in the run-in period briefly covered the role of stress and negative psychological states as potential factors in exacerbating or maintaining painful TMD symptoms, including methods for detecting and managing stress); (4) Self-monitoring of signs and symptoms, enabling participants to detect changes in their physical status in order to reinforce positive self-care behaviors and to call attention to negative factors that might be modified through self-care methods (e.g., detecting effects of parafunctional oral behaviors). (5) Development of a ‘Personal TMD Self-Care Plan,’ a central component of the self-care intervention, which allows the participant, with guidance and assistance from the interventionist, to develop a regular schedule of individually tailored coping behaviors to correct or ameliorate specific physical, psychological, or emotional factors that could exacerbate or maintain TMD symptoms (e.g., specifying times when relaxation, jaw stretching exercises, or monitoring of symptoms would be performed); (6) Supervised practice and reinforcement of prescribed self-care treatments, e.g., observing participant performance of prescribed exercises during regularly scheduled self-care sessions and providing feedback and positive support, using follow-up telephone contacts to elicit changes in symptomatology and compliance with regimens prescribed; (7) Maintenance and relapse prevention, to foster recognition of obstacles to maintaining the Personal TMD Self-Care Plan and to introduce self-initiated corrective behaviors to overcome or reduce such obstacles.
Two manuals were developed for and used in SC. The interventionists’ manual, which standardized conduct during sessions and intervening telephone contacts, included scripted materials, readings and reading feedback forms, exercises, symptom monitoring forms, and personal care plans. A Patient’s Guide to Self Care for TMD, given to each participant, contained TMD education and reading materials as well as blank forms.
Period 2
The second self-care component was based upon a widely used resiliency intervention [44], and was adapted for this project with the assistance of one of its developers (Shatte) and an integrative pain physician, Dr. Heather Tick. Resiliency is a lay-language layperson intervention grounded in cognitive behavioral therapy which has been shown to be useful in treating TMD. The intervention was delivered in the same time frame as in Period 1 (2 in-person sessions, 3 phone calls). A patient self-help manual supported the intervention with monitoring guides and tips, and brief homework and self-assessment exercises. The in-person sessions focused on two key points: (1) the relationship of thoughts to feelings, and how to change thought patterns; (2) the role of thinking traps and how to overcome them. In-person sessions focused on understanding basic concepts, working through scenarios developed from Dr. Tick’s experience with chronic pain patients, and identifying situations to look at in the coming weeks. Follow-up phone calls provided check-ins on the real-world implementation of the lessons, and use of self-help materials.
Per the design, participants could be assigned to SC because they were below the pain cut-point, or could be allocated by minimization if they were above the cut-point. However, practitioners were not informed of these aspects of the design, nor of the source of individual assignments to SC. There was only one SC interventionist at each location (Tucson, Portland), and all participants at that site received intervention from the local interventionist.
Self-care interventionist qualifications and training
The SC interventionists were a retired dentist (Portland) and an experienced health behaviorist who had worked on manualized interventions in other behavioral clinical trials (Tucson). Both components of the intervention had manuals for the interventionists. For the TMD self-care intervention, the interventionists received 8 hours of instruction. Topics included TMD etiology, pathophysiology, and clinical management, delivery of educational materials, behavioral skills training and charting. Quality assurance was implemented via quarterly phone conferences and the trainer was readily available by phone for questions. In addition, there was an in-person review with the interventionists at the end of the first year. For the resiliency component in Period 2, interventionists received 8 hours of training, with follow-up phone conference debriefings after the first few participants and quarterly thereafter. Interventionists tape-recorded randomly chosen sessions for quality control and feedback in both types of sessions.
Study approvals and safety
The Human Subjects Protection Programs (IRBs) at the University of Arizona and the Oregon College of Oriental Medicine approved all procedures affecting participants. The NCCAM Office of Clinical and Regulatory Affairs approved the overall protocol. The herbal protocol operated under an Investigational New Drug status through the FDA. The study was run under the guidance of an independent 5-member Data and Safety Monitoring Board, which met twice yearly and reported its deliberations and findings to the study team, NCCAM and the IRBs, and a Steering Committee that met monthly, which included the NCCAM Project Officer, an external advisor, the key study investigators and project managers.
IRB and DSMB-approved protocols for reporting and adjudicating adverse events were in place at both study sites. Study medical directors were responsible for reviewing all adverse events and recommending action to the Principal Investigator. To assure participant safety in relation to potential mental health problems, all staff members were trained in the implementation of a mental health protocol to assure that any participants apparently manifesting mental health or emotional problems had appropriate resources available.
Outcome measures and data collection procedures
Data for the short-term analyses presented here were collected at baseline (study consent), and weeks 2 (prior to first allocation), 10, and 18. Additional data collection points are part of the long-term follow-up reported elsewhere. The baseline questionnaire was a paper-based form with the entire RDC-TMD Axis II item set, as well as other outcome measures repeated at every subsequent measurement point. A single trained interviewer at the Tucson call center used a computer-assisted telephone interview (CATI) system to collect all of the short-term follow-up data at weeks 2, 10, and 18, the data used for the outcome analyses presented here. Calls were recorded for random quality assurance checks. The interviewer was kept unaware of study design details and blinded to individual participant treatment assignment. Participants were encouraged not to divulge any treatment-related information to the interviewer, and the interviewer was trained to avoid any such discussions. The site-specific project manager was responsible for all necessary treatment-related conversations such as appointment and phlebotomy scheduling. A tracking system in Tucson permitted close monitoring of participant status at both sites in relation to all aspects of the study. Specific study staff had their access restricted to only the information that was needed for their roles, and they could not view other participant-related information. This permitted overall study management while maintaining blinding.
We chose the CATI system for primary data collection in the short-term study phase because of previous difficulty in obtaining timely and complete data in a similar study population in which we were relying on participants to fill out and return paper-based self-reports. At the study consent visit, participants were shown the data collection form for the telephone interviews so that they could be confident that the contacts would be brief. These study phone contacts took 10 minutes on average. The telephone interviews included the study outcomes described below.
The primary study outcome was characteristic facial pain (CFP), the average of worst facial pain, average pain when having pain, and facial pain now, based on the RDC-TMD. The study design group assignment criteria (see Figure 1) were based on worst facial pain, because this was the measure that was available from the previous study to guide this design [45]; because of it’s role in the study we considered it as a second primary outcome. The main secondary outcome was pain interference with social activities. Other RDC-TMD measures, also collected at study baseline, include interference with daily and work activities, and days of pain. The tertiary outcomes also collected in the more abbreviated telephone follow-ups are listed in the Main Outcomes Table below. In addition to the 7 pain items from the RDC-TMD, these included a one-item summary sleep measure (how often do you awaken fresh and rested), a brief depression measure (PHQ2) [23], the Patient Enablement Instrument (PEI) [27], and the Arizona Integrative Outcomes Scale (AIOS), an overall measure of well-being [8; 38]. In addition, at each follow-up each participant provided a complete listing of pain-related medications used in the previous month, including dosage and frequency [20], from which we derived the measure “number of medications” shown here.
Symptoms were monitored in detail at each intervention visit, under DSMB guidance, to identify possible harms. The same form was used in both the SC and TCM arms. It included a checklist of 24 common symptoms, including those that might be caused by herbs, acupuncture, lifestyle changes, or use of any recommended self-care strategies. For each symptom, the participant checked whether or not it had occurred since the last assessment. If so, the participant indicated (1) whether it was new; (2) severity – mild, moderate, severe; (3) whether it was related to treatment; (4) the change since previous assessment – worse, none, better. Practitioners and interventionists had protocols for responding to any severe or worsening symptoms (adverse events). Extensive evaluations were undertaken on these data at each semi-annual DSMB meeting to look for patterns by type of intervention. In addition, participants were asked at each intervention visit and at each follow-up data collection telephone interview whether they had used the emergency room or been admitted to the hospital since the last follow-up. Every affirmative answer was considered an adverse event, and follow-up data collection was initiated per DSMB-approved protocol. Severity and relationship to treatment assessment followed standard NIH definitions; all final decisions regarding severity and relationship to treatment were made by consensus of the two medical directors (Tucson and Portland) with concurrence of the Principal Investigator. Each adverse event was reviewed by the DSMB. The study included a protocol for addressing mental health events and all staff members and practitioners were trained in its use.
Statistical approach
Dynamic allocations to treatment groups at weeks 2 and 10 were accomplished by an automated design-adaptive allocation procedure [1–5; 51] which sequentially balanced the SC and TCM groups with regard to WFP, gender, depression, and age as each person became eligible for allocation. This was done because simulation studies have shown that conventional randomization in small studies is unacceptably inefficient, both at producing balanced treatment groups and accurate effect estimation [5; 51]. Balancing factors were used for adjustment in the primary analysis, again following the results of Aickin [5] and Taves [51], in accordance with the CONSORT statement, in which “minimization” is regarded as equivalent to randomization. Allocations were computer-generated by Dr Aickin using a computer program to which he alone had access, thereby concealing the allocation process from all other project staff. Moreover, participants were allocated in blocks, and an undisclosed feature of the allocation program rendered accurate prediction of allocation extremely unlikely. Allocations were provided to the project managers after data collection and at the time when participants needed to be informed. Staff played no role in generating allocations, nor had any potential to manage or affect the process.
The analysis of the first two dynamic allocations presented here was undertaken on an intent-to-treat basis. Missing data were rare, and were not replaced by imputation. The primary outcome analysis was based entirely on study telephone-administered questionnaires, which were taken to represent the times at which they were intended to be administered in the study (weeks 2, 10, and 18 for the short-term phase). Thus, variations that occurred in the exact timing of study questionnaires were not incorporated into the analysis, since they were driven by the practicalities of participant choice of treatment timing and by study constraints.
The short-term analysis presented here, carried out as specified in the study design, used outcomes from two phases, week 2 to week 10, and week 10 to week 18. For each period, the outcome was expressed as a change score (value end of period minus value start of period). The regression analysis used the change score as the outcome, and explanatory factors were TCM group indicator, age, male indicator, depression, baseline value at week 2, and WFP at week 2 (each centered at its mean). A random effects model took into account that some participants appeared in both time periods (the cluster option of the regress procedure in Stata, Version 9.1). The coefficient of the TCM indicator was the primary effect, for which two-sided p-values were computed. This coefficient reflects the additional improvement due to TCM (above the change due to SC) on the relevant scale for each item. Age, WFP, gender and depression were included based on the findings of Aickin [5] and Taves [51]. The Main Outcomes Table presents results for all outcome measures that were available for evaluating the short-term impact of the interventions. Note that participants assigned to the SC condition with pain levels below the relevant thresholds do not contribute to the analyses for that period.
To provide some indication of the clinical meaningfulness of the overall intervention trajectories, the percent achieving greater than 30% improvement was calculated per trajectory simply by counting those participants whose week 18 value was at least 30% lower than their week 2 value.
Power considerations
Both the study design and the sample size determination were based on the pain trajectories of participants in the pilot study. At power 85% and significance 5% the detectable effect on the primary outcome of WFP change was 1 unit (on the 0–10 scale), determined by applying the current analysis to the pilot data, and factoring up to a sample size of 150. From the results reported below, the actual detectable effect was 0.8 units.
RESULTS
The flow of participants into and through the short-term phase of the study is summarized in detail in the CONSORT diagram (Figure 1). Briefly, 468 potential participants were screened, 336 were eligible for referral to consent, and 244 consented between September 2006 and December 2007. The main reason for lack of consent was study burden. One hundred sixty-eight entered into the study, almost 30% of those initially expressing any interest. The main reasons for not completing run-in were realization of study burden, individuals too busy to complete all study activities, study delays, and inability to contact participants for study activities. The recruitment target of 80 participants per site was exceeded slightly at both sites. Over the two allocation periods, two participants were withdrawn per protocol for medical reasons (one prior to initial allocations, one in second SC continuation group). Of 81 total participants allocated to TCM, we were unable to notify 5, 2 withdrew not related to treatment allocation (illness in family, surgery, etc.), and 1 declined because of the blood work requirement. The remaining 73 participants all received treatment and provided follow-up data. Over the two allocation periods, there were 204 opportunities for participants to be offered SC. Of those, we were unable to notify 4, 8 declined for reasons not related to the treatment group, and 4 refused the assignment and withdrew from the study. One hundred eighty-seven received treatment, with follow-up on 184.
The study design and flow of participants through the study between weeks 2 and 18 is also shown in Figure 1. At the first allocation point, 88 were at or below the WFP cut-point (predefined as WFP =7) and assigned to SC (indicated as (s)), whereas 79 who were above the cut-point were dynamically allocated between SC (S) and TCM (T). In the second period, all first period TCM participants continued with TCM (Tt). Of the remaining participants, 85 continued to have pain above the second cut-point (pre-defined as WFP= 5); 42 were allocated to TCM (ST or sT) and 43 to SC (SS or sS) at the second allocation. Thirty-three participants were at or below the cut-point and continued on SC (27 ss, 6 Ss), and did not contribute to further analyses.
Baseline values for demographic, TCM, and TMD characteristics are shown in Tables 2a and 2b for all participants entering each allocation group in each time period. With regard to RDC-TMD Axis I physical diagnoses, analyses revealed a distribution of Axis I diagnoses in agreement with numerous other studies. The Axis II classifications were also similar to replicated well-published findings that TMD patients do report appreciable amounts of depression, somatization and persistent pain of long duration. However, the proportion of participants in Graded Chronic Pain levels 3 + 4 was higher than anticipated from the previous study. Note that the starting pain level values tend to be higher in the participants allocated at week 2 compared to week 10. This is to be expected, as those with lower pain at baseline were more likely to be assigned to self-care at week 2 and not be allocated until week 10. Full details of TCM baseline TCM diagnoses are provided elsewhere [39].
Table 2.
| a. Demographic characteristics of participants allocated at weeks 2 and 10a | |||||
|---|---|---|---|---|---|
| 1st allocation (week 2) | 2nd allocation (week 10) | ||||
| SC | TCM | SC | TCM | ||
| Number b | 40 | 39 | 43 | 42 | |
| Age | Years [Mean (SD)] | 42.3 (13.5) |
42.9 (13.0) |
43.7 (12.4) |
43.6 (12.0) |
| Percent | |||||
| Gender | Female | 85.0 | 87.2 | 86.0 | 88.1 |
| Ethnic Categories | Hispanic or Latino Not Hispanic or Latino Unknown (individuals not reporting ethnicity) |
10.0 85.0 5.0 |
10.3 84.6 5.2 |
11.6 88.4 0 |
11.9 83.3 4.8 |
| Racial Categories | White Non-white Unknown or not reported |
82.5 5.0 12.5 |
84.6 7.7 7.7 |
86.0 7.0 7.0 |
83.3 4.8 11.9 |
| Education | ≤High School Some College College graduate Post Graduate education |
12.5 37.5 25.0 25.0 |
7.7 38.5 28.2 25.6 |
14.0 23.8 28.6 33.3 |
21.3 31.0 19.1 28.6 |
| Marital Status | Married/ partnered Divorced/ Widowed/ Separated Never married/ partnered Not known |
37.5 27.5 35.0 0 |
59.0 10.2 30.8 0 |
46.5 30.2 23.3 0 |
54.8 21.4 23.8 0 |
| Income ($) | No response <25K 25–50K 50–100K >100K |
5.0 32.5 22.5 27.5 12.5 |
2.6 30.8 35.9 25.6 5.1 |
11.6 23.3 27.9 25.6 11.6 |
4.8 33.3 31.0 21.4 9.5 |
|
TCM differentiations (most salient) |
Liver Qi Constraint Qi & Blood Stagnation Other c |
47.5 37.5 15.0 |
53.8 38.5 7.7 |
34.9 51.2 13.9 |
47.6 38.1 14.3 |
| b Baseline Pain Characteristics for participants allocated at weeks 2 and 10 | |||||
|---|---|---|---|---|---|
| 1st allocation (week 2) | 2nd allocation (week 10) | ||||
| SC | TCM | SC | TCM | ||
| Number | 40 | 39 | 43 | 42 | |
| Variable | Mean (SD) | ||||
| Worst facial pain [0–10] | 8.1(1.4) | 8.1(1.2) | 7.5(1.5) | 7.6(1.5) | |
| Average facial pain [0–10] | 6.0(1.6) | 6.5(1.8) | 5.8(1.7) | 5.6(2.2) | |
| Facial pain today [0–10] | 5.0(2.0) | 5.3(2.2) | 4.5(2.2) | 4.5(2.3) | |
| Characteristic facial pain [0–10](mean of 3 above) | 6.4(1.4) | 6.6(1.4) | 5.9(1.5) | 5.8(2.0) | |
| Days of facial paina | 4.9(1.4) | 4.8(1.3) | 4.7(1.4) | 4.6(1.6) | |
| Amt. interferes with daily activities [0–10] | 4.3(2.3) | 3.8(2.6) | 3.5(2.2) | 3.8(2.7) | |
| Amt. interferes with social activities [0–10] | 3.8(2.5) | 3.3(2.8) | 3.0(2.1) | 3.3(2.7) | |
| Amt. interferes with ability to work [0–10] | 3.8(2.4) | 3.8(2.8) | 3.0(2.3) | 2.9(2.7) | |
| Depression [1–4] | 2.0 (0.8) | 1.8 (0.6) | 1.7 (0.6) | 1.8 (0.8) | |
| Sleep [1–4] | 2.8 (0.8) | 2.5 (0.8) | 2.7 (0.8) | 2.6 (0.8) | |
| N of medications | 2.2 (1.5) | 1.8 (1.5) | 1.8 (1.4) | 1.9 (1.6) | |
| AIOS [0–10] | 5.5 (2.1) | 5.6 (2.0) | 5.6 (1.9) | 5.9 (1.9) | |
| Percent | |||||
| Duration of pain | <1 year 1–10 years >10 years |
10.0 55.0 35.0 |
10.3 43.6 46.1 |
7.0 41.8 51.2 |
4.8 59.5 35.7 |
|
Visited a practitioner for pain treatment |
No Yes |
5.3 94.7 |
18.4 81.6 |
16.3 85.7 |
4.9 95.1 |
| Nature of facial pain | Continuous | 47.5 | 41.0 | 44.2 | 43.9 |
| Intermittent | 52.5 | 58.9 | 55.8 | 56.1 | |
| Graded Chronic Pain score | 0–2 3–4 |
30 70 |
35.9 64.1 |
37.2 62.8 |
42.8 57.1 |
|
TMD Diagnostic Groups (RDC-TMD Axis I) |
Group 1a 1b |
53 41 |
39 42 |
61 25 |
46 41 |
| Any Group 2 | 20 | 15 | 33 | 21 | |
| Group 3a 3b–c |
63 5 |
67 0 |
47 5 |
52 2 |
|
| Group 1 + 3a | 63 | 62 | 44 | 48 | |
None of the between-group differences within allocation periods are significant at p<.05.
Note that per design some of the same people are represented in the baselines for SC in period one, and SC and TCM in period 2.
Full details of other baseline differentiations are found in [39].
1 = 0–3d, 2 = 4–7d, 3 = 8–14d, 4 = 15–21d, 5 = 22–27d, 6 = every day (out of the past month)
Table 3 shows the number of visits participants had in each trajectory in each period. In both TCM and SC, participants completed fewer visits than provided for in the protocol in both time periods, and in general, participants completed fewer treatments in the second period than in the first. However, because of the different durations of visits, time spent per period by participants in TCM and SC visits continued to be similar.
Table 3.
Number of visits* by trajectory in time periods 1 and 2
| Period and trajectory | Mean N of visits (SD) |
Approximate time (hrs) |
N of participants |
|---|---|---|---|
|
Period 1: Assigned to self-care (pain below threshold) (s) |
3.9 (1.4) | 4.0 | 88 |
| Allocated to self-care (S) | 3.3 (1.8) | 3.7 | 40 |
| Allocated to TCM (T)** | 6.4 (3.1) | 4.2 | 39 |
|
Period 2: Continuing self-care: (ss or Ss) |
3.8 (1.8) | 3.9 | 27 |
| Allocated to self-care (sS or SS) | 3.9 (1.7) | 4.0 | 42 |
| Allocated to TCM (sT or ST)** | 6.1 (2.9) | 4.1 | 42 |
| Continuing TCM (Tt) | 4.7 (1.9) | 2.4 | 36 |
Time and attention matching was designed on time, not visit number. In person SC sessions were 1.5 hours (2 per unit), phone calls 0.5 hours (3 per unit); initial TCM visits were 1.5 hours, follow-up sessions were 0.5 hours (6 per unit).
Number of visits was significantly greater in the TCM intervention.
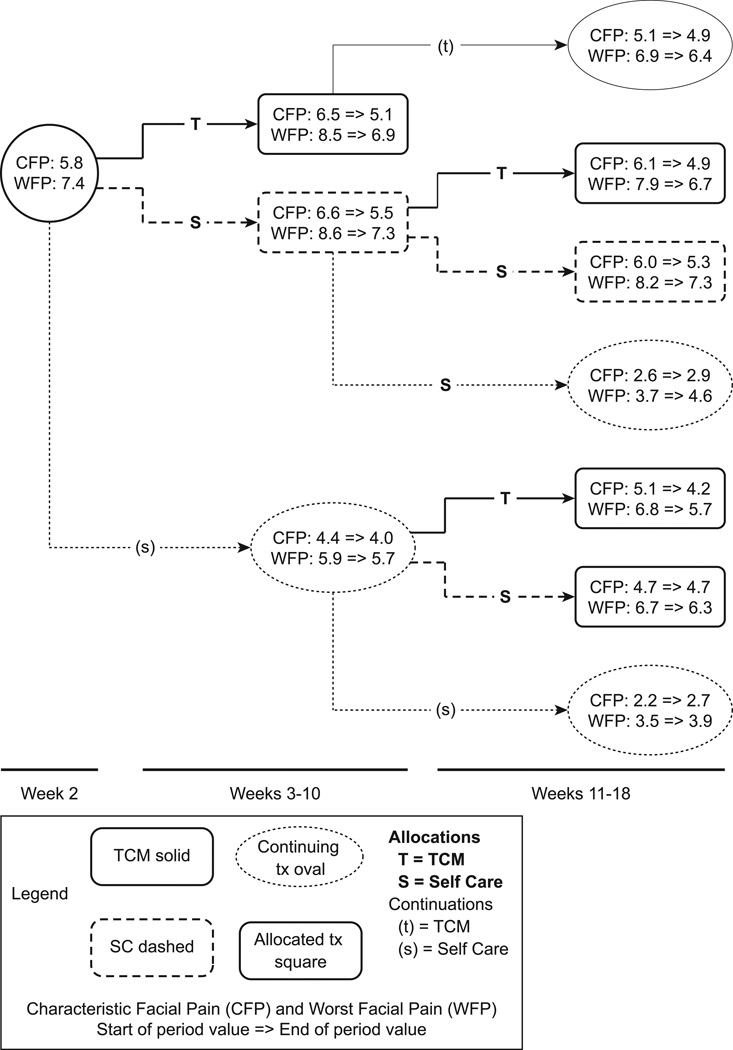
The results of the primary short-term analyses as pre-specified are shown in Figure 2 and Table 4. Figure 2 shows the amount of change for each group under each treatment condition for characteristic facial pain and worst facial pain (primary outcomes); sample sizes in each sub-group are shown in Figure 1. Participants improved during each treatment phase in nearly every treatment condition. Table 4 shows the overall short-term (8-week) effects of TCM in comparison to SC combined over the two treatment periods for allocated participants on all of the evaluated outcome variables. There were statistically significant additional benefits from TCM over SC with regard to our primary outcomes, characteristic facial pain and worst facial pain (−0.60, p = 0.020 and −0.58, p=0.045, respectively). In addition, our pre-specified secondary outcome, "interference with social activities", showed greater improvement in TCM, (−0.81, p=0.016). Statistically significant greater improvements were also seen for TCM in the overall measures of well-being (AIOS) (0.58, p = 0.019), sleep (0.27, p=0.020, 1–4 scale), and Patient Enablement (0.27, p = 0.005). There were no measurable differential benefits from TCM in depression, or the number of medications being taken. Thirteen participants were taking opioids at the start of the study. Detailed evaluation of medication use patterns found no individuals who stopped their opioid medication use during these time periods. Additional secondary analyses evaluated whether TCM differentiation (Liver Qi Constraint, Qi and Blood Stagnation, or Other) at baseline or duration of TMD prior to the study, was related to outcome effects of TCM. Neither had a significant effect on any outcome (data not shown). Within the interventions, there was a trend toward a significant impact of number of visits on pain improvement in TCM but not SC.
Figure 2.
This figure illustrates the main short-term outcomes based on protocol-specified analyses for the primary outcomes, characteristic facial pain and worst facial pain. The statistical analysis that corresponds to the overall changes is provided in Table 4. Note that the facial pain values to the left of the arrow for each group represent values at the start of that period, not at study baseline.
Table 4.
Main Outcomes Table: Results of per protocol analysis of overall short-term effects comparing TCM with Self-Care
| Outcome [possible range of values] | Baseline Average (Week 2)a |
Total TCM effect in comparison to SC b (SDE) [p-value] |
|
|---|---|---|---|
| Primary Outcomes | |||
| Characteristic Facial Pain [0,10] | 6.5 | −0.60 (0.26) [0.020] | |
| Worst Facial Pain [0,10] | 8.5 | −0.58 (0.29) [0.045] | |
| Secondary outcome | |||
| Interfered with Social Activities [0,10] | 3.3 | −0.81 (0.33) [0.016] | |
| Tertiary outcomes | |||
| Average Facial Pain [0,10] | 6.3 | −0.62 (0.27) [0.023] | |
| Facial Pain Now [0,10] | 4.8 | −0.49 (0.33) [0.144] | |
| Days of Facial Painc [0,6] | 5.0 (22–27d) | −0.05 (0.27) [0.841] | |
| Interfered with Daily Activities [0,10] | 4.3 | −0.60 (0.32) [0.070] | |
| Depression [1,4] | 2.0 | -0.11 (0.09) [0.248] | |
| Sleepd [1,4] | 2.6 | −0.26 (0.11) [0.020] | |
| N of Medications | 1.7 | −0.08 (0.13) [0.543] | |
| Patient Enablement [1,4] | 2.4 | 0.27 (0.09) [0.005] | |
| AIOS (overall well-being) [0,10] | 5.9 | 0.58 (0.25) [0.022] | |
Participants allocated to SC or TCM at week 2 or 10
The effect represents the difference between the change scores (TCM-SC), shown in the units associated with each outcome measure
1 = 0–3d, 2 = 4–7d, 3 = 8–14d, 4 = 15–21d, 5 = 22–27d, 6 = every day (out of the past month)
Reverse coded: lower is better
To provide a clinically-related interpretation of these results, we estimated the percent of participants achieving the minimum clinically important difference over 16 weeks in each trajectory, applying the commonly used 30% criterion. In summarizing the results of Table 4 by trajectory, Figure 3 presents the percent of participants achieving, over 16 weeks, at least 30% reduction in our main outcome variables – characteristic facial pain, worst facial pain, and interference with social activities. Individuals are grouped by allocation trajectories as shown in Figures 1 and 2; sample sizes per trajectory are provided in Figure 1. In two of the five trajectories more than two-thirds of the participants achieved greater than 30% improvement in social interference. Both of these trajectories included TCM.
Figure 3.
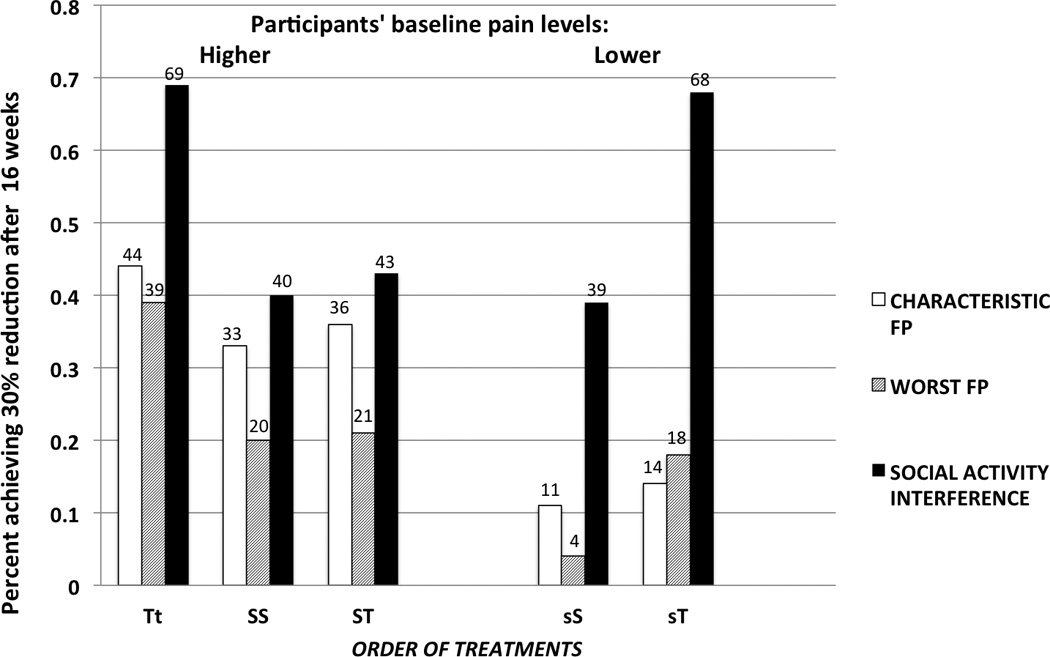
This figure illustrates the percent of allocated participants achieving clinically meaningful response (≥ 30% improvement) through 16 weeks on study intervention (weeks 2–18). Left side of panel: Change for patients with high pain levels at baseline and allocated at first point [Tt allocated to TCM at initial time and continued; SS allocated to self-care and then allocated to self-care again; ST allocated to self-care and then allocated to TCM]. Right side of panel: Change for patients with lower (moderate) pain levels [s] at baseline [sS with moderate baseline pain and allocated at week 8 to self-care; sT with moderate baseline pain and allocated at week 8 to TCM]. Sample sizes for each group are shown in Figure 1.
Statistical and graphical analysis of negative side effects, monitored in both interventions at each treatment visit as described above, revealed neither clinically meaningful nor statistically significant differences between interventions except for occasional and mild GI disturbances associated with herbal formulae which were immediately addressed by the TCM practitioners, or rare mild bruising from acupuncture. The only clinically significant adverse event attributable to an intervention was a mild adverse event – ear cellulitis – attributable to ear acupuncture in a single person who had intermittent ear cellulitis prior to study entry. After this event, the protocol was amended so that all participants were queried about previous cellulitis; if the condition was reported, acupuncture was to be avoided in that area. In fact, none was subsequently reported. No hospitalizations or emergency room visits were determined to be related to any study activities. The blood work collected to evaluate any adverse impacts of herbal treatment demonstrated no episodes of adverse effects.
DISCUSSION
Existing treatment strategies for TMD
Pain relief, sought mainly via biomedical approaches, is the primary therapeutic objective of TMD treatments for both patients and clinicians.[15; 25; 33; 52]. The most common of these conventional treatments are analgesic, anti-inflammatory, anti-depressant and other chronic pain medications, all in use among our participants. Our study implemented a brief psychosocial intervention (SC) modified from that evaluated by Dworkin et al [16] that had been developed for psychosocially functional patients, utilizing self-care with no accompanying usual care. This was efficacious in their patient population, with significant reductions over short- or long-term follow-up for pain and pain-related interference with daily activities [16–18; 22; 28]. Although we had anticipated recruiting a psychologically functional population, in fact our study population included psychosocially dysfunctional participants as well. The SC strategy was shown to be successful for the psychosocially functional participants within our study population, especially for those with moderate pain levels at baseline (ss).
Whole systems TCM care
The present trial is one of only a small number of RCTs designed to assess effectiveness of multiple modalities of traditional Chinese medicine (TCM). In relation to seasonal allergic rhinitis, two different research teams have evaluated the combined use of acupuncture and Chinese herbal therapy [10; 54]. One compared a standardized herbal formula alone to herbs with semi-standardized acupuncture, tailored to TCM diagnosis; the other compared semi-standardized acupuncture plus herbs tailored to TCM diagnosis to sham versions of each. Both studies incorporated design features that moved them in the direction of real-world care. However, we are aware of none besides our own that have evaluated whole system TCM for any chronic pain condition.
The present trial was designed to bring more comprehensive versions of clinical practice into research, from both the TCM and medical pain clinic perspectives. TCM practitioners had the freedom to individually tailor differentiation-specific herbal formulae from an FDA-approved list of 65 herbs. They also had considerable latitude in creating tailored acupuncture protocols within guidelines, and in the use of Chinese massage (Tuina), a technique often found appropriate for musculoskeletal conditions like TMD. Further, as a lesson learned from our pilot study, participants were allowed the choice, in consultation with their practitioner, about how to space their treatment visits. Finally, from the pain clinic perspective, the stepped-care design provided the chance for participants not receiving adequate benefit from biomedical care to explore TCM as a therapeutic option.
Comparative effectiveness research
This trial was designed with the intent of conforming to current views of CER, including the comparison of two viable clinical approaches, measuring outcomes that are important to patients (worst facial pain, characteristic facial pain, and disability), allowing the practitioners flexibility in tailoring the therapy to the patient, assessment of benefits and harms, and including participants who are typical of those who would be seen in a pain clinic [27]. Most importantly, the design allowed for changing the therapeutic approach based on the patient’s response, as would be the case in ordinary clinical practice, but is rarely the case in controlled trials. The analysis presented here was for relatively short-term outcomes (8 and 16 weeks), but in a separate report we will assess longer-term (18 month) data. In our analysis we have also taken into consideration patient subgroups, and examined their trajectories of response to the sequence of treatments they received, again as would be done in real clinical practice. In all of these aspects, we attempted to take into consideration as many as possible of the important aspects of treatment for this patient population. The aim was to present a therapeutic face that was as close to reality as could be done in a research setting, based on findings from a previous study [45]. While there are inherent limitations to how closely a research study can parallel real-world practice, we believe we have shown that steps toward the CER standards can be taken.
Lessons from study implementation
While our prior trial recruited participants as they were referred to a tertiary care TMD specialty clinic, the current study relied on community outreach for participant recruitment, with the TCM component included in the outreach description. Counter to expectations, our recruitment pool had higher pain levels than seen in many tertiary care settings, higher levels of psychosocial impairment as measured by the RDC-TMD Axis II Graded Chronic Pain Scale than the SC intervention was originally intended to manage, and far less prior treatment. Interviews with enrolled participants revealed a commonly expressed opposition to or inability to afford the medications or interventions that they had previously been offered, and the choice instead to just live with the pain. In many cases, they preferred CAM therapies but had not been able to afford them either, and were enthusiastic about an opportunity to participate in this study. With regard to generalizability of the present findings it must be considered that CAM trials, like other clinical trials, are likely to recruit individuals who are interested in the therapy being evaluated. In the present case, the advantage is that this is the population drawn to TCM, which is the population for which the trial is relevant; conversely, this may also be a population that might not choose a CBT-based psychosocial treatment intervention.
Study Limitations
Limitations come from ways in which the study design did not/could not replicate clinical practice. Although we took a step toward clinical practice, the decision of which therapy the participant would receive, and when, was based in the trial allocation process that was only partly responsive to participant needs, and did not permit participant choice. We did not tell participants that those who continued to have substantial pain would all ultimately get TCM, as we were concerned that such information might affect the reported pain levels of participants eager to receive TCM. But as a result, some of the participants who were not allocated to TCM in the second allocation were particularly discouraged. In addition, the SC intervention was not originally designed to be delivered to TMD patients showing high levels of psychosocial disability. The extent to which this affected response to SC by some participants requires further investigation. These aspects may have had some influence on which participants failed to provide follow-up information.
The modest results presented here provide information only on short-term outcomes, and do not include sub-group analyses. However, TCM may provide greater benefits with more treatment visits, and long-term maintenance issues are of great importance in chronic recurring conditions such as TMD. CBT interventions, at least, have been found efficacious in long-term studies [35]. The long-term component of our trial, following participants through and beyond 20 TCM treatments, will shed light on the long-term impact of TCM alone and on TCM preceded by self-care. In addition, the long-term analyses will include the evaluation of variations in outcomes by TMD sub-groups, and by variations in the TCM treatments delivered. However, the design did not include sending those who had completed TCM treatment without self-care (Tt) to self-care after TCM, which limits conclusions on the value of self-care after TCM. This is an appropriate design feature for a future Phase III trial.
Conclusions
The real-world clinic aim addressed in the present trial was to identify effective treatment strategies for patients presenting with different levels of TMD pain. The approach taken here was to continue participants on SC as long as their pain levels were below preset limits, but if pain levels were higher, then to test continuation of SC against a switch to TCM. The implication of this design is that participants with moderate or low pain levels would remain on SC, which was designed for this type of patient, while among those not improving on SC the two conditions (continue SC, change to TCM) would be tested in a conventional fashion. Thus a key feature of our study was to evaluate the effect of TCM on patients for whom SC was not originally designed.
The results of our trial suggest that the stepped-care approach is an effective treatment strategy (Table 4). The present results can provide assurance to clinicians that TMD patients referred for TCM in a community-based model will receive treatment that is safe and is likely to provide short-term relief of pain and improved quality of life. The long-term outcomes of this study, to be presented separately, will provide a more complete picture of the impact of the different treatment trajectories.
In clinical practice for chronic pain, it is rare that patients only receive a single therapy over time. Thus this design, which is built upon a functioning pain clinic model, can help to inform clinical decision-making about potential care trajectories. Further, the fact that we could carry out this type of design, already in use in behavioral medicine research [32], among patients with substantial pain may help to inform clinical effectiveness research designs for other types of chronic pain.
Acknowledgements
We thank our project officer, Partap Khalsa, DC, PhD, for his ongoing involvement and support throughout the project, and critical help in the drafting of this paper, and Richard Nahin, PhD, MPH, for believing in this novel design and supporting us through initial NCCAM hurdles. In relation to the interventions, we thank: Kimberly Huggins, RDH, BS, for her involvement in the modification of the Self-Care intervention, training of practitioners and dentists, and ongoing support; Heather Tick MD and Andrew Shatte PhD for all aspects of the allocation 2 self-care intervention; and our TCM practitioners (Tucson: Linda Stone, Carolyn Shenmen, Leslie Romero, Leslie McGee, Alex Holland; Portland: Zheng Gong, Yan Liu, Ed Chiu, Cita Oudijk, Mark Goldby) for their willingness to fully collaborate. We thank our project managers and staff, Cheryl Glass, Joshua Metlyng, Julie West, and Emery Eaves, our post-doctoral fellow Allison Hopkins, PhD, and our Tucson medical director Ed Paul, MD, for their help that made this possible. Finally, we particularly thank our DSMB for their assistance and involvement in design and implementation and their careful consideration for the safety of our participants throughout this complex project, and the initial reviewers of this article for their helpful comments.
The project was supported by NIH/NCCAM grant U01AT002570.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors have no conflicts of interest to declare.
Contributor Information
Cheryl Ritenbaugh, University of Arizona, Departments of Family and Community Medicine and Anthropology, Tucson, AZ.
Richard Hammerschlag, Oregon College of Oriental Medicine, Portland, OR.
Samuel F Dworkin, University of Washington, Department of Oral Medicine, School of Dentistry, Seattle, WA.
Mikel G Aickin, University of Arizona, Department of Family and Community Medicine, Tucson, AZ.
Scott D Mist, Oregon Health and Science University, School of Nursing and School of Medicine, Portland, OR.
Charles Elder, Kaiser Permanente Center for Health Research, Portland OR.
Richard E Harris, University of Michigan, Chronic Pain and Fatigue Research Center, Ann Arbor, MI.
References
- 1.Aickin M. A program for balancing the allocation of subjects to treatment in a clinical trial. Comput Biomed Res. 1982;15:519–524. doi: 10.1016/0010-4809(82)90014-3. [DOI] [PubMed] [Google Scholar]
- 2.Aickin M. Some large trial properties of minimum likelihood allocation. J Stat Plan Inference. 1983;8:11–20. [Google Scholar]
- 3.Aickin M. Randomization, balance, and the validity and efficiency of design-adaptive allocation methods. J Stat Plan Inference. 2001;94:97–119. [Google Scholar]
- 4.Aickin M. Effect of design-adaptive allocation on inference for a regression parameter: two-group, single and double covariate cases. Stat Probab Lett. 2009;79:16–20. [Google Scholar]
- 5.Aickin M. A simulation study of the validity and efficiency of design-adaptive allocation to two groups in the regresson situation. Int J Biostat. 2009;5(article 19 issue 1) doi: 10.2202/1557-4679.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Badawi E, Mehta N, Forgione A, Lobo S, Zawawi K. Efficacy of pulsed radio frequency energy therapy in temporomandibular joint pain and dysfunction. Cranio. 2004;22:10–20. doi: 10.1179/crn.2004.003. [DOI] [PubMed] [Google Scholar]
- 7.Allen A, Escobar J, Lehrer P, Gara M, Woolfolk R. Psychosocial treatments for multiple unexplained physical symptoms: A review of the literature. Psychosom Med. 2002;64:939–950. doi: 10.1097/01.psy.0000024231.11538.8f. [DOI] [PubMed] [Google Scholar]
- 8.Bell I, Cunningham V, Caspi O, Meek P, Ferro L. Development and validation of a new global well-being outcomes rating scale for integrative medicine research. BMC Complement Altern Med. 2004;4:1. doi: 10.1186/1472-6882-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bensky D, Barolet R. Chinese herbal medicine: formulas and strategies. Seattle: Eastland Press; 1990. [Google Scholar]
- 10.Brinkhaus B, Hummelsberger J, Kohnen R, Seufert J, Hempen CH, Leonhardy H, Nögel R, Joos S, Hahn E, Schuppan D. Acupuncture and Chinese herbal medicine in the treatment of patients with seasonal allergic rhinitis: a randomized-controlled clinical trial. Allergy. 2004;59:953–960. doi: 10.1111/j.1398-9995.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 11.Campbell C, Weisner C, LeResche L, Ray G, Saunders K, Sullivan M, Banta-Green C, Merrill J, Silverberg M, Boudreau D, Satre D, Von Korff M. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100:2541–2547. doi: 10.2105/AJPH.2009.180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Chen T. Chinese medical herbology and pharmacology. City of Industry, CA: Art of Medicine Press; 2004. [Google Scholar]
- 13.DeBar L, Vuckovic N, Schneider J, Ritenbaugh C. Use of complementary and alternative medicine for temporomandibular disorders. J Orofac Pain. 2003;17:224–236. [PubMed] [Google Scholar]
- 14.Dworkin S. Psychosocial impact of orofacial pain. In: Turp J, Sommer C, Hugger A, editors. The puzzle of orofacial pain. Basel: Karger AG; 2007. [Google Scholar]
- 15.Dworkin S, Truelove E. Temporomandibular disorders. In: Rakel R, editor. Conn’s current therapy. New York: W.B. Saunders; 1997. [Google Scholar]
- 16.Dworkin SF, Huggins KH, Wilson L, Mancl L, Turner JA, Massoth D, LeResche L, Truelove E. A randomized clinical trial using research diagnostic criteria for temporomandibular disorders-axis II to target clinic cases for a tailored self-care TMD treatment program. J Orofac Pain. 2002;16:48–63. [PubMed] [Google Scholar]
- 17.Dworkin SF, Turner JA, Mancl L, Wilson L, Massoth D, Huggins KH, LeResche L, Truelove E. A randomized clinical trial of a tailored comprehensive care treatment program for temporomandibular disorders. J Orofac Pain. 2002;16:259–276. [PubMed] [Google Scholar]
- 18.Dworkin SF, Turner JA, Wilson L, Massoth D, Whitney C, Huggins KH, Burgess J, Sommers E, Truelove E. Brief group cognitive-behavioral intervention for temporomandibular disorders. Pain. 1994;59:175–187. doi: 10.1016/0304-3959(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 19.Dworkin SF, Von Korff M, Le Resche L. Multiple pains and psychiatric disturbance: an epidemiologic investigation. Arch Gen Psychiatry. 1990;47:239–244. doi: 10.1001/archpsyc.1990.01810150039007. [DOI] [PubMed] [Google Scholar]
- 20.Elder C, Ritenbaugh C, Aickin M, Hammerschlag R, Dworkin S, Mist S, Harris RE. Reduction in medication use associated with traditional Chinese medicine for chronic pain. Perm J. 2012;16:18–23. doi: 10.7812/tpp/12.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gatchel R, Turk D, editors. Psychological approaches to pain management: a practitioner's handbook. New York, NY: Guilford Press; 2002. [Google Scholar]
- 22.Gatchel RJ, Stowell AW, Wildenstein L, Riggs R, Ellis E., III Efficacy of an early intervention for patients with acute temporomandibular disorder-related pain: a one-year outcome study. J Am Dent Assoc. 2006;137:339–347. doi: 10.14219/jada.archive.2006.0183. [DOI] [PubMed] [Google Scholar]
- 23.Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22:1596–1602. doi: 10.1007/s11606-007-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goddard G, Karibe H, McNeill C, Villafuerte E. Acupuncture and sham acupuncture reduce muscle pain in myofascial pain patients. J Orofac Pain. 2002;16:71–76. [PubMed] [Google Scholar]
- 25.Greene CS. Managing TMD patients: initial therapy is the key. J Am Dent Assoc. 1992;123:43–45. doi: 10.14219/jada.archive.1992.0188. [DOI] [PubMed] [Google Scholar]
- 26.Howie JG, Heaney DJ, Maxwell M, Walker JJ. A comparison of a Patient Enablement Instrument (PEI) against two established satisfaction scales as an outcome measure of primary care consultations. Fam Pract. 1988;15:165–171. doi: 10.1093/fampra/15.2.165. [DOI] [PubMed] [Google Scholar]
- 27.Institute of Medicine (IOM) Initial national priorities for comparative effectiveness research. Washington DC: The Institute of Medicine, The National Academies Press; 2009. pp. 37–39. [Google Scholar]
- 28.Jerjes W, Upile T, Abbas S, Kafas P, Vourvachis M, Rob J, Mc Carthy E, Angouridakis N, Hopper C. Muscle disorders and dentition-related aspects in temporomandibular disorders: controversies in the most commonly used treatment modalities. Int Arch Med. 2008;1:1–13. doi: 10.1186/1755-7682-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson A, Wenneberg B, Wagersten C, Haraldson T. Acupuncture in treatment of facial muscular pain. Acta Odontol Scand. 1991;49:153–158. doi: 10.3109/00016359109005900. [DOI] [PubMed] [Google Scholar]
- 30.Kulekcioglu S, Sivrioglu K, Ozcan O, Parlak M. Effectiveness of low-level laser therapy in temporomandibular disorder. Scand J Rheumatol. 2003;32:114–118. doi: 10.1080/03009740310000139. [DOI] [PubMed] [Google Scholar]
- 31.La Touche R, Goddard G, De-la-Hoz JL, Wang K, Paris-Alemany A, Angulo-Diaz-Parreo S, Mesa J, Hernandez M. Acupuncture in the treatment of pain in temporomandibular disorders: a systematic review and meta-analysis of randomized controlled trials. Clin J Pain. 2010;26:541–550. doi: 10.1097/AJP.0b013e3181e2697e. [DOI] [PubMed] [Google Scholar]
- 32.Lei H, Nahum-Shani I, Lynch K, Oslin D, Murphy SA. A "SMART" design for building individualized treatment sequences. Annual Review of Clinical Psychology. 2012;8:14.1–14.28. doi: 10.1146/annurev-clinpsy-032511-143152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeResche L, Drangsholt M. Temporomandibular disorders. In: Goldman M, Hatch M, editors. Women and health. San Diego: Academic Press; 2000. pp. 1120–1128. [Google Scholar]
- 34.List T, Helkimo M, Andersson S, Carlsson G. Acupuncture and occlusal splint therapy in the treatment of craniomandibular disorders. Part I. A comparative study. Swed Dent J. 1992;16:125–141. [PubMed] [Google Scholar]
- 35.Litt MD, Shafer DM, Kreutzer DL. Brief cognitive-behavioral treatment for TMD pain: Long-term outcomes and moderators of treatment. Pain. 2010;151:110–116. doi: 10.1016/j.pain.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manfredini D, Ahlberg J, Winocur E, Guarda-Nardini L, Lobbezoo F. Correlation of RDC/TMD axis I diagnoses and axis II pain-related disability. A multicenter study. Clin Oral Investig. 2011;15:749–56. doi: 10.1007/s00784-010-0444-4. [DOI] [PubMed] [Google Scholar]
- 37.Manfredini D, Marini M, Pavan C, Pavan L, Guarda-Nardini L. Psychosocial profiles of painful TMD patients. J Oral Rehabil. 2009;36:193–198. doi: 10.1111/j.1365-2842.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- 38.Menk Otto L, Howerter A, Bell I, Jackson N. Exploring measures of whole person wellness: integrative well-being and psychological flourishing. Explore. 2010;6:364–370. doi: 10.1016/j.explore.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mist S, Ritenbaugh C, Aickin M. Effects of questionnaire-based diagnosis and training on inter-rater reliability among practitioners of traditional Chinese medicine. J Altern Complement Med. 2009;15:703–709. doi: 10.1089/acm.2008.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohrbach R, Dworkin SF. Five-year outcomes in TMD: relationship of changes in pain to changes in physical and psychological variables. Pain. 1998;74:315–326. doi: 10.1016/s0304-3959(97)00194-2. [DOI] [PubMed] [Google Scholar]
- 41.Peroz I, Chun Y-H, Karageorgi G, Schwerin C, Bernhardt O, Roulet J-F, Freesmeyer WB, Meyer G, Lange K-P. A multicenter clinical trial on the use of pulsed electromagnetic fields in the treatment of Temporomandibular disorders. J Prosthet Dent. 2004;91:180–187. doi: 10.1016/j.prosdent.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Raphael K, Klausner J, Nayak S, Marbach J. Complementary and alternative therapy use by patients with myofascial Temporomandibular disorders. J Orofac Pain. 2003;17:36–41. [PubMed] [Google Scholar]
- 43.Raustia A, Pohjola R, Virtanen K. Acupuncture compared with stomatognathic treatment for TMJ dysfunction. Part I: A randomized study. J Prosthet Dent. 1985;54:581–585. doi: 10.1016/0022-3913(85)90440-8. [DOI] [PubMed] [Google Scholar]
- 44.Reivich K, Shatte A. The resilience factor: 7 essential skills for overcoming life's inevitable obstacles. New York: Broadway Books; 2003. [Google Scholar]
- 45.Ritenbaugh C, Hammerschlag R, Calabrese C, Mist S, Aickin M, Sutherland E, Leben J, deBar L, Elder C, Dworkin SF. A pilot whole systems clinical trial of traditional Chinese medicine and naturopathic medicine for the treatment of temporomandibular disorders. J Altern and Complement Med. 2008;14:475–487. doi: 10.1089/acm.2007.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosted P. Practical recommendations for the use of acupuncture in the treatment of temporomandibular disorders based on the outcome of published controlled studies. Oral Dis. 2001;7:109–115. [PubMed] [Google Scholar]
- 47.Schmid-Schwap M, Simma-Kletschka I, Stockner A, Sengstbratl M, Gleditsch J, Kundi M, Piehslinger E. Oral acupuncture in the therapy of craniomandibular dysfunction syndrome – a randomized controlled trial. Wien Klin Wochenschr. 2006;118:36–42. doi: 10.1007/s00508-005-0501-1. [DOI] [PubMed] [Google Scholar]
- 48.Shen YF, Goddard G. The short-term effects of acupuncture on myofascial pain patients after clenching. Pain Pract. 2007;7:256–264. doi: 10.1111/j.1533-2500.2007.00140.x. [DOI] [PubMed] [Google Scholar]
- 49.Smith P, Mosscrop D, Davies S, Sloan P, Al-Ani Z. The efficacy of acupuncture in the treatment of temporomandibular joint myofascial pain: a randomised controlled trial. J Dent. 2007;35:259–267. doi: 10.1016/j.jdent.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 50.Suvinen TI, Reade PC, Kemppainen P, Könönen M, Dworkin SF. Review of aetiological concepts of temporomandibular pain disorders: towards a biopsychosocial model for integration of physical disorder factors with psychological and psychosocial illness impact factors. Eur J Pain. 2005;9:613–633. doi: 10.1016/j.ejpain.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Taves D. Minimization: A new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15:443–453. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- 52.Truelove E, Huggins KH, Mancl L, Dworkin SF. The efficacy of traditional, low-cost and nonsplint therapies for temporomandibular disorder: a randomized controlled trial. J Am Dent Assoc. 2006;137:1099–1107. doi: 10.14219/jada.archive.2006.0348. [DOI] [PubMed] [Google Scholar]
- 53.Von Korff M, Dworkin SF, Le Resche L, Kruger A. An epidemiologic comparison of pain complaints. Pain. 1988;32:173–183. doi: 10.1016/0304-3959(88)90066-8. [DOI] [PubMed] [Google Scholar]
- 54.Xue C, Thien F, Zhang J, Yang W, DaCosta C, Li C. Effect of adding a Chinese herbal preparation to acupuncture for seasonal allergic rhinitis: randomised double-blind controlled trial. Hong Kong Med J. 2003;9:427–434. [PubMed] [Google Scholar]
- 55.Yap A, Chua E, Dworkin SF, Tan H, Tan K. Multiple pains and psychosocial functioning/psychologic distress in TMD patients. Int J Prosthodont. 2002;15:461–466. [PubMed] [Google Scholar]