Abstract
Childhood presentations of ataxia, an impairment of balance and coordination caused by damage to or dysfunction of the cerebellum, can often be challenging to diagnose. Presentations tend to be clinically heterogeneous but key considerations may vary based on the child's age at onset, the course of illness, and subtle differences in phenotype. Systematic investigation is recommended for efficient diagnosis. In this review, we outline common etiologies and describe a comprehensive approach to the evaluation of both acquired and genetic cerebellar ataxia in children.
Keywords: ataxia, autosomal recessive, cerebellum, children, spinocerebellar ataxia
Introduction
Balance and coordination problems in children can point to a variety of underlying causes and may involve elements of the central or peripheral nervous system. When unrelated to muscle weakness, this symptom is referred to as ataxia and generally involves disorders affecting the vestibular system, the cerebellum, and/or the peripheral sensory nerves. In this review, we will focus primarily on disorders affecting the cerebellum that result in impairment due to disturbance of the regulatory system that modulates motor commands based on the integration of multimodal sensory input from the environment.1 This lack of corrective feedback significantly impacts rhythmic and coordinated motor activity,1 leading to characteristic clinical findings such as an unsteady or wobbling gait, dysmetria, dysarthria, dysphagia, and various abnormalities of eye movement. In slowly progressive cases, children may initially complain of muscle weakness or feelings of dizziness, and a detailed clinical evaluation of coordination should always be performed as part of a comprehensive neurological examination to ensure that cerebellar function is normal.
The causes of cerebellar ataxia are myriad,2-7 and can be divided into 3 categories: acquired, hereditary, and idiopathic. For the purposes of this discussion, we will focus on the acquired and hereditary causes. Specific historical or clinical features can direct physicians in their evaluation6 and a thorough history of illness and clinical examination is extremely important. Subsequent workup should be tailored toward the most likely etiologies, with the caveat that acquired causes must always be preeminent in the clinician's mind, as early detection and treatment will maximize good outcomes. If cerebellar damage is allowed to proceed unchecked, the outcome may be devastating for the child, with significant residual neurological deficits over his or her lifetime. In this article, we discuss the various causes of cerebellar ataxia and suggest ways to efficiently maximize their evaluation and diagnosis.
Acquired Causes of Cerebellar Ataxia in Children
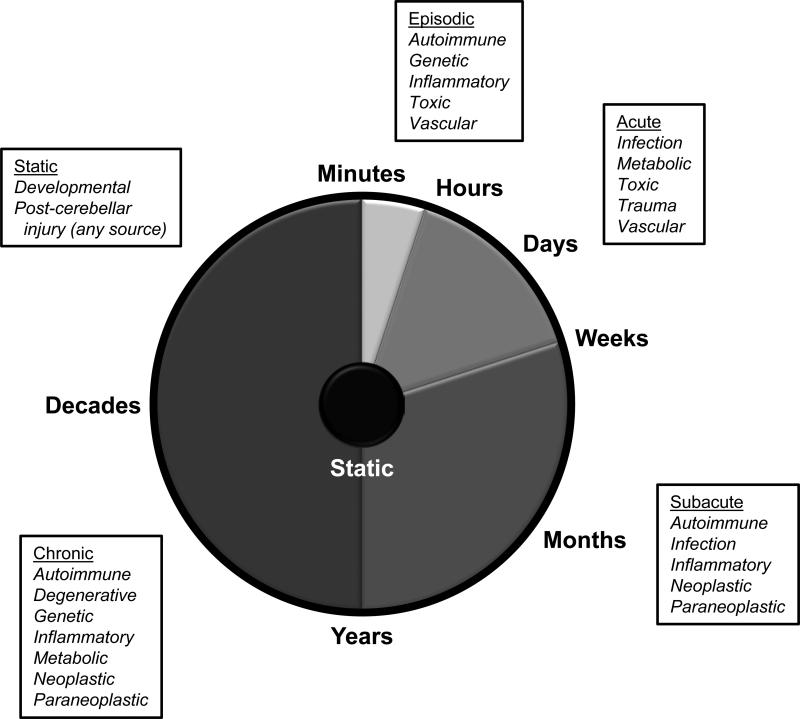
A wide variety of disorders can lead to clinical ataxia, and virtually the entire categorical spectrum of medical disease must be considered to some degree.2-7 Tempo of illness (Figure 1) can be useful in developing a differential diagnosis highlighting different etiologies showing congenital, acute/subacute, and chronic courses, as discussed below. Prompt identification of acquired etiologies in progressive ataxic disorders (Figure 2) is paramount, as corrective treatments may halt the degenerative process and preserve cerebellar functioning.2
Figure 1.
Differential diagnosis of ataxia for given rates of disease progression. Various tempos of cerebellar disease are shown with common etiologies for consideration indicated.
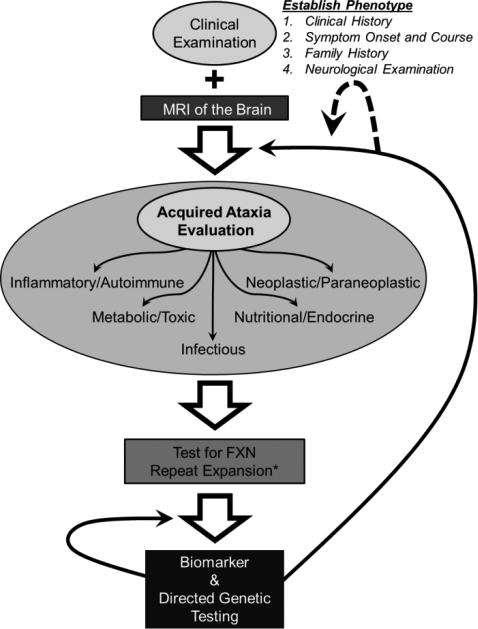
Figure 2.
Evaluation of the child with cerebellar ataxia. All patients require a through clinical examination to establish the phenotype of their disease. This includes a complete history of illness with onset of symptoms and detailed course, family history, and neurological examination. MRI of the brain can provide additional detail as well as identify certain vascular, developmental, traumatic, and neoplastic etiologies, among others. A detailed evaluation of acquired causes, particularly those amenable to treatment, follows. Such evaluation should be targeted to etiologies suggested from the clinical examination. If a genetic etiology is suspected, all patients should be tested for repeat expansion in the frataxin (FXN) gene. FXN sequencing for point mutations should only be performed in patients with an identified expansion on one allele (*). If normal, biomarker screening should be performed and, if negative, followed by genetic testing directed toward the identified phenotype. If unsuccessful, re-evaluation can include additional genetic or acquired testing (black arrows), particularly if new symptoms arise, prompting a re-definition of the patient's phenotype (dashed arrow).
Congenital Causes
When evaluating a patient for the first time, determining whether a coordination problem is congenital can often be difficult. Although congenital ataxias are static and nonprogressive, children with early-onset progressive ataxia and cerebellar atrophy may be difficult to distinguish, especially when one considers that congenital patients may show cerebellar hypoplasia on magnetic resonance imaging (MRI).8 Obtaining imaging early in cases of ataxia observed before the age of 5 years provides a baseline that can be helpful in evaluating older children for progression of any observed cerebellar atrophy. Historical information is also extremely valuable, as congenital patients frequently come to attention around the time of ambulation, but symptoms may have been present earlier. Conversely, subtle congenital cases can often be overlooked, particularly in new parents who may interpret mild gait or hand ataxia as normal in a toddler. Descriptions of play activity, feeding behavior, etc. may help identify clues to the initial appearance of ataxia findings. As video technology has become a more widely available feature on basic cameras and cellular phones, many families can provide brief videos of children at different time points for objective clinical assessment in the normal home environment.
Additional acquired causes of congenital ataxia can include secondary damage from neonatal hypoxic-ischemic encephalopathy or intrauterine strokes.8 Posterior fossa malformations, such as Dandy-Walker syndrome, or cerebellar dysgenesis can also be responsible,9,10 but can often be identified by MRI. Cerebral palsy is also a consideration, but such a diagnosis must be well-documented, as it can often be a common mislabel applied to patients with other early-onset forms of ataxia, such as genetic disease.11 This illustrates an important point when initially assessing older children or adults with ataxia who carry congenital diagnoses such as cerebral palsy — does the diagnosis remain well-supported over time? In some unfortunate cases, patients with early-onset progressive disease, originally labeled as congenital, may not be re-evaluated until much later in life as their diagnosis is passed from physician to physician over time (Figure 3).
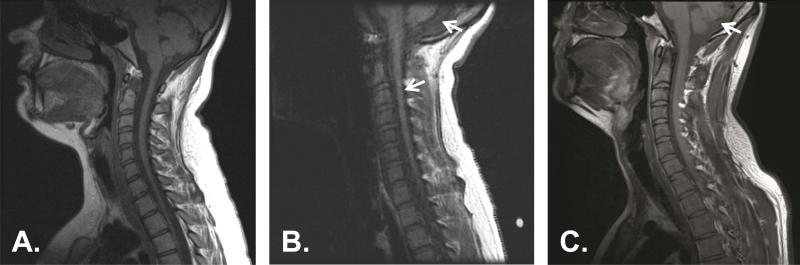
Figure 3.
Neuroimaging in acquired and genetic cerebellar ataxia and diagnostic mislabeling. Sagittal T1-weighted MRI of the cervical spine and posterior fossa is shown for 3 adolescent patients. (A) Unaffected individual showing normal cerebellar vermis and cervical spinal cord. (B) A patient with genetically-proven autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS), demonstrating atrophy of the midline cerebellar vermis and thinning of the cervical spinal cord (arrows). This patient presented having carried a diagnosis of cerebral palsy throughout her entire childhood. (C) A patient with autoimmune-mediated ataxia showing midline cerebellar vermian atrophy (arrow). This patient presented with a clinical diagnosis of Friedreich ataxia despite acute and severe symptom onset and had a subsequently negative gene test . Acquired workup showed mild elevations of several autoantibodies but was otherwise unremarkable, so the patient was given an empiric course of steroids, resulting in stabilization of symptoms over the following year.
Acute Causes
In children with an abrupt onset of cerebellar ataxia, various causes must be considered.12 Trauma, intoxication, infection, and stroke are 4 areas of particular concern owing to their serious nature and potential complications. Evaluation by neuroimaging can be helpful in determining a diagnosis and, in general, MRI is the modality of choice, given the increased detail of the posterior fossa it provides.12
Ataxia can be a common finding after concussion but warrants detailed evaluation when occurring immediately after trauma.13 Neck trauma should also be noted,12 as posterior circulation strokes in children are typically due to vertebral artery dissection.14 Intoxication is common as well, particularly in adolescents who ingest substances for recreational purposes (eg, prescription or illicit drugs, alcohol, organic solvents, etc.).12 In younger children, accidental ingestion (eg, anticonvulsants, benzodiazepines, etc.) must also be considered.12 Mental status changes are often associated,12 and diagnostic evaluation should include urine and blood toxicology screening as well as tests for common medications that cause ataxia.
Postinfectious etiologies can lead to cerebellar dysfunction, presumably through an autoimmune-mediated inflammatory cerebellitis.12,15 Viral causes (eg, enterovirus, Epstein-Barr, hepatitis A, herpes simplex, influenza, measles, mumps, parvovirus B19, varicella) are most common, but bacterial cases do occur as well (eg, legionella or mycoplasma).12,15 In general, this symptom is self-limiting and resolves,12 but patients should be monitored for sequelae requiring symptomatic treatment, and some patients do retain permanent deficits.
Subacute and Chronic Causes
Important categories of disease represented in patients presenting with subacute or chronic disease include nutritional and endocrine disorders, inflammatory or autoimmune disease, infections, and neoplastic/paraneoplastic disease. Nutritional causes can include various deficiencies (eg, vitamin B12, vitamin E, folate, copper, etc.)2,3,5 and these should be screened for thoroughly, particularly in patients with restricted diets or malnutrition. Diabetes is a common cause of ataxia in adults, often due to a sensory neuropathy,2,3,5 and consideration in children is important as well, both because of the effects on the peripheral nervous system and the observation that some genetic ataxias (most notably Friedreich ataxia16) can be associated with the disease.
Autoimmune disease is likely underdiagnosed in adults with clinical ataxia but there is limited literature on association with ataxia in children. This should be screened for using a test for antinuclear antibodies, at a minimum, particularly if there are overt systemic findings consistent with autoimmune disease. The observation that mild elevations can be normal in some children is an important caveat.17 The observance of other autoantibodies associated with cerebellar ataxia (eg, anti-thyroid, anti-gliadin, anti-glutamic acid decarboxylase, others), although not well-documented in children with ataxia,18 should warrant further investigation if observed.2-5 While the precise role of these antibodies in ataxia is unclear, their detection may be relevant to the diagnostic workup should all other testing prove to be unremarkable. Evaluation and treatment of diseases typically associated with these antibodies is generally the initial course of management, but in cases with isolated antibodies and progressive ataxia, an empiric course of steroids or other treatments could be considered if not otherwise contraindicated (Figure 3).19
Neoplastic and paraneoplastic conditions can occur in cases with slowly developing cerebellar ataxia with or without additional neurological features. Posterior fossa tumors are common tumors in children, often featuring ataxia, and early recognition and diagnosis is important for treatment.20 In patients under 3 years, the opsoclonus-myoclonus-ataxia syndrome is important to recognize, as it is often associated with neuroblastoma.20 Other cancers can also be associated with childhood ataxia,12 but leukemia is notable, as children with ataxia telangiectasia are at a significantly increased risk for developing this disease.21
Genetic Causes of Cerebellar Ataxia in Children
Genetic Ataxias
In adults, genetic ataxias are often best thought of as diagnoses of exclusion because, with the exception of ongoing clinical trials, there are no established therapies to treat the disorders.2,3,5,7,22,23 In contrast, a few rare childhood-onset genetic ataxias have established treatments that can, in some cases, arrest or slow the disease course if identified and treated early.2,23 It is therefore important for the physician to recognize these cases so therapy can begin. Unfortunately, for the majority of genetic ataxias, including the most common, established treatments are still lacking, so screening must follow or be coupled with an evaluation for acquired causes to ensure that treatable etiologies have been excluded.2 Symptomatic therapies are otherwise the mainstay of treatment but physical therapy, in particular, can be quite effective in restoration and maintenance of function, even in progressive disease.2,24,25
Prior to testing, all patients suspected of having genetic disease require genetic counseling and consideration for referral to a genetics or neurogenetics specialist if there is diagnostic confusion. The use of genetic panels to evaluate large numbers of genes is, in general, not recommended because of high cost and poor diagnostic efficiency.2,22 This disparity will likely further widen as more genes are added to such panels.26
Autosomal Recessive Ataxias
Diseases showing an autosomal recessive pattern of inheritance are the most common class of genetic ataxia seen in children, with typical onset before the age of 20 years.2,23 Milder variants of these disorders can present in adulthood, so they remain a consideration in older individuals as well.2,23 Incidence is approximately 4 cases per 100000 persons worldwide.2,23 Although family history is often absent, the presence of multiple affected siblings and/or consanguinity can suggest this form of inheritance.27
Clinically, autosomal recessive genetic ataxias typically develop as slowly progressive and symmetrical gait and limb ataxia, often associated with sensory or sensorimotor polyneuropathy.2,23 Involvement of other organ systems outside the central nervous system can often be seen as well, and can be useful diagnostically.2,23 Classification of the autosomal recessive genetic ataxias is particularly challenging. Many schemes classify these conditions based upon the involved gene or the molecular mechanism of pathogenesis,5,7 which, although helpful in grouping disorders for research purposes, is less helpful clinically. We previously have developed a clinically-based classification scheme,23 but since its development have observed that, as new disorders are rapidly being discovered, certain categories have become overbalanced and less useful to the clinician. The same has been observed for clinical schemes involving the autosomal dominant ataxias.22,28 We currently use a scheme defining age of onset as an initial classifier coupled with key phenotypic features (Table 1) and have found this to be useful. As mentioned above, however, onset can vary among these conditions (eg, virtually all childhood-onset genetic ataxias have been found as rare adult-onset forms), but disorders with a typical adult age of onset are considered elsewhere.2 Evaluation by a geneticist or neurogeneticist with experience in cerebellar ataxia should be considered in cases where diagnosis proves elusive.
Table 1.
Autosomal Recessive Cerebellar Ataxias
| DISEASE | LOCUS | GENE | YEAR IDENTIFIED | PROTEIN | FUNCTION | KEY CLINICAL FEATURES | |
|---|---|---|---|---|---|---|---|
| Class I: Friedreich Ataxia | |||||||
| Friedreich ataxia | FRDA | 9q21.11 | FXN | 1996 | frataxin | mitochondrial metabolism | cardiomyopathy polyneuropathy |
| Class II: Early-onset | |||||||
| Ataxia telangiectasia | AT | 11q22.3 | ATM | 1995 | ataxia-telangiectasia mutated | DNA repair | oculomotor apraxia telangiectasias |
| Ataxia telangiectasia-like disorder | ATLD | 11q21 | MRE11A | 1999 | meiotic recombination-11 | DNA repair | oculomotor apraxia |
| Autosomal recessive ataxia of Charlevoix-Saguenay | ARSACS | 13q12 | SACS | 2000 | sacsin | protein folding and/or quality control | hyper-reflexia pyramidal signs |
| Cayman ataxia | CA | 19p13.3 | ATCAY | 2003 | caytaxin | glutamate signaling | psychiatric symptoms pure cerebellar |
| Infantile-onset spinocerebellar ataxia | IOSCA | 10q24 | C10orf2 | 2005 | twinkle, twinky | mitochondrial DNA metabolism | extrapyramidal signs polyneuropathy |
| Marinesco-Sjögren syndrome | MSS | 5q31 | SIL1 | 2005 | BiP-associated protein | protein folding and/or quality control | cataracts myopathy |
| Autosomal recessive cerebellar ataxia, type 2 | ARCA2 | 1q42.13 | ADCK3 | 2008 | aarF domain-containing kinase-3 | CoQ10 synthesis, mitochondrial metabolism | intellectual disability pure cerebellar |
| Dysequilibrium syndrome | DES | 9p24 | VLDLR | 2008 | very low density lipoprotein receptor | intracellular signaling | intellectual disability quadrupedal gait |
| 8q12.1 | CA8 | 2009 | carbonic anhydrase VIII | intracellular calcium signaling | |||
| Peroxin-associated ataxias | PEX | 8q21.1 | PEX2 | 2011 | peroxin 2 | peroxisomal metabolism | pure cerebellar |
| 1p36.32 | PEX10 | 2010 | peroxin 10 | peroxisomal metabolism | polyneuropathy | ||
| 11p11.2 | PEX16 | 2010 | peroxin 16 | peroxisomal metabolism | cataracts hyper-reflexia | ||
| Rundataxin ataxia | RDTX | 3q29 | KIAA0226 | 2010 | rundataxin | autophagosome function and/or endocytic trafficking | epilepsy intellectual disability |
| Class III: Adolescent-onset | |||||||
| Late-onset Tay-Sachs | LOTS | 15q23 | HEXA | 1986 | hexosaminidase A | ganglioside metabolism | extrapyramidal signs psychiatric symptoms |
| Cerebrotendinous xanthomatosis | CTX | 2q35 | CYP27A1 | 1991 | sterol 27-hydroxylase | bile acid metabolism | cataracts tendon xanthomas |
| Abetalipoproteinemia | ABL | 4q24 | MTTP | 1993 | microsomal triglyceride transfer protein | lipoprotein metabolism | lipid malabsorption pigmentary retinopathy |
| Ataxia with vitamin E deficiency | AVED | 8q12.3 | TTPA | 1995 | alpha-tocopherol transfer protein | vitamin E metabolism | pigmentary retinopathy polyneuropathy |
| Refsum disease | RD | 10p13 | PHYH | 1997 | phytanoyl-CoA hydroxylase | fatty acid metabolism | anosmia pigmentary retinopathy |
| 6q23.3 | PEX7 | 2003 | peroxin-7 | peroxisomal import | |||
| Ataxia with oculomotor apraxia, type 1 | AOA1 | 9p13.3 | APTX | 2001 | aprataxin | DNA repair | oculomotor apraxia polyneuropathy |
| Ataxia with oculomotor apraxia, type 2 | AOA2 | 9q34.13 | SETX | 2004 | senataxin | DNA repair, transcription RNA processing | oculomotor apraxia polyneuropathy |
| Spinocerebellar ataxia with axonal neuropathy | SCAN1 | 14q32.11 | TDP1 | 2002 | tyrosyl-DNA phoshodiesterase-1 | DNA repair | polyneuropathy |
| DNA polymerase gamma disorders | POLG | 15q25 | POLG | 2004 | DNA polymerase gamma | mitochondrial DNA metabolism | dementia epilepsy |
| Spinocerebellar ataxia autosomal recessive, type 10 | SCAR10 | 3p22.1 | ANO10 | 2010 | anoctamin 10 | Ca++ activated chloride channel | hyper-reflexia motor neuron signs |
| Autosomal recessive spastic ataxia with leukoencephalopathy | ARSAL | 2q33.1 | MARS2 | 2012 | mitochondrial methionyl-tRNA synthetase 2 | mitochondrial protein synthesis | hyper-reflexia pyramidal signs |
| Class IV: Adult-onset | |||||||
| Autosomal recessive cerebellar ataxia, type1 | ARCA1 | 6q25 | SYNE1 | 2007 | synaptic nuclear envelope protein-1 | cerebellar architecture | pure cerebellar |
| AMACR deficiency | AMACR | 5p13 | AMACR | 2011 | alpha-methylacyl-CoA racemase | peroxisomal metabolism | epilepsy dementia |
| Spinocerebellar ataxia autosomal recessive, type 11 | SCAR11 | 1q32.2 | SYT14 | 2011 | synaptotagmin 14 | membrane trafficking | psychomotor retardation |
Year identified refers to initial publication linking disease and gene in a search of the PubMed database. Listed protein functions are based on available literature and are presumptive in some cases. The key clinical features listed are suggested to aid in differential diagnosis and are not meant to represent all clinical features associated with the disease.
Class I: Friedreich Ataxia
Friedreich ataxia is the most common autosomal recessive cerebellar ataxia and the most common hereditary ataxia overall, comprising nearly 50% of all recessive ataxias.2,16,23 Because it is much more common than all other autosomal recessive forms and can exhibit multiple variant presentations,2,16,23 we recommend screening all patients suspected of having autosomal recessive cerebellar ataxia for this disorder prior to any other genetic testing (Figure 2). Typical cases are characterized by gait and limb ataxia, neuropathy affecting the posterior columns, areflexia, pyramidal weakness of the lower extremities, and upgoing toes.2,16,23 Associated conditions include pes cavus, scoliosis (70%), diabetes (10%), and cardiomyopathy.2,16,23 MRI often shows a normal cerebellum early in the course but a thin cervical spinal cord.2,23 Cerebellar atrophy can be seen late.29 The disease is caused by GAA repeat expansion in the frataxin gene, leading to reduced gene expression and impaired mitochondrial iron metabolism (Table 1).2,16,23,30 The disease can be quite disabling, with many patients wheelchair-bound by 15 years post-diagnosis.16
Class II: Early-Onset Ataxia
This class of recessive disorders is characterized by onset before the age of 5 years. In patients seen early in the course of the disease, it may be difficult to determine whether the disease is congenital unless progression has been clearly observed (eg, normal walking was attained and then subsequently became ataxic). Patients with disorders in this group are the most likely to carry a mislabeled diagnosis (eg, cerebral palsy, cerebellar hypoplasia, etc.) when seen later in life (Figure 3).
The most common member of the class is ataxia telangiectasia, caused by mutations of the ATM gene, which encodes a serine/threonine kinase involved in DNA damage repair (Table 1).2,21,23 This is likely the second most common autosomal recessive ataxia in most populations.21,29 In addition to gait and limb ataxia, key clinical features include oculomotor apraxia and oculocutaneous telangiectasias.2,21,23 Diagnosis can be aided by increased radiosensitivity, usually performed with cell lines derived from blood or skin, and elevation of α-fetoprotein in the blood.2,21,23 MRI often shows atrophy of the cerebellum and brainstem and children are at increased risk for the development of leukemia or lymphoma.2,21,23
Other members of this class are rare but should be tested for, if possible, in patients with clinically appropriate phenotypes (Table 1). In particular, the recently identified peroxin-associated ataxias (and Refsum disease) show abnormalities in levels of very long chain fatty acids,2,23,31-33 making this a useful biomarker in lieu of genetic testing. This may also be important for management, as dietary modification may be a potential treatment.32
Class III: Adolescent-Onset Ataxia
The disorders in this group typically have an average age of onset in early- to mid-adolescence, and most symptoms appear before age 20.2,23 The diseases in this class vary in prevalence worldwide, but one of the more common is autosomal recessive ataxia with oculomotor apraxia type 2 or AOA2, caused by mutation in the senataxin gene, SETX (Table 1).2,23 Clinically, this disease can present similarly to both Friedreich ataxia, with gait ataxia associated with sensorimotor neuropathy, and ataxia telangiectasia, with oculomotor apraxia (in about 50%) and elevated alpha-fetoprotein.2,23 MRI shows cerebellar vermian atrophy.2,23 Disease is caused by a variety of mutations in the SETX gene,34,35 and the gene itself is quite polymorphic, which can challenge the interpretation of genetic testing.36
Several other members of this category can be identified through biomarker evaluations from blood (Table 2), including late-onset Tay-Sachs (hexosaminidase A enzyme activity), cerebrotendinous xanthomatosis (cholestanol levels), abetalipoproteinemia (acanthocytes and deficiency in fat-soluble vitamins, including vitamin E), ataxia with vitamin E deficiency, and Refsum disease. Because replacement or dietary therapies can be clinically valuable in many of these cases,23 biomarker testing is essential in this age group prior to genetic testing.
Table 2.
Biomarkers Associated With Autosomal Recessive Cerebellar Ataxias
| Biomarker | Abnormality | Disease |
|---|---|---|
| Acanthocytes | Present | ABL |
| Albumin | Reduced | AOA1 |
| SCAN1 | ||
| α-fetoprotein | Elevated | AOA2 |
| AT | ||
| Cholestanol | Elevated | CTX |
| Cholesterol | Elevated | AOA1 |
| AOA2 | ||
| SCAN1 | ||
| Hexosaminidase A | Reduced | LOTS |
| Immunoglobulins | Reduced | AT |
| ATLD | ||
| Lactate | Elevated | ARCA2 |
| Radiosensitivity | Present | AT |
| ATLD | ||
| Very long chain fatty acids | Elevated | PEX |
| RD | ||
| Vitamin E | Reduced | ABL |
| AVED |
Abbreviations: ABL, abetalipoproteinemia; AOA1, ataxia with oculomotor apraxia, type 1; AOA2, ataxia with oculomotor apraxia, type 2; ARCA2, autosomal recessive cerebellar ataxia, type 2; AT, ataxia telangiectasia; ATLD, ataxia telangiectasia-like disorder; AVED, ataxia with vitamin E deficiency; CTX, cerebrotendinous xanthomatosis; LOTS, late-onset Tay-Sachs; PEX, peroxin-associated ataxias; RD, Refsum disease; SCAN1, spinocerebellar ataxia with axonal neuropathy.
Autosomal Dominant Ataxias
Although equally as common as the autosomal recessive diseases, genetic ataxias inherited in an autosomal dominant fashion (the spinocerebellar ataxias) are most often seen in adults ages 20 years to 50 years.2,3,22 Because many of these are caused by triplet repeat expansions, extremely large expansions resulting from anticipation can result in onset at a much earlier age.2,22,27 This is most common in children for dentatorubral pallidoluysian atrophy, spinocerebellar ataxia type 2, and spinocerebellar ataxia type 7.22 In addition, a few of the rarer spinocerebellar ataxias have a typical childhood onset, including spinocerebellar ataxia type 18, spinocerebellar ataxia type 21, spinocerebellar ataxia type 28, and spinocerebellar ataxia type 29.2 Of these, only spinocerebellar ataxia type 28, caused by mutation of the AFG3L2 gene, is available for commercial testing.2 Typically a family history consistent with a dominant ataxic disorder is present but, in some cases, the affected parent may have only mild symptoms. Consideration may also be appropriate if the child is adopted or a parent has died early. Broad genetic screening for autosomal dominant cerebellar ataxias is not routinely recommended in children or those without a definite family history of disease.3,22,36
Other Genetic Considerations
Several leukodystrophies (eg, Krabbe, metachromatic, etc.) can present with cerebellar ataxia and should be considered if phenotypically appropriate.5,7 Joubert syndrome, X-linked adrenoleukodystrophy, Wilson disease, Niemann-Pick type C, and childhood ataxia with central nervous system hypomyelination/vanishing white matter disease are other considerations in this age group as well.5,7 Often other associated features can direct the clinician toward one of these conditions; for example, some of these disorders have characteristic clinical features (eg, Kayser-Fleischer rings in Wilson disease), neuroimaging hallmarks (eg, the molar tooth sign in Joubert syndrome), or less specific findings, such as the white matter hyperintensities seen in leukodystrophies, that are uncommon in other genetic ataxias.
Discussion
Evaluating the child with ataxia requires a systematic and comprehensive approach so that treatment, if available, can be instituted as expediently as possible. This requires a detailed clinical history, with particular attention to age of onset and family history, and a complete neurological evaluation with a physical examination to assess for other observable systemic features if present. Differential diagnosis often involves the careful consideration of acquired and hereditary conditions and the workup requires careful planning to maximize patient and clinical resources. The systematic approach outlined here can help facilitate this evaluation, particularly for acquired causes of childhood cerebellar ataxia; however, tailoring to the situation is necessary given the uniqueness of each individual patient.
In chronic progressive childhood cerebellar disease, genetic mutations must often be considered. The autosomal recessive genetic ataxias are rapidly expanding in number, particularly in recent years with the implementation of new technologies to enhance genetic sequencing (Table 1).37 Molecularly, as a group, these ataxias result from loss of function in cellular or metabolic pathways crucial to the functioning of cerebellar neurons.7,23 Important questions for future research include why, despite such a diversity of molecular functions and expression in many cell types besides neurons,7,23 the clinical phenotypes exhibited in these disorders are so homogenous and specific to the cerebellum. With most familial ataxia cases worldwide lacking genetic diagnosis,2 it is likely we will see continued expansion in the numbers of genes implicated in these disorders in the years to come.
Acknowledgments
This work is based on a presentation given by the author at the Neurobiology of Disease in Children Symposium: Childhood Ataxia, in conjunction with the 40th Annual Meeting of the Child Neurology Society, Savannah, Georgia, October 26, 2011. Supported by grants from the National Institutes of Health (2R13NS040925-14 Revised), the National Institutes of Health Office of Rare Diseases Research, the Child Neurology Society, and the National Ataxia Foundation.
Financial Disclosure/Funding:
Support provided by National Institute of Mental Health grant K08MH86297 (B.L.F.).
Footnotes
Declaration of Conflicting Interests: The author reports no conflicts of interest.
Author Contributions/Roles:
B.L.F. was responsible for the concept, design, and drafting of this manuscript.
References
- 1.Manto M. Mechanisms of human cerebellar dysmetria: experimental evidence and current conceptual bases. J Neuroeng Rehabil. 2009;6:10. doi: 10.1186/1743-0003-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fogel BL, Perlman S. Cerebellar disorders: Balancing the approach to cerebellar ataxia. In: Gálvez-Jiménez N, Tuite PJ, editors. Uncommon Causes of Movement Disorders. 1st ed Cambridge University Press; 2011. pp. 198–216. [Google Scholar]
- 3.Fogel BL, Perlman S. An approach to the patient with late-onset cerebellar ataxia. Nat Clin Pract Neurol. 2006;2:629–635. doi: 10.1038/ncpneuro0319. quiz 1 p following 635. [DOI] [PubMed] [Google Scholar]
- 4.Klockgether T. Sporadic ataxia with adult onset: classification and diagnostic criteria. Lancet Neurol. 2010;9:94–104. doi: 10.1016/S1474-4422(09)70305-9. [DOI] [PubMed] [Google Scholar]
- 5.Brusse E, Maat-Kievit JA, van Swieten JC. Diagnosis and management of early-and late-onset cerebellar ataxia. Clin Genet. 2007;71:12–24. doi: 10.1111/j.1399-0004.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 6.Benini R, Ben Amor IM, Shevell MI. Clinical clues to differentiating inherited and noninherited etiologies of childhood ataxias. J Pediatr. 2012;160:152–157. doi: 10.1016/j.jpeds.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 7.Manto M, Marmolino D. Cerebellar ataxias. Curr Opin Neurol. 2009;22:419–429. doi: 10.1097/WCO.0b013e32832b9897. [DOI] [PubMed] [Google Scholar]
- 8.Poretti A, Wolf NI, Boltshauser E. Differential diagnosis of cerebellar atrophy in childhood. Eur J Paediatr Neurol. 2008;12:155–167. doi: 10.1016/j.ejpn.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Steinlin M. Non-progressive congenital ataxias. Brain Dev. 1998;20:199–208. doi: 10.1016/s0387-7604(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 10.Boddaert N, Desguerre I, Bahi-Buisson N, et al. Posterior fossa imaging in 158 children with ataxia. J Neuroradiol. 2010;37:220–230. doi: 10.1016/j.neurad.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Lynch DA, Farmer JM, editors. Neurogenetics: Scientific and Clinical Advances. 1st ed. Marcel Dekker; New York: 2006. [Google Scholar]
- 12.Ryan MM, Engle EC. Acute ataxia in childhood. J Child Neurol. 2003;18:309–316. doi: 10.1177/08830738030180050901. [DOI] [PubMed] [Google Scholar]
- 13.Luckhoff C, Starr M. Minor head injuries in children – an approach to management. Aust Fam Physician. 2010;39:284–287. [PubMed] [Google Scholar]
- 14.Ganesan V, Chong WK, Cox TC, et al. Posterior circulation stroke in childhood: risk factors and recurrence. Neurology. 2002;59:1552–1556. doi: 10.1212/01.wnl.0000033092.87560.1a. [DOI] [PubMed] [Google Scholar]
- 15.Salas AA, Nava A. Acute cerebellar ataxia in childhood: initial approach in the emergency department. Emerg Med J. 2010;27:956–957. doi: 10.1136/emj.2009.079376. [DOI] [PubMed] [Google Scholar]
- 16.Pandolfo M. Friedreich ataxia: the clinical picture. J Neurol. 2009;256(Suppl 1):3–8. doi: 10.1007/s00415-009-1002-3. [DOI] [PubMed] [Google Scholar]
- 17.Wananukul S, Voramethkul W, Kaewopas Y, Hanvivatvong O. Prevalence of positive antinuclear antibodies in healthy children. Asian Pac J Allergy Immunol. 2005;23:153–157. [PubMed] [Google Scholar]
- 18.Ruggieri M, Incorpora G, Polizzi A, et al. Low prevalence of neurologic and psychiatric manifestations in children with gluten sensitivity. J Pediatr. 2008;152:244–249. doi: 10.1016/j.jpeds.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa N, Kobayashi M. Recurrent acute cerebellar ataxia associated with anti-cardiolipin antibodies. Brain Dev. 2010;32:588–591. doi: 10.1016/j.braindev.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Gorman MP. Update on diagnosis, treatment, and prognosis in opsoclonusmyoclonus-ataxia syndrome. Curr Opin Pediatr. 2010;22:745–750. doi: 10.1097/MOP.0b013e32833fde3f. [DOI] [PubMed] [Google Scholar]
- 21.Perlman SL, Boder Deceased E, Sedgewick RP, Gatti RA. Ataxia-telangiectasia. Handb Clin Neurol. 2012;103:307–332. doi: 10.1016/B978-0-444-51892-7.00019-X. [DOI] [PubMed] [Google Scholar]
- 22.Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9:885–894. doi: 10.1016/S1474-4422(10)70183-6. [DOI] [PubMed] [Google Scholar]
- 23.Fogel BL, Perlman S. Clinical features and molecular genetics of autosomal recessive cerebellar ataxias. Lancet Neurol. 2007;6:245–257. doi: 10.1016/S1474-4422(07)70054-6. [DOI] [PubMed] [Google Scholar]
- 24.Ilg W, Synofzik M, Brotz D, et al. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology. 2009;73:1823–1830. doi: 10.1212/WNL.0b013e3181c33adf. [DOI] [PubMed] [Google Scholar]
- 25.Ilg W, Brotz D, Burkard S, et al. Long-term effects of coordinative training in degenerative cerebellar disease. Mov Disord. 2010;25:2239–2246. doi: 10.1002/mds.23222. [DOI] [PubMed] [Google Scholar]
- 26.Fogel BL, Lee JY, Lane J, et al. Mutations in rare ataxia genes are uncommon causes of sporadic cerebellar ataxia. Mov Disord. 2012 doi: 10.1002/mds.24064. epub ahead of print. doi: 10.1002/mds.24064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fogel BL, Geschwind DH. Clinical Neurogenetics. In: Daroff R, Fenichel G, Jankovic J, Mazziotta J, editors. Neurology in Clinical Practice. 6th ed. Elsevier; In press. [Google Scholar]
- 28.Duenas AM, Goold R, Giunti P. Molecular pathogenesis of spinocerebellar ataxias. Brain. 2006;129:1357–1370. doi: 10.1093/brain/awl081. [DOI] [PubMed] [Google Scholar]
- 29.Anheim M, Fleury M, Monga B, et al. Epidemiological, clinical, paraclinical and molecular study of a cohort of 102 patients affected with autosomal recessive progressive cerebellar ataxia from Alsace, Eastern France: implications for clinical management. Neurogenetics. 2010;11:1–12. doi: 10.1007/s10048-009-0196-y. [DOI] [PubMed] [Google Scholar]
- 30.Pandolfo M, Pastore A. The pathogenesis of Friedreich ataxia and the structure and function of frataxin. J Neurol. 2009;256(Suppl 1):9–17. doi: 10.1007/s00415-009-1003-2. [DOI] [PubMed] [Google Scholar]
- 31.Sevin C, Ferdinandusse S, Waterham HR, et al. Autosomal recessive cerebellar ataxia caused by mutations in the PEX2 gene. Orphanet J Rare Dis. 2011;6:8. doi: 10.1186/1750-1172-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regal L, Ebberink MS, Goemans N, et al. Mutations in PEX10 are a cause of autosomal recessive ataxia. Ann Neurol. 2010;68:259–263. doi: 10.1002/ana.22035. [DOI] [PubMed] [Google Scholar]
- 33.Ebberink MS, Csanyi B, Chong WK, et al. Identification of an unusual variant peroxisome biogenesis disorder caused by mutations in the PEX16 gene. J Med Genet. 2010;47:608–615. doi: 10.1136/jmg.2009.074302. [DOI] [PubMed] [Google Scholar]
- 34.Fogel BL, Perlman S. Novel mutations in the senataxin DNA/RNA helicase domain in ataxia with oculomotor apraxia 2. Neurology. 2006;67:2083–2084. doi: 10.1212/01.wnl.0000247661.19601.28. [DOI] [PubMed] [Google Scholar]
- 35.Fogel BL, Lee JY, Perlman S. Aberrant splicing of the senataxin gene in a patient with ataxia with oculomotor apraxia type 2. Cerebellum. 2009;8:448–453. doi: 10.1007/s12311-009-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fogel BL. Interpretation of genetic testing: variants of unknown significance. Continuum Lifelong Learning in Neurology. 2011;17:347–352. doi: 10.1212/01.CON.0000396975.87637.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metzker ML. Sequencing technologies – the next generation. Nat Rev Genet. 2010;11:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]