Abstract
Objectives
Gallbladder and cholangiocarcinomas represent a heterogeneous group of malignant diseases that commonly present at an advanced stage and that have limited therapeutic options. Based on the role of the Ras-Raf-Mek-Erk pathway and the VEGF axis in biliary carcinomas, we conducted a phase II study of sorafenib in patients with advanced biliary cancers.
Methods
Eligible patients had no prior therapy for metastatic or unresectable disease. Sorafenib was administered at 400 mg po twice daily continuously.
Results
The study was terminated after the first stage of accrual due to failure to meet the primary objective. A confirmed response rate of 0% (0% – 11%) was observed. Thirty-nine percent of patients demonstrated stable disease (including 2 with unconfirmed PR). PFS was 3 months (95% CI: 2–4 months) and OS 9 months (95% CI: 4–12 months). The most common grade 3 and 4 toxicities included venous hand-foot skin reaction (13%), bilirubin elevation (13%), thromboembolism (10%), and AST/ALT elevation (10%) and elevated alkaline phosphatase (10%).
Conclusion
While treatment with sorafenib did not result in objective responses, patients with biliary cancers receiving this drug had some therapeutic benefit. Additional studies with sorafenib in combination with chemotherapy or other targeted agents may be warranted.
Keywords: sorafenib, gallbladder cancer, cholangiocarcinoma, biliary cancer
INTRODUCTION
Gallbladder and cholangiocarcinomas constitute a heterogeneous group of tumors that arise in the gallbladder, the intrahepatic bile ducts (intrahepatic cholangiocarcinoma, IHCC), the biliary bifurcation (hilar cholangiocarcinoma), or distally in the biliary tree (extrahepatic cholangiocarcinoma, EHCC). Epidemiologic studies have shown a recent increase in the incidence of cholangiocarcinoma in the United States and in various parts of the world [1]; for example, McGlynn and colleagues [2] reported an approximate doubling in the rates of hepatocellular carcinoma and intrahepatic cholangiocarcinoma between 1976 and 2000 based on the Surveillance Epidemiology and End Results program.
Most patients with gallbladder cancer or cholangiocarcinoma present with advanced disease that is not amenable to surgical resection, a situation in which the administration of palliative chemotherapy has become common practice. Historically, the data regarding the benefit of palliative chemotherapy was derived from small phase II studies with heterogeneous patient populations; the most commonly used agents included 5-fluorouracil (5FU) and gemcitabine which were administered alone or in combination with other drugs such as cisplatin, taxanes or etoposide [3–6]. More recently, the combination of gemcitabine and cisplatin was evaluated in a randomized phase III study and was superior to gemcitabine alone as manifested by an improved progression-free survival (PFS) and overall survival (OS) [7].
Given the modest efficacy of the various cytotoxic chemotherapy combinations, recent efforts have focused on improving the understanding of the molecular carcinogenesis underlying biliary cancers. These efforts have lead to the identification of several genes that may play a role in the development of biliary cancers and that present potential therapeutic targets. Tannapfel and colleagues [8] demonstrated that BRAF gene mutations were detected in 15 out of 69 (22%) human biliary carcinoma specimens. A mutation locus in nucleotide 1796 accounted for 11 out of 15 mutations. All cholangiocarcinomas with a BRAF mutation exhibited stronger immunostaining of the MAPK protein, with a median of 69% positive tumor cells. Yoon and associates [9] showed that Raf-1 inhibitors rendered cholangiocarcinoma cells more susceptible to apoptosis by blocking the increase in Mcl-1, an anti-apoptotic protein. Another gene that is thought to be involved in biliary carcinomas is the vascular endothelial growth factor receptor (VEGF). Benckert and colleagues [10] found VEGF to be expressed in 19 out of 19 tumor specimens from patients with cholangiocarcinoma. In a retrospective evaluation of 236 cases of cholangiocarcinoma, VEGF was overexpressed in 53.8% and 59.2% of intrahepatic and extrahepatic cholangiocarcinomas respectively [11].
Sorafenib is a multi-targeted kinase inhibitor of vascular endothelial growth factor receptors (VEGFR) 2 and 3, platelet derived growth factor receptor and Raf kinase. The anti-tumor effect of Sorafenib is thought to be mediated through its inhibition of the Ras-Raf-Erk pathway involved in cell proliferation as well as its inhibition of VEGFR2 related angiogenesis [12]. This phase II study was designed to test the hypothesis that inhibition of the Ras-Raf pathway as well as the VEGF axis in patients with biliary cancers would result in significant tumor responses and improved progression free survival. The primary objective was to determine the confirmed objective response rate in patients with advanced biliary cancers treated with sorafenib 400 mg twice a day. Secondary endpoints included PFS, OS, and adverse event profile.
MATERIALS AND METHODS
Eligibility Criteria
Patients eligible for this study (ClinicalTrials.govIdentifier:NCT00238212) had cytologically or pathologically confirmed diagnosis of gallbladder carcinoma or cholangiocarcinoma that was surgically unresectable or metastatic. Measurable disease was required. While prior therapy for unresectable or metastatic disease was not allowed, previous chemotherapy or radiation therapy administered in the neoadjuvant or adjuvant settings were permitted, but must have been completed at least 12 months prior to the documentation of recurrence. Other eligibility criteria included a Zubrod performance status of 0 to 1, adequate hepatic function with a total bilirubin up to 3x the upper limit of normal; AST or ALT levels ≤ 2.5 the upper limit of normal or ≤ 5x upper limit of normal in the presence of liver metastases; creatinine ≤ 1.5x the upper limit of normal or creatinine clearance ≥ 60 ml/min; adequate bone marrow function indicated by a leukocyte count ≥ 3000/mcl, absolute neutrophil count ≥ 1000/mcL, and platelet count ≥ 100,000/mcL; prothrombin time and partial thromboplastin times ≤ ULN.
Sorafenib Administration
All patients received sorafenib 400 mg orally twice daily on a continuous basis. One treatment cycle was 28 days. Sorafenib was supplied by the division of Cancer Treatment and Diagnosis, National Cancer Institute (Bethesda, MD). Treatment was held for grade 2 mucositis and any grade 3 or 4 toxicity, including diarrhea, dermatologic toxicities, AST/ALT or bilirubin elevation. In the case of hypertension, sorafenib was held for grade 2 symptomatic or persistent hypertension, for diastolic blood pressure ≥ 110 mmHg, or grade 3 hypertension; treatment was restarted with one dose level reduction once diastolic BP was ≤ 100 mmHg and patient had been started on antihypertensive therapy. The dose reductions of sorafenib were defined pre-study with dose level -1 being 400 mg once daily, and dose level -2 being 400 mg every other day. Patients requiring treatment interruption for more than 4 weeks or requiring more than 2 dose reductions were removed from protocol treatment.
Disease Assessment
Patient response was assessed every 8 weeks using the Response Evaluation Criteria in Solid Tumors classification (RECIST) 1.0. Measurable disease was defined as at least one lesion for which the longest diameter could be accurately measured as ≥ 1 cm using spiral computed tomography, or ≥ 2 cm using conventional computed tomography or magnetic resonance imaging. All other lesions, including ascites and pleural effusions, were considered non-measurable.
Patients who met stable disease criteria at least once after study entry at a minimum interval of 6 weeks were considered to have achieved disease stabilization. A best response of partial response (PR) required two or more objective statuses of PR or better at a minimum of 4 weeks apart. Similarly, a best response of complete response required two or more objective statuses of complete response at a minimum of 4 weeks apart. Progression free survival (PFS) was calculated from date of registration to date of first observation of progressive disease, death due to any cause, or symptomatic deterioration. Patients last known to be alive and progression free are censored at last date of contact.
Toxicity Assessment
Patients were evaluated for treatment-related toxicity at a minimum of every 14 days as per the National Cancer Institute Common Terminology Criteria for adverse events version 3.0. The worst grade of toxicity per patient was recorded in each cycle. Blood pressure was monitored weekly for the first 4 weeks and every 14 days thereafter.
Statistical Considerations
The primary endpoint of the trial was to assess the response probability (confirmed complete and partial responses). Secondary endpoints included overall survival (OS) and progression free survival. It was assumed that sorafenib would be of interest for further study if the overall true response probability was 20% or more, and of no further interest if it were 5% or less. A two-stage design was used to evaluate response [13]. If, after the first 25 patients were accrued, we observed at least one response, the study was to accrue an additional 25 patients to the second stage. Sorafenib was to be considered of interest if 6 or more responses were noted out of the total of 50 patients.
RESULTS
Patient Characteristics
A total of 36 patients were accrued over a period of 10 months. Five patients were ineligible due to elevated coagulation parameters. The median age for eligible patients was 57.8 years (range of 33 to 81), 15 patients (48%) were male, 13 patients (42%) had Zubrod performance status of 0 and 18 (58%) had a performance status of 1 (Table 1).
Table 1.
patient baseline characteristics
| Characteristics | Patients (n=31) |
|---|---|
|
| |
| Age (years) | |
| Median | 57 years |
| Range | 33 yrs 81 yrs |
|
| |
| Gender | |
| Male | 15 (48%) |
| Female | 16 (52%) |
|
| |
| Race | |
| Asian | 0 |
| Black | 4 (13%) |
| Caucasian | 24 (77%) |
| Multi-racial | 1 (3%) |
| Native American | 1 (3%) |
| Unknown | 1 (3%) |
| Hispanic | |
| Yes | 1 (3%) |
| No | 26 (84%) |
| Unknown | 4 (13%) |
|
| |
| Primary Site | |
| Gallbladder | 12 (39%) |
| Cholangiocarcinoma | 19 (61%) |
|
| |
| Prior adjuvant chemotherapy | 1 (3%) |
|
| |
| Zubrod PS | |
| 0 | 13 (42%) |
| 1 | 18 (58%) |
Sorafenib Administration
The median number of cycles administered was 2 with a range of 1 to 8. Reasons for discontinuation of treatment included progressive disease (20 patients; 66.7 %), adverse events (9 patients; 29 %), death (1 patient; 3 %), and investigator indicating progressive disease not meeting RECIST criteria (1 patient; 3%)
Toxicity
All eligible patients were assessable for toxicity. One patient died after having grade 4 pulmonary embolism, as well as grade 4 atrial fibrillation. Grade 3 and 4 toxicities were noted in 20 additional patients (66.7%) (Table 2). The most common grade 4 toxicity was venous thrombosis/embolism in 3 patients (10%). Other grade 4 toxicities occurred in 1 patient each and included hypertension with reversible posterior leukoencephalopathy syndrome, fatigue, bilirubin elevation, ALT elevation, and cardiac arrhythmia. The most common grade 3 toxicities were hand-foot syndrome in 4 patients (13%), bilirubin elevation in 3 patients (10%), AST/ALT elevation in 3 patients (10%), gastrointestinal bleeding in 2 patients (6%), nausea in 2 patients (6%), vomiting in 2 patients (6%), and rash in 2 patients (6%).
Table 2.
Grade 3 and 4 adverse events related to study drug that occurred in ≥ 2 patients
| Patients (N = 31) | |
|---|---|
| Non-hematologic | |
| Venous thromboembolism | 3(10%) |
| Bilirubin elevation | 4 (13%) |
| Hand-foot skin reaction | 4 (13%) |
| AST/ALT elevation | 3 (10%) |
| Abdominal pain | 3 (10%) |
| Rash | 2 (6%) |
| Gastrointestinal bleeding | 2 (6%) |
| Fatigue | 2 (6%) |
| Hypertension | 2 (6%) |
| Nausea | 2 (6%) |
| Vomiting | 2 (6%) |
Efficacy
The study was terminated after the first stage of accrual because of failure to meet the requirement of a minimum of one confirmed PR. Response was adequately assessed in 25 out of 31 patients; five patients were removed from treatment prior to the first disease assessment. No confirmed responses were noted. Two patients (6%) achieved unconfirmed PR. Responses were not confirmed because one patient died with pulmonary embolism prior to confirmation of PR and the other had progression of disease on the subsequent scan. Ten (32%) patients had stable disease with a median time to progression of 4.4 months (95% CI: 3–7 months). Fourteen patients (45%) had progression of disease. All 31 patients have died with a median OS of 9 months (95% CI: 4–12 months) (Fig. 1) and median PFS of 3 months (95% CI: 2 to 4 months) (Fig. 2).
Fig. 1.
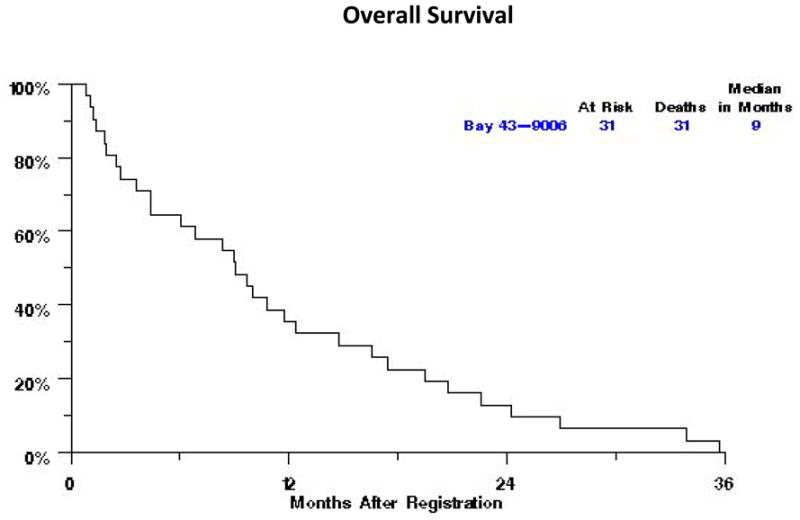
Overall survival for the patients treated with single agent Sorafenib
Fig. 2.
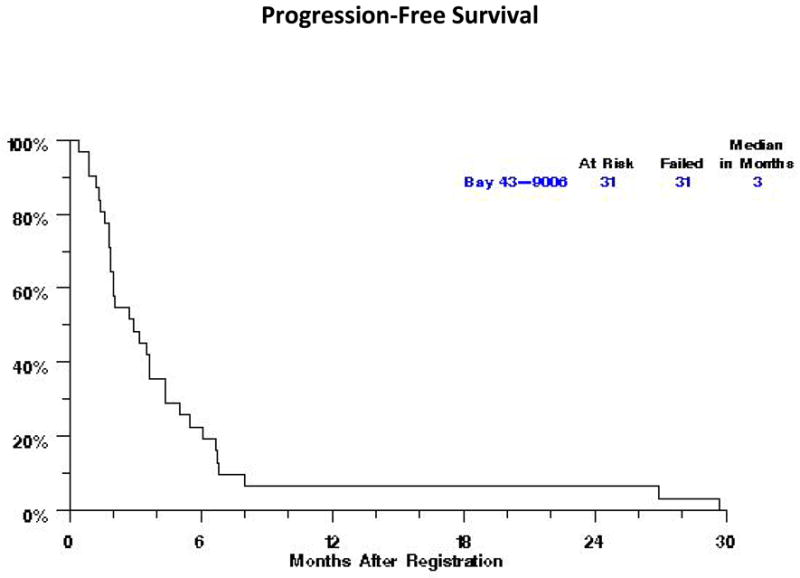
Progression-free survival for the patients treated with singleagent Sorafenib
DISCUSSION
Advanced biliary cancers pose a significant therapeutic challenge for multiple reasons that include the limited efficacy of cytotoxic chemotherapy, rarity of the disease leading to difficulties in conducting large randomized studies, and heterogeneity of the disease. Sorafenib has manifested anti-tumor activity in various cholangiocarcinoma cell lines and in xenograft models; the activity appears to have been mediated through the inhibition of the JAK/STAT3 signaling axis and the sensitization of cells to tumor necrosis factor related apoptosis-inducing ligand-mediated apoptosis [14, 15]. This phase II study was designed to explore the efficacy of sorafenib, as a single agent, in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. The study was closed after the first stage of accrual because it did not meet the predetermined minimum requirement of one confirmed complete or partial response in the first 25 patients based on a two-stage design. While the study failed to meet its primary endpoint of response rate, the patients accrued had a PFS of 3 months and an OS of 9 months.
In retrospect, selection of objective response rate as the primary endpoint of this trial was not optimal for the evaluation of the single agent activity of a biologic targeted drug such as sorafenib. This issue is clearly highlighted in the treatment of advanced hepatocellular carcinoma, where sorafenib improved survival without manifesting a significant objective response rate [16]. Therefore, it is possible that the discontinuation of our study due to the absence of a confirmed objective response in the first stage may have prevented a more accurate assessment of the activity of sorafenib as a single agent in advanced biliary cancers. The stable disease rate of 39% (including two unconfirmed partial responses) and the interesting survival outcomes may be suggestive of a modest degree of anti-tumor activity for sorafenib. More specifically, the PFS of 3 months and OS of 9 months are within the range reported for several combinations of cytotoxic drugs. The combination of gemcitabine and capecitabine, evaluated within the same national cooperative group (SWOG0202), resulted in a stable disease rate of 27% and a median overall survival of 7 months [17]. In a meta-analysis of 104 trials evaluating a variety of chemotherapy regimens for patients with advanced biliary cancers, Eckel and associates [6] reported a median TTP of 4.1 months (60 trials, 1543 patients) and a median OS of 8.2 months (82 trials, 2197 patients). In contrast, the potential single agent activity of sorafenib appears more limited in comparison with the outcomes noted with the combination of gemcitabine and cisplatin in the randomized phase III study against gemcitabine alone [7].
Another phase II study of sorafenib as a single agent in patients with advanced cholangiocarcinoma has been performed with the primary endpoint of disease control, defined as lack of progression, at 12 weeks. With 46 patients enrolled and 24 of them (52%) evaluated for response, the authors reported a disease control rate of 32%. However, the median PFS and OS were 2.3 and 4.4 months respectively. One possible explanation for the inferior survival outcomes noted by Bengala and associates [18] in this study is the fact that 56% of patients had one or more prior lines of chemotherapy prior to being treated with sorafenib on study. Of note, patients with ECOG performance status of 0 had a PFS of 5.7 months and an OS of 8.8 months.
Our study has several limitations, including the small number of patients and the high rate of grade 3 and 4 toxicities. The 67% rate of grade 3 or higher adverse events and the 29% rate of treatment discontinuation secondary to toxicity are of concern and may have been averted by more strict eligibility criteria such as a bilirubin level that is within the upper limits of normal. Furthermore, this rate of adverse events in a small phase II study may have impacted our ability to accurately assess the anti-tumor activity of sorafenib. Another limitation of our study and others that have evaluated molecularly targeted agents is the incorporation of all biliary cancers into one group, irrelevant of the site of origin. The response rate and survival outcomes appear to be different among patients with IHCC versus gallbladder carcinoma versus EHCC. Several clinical trials evaluating cytotoxic chemotherapy suggest that response rate is higher in patients with gallbladder carcinoma and EHCC in comparison to IHCC, and PFS is higher in patients with EHCC [19–21]. In addition to these clinical observations, recent investigations into the molecular biology of biliary tract cancers support the clinical observation of differential outcome based on location. In a study of 128 patients who underwent resection for biliary cancers, significant differences in p27, cyclin D1, and Bcl2 expression were observed according to anatomic location, all of which were proportionally higher in IHCC [22]. In a different study by Yoshikawa and associates [11], the expression of EGFR was 27% in IHCC versus 19% in EHCC; similarly, the expression of HER2 was 0.9% in IHCC versus 8.5% in EHCC. These data should provide a strong rational for the stratification of patients based on tumor location in future studies, especially ones that incorporate molecularly targeted therapies. Our study was not designed and powered to explore differences in outcome based on the site of origin of the tumor, but an exploratory analysis indicated a PFS of 4 months for the 12 patients with gallbladder carcinoma and 2 months for the 19 patients with cholangiocarcinoma including both IHCC and EHCC (data not shown).
We conclude that sorafenib has marginal to limited activity as a single agent in patients with advanced cholangiocarcinoma. However, the preclinical data and the suggestion of clinical efficacy in our small phase II study lend support to further evaluation of sorafenib in combination with other targeted agents or in combination with cytotoxic chemotherapy.
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, NIH, DHHS: CA32102, CA38926, CA20319, CA105409, CA37981, CA16385, CA46441, CA35090, CA35431, CA46282, CA58882, CA45807, CA35176, CA67575, CA45560, CA45808, CA63844, CA12644, CA11083
References
- 1.Cardinale V, et al. Intra-hepatic and extra-hepatic cholangiocarcinoma: New insight into epidemiology and risk factors. World J Gastrointest Oncol. 2010;2(11):407–16. doi: 10.4251/wjgo.v2.i11.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1198–203. doi: 10.1158/1055-9965.EPI-05-0811. [DOI] [PubMed] [Google Scholar]
- 3.Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13(4):415–23. doi: 10.1634/theoncologist.2007-0252. [DOI] [PubMed] [Google Scholar]
- 4.Choi CW, et al. Effects of 5-fluorouracil and leucovorin in the treatment of pancreatic-biliary tract adenocarcinomas. Am J Clin Oncol. 2000;23(4):425–8. doi: 10.1097/00000421-200008000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Ducreux M, et al. A randomised phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur J Cancer. 2005;41(3):398–403. doi: 10.1016/j.ejca.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96(6):896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valle J, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 8.Tannapfel A, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52(5):706–12. doi: 10.1136/gut.52.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon JH, et al. Bile acids inhibit Mcl-1 protein turnover via an epidermal growth factor receptor/Raf-1-dependent mechanism. Cancer Res. 2002;62(22):6500–5. [PubMed] [Google Scholar]
- 10.Benckert C, et al. Transforming growth factor beta 1 stimulates vascular endothelial growth factor gene transcription in human cholangiocellular carcinoma cells. Cancer Res. 2003;63(5):1083–92. [PubMed] [Google Scholar]
- 11.Yoshikawa D, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98(2):418–25. doi: 10.1038/sj.bjc.6604129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilhelm SM, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 13.Green SJ, Dahlberg S. Planned versus attained design in phase II clinical trials. Stat Med. 1992;11(7):853–62. doi: 10.1002/sim.4780110703. [DOI] [PubMed] [Google Scholar]
- 14.Blechacz BR, et al. Sorafenib inhibits signal transducer and activator of transcription-3 signaling in cholangiocarcinoma cells by activating the phosphatase shatterproof 2. Hepatology. 2009;50(6):1861–70. doi: 10.1002/hep.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugiyama H, et al. Potent in vitro and in vivo antitumor activity of sorafenib against human intrahepatic cholangiocarcinoma cells. J Gastroenterol. 2011 doi: 10.1007/s00535-011-0380-3. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal S, Blanke C, Lenz HJ. SWOG S0202: A phase II trial of gemcitabine and capecitabine in patients (pts) with unresectable or metastatic gallbladder cancer or cholangiocarcinoma. Abstract # 4131. Proceedings of the American Society of Clinical Oncology. 2006 [Google Scholar]
- 18.Bengala C, et al. Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer. 2010;102(1):68–72. doi: 10.1038/sj.bjc.6605458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yonemoto N, et al. A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol. 2007;37(11):843–51. doi: 10.1093/jjco/hym116. [DOI] [PubMed] [Google Scholar]
- 20.Nehls O, et al. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br J Cancer. 2008;98(2):309–15. doi: 10.1038/sj.bjc.6604178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harder J, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer. 2006;95(7):848–52. doi: 10.1038/sj.bjc.6603334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarnagin WR, et al. Differential cell cycle-regulatory protein expression in biliary tract adenocarcinoma: correlation with anatomic site, pathologic variables, and clinical outcome. J Clin Oncol. 2006;24(7):1152–60.f. doi: 10.1200/JCO.2005.04.6631. [DOI] [PubMed] [Google Scholar]


