Abstract
Xylem cavitation in winter and recovery from cavitation in the spring were visualized in two species of diffuse-porous trees, Betula platyphylla var. japonica Hara and Salix sachalinensis Fr. Schm., by cryo-scanning electron microscopy after freeze-fixation of living twigs. Water in the vessel lumina of the outer three annual rings of twigs of B. platyphylla var. japonica and of S. sachalinensis gradually disappeared during the period from January to March, an indication that cavitation occurs gradually in these species during the winter. In April, when no leaves had yet expanded, the lumina of most of the vessels of both species were filled with water. Many vessel lumina in twigs of both species were filled with water during the period from the subsequent growth season to the beginning of the next winter. These observations indicate that recovery in spring occurs before the onset of transpiration and that water transport through twigs occurs during the subsequent growing season. We found, moreover, that vessels repeat an annual cycle of winter cavitation and spring recovery from cavitation for several years until irreversible cavitation occurs.
In plants “cavitation” refers to the formation of a cavity within a body of water, usually in the xylem water-transport system (Milburn, 1991). All conduits for the transport of water in plants are potentially vulnerable to cavitation. Cavitation disrupts water transport and long-term consequences can include reduced growth and dieback of the plant. Cavitation occurs mainly in response to water stress or freezing (Tyree and Sperry, 1989), and cavitation caused by water stress has been explained in terms of air-seeding, as follows. When the tension inside xylem conduits exceeds a certain critical value, air is sucked into the lumina of water-filled conduits from adjacent gas-filled conduits through the pores of intervascular pit membranes (Zimmermann, 1983; Sperry and Tyree, 1988). By contrast, the cavitation that is caused by freezing seems to occur as a result of the low solubility of gases in ice. When frozen sap containing air bubbles thaws under tension, air bubbles expand in the xylem conduits and cavitation occurs (Zimmermann, 1983; Sperry, 1993).
In dicotyledonous trees the region of the xylem that functions in water transport varies among species. In ring-porous trees it is the earlywood vessels of the current-year xylem that are mainly involved in water transport (Chaney and Kozlowski, 1977; Ellmore and Ewers, 1986). The earlywood vessels lose the capacity to function in the transport of water by the end of each winter, and no refilling with water occurs in these vessels during the following spring (Zimmermann, 1983). Consequently, earlywood vessels play a role in water transport during a single growth season. The cavitation that occurs in earlywood vessels of ring-porous trees is believed to be irreversible.
In diffuse-porous trees, by contrast to ring-porous trees, water transport generally occurs in a larger part of the sapwood (Greenidge, 1958; Chaney and Kozlowski, 1977). This observation indicates that vessels in diffuse-porous trees may continue to function as pathways for water transport for several years. Two possibilities might explain how water transport is maintained for several years in the vessels of diffuse-porous trees. One possibility is that newly formed vessels might retain the ability to function in water transport for several years in the absence of cavitation until irreversible cavitation occurs. Alternatively, functional vessels might repeat a cycle of cavitation and recovery for several years until irreversible cavitation occurs. However, these possibilities have not yet been examined.
Seasonal changes in water transport have been characterized mainly in terms of hydraulic conductivity (Sperry et al., 1988; Cochard and Tyree, 1990). It has been reported that in diffuse-porous trees hydraulic conductivity is lower in the winter than in other seasons (Sperry and Sullivan, 1992; Magnani and Borghetti, 1995), an indication that water transport is disrupted during the winter in diffuse-porous trees. This method has yielded important information about quantitative analysis in seasonal changes in water transport, but it is indirect and does not reveal the location of cavitated vessels in xylem or the state of water inside vessels (McCully et al., 1998). In particular, it is difficult to reveal whether air occupies the entire lumina of vessels or whether only small air bubbles are present inside the vessels.
Cryo-SEM allows fixation of water as ice crystals in cell lumina and visualization of the precise location of water in the xylem of living trees (Ohtani and Fujikawa, 1990; Utsumi et al., 1996). However, during the growing season, water moves from roots to leaves as a consequence of the negative pressure that is created in the leaf xylem by transpiration. Zimmermann and Brown (1971) proposed that the water in vessels is replaced by air when the pressure in these conduits falls below atmospheric pressure and the conduits are exposed to air. To prevent this phenomenon during cutting of twigs, we froze the twigs of living trees in LN2, and stored the samples at subzero temperatures prior to cryo-SEM. In our previous study, in which we applied these techniques for an examination of the seasonal progression of xylem cavitation in Fraxinus mandshurica var. japonica, we obtained valuable information about the process and timing of cavitation in the earlywood vessels of a species of ring-porous trees (Utsumi et al., 1996). In the present study we attempted to visualize seasonal changes in the distribution of water in two species of diffuse-porous trees, B. platyphylla var. japonica Hara and S. sachalinensis Fr. Schm., by cryo-SEM after freeze-fixation of living twigs to characterize the seasonal progression of xylem cavitation.
MATERIALS AND METHODS
Plant Materials and Sampling
Two mature trees of Betula platyphylla var. japonica Hara (height, about 8 m; diameter at breast height, about 15 cm) and of Salix sachalinensis Fr. Schm. (height, about 15 m; diameter at breast height, about 40 cm) growing on the campus of Hokkaido University (Sapporo, Japan) were used. Twig internodes with three to four annual rings were collected at monthly intervals from the beginning of March, 1996, to the end of April, 1996, and from the beginning of November, 1996, to the end of April, 1997. The sample twigs for summer measurements were collected in August, 1996, only. Four twigs per species were obtained in each sampling time. In a preliminary investigation, we found that several sample trees had similar moisture contents.
Samples were collected after the xylem sap had been fixed by freezing the twigs on living trees by the previously described procedure (Utsumi et al., 1996). Watertight collars were made with plastic funnels as containers for LN2, and fitted to the sample twigs. Then, the collars were filled with LN2 and the twigs were allowed to freeze for approximately 5 min. Twigs that contained completely frozen regions (over 5 cm in length) were immediately removed from the sample trees and stored in a container with LN2.
Cryo-SEM
The sample twigs that had been stored in LN2 were transferred to a low-temperature room kept at −20°C and were divided into small specimens (1 cm in length). These specimens were cut into small blocks (5 × 5 × 5 mm3) that included part of the outer three annual rings and phloem. One to four blocks were selected from each twig. Transverse, tangential, or radial surfaces of the each block were cleanly cut with steel blades on a sliding microtome to expose cell lumina (Sano et al., 1993, 1995). Then, the blocks were attached to specimen holders with a drop of glycerol and further fastened with a screw. The specimen holder with the sample immersed in LN2 was transferred to a system for cryo-SEM (model JSM840-a, Jeol) and was fixed on the cold stage of the freeze-etching unit, which was maintained under a vacuum of approximately 1 × 10−4 Pa and equilibrated to −108°C. The specimen was freeze-etched under these conditions for about 10 min to eliminate contamination by frost, and then it was rotary-shadowed with a platinum-carbon pellet. The sample was transferred to the cold stage of the SEM, which was kept at about −160°C, and secondary electron images were observed and recorded at an accelerating voltage of 5 kV (Fujikawa et al., 1988).
To evaluate seasonal occurrence of cavitation quantitatively, 1.5- × 1.5-mm2 areas containing more than 100 vessel elements of the outer two annual rings were selected from the cryo-SEM photographs of the transverse surface of each sample. The number of water-filled and cavitated vessel elements were counted and the percentage of cavitated vessel elements was determined.
Environmental Temperatures
Daily minimum and maximum air temperatures and the temperature of the soil 10 cm below the surface of the ground were recorded at the Experimental Farm of Hokkaido University, which is located about 1 km from the site where sample trees were growing from March, 1996, to March, 1997, for evaluation of the relationship between cavitation and freezing.
RESULTS
Seasonal Changes in the Distribution of Water in Twigs
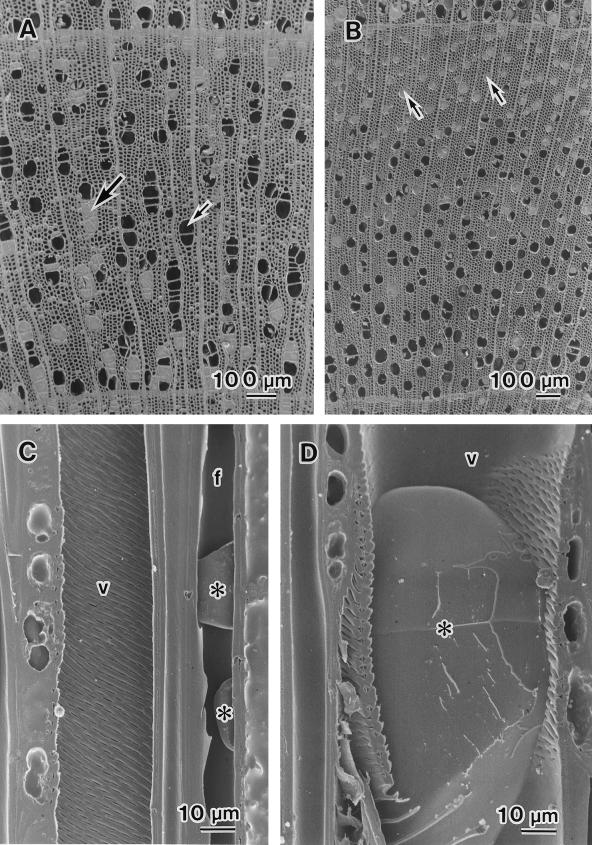
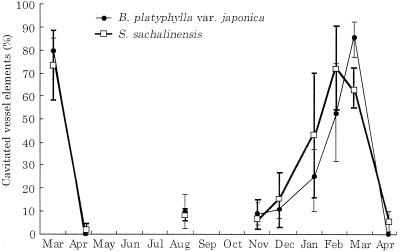
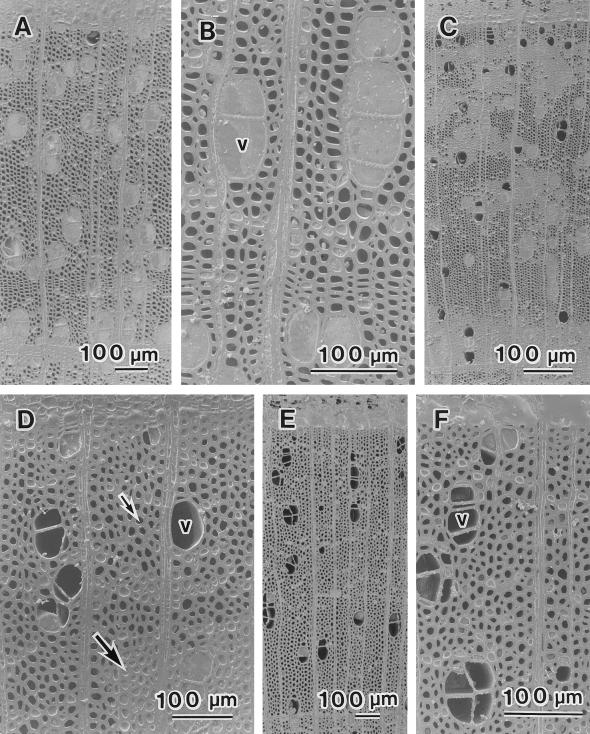
The distribution of water in twigs was visualized by cryo-SEM. Seasonal changes in the distribution of water in vessels and fibers of twigs of B. platyphylla var. japonica and S. sachalinensis were similar, with the exception of those in March, when no leaves had yet expanded and current-year xylem had not yet differentiated. No differences in the distribution of water were found among annual rings. Figure 1A shows an example of xylem from the previous year's growth from B. platyphylla var. japonica. Some vessels were filled with water (large arrow), whereas others were completely empty (small arrow). Most fibers had no water in their lumina. Similarly, Figure 1B shows an example of xylem from the previous year's growth from S. sachalinensis. Both water-filled vessels and empty vessels were found. Vessels near the terminal zone of the annual ring, however, consistently had water in their lumina (arrows). On tangential surfaces, almost all vessels were found to be cavitated (Fig. 1, C and D). Some vessels were completely empty (Fig. 1C, v), whereas other vessels still had some water in their lumina (Fig. 1D, *). Many fiber lumina were empty, but a few fibers had some water in their lumina (Fig. 1C, *). The percentage of cavitated vessel elements was about 80% in B. platyphylla var. japonica and about 70% in S. sachalinensis in a given area of the outer two annual rings (Fig. 2).
Figure 1.
Cryo-SEM photographs of Betula platyphylla var. japonica and Salix sachalinensis in March. A, Transverse surface of the xylem from the previous year's growth of B. platyphylla var. japonica. Some vessels were filled with water (large arrow), whereas others were completely empty (small arrow). B, Transverse surface of the xylem from the previous year's growth of S. sachalinensis. Some vessels were filled with water; others contained no water. Vessels near the terminal zone of an annual ring contained water in their lumina (arrows). C, Tangential surface of B. platyphylla var. japonica. Vessels (v) contained no water. Drops of water (*) were present in fiber lumina (f). D, Tangential surface of B. platyphylla var. japonica. A drop of water (*) can be seen in a vessel lumen (v).
Figure 2.
Seasonal changes of percentage of cavitated vessel elements during the period from March, 1996, to April, 1997. Means for four twigs per one species are shown with 95% confidence intervals.
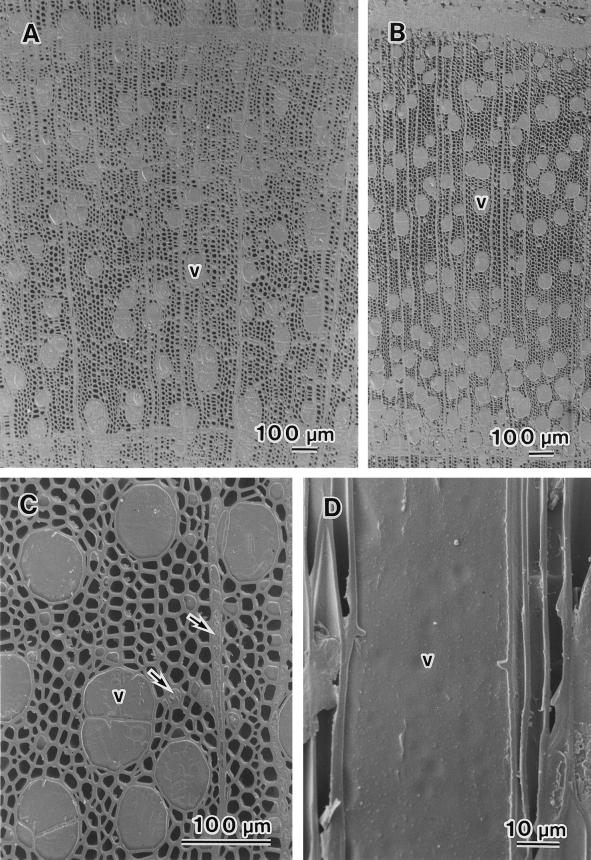
In April, when the leaves had still not yet expanded, almost all of the vessels of B. platyphylla var. japonica (Fig. 3A) and S. sachalinensis (Fig. 3B) were filled with water. Cell division in the cambium had begun in S. sachalinensis, but not in B. platyphylla var. japonica. Figure 3C shows an example of the xylem from the previous year's growth of S. sachalinensis. The vessel lumina were completely filled with water and the lumina of axial and ray parenchyma cells were filled with cytoplasm (arrows). In contrast to samples collected in March, almost all vessel lumina were completely filled with water, as seen in the radial view (Fig. 3D, v). The percentage of cavitated vessel elements was near 0% in both species (Fig. 2).
Figure 3.
Cryo-SEM photographs of B. platyphylla var. japonica and S. sachalinensis in April. A, Transverse surface of the xylem from the previous year's growth of B. platyphylla var. japonica. All vessels (v) were filled with water. B, Transverse surface of the xylem from the previous year's growth of S. sachalinensis. All vessels were filled with water. C, Transverse surface of S. sachalinensis. All vessels were filled with water. Axial and ray parenchyma cells were filled with cytoplasm (arrows). D, Radial surface of S. sachalinensis. The vessel shown was completely filled with water.
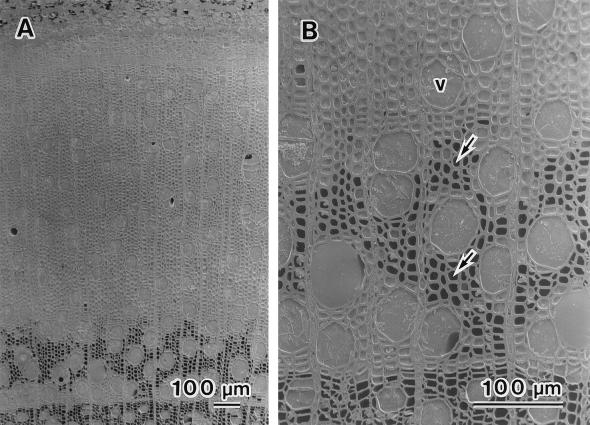
In August, leaves were fully expanded and new wood was being formed. In the xylem that had been formed during the previous year and the year prior to that, most vessels were filled with water, but a few vessels were completely or partially cavitated. Most wood fibers had no water in their lumina. In the newly forming current-year xylem, most vessels and wood fibers were filled with either water or cytoplasm (Fig. 4A). Wood fibers without water in their lumina were found only in the initial zone of the current-year's annual ring (Fig. 4B, arrows). The percentage of cavitated vessel elements was about 10% in both species (Fig. 2).
Figure 4.
Cryo-SEM photographs of S. sachalinensis in August. A, Transverse surface of the newly forming current-year xylem. Most vessels and fibers were filled with water or cytoplasm. B, Initial zone of newly forming current-year xylem. All vessels (v) were filled with water. Fibers without water in their lumina were also found (arrows).
During the period from November to February, when all leaves had fallen, the percentage of cavitated vessel elements gradually increased (Fig. 2). The amount of water retained within vessels differed among the cavitated vessels, but such differences were independent of the time of sampling. In November, many vessels were filled with water, whereas almost all wood fiber lumina were empty (Fig. 5, A and B). No apparent differences were observed between November and December. In January, the numbers of cavitated vessel elements increased (Figs. 2 and 5C). Furthermore, both water-filled wood fibers (Fig. 5D, large arrow) and empty fibers were observed (Fig. 5D, small arrow). In February, the number of cavitated vessel elements increased still further (Figs. 2 and 5E). Almost no wood fibers had water in their lumina (Fig. 5F). Resembling results from 1996, most vessel lumina had no water in March, 1997, and were refilled with water in April, 1997 (Fig. 2).
Figure 5.
Cryo-SEM photographs of B. platyphylla var. japonica in winter. A, Transverse surface of current-year xylem in November. Most vessels were filled with water, whereas almost all fiber lumina were empty. B, Current-year xylem in November. Vessels (v) were filled with water. C, Transverse surface of previous-year xylem in January. Some vessels were filled with water but others contained no water. D, Previous-year xylem in January. Both water-filled fibers (large arrow) and empty fibers (small arrow) were present. E, Transverse surface of previous-year xylem in February. Most vessels were empty. F, Previous-year xylem in February. Most vessels and fibers contained no water.
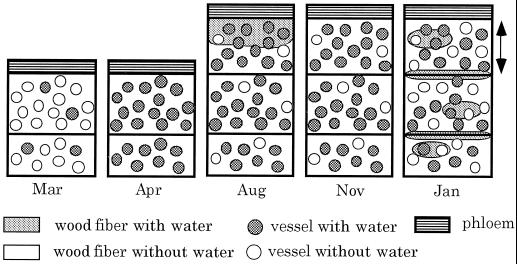
The results of this study are schematically illustrated in Figure 6.
Figure 6.
A schematic representation of seasonal changes in the distribution of water in vessel and wood-fiber lumina of B. platyphylla var. japonica. The double-headed arrow shows a newly formed annual ring.
Environmental Temperatures
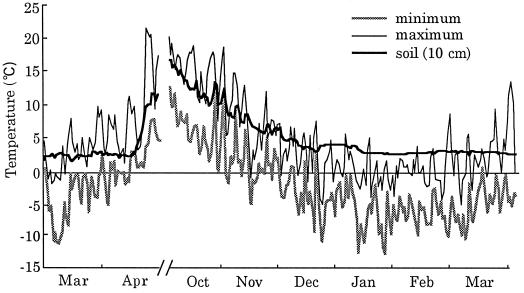
To study the relationship between cavitation and stress due to freezing, we analyzed seasonal change in air and soil temperatures. Figure 7 shows the daily maximum and minimum air temperatures, as well as soil temperatures, recorded at the Experimental Farm of Hokkaido University from March, 1996, to March, 1997. From March, 1996, to April, 1996, the temperature profiles (including that of the soil) increased rapidly. The first subzero minimum temperature was recorded in late October, and the first subzero maximum temperature was recorded in mid November, 2 d before the sampling date. During the subsequent winter, the daily minimum air temperature decreased to and remained below −10°C on 12 separate days. No subzero soil temperatures were recorded during this period.
Figure 7.
Changes in air and soil temperatures during the period from March, 1996, to April, 1996, and October, 1996, to March, 1997. Soil temperatures were measured 10 cm beneath the surface of the soil.
DISCUSSION
From January to March, the number of cavitated vessels increased steadily in the outer three annual rings of twigs of B. platyphylla var. japonica and S. sachalinensis (Figs. 1, 2, and 5). In January, cavitation was found in only a few vessels but by March, the number of cavitated vessels had greatly increased. These results indicate that xylem cavitation starts in early winter and progresses steadily during the winter in both of the species examined. Gradual progression of cavitation is in contrast to the simultaneous cavitation of earlywood vessel in ring-porous trees. In our previous study, which was designed to monitor the seasonal progression of cavitation in F. mandshurica var. japonica (a ring-porous tree) and which was performed by the same methods used in this study, all of the earlywood vessels were simultaneously cavitated in early winter (Utsumi et al., 1996).
During the period from December to March, the daily minimum air temperature fell below −10°C on several occasions (Fig. 7). This infrequent incidence of low temperatures might have been sufficient to freeze the sap in vessel lumina; in fact, it is likely that the sap was frozen and thawed several times during the winter. In diffuse-porous trees, repeated cycles of freezing and thawing might result in the progression of cavitation. Differences in the progression of cavitation between diffuse-porous trees and ring-porous trees have been observed by many investigators in studies of the seasonal progression of cavitation by determinations of hydraulic conductivity. In dicotyledonous trees, hydraulic conductivity decreases in the winter (Sperry and Sullivan, 1992; Magnani and Borghetti, 1995; Pockman and Sperry, 1997). The extent of the loss of hydraulic conductivity depends on the species. In general, loss of hydraulic conductivity is less extensive in diffuse-porous than in ring-porous trees (Sperry, 1993; Hacke and Sauter, 1996). Moreover, in a study of ring-porous trees, the first hard frost induced an abrupt loss of hydraulic conductivity (Cochard and Tyree, 1990).
Such differences in the progression and extent of cavitation caused by freezing appear to be due to differences in conduit diameter (Tyree et al., 1994; Cochard and Tyree, 1990). Ewers (1985) examined the stability of freezing-induced air bubbles in water contained in glass capillary tubes of various diameters. In these experiments, when the water in narrow capillary tubes was frozen and thawed, air bubbles formed during freezing and disappeared soon after thawing. These capillary tubes were of similar diameter to the large earlywood tracheids of conifers. By contrast, air bubbles formed during freezing in large glass capillary tubes, with diameters similar to those of earlywood vessels of ring-porous trees, were present for a long time after thawing. Evidence suggesting similar tendencies has been obtained in other plant materials. It has been reported that most conifers are entirely resistant to cavitation caused by freeze-thaw cycles, whereas dicotyledonous trees are vulnerable to such cavitation (Hammel, 1967; Sperry et al., 1994). The decrease in hydraulic conductivity in diffuse-porous trees was reported to be smaller than that in ring-porous trees during the winter (Sperry and Sullivan, 1992). In the present study vessels near the terminal zone of the annual rings of S. sachalinensis, which had relatively small diameters, retained water in March (Fig. 1B), although about 70% of vessel elements were cavitated in the outer two annual rings (Fig. 2).
Our cryo-SEM observations provide some crucial information about the process and timing of refilling of vessels. In April, when the leaves had not yet expanded, almost all of the vessel lumina of twigs of B. platyphylla var. japonica and S. sachalinensis were completely refilled with water (Fig. 3). Most of these refilled vessels retained water in their lumina in August. These results indicate that spring recovery from winter cavitation prior to leaf expansion and water transport occurs in most vessels in twigs during the subsequent growth season. In general, water transport in plants has mainly been explained by the so-called cohesion theory (Dixon, 1914; Kramer and Kozlowski, 1960; Kozlowski, 1961). Leaves had not yet expanded in April, however, when the vessels had already been refilled with water. Recovery from winter cavitation clearly occurred before the onset of transpiration in both species examined.
Root pressure is considered to play a major role in the spring recovery from xylem cavitation (Sholander et al., 1955; Sperry et al., 1987, 1988). Positive pressures have been measured in many woody species (O'Malley and Milburn, 1983; Steudle, 1994; Hacke and Sauter, 1996). Such positive root pressure could dissolve the gas in the sap and push undissolved gas out of the vessels until cavitated vessels become filled with water (Sperry et al., 1987). However, it has been noted that root pressure is insufficient to push water up to the crown of a mature tall tree (Steudle, 1995). In addition, recovery from cavitation in the absence of root pressure has been suggested in conifers (Borghetti et al., 1991; Grace, 1993; Sperry, 1993). Borghetti et al. (1991) suggested that capillary action might help to refill tracheids. In our experiments no measurements were made of the root pressure, therefore it is not possible to state the mechanisms of the refilling of cavitated vessels. If spring recovery from winter cavitation was caused by root pressure, refilling of cavitated vessels would progress upward from the basal portion of trunk to crown. To evaluate the contribution of root pressure to the refilling, it is necessary to examine the differences in the distribution of water among heights.
We observed changes in the distribution of water in the wood fibers of the newly forming current-year xylem during the growth season. In August, water or cytoplasm was present in lumina of newly forming wood fiber, and had disappeared by early winter. The disappearance of water from the wood fibers started in the initial zone of the current-year annual ring and progressed toward the terminal zone of the annual ring (Fig. 4). A similar result was observed in F. mandshurica var. japonica (Utsumi et al., 1996). The disappearance of water from wood fibers would be associated with their maturation.
We also found changes in the distribution of water in mature wood fibers in winter. In January, when the current-year xylem had completely matured and some vessels had been cavitated, some fiber lumina were refilled with water. This phenomenon was not confined to the current-year xylem but was also observed in older annual rings. Utsumi et al. (1996) reported that in F. mandshurica var. japonica, wood fibers surrounding current-year earlywood vessels were refilled with water when these vessels were cavitated in November. They proposed that water might migrate from newly formed earlywood vessels to the wood fibers around the vessels when cavitation occurred. In B. platyphylla var. japonica and S. sachalinensis, it is also possible that water might move from vessels to wood fibers when cavitation occurs in winter, as seems to be the case in F. mandshurica var. japonica. However, the wood fibers that surrounded vessels were not always filled with water in the two species examined in this study (Fig. 5, C and D). The appearance of water-filled wood fibers seemed to be unrelated to the arrangement of vessels. More detailed information about the relationship between the cavitation of vessels and the refilling of wood fibers with water might provide some clues to the mechanisms of cavitation that is caused by freezing.
Abbreviations:
- LN2
liquid nitrogen
- SEM
scanning electron microscopy
Footnotes
This study was supported in part by a Grant-in-Aid for Scientific Research (no. 08456083) from the Ministry of Education, Science, and Culture, Japan.
LITERATURE CITED
- Borghetti M, Edwards WRN, Grace J, Jarvis PG, Raschi A. The refilling of embolized xylem in Pinus sylvestris. L. Plant Cell Environ. 1991;14:357–369. [Google Scholar]
- Chaney WR, Kozlowski TT. Patterns of water movement in intact and excised stems of Fraxinus americana and Acer saccharum. Ann Bot. 1977;41:1093–1100. [Google Scholar]
- Cochard H, Tyree MT. Xylem dysfunction in Quercus: vessel sizes, tyloses, cavitation and seasonal changes in embolism. Tree Physiol. 1990;6:393–407. doi: 10.1093/treephys/6.4.393. [DOI] [PubMed] [Google Scholar]
- Dixon HH (1914) Transpiration and the Ascent of Sap in Plants. Macmillan and Co., Ltd., London
- Ellmore GS, Ewers FW. Fluid flow in the outermost xylem increment of a ring-porous tree, Ulmus americana. Am J Bot. 1986;73:1771–1774. [Google Scholar]
- Ewers FW. Xylem structure and water conduction in conifer trees, dicot trees, and lianas. IAWA Bull. 1985;6:309–317. [Google Scholar]
- Fujikawa S, Suzuki T, Ishikawa T, Sakurai S, Hasegawa Y. Continuous observation of frozen biological materials with cryo-scanning electron microscope and freeze-replica by a new cryo-system. J Electron Microsc. 1988;37:315–322. [PubMed] [Google Scholar]
- Grace J. Refilling of embolised xylem. In: Borghetti M, Grace J, Raschi A, editors. Water Transport in Plants under Climatic Stress. Cambridge, UK: Cambridge University Press; 1993. pp. 52–62. [Google Scholar]
- Greenidge KNH (1958) Rates and patterns of moisture movement in trees. In KV Thimann, eds, The Physiology of Forest Trees. The Ronald Press, New York, pp 19–41
- Hacke U, Sauter JJ. Xylem dysfunction during winter and recovery of hydraulic conductivity in diffuse-porous and ring-porous trees. Oecologia. 1996;105:435–439. doi: 10.1007/BF00330005. [DOI] [PubMed] [Google Scholar]
- Hammel HT. Freezing of xylem sap without cavitation. Plant Physiol. 1967;42:55–66. doi: 10.1104/pp.42.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski TT. The movement of water in trees. For Sci. 1961;7:177–192. [Google Scholar]
- Kramer PJ, Kozlowski TT (1960) Physiology of Trees. McGraw- Hill, New York
- Magnani F, Borghetti M. Interpretation of seasonal changes of xylem embolism and plant hydraulic resistance in Fagus sylvatica. Plant Cell Environ. 1995;18:689–696. [Google Scholar]
- McCully ME, Huang CX, Ling LEC. Daily embolism and refilling of xylem vessels in the roots of field-grown maize. New Phytol. 1998;138:327–342. doi: 10.1046/j.1469-8137.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- Milburn JA. Cavitation and embolisms in xylem conduits. In: Raghavendra AS, editor. Physiology of Trees. Publications, New York: Wiley-Interscience; 1991. pp. 163–174. [Google Scholar]
- Ohtani J, Fujikawa S. Cryo-SEM observation on vessel lumina of a living tree: Ulmus davidiana var. japonica. IAWA Bull. 1990;11:183–194. [Google Scholar]
- O'Malley PER, Milburn JA. Freeze-induced fluctuations in xylem sap pressure in Acer pseudoplatanus. Can J Bot. 1983;61:3100–3106. [Google Scholar]
- Pockman WT, Sperry JS. Freezing-induced xylem cavitation and the northern limit of Larrea tridentata. Oecologia. 1997;109:19–27. doi: 10.1007/s004420050053. [DOI] [PubMed] [Google Scholar]
- Sano Y, Fujikawa S, Fukazawa K. Studies on mechanisms of frost crack formation in tree trunks. Japanese Journal of Freezing and Drying. 1993;39:13–21. [Google Scholar]
- Sano Y, Fujikawa S, Fukazawa K. Trees. 1995;9:261–268. [Google Scholar]
- Sholander PF, Love WE, Kanwisher JW. The rise of sap in tall grapevine. Plant Physiol. 1955;30:93–104. doi: 10.1104/pp.30.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS (1993) Winter xylem embolism and spring recovery in Betula cordifolia, Fagus grandifolia, Abies balsamera and Picea rubens. In M Borghetti, J Grace, A Raschi, eds, Water Transport in Plants under Climatic Stress. Cambridge University Press, Cambridge, UK, pp 86–98
- Sperry JS, Donnelly JR, Tyree MT. Seasonal occurrence of xylem embolism in sugar maple (Acer saccharum) Am J Bot. 1988;75:1212–1218. [Google Scholar]
- Sperry JS, Holbrook NM, Zimmermann MH, Tyree MT. Spring filling of xylem vessels in wild grapevine. Plant Physiol. 1987;83:414–417. doi: 10.1104/pp.83.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS, Nichols KL, Sullivan JEM, Eastlack SE. Xylem embolism in ring-porous, diffuse-porous and coniferous trees of northern Utah and interior Alaska. Ecology. 1994;75:1736–1752. [Google Scholar]
- Sperry JS, Sullivan JEM. Xylem embolism in response to freeze-thaw cycles and water stress in ring-porous, diffuse-porous, and conifer species. Plant Physiol. 1992;100:605–613. doi: 10.1104/pp.100.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS, Tyree MT. Mechanism of water stress-induced xylem embolism. Plant Physiol. 1988;88:581–587. doi: 10.1104/pp.88.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E. Water transport across roots. Plant Soil. 1994;167:79–90. [Google Scholar]
- Steudle E. Trees under tension. Nature. 1995;378:663–664. [Google Scholar]
- Tyree MT, Davis SD, Cochard H. Biophysical perspectives of xylem evolution: is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction? IAWA J. 1994;15:335–360. [Google Scholar]
- Tyree MT, Sperry JS. Vulnerability of xylem to cavitation and embolism. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:19–38. [Google Scholar]
- Utsumi Y, Sano Y, Ohtani J, Fujikawa S. Seasonal changes in the distribution of water in the outer growth rings of Fraxinus mandshurica var. japonica: a study by cryo-scanning electron microscopy. IAWA J. 1996;17:113–124. [Google Scholar]
- Zimmermann MH. Xylem Structure and the Ascent of Sap. Berlin: Springer-Verlag; 1983. [Google Scholar]
- Zimmermann MH, Brown CL. Trees: Structure and Function. Berlin: Springer-Verlag; 1971. [Google Scholar]