Abstract
Background
1,2,4-Triazole derivatives have received much attention due to their versatile biological properties including antibacterial, antifungal, anticonvulsant, antiinflammatory, anticancer, and antiproliferative properties. 1,2,4-Triazole nucleus has been incorporated into a wide variety of therapeutically interesting molecules to transform them into better drugs. Schiff bases of 1,2,4-triazoles have also been found to possess extensive biological activities. On the other hand, γ-substituted butenolide moiety represents a biological important entity that is present in numerous biologically active natural products.
Results
We have described herein the synthesis of 12 hybrid 1,2,4-triazole Schiff bases bearing γ-substituted butenolide moiety. These compounds were synthesized by utilizing the tandem asymmetric Michael addition/elimination reaction as the key step. All the new compounds were evaluated for their in vitro anticancer activity.
Conclusions
Tandem asymmetric Michael addition/elimination approach has offered an easy access to new chiral 1,2,4-triazole compounds 7a-7l. All these chiral 1,2,4-triazole derivatives exhibited good anticancer activities towards Hela. Of all the tested compounds, the chiral compound 7l with an IC50 of 1.8 μM was found to be the most active.
Keywords: 1,2,4-triazole; Schiff base; γ-butenolide; A activity; HeLa cells
Background
Cancer, a diverse group of diseases characterized by the proliferation and spread of abnormal cells, is a major worldwide problem. Therefore, the discovery and development of new potent and selective anticancer drugs are of high importance in modern cancer research.
1,2,4-Triazole derivatives have received much attention due to their versatile biological properties including antibacterial, antifungal, anticonvulsant, antiinflammatory, anticancer, and antiproliferative properties [1-10]. 1,2,4-Triazole nucleus has been incorporated into a wide variety of therapeutically interesting molecules to transform them into better drugs [11-13]. Schiff bases of 1,2,4-triazoles have also been found to possess extensive biological activities [14-18]. On the other hand, γ-substituted butenolide moiety represents a biological important entity that is present in numerous biologically active natural products [19-24].
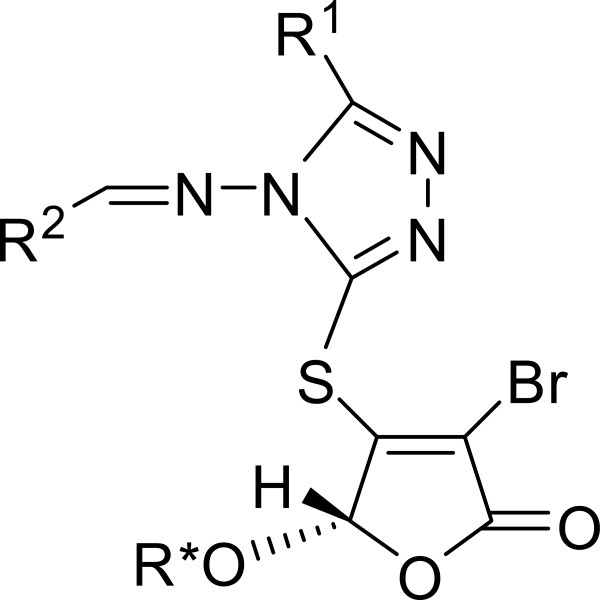
Recently, we reported on the synthesis of a series of hybrid 1,3,4-thiadiazoles derivatives possessing γ-substituted butenolide moiety, which exhibited good anticancer activities against cervical cancer cells [25]. In continuation of our studies on the identification of potential active antitumor compounds, herein we report the synthesis and evaluation of a new series of hybrid 1,2,4-triazole Schiff bases bearing γ-substituted butenolide moiety as potential anticancer agents (Figure 1). To the best of authors’ knowledge, the synthesis and anticancer activities of this types of compounds have not been reported so far.
Figure 1.
The general structure of target compounds.
Results and discussion
The enantiomerically pure γ-substituted butenolides 1 were synthesized via acetalization of mucobromic acid by employing (−)-menthol and (+)-borneol as a chiral auxiliary, respectively, and followed by resolution of the resulting diastereomers [25-27].
The 1,2,4-triazole Schiff bases 6 were synthesized by condensation 4-amino-5-substituted-4H-1,2,4-triazol-3-thiols 5 with aromatic aldehydes in glacial acetic acid (Scheme 1) [14]. The 4-amino-5-substituted-4H-1,2,4-triazol-3-thiols 5 were prepared according to the previous procedure [28,29]. When R1 is methyl, the compound 5a was prepared by heating a mixture of thiocarbohydrazide with acetic acid [28]. When R1 are aryl, a different procedure was employed as aromatic carboxylic acids are generally solid, have high melting points, and are difficult to react with thiocarbohydrazide fully [29]. Thus, staring from aromatic carboxylic acid esters 2, the aroyl hydrazides 3 were obtained by reaction with hydrazine in EtOH. Treatment of the aroyl hydrazides 3 with CS2 under a basic condition (KOH/EtOH) gave the corresponding potassium aroyl dithiocarbazates 4. Then, the resulting compounds 4 were cyclized with hydrazine to provide the compounds 5b–d in good yields.
Scheme 1.
Synthesis of 1,2,4-triazole Schiff bases 6.
The target compounds 7a–l were prepared via tandem Michael addition–elimination reaction of γ-substituted butenolides 1 with 5-substituted 1,2,4-triazole Schiff bases 6 under phase-transfer catalysis conditions (Scheme 2).
Scheme 2.
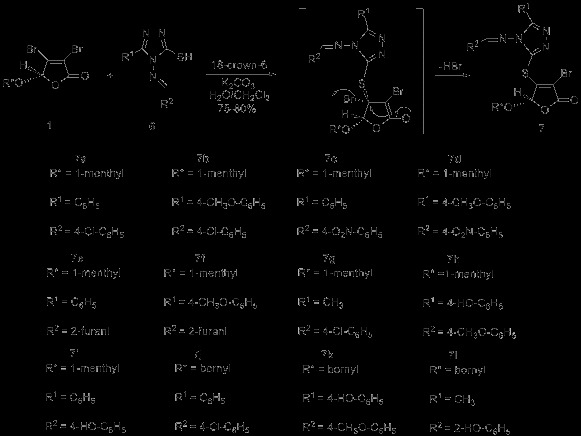
Synthesis of target compounds 7a–l.
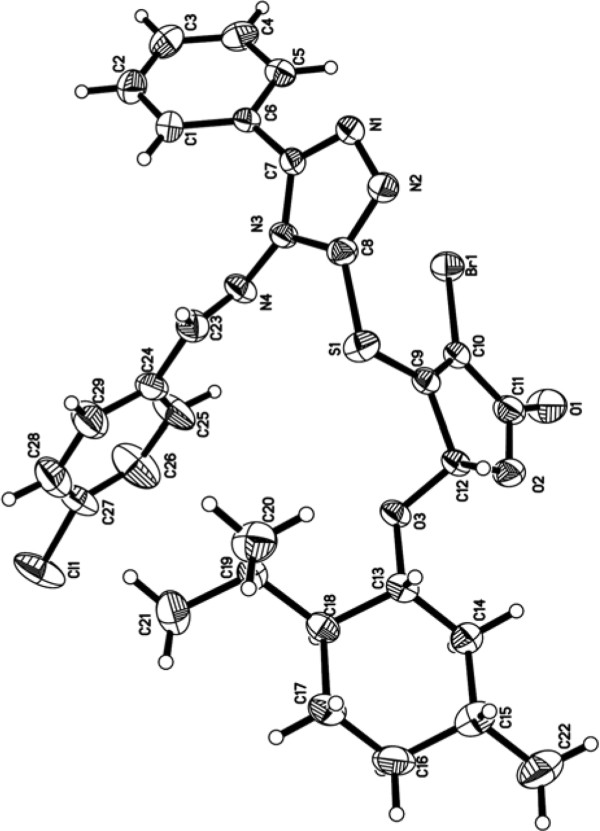
The structures of these new compounds 7a–l were characterized with IR, 1H, 13 C NMR, and LC-MS spectra. In addition, the molecular structure of 7a was unambiguously confirmed through X-ray crystallography (Figure 2).a
Figure 2.
ORTEP view of the crystal structure of compound 7a.
All newly synthesized compounds 7a–l were initially evaluated for their in vitro anticancer activities against cervical cancer cell lines (HeLa) using the MTT assay, and the results were summarized in Table 1. All the compounds 7a–l displayed good inhibition activities on HeLa cell lines. Of all the studied compounds, the compound 7l exhibited the best inhibitory activity with an IC50 of 1.8 μM.
Table 1.
In vitroanticancer activities against HeLa cell lines with compounds 7a–l (n= 3)
| Compound | IC50(μM) | Compound | IC50(μM) |
|---|---|---|---|
|
7a |
19.7 |
7h |
7.1 |
|
7b |
4.4 |
7i |
3.7 |
|
7c |
11.6 |
7j |
4.5 |
|
7d |
11.2 |
7k |
6.2 |
|
7e |
6.8 |
7l |
1.8 |
|
7f |
5.1 |
DDP (Cisplatin) |
2.6 |
| 7g | 8.2 |
The IC50 values represent the compound concentration (μM) required to inhibit tumor cell proliferation by 50%.
Then, the growth inhibition rates of HeLa cell lines with compounds 7a–l at different concentrations (0.1–20 μM) were evaluated (Table 2). After being treated with 20 μg/mL compound 7l for 24 h, the growth inhibition rate was the highest (90.0%).
Table 2.
Growth inhibition rates of HeLa cell lines with compounds 7a–l at different concentrations
|
Compounds |
|
|
Inhibition rates (%) |
|
|
|---|---|---|---|---|---|
| 1.25 μM | 2.5 μM | 5 μM | 10 μM | 20 μM | |
|
7a |
1.2 |
8.7 |
16.1 |
30.2 |
41.9 |
|
7b |
26.2 |
30.2 |
53.8 |
65.2 |
85.7 |
|
7c |
9.4 |
35.3 |
21.3 |
52.3 |
60.2 |
|
7d |
17.9 |
11.0 |
34.8 |
47.7 |
65.5 |
|
7e |
10.9 |
11.4 |
24.3 |
76.0 |
85.2 |
|
7f |
10.3 |
27.4 |
56.9 |
73.4 |
85.1 |
|
7g |
18.0 |
30.9 |
43.4 |
49.7 |
67.1 |
|
7h |
8.3 |
14.3 |
40.7 |
77.5 |
71.7 |
|
7i |
14.3 |
35.6 |
67.8 |
85.4 |
87.7 |
|
7j |
24.0 |
32.5 |
54.1 |
67.6 |
81.2 |
|
7k |
14.8 |
36.2 |
60.0 |
56.7 |
67.3 |
| 7l | 47.5 | 45.4 | 74.1 | 88.6 | 90.0 |
Experimental
All the chemicals were used as-received without further purification unless otherwise stated. IR spectra were recorded on a FTIR-8400S spectrometer as KBr disks. 1H NMR and 13 C NMR spectra were obtained with a Bruker Avance III 400 MHz spectrometer in chloroform-d (CDCl3) and tetramethylsilane was used as an internal standard. Diffraction measurement was made on a Bruker AXS SMART 1000 CCD diffractometer with graphite-monochromatized Mo Kα radiation (λ = 0.71073 Å). All the melting points were determined on a WRS-1B digital melting point apparatus and are uncorrected. Thin-layer chromatography (TLC) was carried out on silica GF254 plates (Qingdao Haiyang Chemical Co., Ltd., China).
General procedure for the synthesis of compounds 7
To an aqueous solution of dichloromethane was sequentially added the compounds 1 (1.0 mmol), potassium carbonate (1.0 mmol), 18-crown-6 (0.1 mmol), and the compounds 6 (1.1 mmol). The resulting mixture was stirred at room temperature, and the reaction was monitored by TLC. On completion of the reaction (10–20 h), the mixture was exacted and the organic layer was washed with saturated brine. Then the organic layer was dried over anhydrous MgSO4, filtered, and concentrated in vacuo The purification of the residue by silica gel column chromatography or crystallizations yielded the desired compounds 7a-l in 65-89% yields (For the characterization of compound 7a-7l, please see the Additional file 1: Supporting Information). Compound 7 l: white solid, 76% yield, [α]D20 = −37.2 (c = 0.5 M, CHCl3). mp 131–132°C. IR (KBr) 3210, 1780, 1603, 1523, 1440, 1421, 1319, 1212, 1134, 993 cm-1. 1H NMR (400 MHz, CDCl3) 10.04 (s, 1H), 8.73 (s, 1H), 7.59-7.04 (m, 2H), 7.14-7.06 (m, 2H), 6.20 (s, 1H), 3.81 (m, 1H), 2.59 (s, 3H), 2.25-2.22 (m, 1H), 1.69-1.09 (m, 6H), 0.78-0.74 (m, 6H), 0.53 (s, 3H). 13 C NMR (100 MHz, CDCl3) 170.4, 164.0, 160.3, 152.9, 151.0, 138.2, 136.3, 133.7, 120.6, 118.1, 115.4, 112.8, 103.1, 88.8, 49.3, 47.6, 44.7, 36.7, 27.9, 26.5, 19.5, 18.7, 13.3, 11.2. HRMS calcd. for C24H27Br N4O4S [M]+: 546.0936, found 546.0933.
Pharmacology
Cells (1 × 104 in 100 μL) were seeded on 96-well plates in triplicate. Following a 24-h culture at 37°C, the medium was replaced with fresh medium at various concentrations (1.25, 2.5, 5, 10, 20 μg/mL) of compounds 7a–l in a final volume of 110 μL. At the same time, set drug-free medium negative control well, and solvent control well of the same volume of dimethyl sulfoxide (DMSO). Cells were incubated at 37°C for 24 h. Then, 20 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (2 mg/mL in a phosphate buffer solution) was added to each well, incubated for an additional 4 h, the plates were centrifuged at 1000 r/min for 10 min, then the medium was removed. MTT formazan precipitates were dissolved in 100 μL of DMSO, shaken mechanically for 10 min and then read immediately at 492 nm in a plate reader (Opsys MR, Denex Technology, USA).
Conclusions
In summary, a new type of chiral 1,2,4-triazole Schiff bases bearing γ-substituted butenolide moiety have been synthesized and their in vitro anticancer activities against have been evaluated. These chiral 1,2,4-triazole derivatives exhibited good anticancer activities towards HeLa. The compound 7l with an IC50 of 1.8 μM was found to be the most active. Further studies of anticancer activities of these compounds are in progress in our group.
Endnote
aThe molecular structure of the product 7a was determined by means of X-ray crystallographic studies. CCDC 829447 (7a) contains the supplementary crystallographic data for this article. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ZS and XL carried out the design of the project, and drafted the manuscript. XL and HL synthesized target compounds. XZ evaluated in vitro anticancer activities against cervical cancer cell lines (HeLa). All authors read and approved the final manuscript.
Supporting information available
Experimental procedures, spectral data of new compounds.
Supplementary Material
Supporting Information Available. Experimental procedures, spectral data of new compounds.
Contributor Information
Xiang Li, Email: ningda119@163.com.
Xue-Qiang Li, Email: lixq@nxu.edu.cn.
He-Mei Liu, Email: pengfangzhi@ynu.edu.cn.
Xue-Zhang Zhou, Email: 20070114@ynu.edu.cn.
Zhi-Hui Shao, Email: zhihui_shao@hotmail.com.
Acknowledgments
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (20962023, 21062014, 21162034), the Major State Basic Research Development Program of China (2007CB21602), the Program for New Century Excellent Talents in University (NCET-10-0907), the Key Project of Chinese Ministry of Education (210237), and the Natural Science Foundation of Ningxia Province of China (NZ0606).
References
- Sztanke K, Tuzimski T, Rzymowska J, Pasternak K, Kandefer-Szerszen M. Synthesis, determination of the lipophilicity, anticancer and antimicrobial properties of some fused 1,2,4-triazolederivatives. Eur J Med Chem. 2008;43:404–419. doi: 10.1016/j.ejmech.2007.03.033. [DOI] [PubMed] [Google Scholar]
- Sadana AK, Mirza Y, Aneja KR, Prakash O. Hypervalent iodine mediated synthesis of 1-aryl/hetryl-1,2,4-triazolo[4,3-a]pyridines and 1-aryl/hetryl5-methyl-1,2,4-triazolo[4,3-a]quinolines as antibacterial agents. Eur J Med Chem. 2003;38:533–536. doi: 10.1016/S0223-5234(03)00061-8. [DOI] [PubMed] [Google Scholar]
- Amir M, Kumar H, Javed SA. Condensed bridge head nitrogen heter-ocyclic system: synthesis and pharmacological activities of 1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazole derivatives of ibuprofen and biphenyl-4-yloxyaceticacid. Eur Med Chem. 2008;43:2056–2066. doi: 10.1016/j.ejmech.2007.09.025. [DOI] [PubMed] [Google Scholar]
- Turan-Zitouni G, Kaplancıklı ZA, Yıldız MT, Chevallet P, Kaya D. Synthesis and antimicrobial activity of 4-phenyl/cyclohexyl-5-(1-phenoxyethyl)-3-[N-(2-thiazolyl)acetamido]thio-4H-1,2,4-triazole derivatives. Eur J Med Chem. 2005;40:607–613. doi: 10.1016/j.ejmech.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Mavrova AT, Wesselinova D, Tsenov YA, Denkova P. Synthesis, cytotoxicity and effects of some1,2,4-triazole and 1,3,4-thiadiazole derivatives on immunocompetent cells. Eur J Med Chem. 2009;44:63–69. doi: 10.1016/j.ejmech.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Al-Soud YA, Al-Masoudi NA, Ferwanah AE-RS. Synthesis and properties of new substituted 1,2,4-triazoles: potential antitumor agents. Bioorg Med Chem. 2003;11:1701–1708. doi: 10.1016/S0968-0896(03)00043-9. [DOI] [PubMed] [Google Scholar]
- Almasirad A, Tabatabai SA, Faizi M, Kebriaeezadeh A, Mehrabi N, Dalvandi A, Shafiee A. Synthesis and anticonvulsant activity of new 2-substituted-5-[2-(2-fluorophenoxy)phenyl]-1,3,4-oxadiazoles and 1,2,4-triazoles. Bioorg Med Chem Lett. 2004;14:6057–6059. doi: 10.1016/j.bmcl.2004.09.072. [DOI] [PubMed] [Google Scholar]
- Padmavathi V, Thriveni P, Reddy GS, Deepti D. Synthesis and antimicrobial activity of novel sulfone-linked bis heterocycles. Eur J Med Chem. 2008;43:917–924. doi: 10.1016/j.ejmech.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Bhat KS, Poojary B, Prasad DJ, Naik P, Holla BS. Synthesis and antitumor activity studies of some new fused 1,2,4-triazole derivatives carrying 2,4-dichloro-5-fluorophenylmoiety. Eur J Med Chem. 2009;44:5066–5070. doi: 10.1016/j.ejmech.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Romagnoli R, Baraldi PG, Cruz-Lopez O, Cara CL, Carrion MD, Brancale A, Hamel E, Chen L, Bortolozzi R, Basso G, Viola G. Synthesis and antitumor activity of 1,5-disubstituted 1,2,4-triazoles as cis-restricted combretastatin analogues. J Med Chem. 2010;53:4248–4258. doi: 10.1021/jm100245q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Lou H, Gao Y, Fan P, Ma B, Ge W, Wang X. Liquid chromatography-tandem mass spectrometric method for the analysis of fluconazole and evaluation of the impact of phenolic compounds on the concentration of fluconazole in Candida albicans. J Pharm Biomed Anal. 2004;34:1117–1124. doi: 10.1016/j.jpba.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Clemons M, Coleman RE, Verma S. Aromatase inhibitors in the adjuvant setting: bringing the gold to a standard. Cancer Treat Rev. 2004;30:325–332. doi: 10.1016/j.ctrv.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Johnston GAR. Medicinal chemistry and molecular pharmacology of GABAC receptors. Curr Top Med Chem. 2002;2:903–913. doi: 10.2174/1568026023393453. [DOI] [PubMed] [Google Scholar]
- Li Z, Gu Z, Yin K, Zhang R, Deng Q, Xiang J. Synthesis of substituted-phenyl-1,2,4-triazol-3-thione analogues with modified d-glucopyranosyl residues and their antiproliferative activities. Eur J Med Chem. 2009;44:4716–4720. doi: 10.1016/j.ejmech.2009.05.030. [DOI] [PubMed] [Google Scholar]
- Holla BS, Veerendra B, Shivananda MK, Poojary B. Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur J Med Chem. 2003;38:759–767. doi: 10.1016/S0223-5234(03)00128-4. [DOI] [PubMed] [Google Scholar]
- Bayrak H, Demirbas A, Karaoglu SA, Demirbas N. Synthesis of some new 1,2,4-triazoles, their Mannich and Schiff bases and evaluation of their antimicrobial activities. Eur J Med Chem. 2009;44:1057–1066. doi: 10.1016/j.ejmech.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Bagihalli GB, Avaji PG, Patil SA, Badami PS. Synthesis, spectra characterization, in vitro anti bacterial, antifungal and cytotoxic activities of Co(II), Ni(II) and Cu(II) complexes with 1,2,4-triazole Schiff bases. Eur J Med Chem. 2008;43:2639–2649. doi: 10.1016/j.ejmech.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Bekircan O, Bektas H. Synthesis of new bis-1,2,4-triazole derivatives. Molecules. 2006;11:469–477. doi: 10.3390/11060469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekera SP, McCarthy PJ, Kelly-Borges M, Lobkovsky E, Clardy J. Dysidiolide: a novel protein phosphatase inhibitor from the Caribbean sponge dysidea etheria de laubenfels. J Am Chem Soc. 1996;118:8759–8760. doi: 10.1021/ja961961+. [DOI] [Google Scholar]
- Avcibasi H, Anil H. Four terpenoids from Cedrus libanotica. Phytochemistry. 1987;26:2852–2854. doi: 10.1016/S0031-9422(00)83605-5. [DOI] [Google Scholar]
- Miles DH, Chittawong V, Lho D-S, Payne AM, de La Cruz AA, Gomez ED, Weeks JA, Atwood JL. Novel sesquiterpene lactones from the mangrove plant Heritiera littoralis. J Nat Prod. 1991;54:286–289. doi: 10.1021/np50073a036. [DOI] [Google Scholar]
- Marcos IS, Escola MA, Moro RF, Basabe P, Diez D, Sanz F, Mollinedo F, de la Iglesia-Vicente J, Sierrac BG, Urones JG. Synthesis of novel antitumour alanalogues of dysidiolide from ent-halimicacid. Bioorg Med Chem. 2007;15:5719–5737. doi: 10.1016/j.bmc.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Dodo K, Sugimoto Y, Aoyagi Y, Yamada Y, Hashimoto Y, Shirai R. Synthesis of the novel analogues of dysidiolide and their structure–activity relationship. Bioorg Med Chem Lett. 2000;10:2571–2574. doi: 10.1016/S0960-894X(00)00527-8. [DOI] [PubMed] [Google Scholar]
- Brohm D, Philippe N, Metzger S, Bhargava A, Muller O, Lieb F, Waldmann H. Solid-phase synthesis of dysidiolide-derived protein phosphatase inhibitors. J Am Chem Soc. 2002;124:13171–13178. doi: 10.1021/ja027609f. [DOI] [PubMed] [Google Scholar]
- Wei M, Feng L, Li X, Zhou X, Shao Z. Synthesis of new chiral 2,5-disubstituted 1,3,4-thiadiazoles possessing gamma-butenolide moiety and preliminary evaluation of in vitro anticancer activity. Eur J Med Chem. 2009;44:3340–3344. doi: 10.1016/j.ejmech.2009.03.023. [DOI] [PubMed] [Google Scholar]
- van Oeveren A, Jansen JFGA, Feringa BL. Enantioselective synthesis of natural dibenzylbutyrolactone lignans (−)-Enterolactone, (−)-Hinokinin, (−)-Pluviatolide, (−)-Enterodiol, and Furofuran Lignan (−)-Eudesmin via tandem conjugate addition to gamma-alkoxybutenolides. J Org Chem. 1994;59:5999–6007. doi: 10.1021/jo00099a033. [DOI] [Google Scholar]
- Chen Q, Geng Z, Huang B. Synthesis of enantiomerically pure 5-(l-menthyloxy)-3,4-dibromo-2(5 H)-furanone and its tandem asymmetric Michael addition–elimination reaction. Tetrahedron Asymmetry. 1995;6:401–404. doi: 10.1016/0957-4166(95)00024-J. [DOI] [Google Scholar]
- Smicius R, Burbuliene MM, Jakubkiene V, Udrwėnaitė E, Vainilavičius P. Convenient way to 5-substituted 4-amino-2,3-dihydro-4H-1,2,4-triazole-3-thiones. J Heterocyclic Chem. 2007;44:279–284. doi: 10.1002/jhet.5570440201. [DOI] [Google Scholar]
- Reid JR, Heindel ND. Improved synthesis of 5-substituted −4-amino-3-mercapto-4H-1,2,4-triazoles. J Heterocyclic Chem. 1976;13:925–926. doi: 10.1002/jhet.5570130450. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available. Experimental procedures, spectral data of new compounds.