Abstract
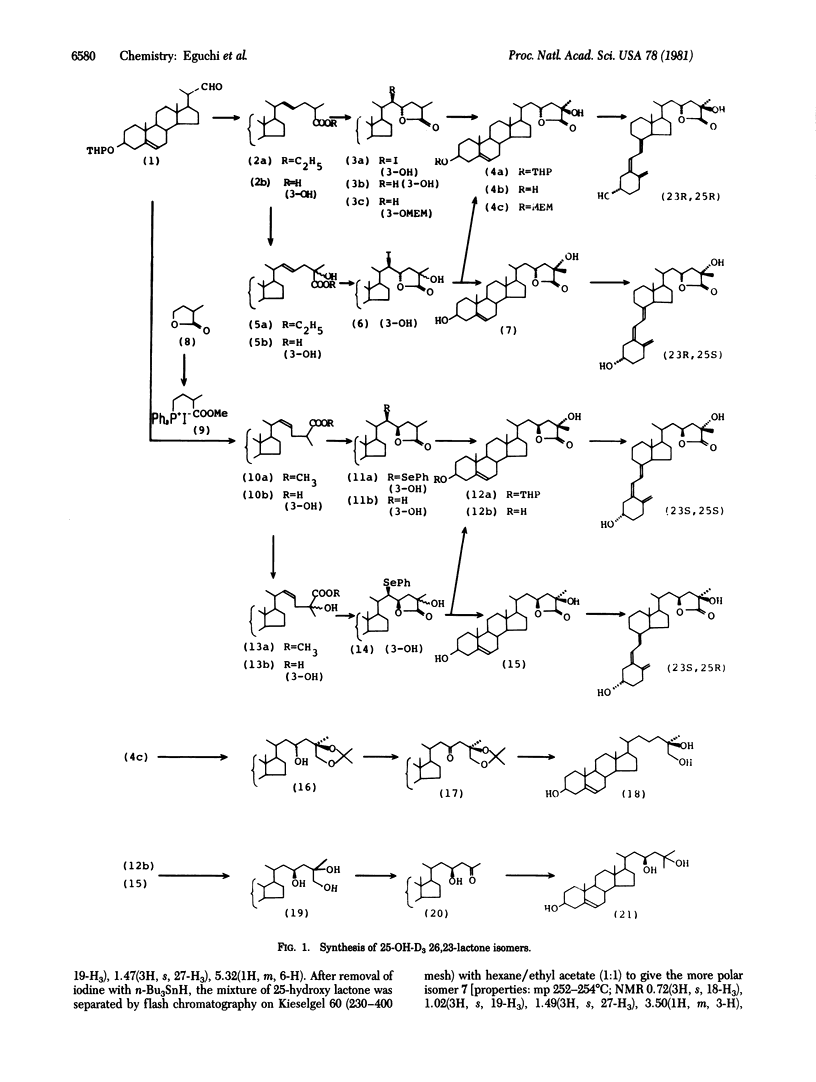
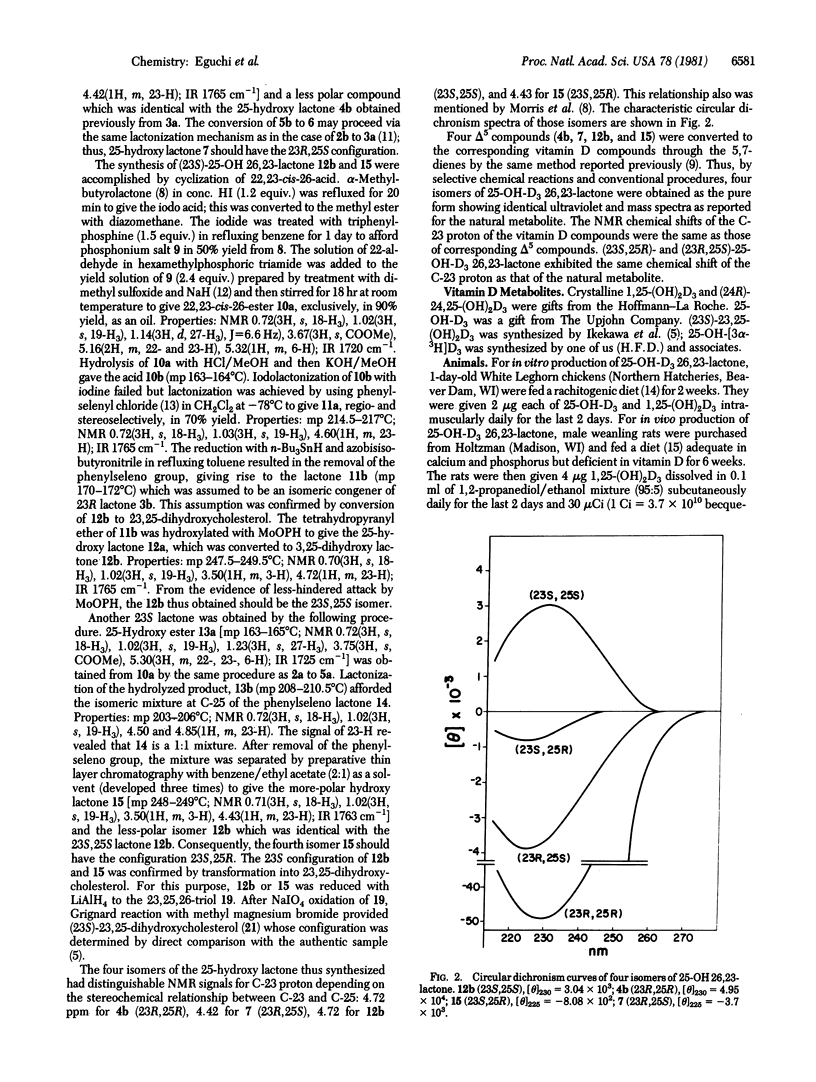
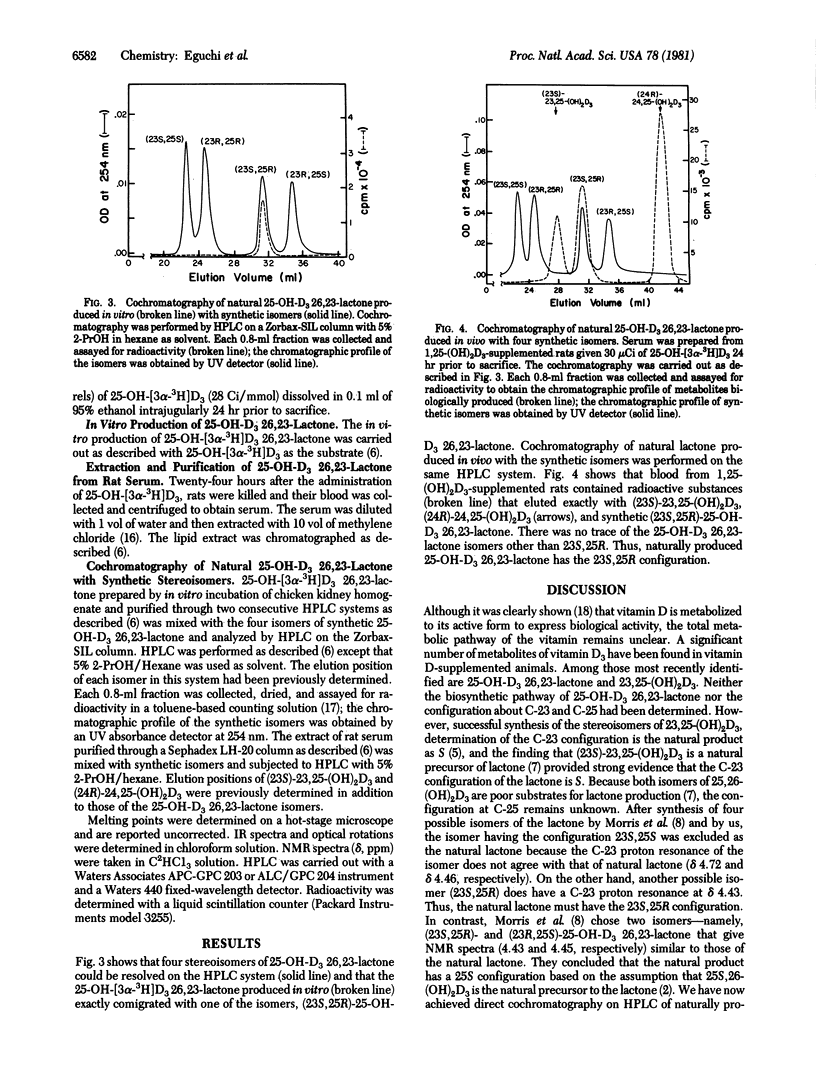
The four stereoisomers of 25-hydroxyvitamin D3 26,23-lactone were synthesized by a stereoselective lactonization method. Natural 25-hydroxyvitamin D3 26,23-lactone was produced from 25-hydroxy-[3α-3H]vitamin D3 by in vitro incubation of kidney homogenate prepared from vitamin D-supplemented chickens or was isolated from the serum of rats given 1,25-dihydroxyvitamin D3 and 25-hydroxy-[3α-3H]vitamin D3. The four synthetic isomers and the naturally produced 25-hydroxyvitamin D3 26,23-lactone were subjected to high-performance liquid chromatography in a system capable of separating the four isomers. The natural lactone comigrated with synthetic (23S,25R)-25-hydroxyvitamin D3 26,23-lactone, establishing it as the natural vitamin D3 metabolite.
Keywords: vitamin D metabolite, iodolactonization, phenylselenolactonization
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeLuca H. F. William C. Rose lectureship in biochemistry and nutrition. Some new concepts emanating from a study of the metabolism and function of Vitamin D. Nutr Rev. 1980 May;38(5):169–182. doi: 10.1111/j.1753-4887.1980.tb05887.x. [DOI] [PubMed] [Google Scholar]
- Eisman J. A., Hamstra A. J., Kream B. E., DeLuca H. F. 1,25-Dihydroxyvitamin D in biological fluids: a simplified and sensitive assay. Science. 1976 Sep 10;193(4257):1021–1023. doi: 10.1126/science.1085035. [DOI] [PubMed] [Google Scholar]
- Hollis B. W., Roos B. A., Lambert P. W. 25,26-Dihydroxycholecalciferol: a precursor in the renal synthesis of 25-hydroxycholecalciferol-26,23-lactone. Biochem Biophys Res Commun. 1980 Jul 31;95(2):520–528. doi: 10.1016/0006-291x(80)90815-3. [DOI] [PubMed] [Google Scholar]
- Neville P. F., DeLuca H. F. The synthesis of [1,2-3H]vitamin D3 and the tissue localization of a 0.25-mu-g (10 IU) dose per rat. Biochemistry. 1966 Jul;5(7):2201–2207. doi: 10.1021/bi00871a007. [DOI] [PubMed] [Google Scholar]
- Omdahl J., Holick M., Suda T., Tanaka Y., DeLuca H. F. Biological activity of 1,25-dihydroxycholecalciferol. Biochemistry. 1971 Jul 20;10(15):2935–2940. doi: 10.1021/bi00791a022. [DOI] [PubMed] [Google Scholar]
- Redel J., Bazely N., Tanaka Y., DeLuca H. F. The absolute configuration of the natural 25,26-dihydroxycholecalciferol. FEBS Lett. 1978 Oct 15;94(2):228–230. doi: 10.1016/0014-5793(78)80943-0. [DOI] [PubMed] [Google Scholar]
- Suda T., DeLuca H. F., Tanaka Y. Biological activity of 25-hydroxyergocalciferol in rats. J Nutr. 1970 Sep;100(9):1049–1052. doi: 10.1093/jn/100.9.1049. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., DeLuca H. F., Schnoes H. K., Ikekawa N., Eguchi T. 23,25-Dihydroxyvitamin D3: a natural precursor in the biosynthesis of 25-hydroxyvitamin D3-26,23-lactone. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4805–4808. doi: 10.1073/pnas.78.8.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Wichmann J. K., Paaren H. E., Schnoes H. K., DeLuca H. F. Role of kidney tissue in the production of 25-hydroxyvitamin D3-26,23-lactone and 1 alpha, 25-dihydroxyvitamin D3-26,23-lactone. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6411–6414. doi: 10.1073/pnas.77.11.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Wichmann J. K., Schnoes H. K., DeLuca H. F. Isolation and identification of 23,25-dihydroxyvitamin D3, an in vivo metabolite of vitamin D3. Biochemistry. 1981 Jun 23;20(13):3875–3879. doi: 10.1021/bi00516a032. [DOI] [PubMed] [Google Scholar]
- Wichmann J. K., DeLuca H. F., Schnoes H. K., Horst R. L., Shepard R. M., Jorgensen N. A. 25-Hydroxyvitamin D3 26,23-lactone: a new in vivo metabolite of vitamin D. Biochemistry. 1979 Oct 30;18(22):4775–4780. doi: 10.1021/bi00589a002. [DOI] [PubMed] [Google Scholar]


