Abstract
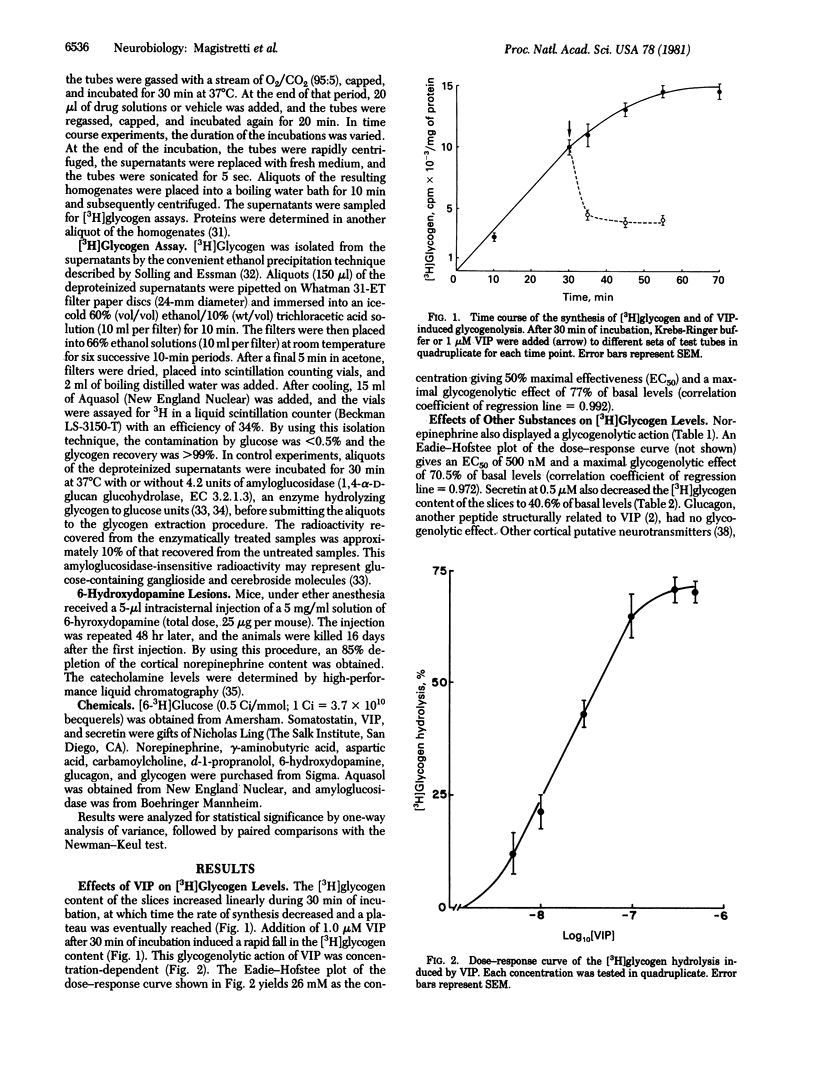
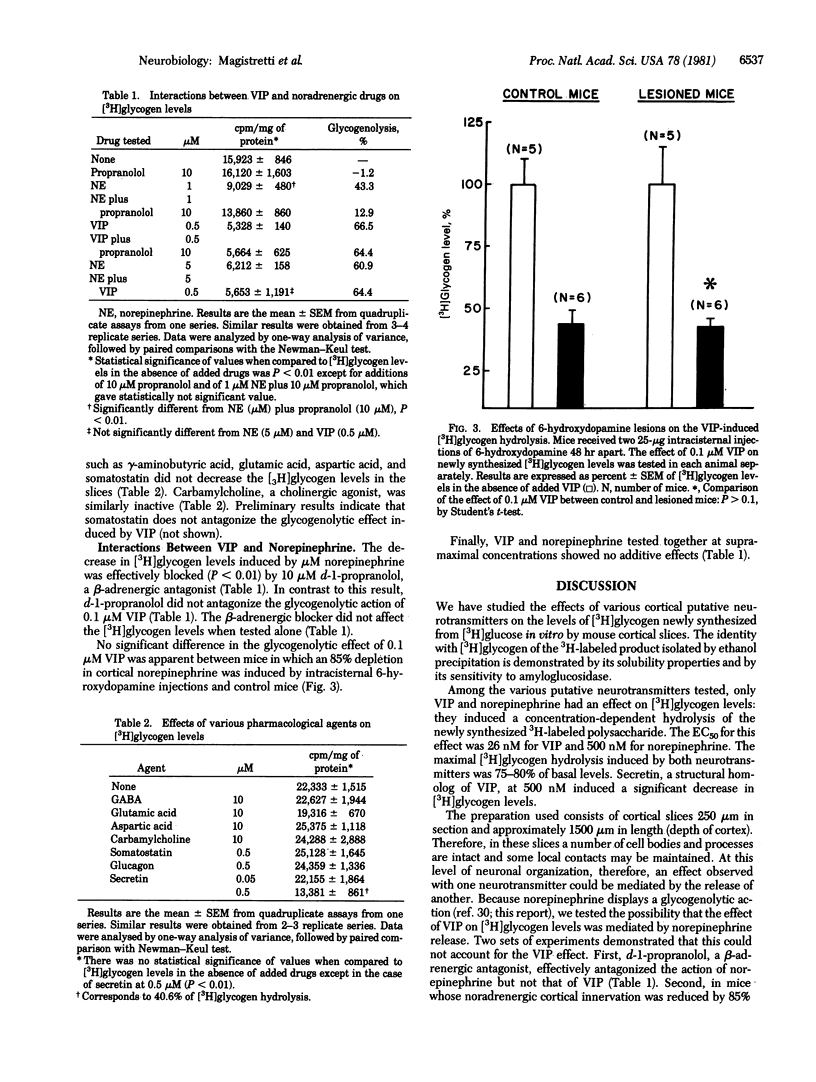
Mouse cerebral cortex slices will synthesize [3H]glycogen in vitro. Vasoactive intestinal polypeptide (VIP) stimulates the enzymatic breakdown of this [3H]glycogen. The concentration giving 50% of maximum effectiveness (EC50) is 26 nM. Under the same experimental conditions norepinephrine also induces a concentration-dependent [3H]glycogen hydrolysis with an EC50 of 500 nM. The effect of VIP is not mediated by the release of norepinephrine because it is not blocked by the noradrenergic antagonist d-1-propranolol and is still present in mice in which an 85% depletion of norepinephrine was induced by intracisternal 6-hydroxydopamine injections. Other cortical putative neurotransmitters such as gamma-aminobutyric acid, aspartic acid, glutamic acid, somatostatin, and acetylcholine (tested with the agonist carbamylcholine) do not induce a breakdown of [3H]glycogen. This glycogenolytic effect of VIP and norepinephrine, presumed to be mediated by cyclic AMP formation, should result, at the cellular level, in an increased glucose availability for the generation of phosphate-bound energy. Given the narrow radial pattern of arborization of the intracortical VIP neuron and the tangential intracortical trajectory of the noradrenergic fibers, these two systems may function in a complementary fashion: VIP regulating energy metabolism locally, within individual columnar modules, and norepinephrine exerting a more global effect that spans adjacent columns.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbezat G. O., Grossman M. I. Intestinal secretion: stimulation by peptides. Science. 1971 Oct 22;174(4007):422–424. doi: 10.1126/science.174.4007.422. [DOI] [PubMed] [Google Scholar]
- Bloom F. E. The role of cyclic nucleotides in central synaptic function. Rev Physiol Biochem Pharmacol. 1975;74:1–103. doi: 10.1007/3-540-07483-x_19. [DOI] [PubMed] [Google Scholar]
- Browning E. T., Schwartz J. P., Breckenridge B. M. Norepinephrine-sensitive properties of C-6 astrocytoma cells. Mol Pharmacol. 1974 Jan;10(1):162–174. [PubMed] [Google Scholar]
- COGGESHALL R. E., FAWCETT D. W. THE FINE STRUCTURE OF THE CENTRAL NERVOUS SYSTEM OF THE LEECH, HIRUDO MEDICINALIS. J Neurophysiol. 1964 Mar;27:229–289. doi: 10.1152/jn.1964.27.2.229. [DOI] [PubMed] [Google Scholar]
- Deschodt-Lanckman M., Robberecht P., Christophe J. Characterization of VIP-sensitive adenylate cyclase in guinea pig brain. FEBS Lett. 1977 Nov 1;83(1):76–80. doi: 10.1016/0014-5793(77)80645-5. [DOI] [PubMed] [Google Scholar]
- Edwards C., Nahorski S. R., Rogers K. J. In vivo changes of cerebral cyclic adenosine 3',5'-monophosphate induced by biogenic amines: association with phosphorylase activation. J Neurochem. 1974 Apr;22(4):565–572. doi: 10.1111/j.1471-4159.1974.tb06896.x. [DOI] [PubMed] [Google Scholar]
- Emson P. C., Fahrenkrug J., Schaffalitzky de Muckadell O. B., Jessell T. M., Iversen L. L. Vasoactive intestinal polypeptide (VIP): vesicular localization and potassium evoked release from rat hypothalamus. Brain Res. 1978 Mar 17;143(1):174–178. doi: 10.1016/0006-8993(78)90762-x. [DOI] [PubMed] [Google Scholar]
- Emson P. C., Lindvall O. Distribution of putative neurotransmitters in the neocortex. Neuroscience. 1979;4(1):1–30. doi: 10.1016/0306-4522(79)90215-x. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J., Schaffalitzky de Muckadell O. B. Distribution of vasoactive intestinal polypeptide (VIP) in the porcine central nervous system. J Neurochem. 1978 Dec;31(6):1445–1451. doi: 10.1111/j.1471-4159.1978.tb06571.x. [DOI] [PubMed] [Google Scholar]
- Felice L. J., Felice J. D., Kissinger P. T. Determination of catecholamines in rat brain parts by reverse-phase ion-pair liquid chromatography. J Neurochem. 1978 Dec;31(6):1461–1465. doi: 10.1111/j.1471-4159.1978.tb06573.x. [DOI] [PubMed] [Google Scholar]
- Foote S. L., Freedman R., Oliver A. P. Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res. 1975 Mar 21;86(2):229–242. doi: 10.1016/0006-8993(75)90699-x. [DOI] [PubMed] [Google Scholar]
- Garbarg M., Barbin G., Feger J., Schwartz J. C. Histaminergic pathway in rat brain evidenced by lesions of the medial forebrain bundle. Science. 1974 Nov 29;186(4166):833–835. doi: 10.1126/science.186.4166.833. [DOI] [PubMed] [Google Scholar]
- Giachetti A., Said S. I., Reynolds R. C., Koniges F. C. Vasoactive intestinal polypeptide in brain: localization in and release from isolated nerve terminals. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3424–3428. doi: 10.1073/pnas.74.8.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., O'Toole A. G. The properties of glycogen synthetase and regulation of glycogen biosynthesis in rat brain. J Biol Chem. 1969 Jun 10;244(11):3053–3061. [PubMed] [Google Scholar]
- Jörnvall H., Carlström A., Pettersson T., Jacobsson B., Persson M., Mutt V. Structural homologies between prealbumin, gastrointestinal prohormones and other proteins. Nature. 1981 May 21;291(5812):261–263. doi: 10.1038/291261a0. [DOI] [PubMed] [Google Scholar]
- Kerins C., Said S. I. Hyperglycemic and glycogenolytic effects of vasoactive intestinal polypeptide. Proc Soc Exp Biol Med. 1973 Mar;142(3):1014–1017. doi: 10.3181/00379727-142-37165. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsson L. I., Fahrenkrug J., Schaffalitzky De Muckadell O., Sundler F., Håkanson R., Rehfeld J. R. Localization of vasoactive intestinal polypeptide (VIP) to central and peripheral neurons. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3197–3200. doi: 10.1073/pnas.73.9.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidov H. G., Grzanna R., Molliver M. E. The serotonin innervation of the cerebral cortex in the rat--an immunohistochemical analysis. Neuroscience. 1980;5(2):207–227. doi: 10.1016/0306-4522(80)90099-8. [DOI] [PubMed] [Google Scholar]
- Lorén I., Emson P. C., Fahrenkrug J., Björklund A., Alumets J., Håkanson R., Sundler F. Distribution of vasoactive intestinal polypeptide in the rat and mouse brain. Neuroscience. 1979;4(12):1953–1976. doi: 10.1016/0306-4522(79)90068-x. [DOI] [PubMed] [Google Scholar]
- MEYER W. L., FISCHER E. H., KREBS E. G. ACTIVATION OF SKELETAL MUSCLE PHOSPHORYLASE B KINASE BY CA. Biochemistry. 1964 Aug;3:1033–1039. doi: 10.1021/bi00896a004. [DOI] [PubMed] [Google Scholar]
- Makhlouf G. M., Zfass A. M., Said S. I., Schebalin M. Effects of synthetic vasoactive intestinal peptide (VIP), secretin and their partial sequences on gastric secretion. Proc Soc Exp Biol Med. 1978 Apr;157(4):565–568. doi: 10.3181/00379727-157-40097. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Bloom F. E. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Morrison J. H., Grzanna R., Molliver M. E., Coyle J. T. The distribution and orientation of noradrenergic fibers in neocortex of the rat: an immunofluorescence study. J Comp Neurol. 1978 Sep 1;181(1):17–39. doi: 10.1002/cne.901810103. [DOI] [PubMed] [Google Scholar]
- Morrison J. H., Molliver M. E., Grzanna R., Coyle J. T. The intra-cortical trajectory of the coeruleo-cortical projection in the rat: a tangentially organized cortical afferent. Neuroscience. 1981;6(2):139–158. doi: 10.1016/0306-4522(81)90051-8. [DOI] [PubMed] [Google Scholar]
- Morrison J. H., Molliver M. E., Grzanna R. Noradrenergic innervation of cerebral cortex: widespread effects of local cortical lesions. Science. 1979 Jul 20;205(4403):313–316. doi: 10.1126/science.451605. [DOI] [PubMed] [Google Scholar]
- Mutt V., Carlquist M., Tatemoto K. Secretin-like bioactivity in extracts of porcine brain. Life Sci. 1979 Nov 12;25(20):1703–1707. doi: 10.1016/0024-3205(79)90472-7. [DOI] [PubMed] [Google Scholar]
- Nahorski S. R., Rogers K. J. An enzymic fluorometric micro method for determination of glycoen. Anal Biochem. 1972 Oct;49(2):492–497. doi: 10.1016/0003-2697(72)90453-8. [DOI] [PubMed] [Google Scholar]
- Passonneau J. V., Crites S. K. Regulation of glycogen metabolism in astrocytoma and neuroblastoma cells in culture. J Biol Chem. 1976 Apr 10;251(7):2015–2022. [PubMed] [Google Scholar]
- Passonneau J. V., Lauderdale V. R. A comparison of three methods of glycogen measurement in tissues. Anal Biochem. 1974 Aug;60(2):405–412. doi: 10.1016/0003-2697(74)90248-6. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Kirkpatrick J. R., Said S. I. Vasoactive intestinal polypeptide excitation of central neurons. Can J Physiol Pharmacol. 1978 Apr;56(2):337–340. doi: 10.1139/y78-052. [DOI] [PubMed] [Google Scholar]
- Piper P. J., Said S. I., Vane J. R. Effects on smooth muscle preparations of unidentified vasoactiv peptides from intestine and lung. Nature. 1970 Mar 21;225(5238):1144–1146. doi: 10.1038/2251144a0. [DOI] [PubMed] [Google Scholar]
- Quach T. T., Rose C., Schwartz J. C. [3H]Glycogen hydrolysis in brain slices: responses to neurotransmitters and modulation of noradrenaline receptors. J Neurochem. 1978 Jun;30(6):1335–1341. doi: 10.1111/j.1471-4159.1978.tb10464.x. [DOI] [PubMed] [Google Scholar]
- Quik M., Iversen L. L., Bloom S. R. Effect of vasoactive intestinal peptide (VIP) and other peptides on cAMP accumulation in rat brain. Biochem Pharmacol. 1978;27(18):2209–2213. doi: 10.1016/0006-2952(78)90079-5. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Relationships between calcium and cyclic nucleotides in cell activation. Physiol Rev. 1977 Jul;57(3):421–509. doi: 10.1152/physrev.1977.57.3.421. [DOI] [PubMed] [Google Scholar]
- Robberecht P., De Neef P., Lammens M., Deschodt-Lanckman M., Christophe J. P. Specific binding of vasoactive intestinal peptide to brain membranes from the guinea pig. Eur J Biochem. 1978 Sep 15;90(1):147–154. doi: 10.1111/j.1432-1033.1978.tb12585.x. [DOI] [PubMed] [Google Scholar]
- Said S. I., Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970 Sep 18;169(3951):1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- Said S. I., Rosenberg R. N. Vasoactive intestinal polypeptide: abundant immunoreactivity in neural cell lines and normal nervous tissue. Science. 1976 May 28;192(4242):907–908. doi: 10.1126/science.1273576. [DOI] [PubMed] [Google Scholar]
- Schebalin M., Said S. I., Makhlouf G. M. Stimulation of insulin and glucagon secretion by vasoactive intestinal peptide. Am J Physiol. 1977 Feb;232(2):E197–E200. doi: 10.1152/ajpendo.1977.232.2.E197. [DOI] [PubMed] [Google Scholar]
- Segal M., Bloom F. E. The action of norepinephrine in the rat hippocampus. IV. The effects of locus coeruleus stimulation on evoked hippocampal unit activity. Brain Res. 1976 May 14;107(3):513–525. doi: 10.1016/0006-8993(76)90141-4. [DOI] [PubMed] [Google Scholar]
- Sims K. B., Hoffman D. L., Said S. I., Zimmerman E. A. Vasoactive intestinal polypeptide (VIP) in mouse and rat brain: an immunocytochemical study. Brain Res. 1980 Mar 17;186(1):165–183. doi: 10.1016/0006-8993(80)90263-2. [DOI] [PubMed] [Google Scholar]
- Solling H., Esmann V. A sensitive method of glycogen determination in the presence of interfering substances utilizing the filter-paper technique. Anal Biochem. 1975 Oct;68(2):664–668. doi: 10.1016/0003-2697(75)90667-3. [DOI] [PubMed] [Google Scholar]
- Taylor D. P., Pert C. B. Vasoactive intestinal polypeptide: specific binding to rat brain membranes. Proc Natl Acad Sci U S A. 1979 Feb;76(2):660–664. doi: 10.1073/pnas.76.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Passonneau J. V. Factors affecting the turnover of cerebral glycogen and limit dextrin in vivo. J Neurochem. 1973 Jun;20(6):1543–1554. doi: 10.1111/j.1471-4159.1973.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Wilkening D., Makman M. H. Activation of glycogen phosphorylase in rat caudate nucleus slices by L-isopropylnorepinepherine and dibutyryl cyclic AMP. J Neurochem. 1977 May;28(5):1001–1007. doi: 10.1111/j.1471-4159.1977.tb10662.x. [DOI] [PubMed] [Google Scholar]
- Wolfe D. E., Nicholls J. G. Uptake of radioactive glucose and its conversion to glycogen by neurons and glial cells in the leech central nervous system. J Neurophysiol. 1967 Nov;30(6):1593–1609. doi: 10.1152/jn.1967.30.6.1593. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Regulation by secretin, vasoactive intestinal peptide, and somatostatin of cyclic AMP accumulation in cultured brain cells. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6907–6911. doi: 10.1073/pnas.77.11.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]



