Abstract
Hand-foot-and-mouth disease (HFMD) and herpangina are commonly prevalent illness in young children. They are similarly characterized by lesions on the skin and oral mucosa. Both diseases are associated with various enterovirus serotypes. In this study, enteroviruses from patients with these diseases in Korea in 2009 were isolated and analyzed. Demographic data for patients with HFMD and herpangina were compared and all enterovirus isolates were amplified in the VP1 region by reverse transcription-polymerase chain reaction and sequenced. Among the enterovirus isolates, prevalent agents were coxsackievirus A16 in HFMD and coxsackievirus A5 in herpangina. More prevalent months for HFMD were June (69.2%) and May (11.5%), and June (40.0%) and July (24.0%) for herpangina. Age prevalence of HFMD patients with enterovirus infection was 1 year (23.1%), 4 years (19.2%), and over 5 years (19.2%). However, the dominant age group of herpangina patients with enterovirus infection was 1 year (48.0%) followed by 2 years (28.0%). Comparison of pairwise VP1 nucleotide sequence alignment of all isolates within the same serotypes revealed high intra-type variation of CVA2 isolates (84.6–99.3% nucleotide identity). HFMD and herpangina showed differences in demographic data and serotypes of isolated enteroviruses, but there was no notable difference in amino acid sequences by clinical syndromes in multiple comparison of the partial VP1 gene sequence.
Keywords: Herpangina, Hand-foot-and-mouth disea1se, Enteroviruses
Introduction
Hand-foot-and-mouth disease (HFMD) and herpangina, which commonly affect young children, are enterovirus infections causing a variety of exanthems. HFMD is a self-limiting exanthematous eruption characterized by vesicles in the oral cavity, mainly in the buccal mucosa and tongue, and peripherally distributed cutaneous lesions on the hands and feet. Herpangina produces multiple oral ulcers affecting predominantly the posterior part of the oral cavity only [1-3]. These diseases are associated with different strains of enteroviruses, such as coxsackievirus A (CVA) 2, 5, 6, 10, 16; coxsackievirus B (CVB) 1, 2, 5; and enterovirus (EV) 71 [4-8].
Human enterovirus (HEV) genera containing the CVA, CVB, echovirus (ECV), and EV serotypes are transmitted mainly via the fecal-oral route and by contact with throat discharges or fluid from blisters [9]. Generally, HEV outbreaks peak during the summer and early fall, and various serotypes are often associated with a single outbreak [10]. Since 1993, when nationwide surveillance began in Korea, there have been reports of summer outbreaks of enteroviruses caused by ECV 5, 6, 7, 9, 13, 18, and 30; CVA 24; CVB 3 and 5; and EV 71 [11,12]. Especially, outbreaks of HFMD and herpangina caused by HEV infection were reported in 2009 in Korea [13,14].
Diagnosis of HEV infections is based on amplification of a highly conserved 5’ non-coding region (NCR) that is widely-targeted in diagnostic procedures [15,16]. In addition to traditional virological methods to serotype HEV, reverse transcription-polymerase chain reaction (RT-PCR) based on amplification of the VP1 region have been recently developed [17-19]. Because the VP1 region is one of the main exposed regions of the viral capsid and has been suggested to include a serotype specific antigenic neutralization site, the BC loop in this region has been implicated with viral antigenicity, and substitutions resulting in conformational changes in this region are believed to play a role in host adaptation for HEVs [20,21].
In this study, to compare epidemic patterns of HFMD and herpangina, specimens from patients with HFMD and herpangina disease were collected and analyzed along with demographic data. Molecular detection by 5’ NCR RT-PCR and sequencing of the VP1 region of HEVs were carried out.
Materials and methods
Diagnostic definitions
Herpangina was defined as the presence of oral ulcers on the anterior tonsillar pillars, soft palate, buccal mucosa, or uvula. Patients with HFMD had oral ulcers on the tongue or the buccal mucosa, and vesicular rashes over the palms, soles, knees, or buttocks [22]. This study was conducted in accordance with ethical principles as formulated in the World Medical Association Declaration of Helsinki and approved by the institutional review board (IRB No. 2012–48) of the Ethical Committee of Soonchunhyang University Cheonan Hospital. Additionally, informed consent was obtained from the parents on the patients’ behalf who participated in the study and the parents of participants also gave their consent to publish the data.
Detection of enterovirus and molecular typing
Stool specimens from 29 HFMD and 32 herpangina patients were collected from collaborative JeongGene Pediatrics hospital in Chungnam province, Korea during 2009. The testing algorithm for detection and molecular typing of HEV has been previously described [12]. Viral RNA was extracted from the supernatant of infected cells using magnetic beads (Toyobo, Osaka, Japan). Extracted RNA was dissolved in 50 μL of nuclease-free water and stored at −70°C until used for RT-PCR.
HEVs were assayed for in clinical samples using a AccuPower® Enterovirus Real-Time RT-PCR Kit (Bioneer, Daejeon, Korea) based on 5’ NCR of highly conserved region in the HEV genome, according to the manufacturer’s instructions [23]. If the sample was positive, it was performed semi-nested PCR in VP1 coding region for molecular typing. Semi-nested PCR condition and primer sequences amplifying the VP1 coding region were described previously [18]. In the initial PCR, a 50 μL reaction mix containing 0.2 μM of primers 224 and 222, 2 U of Taq DNA polymerase (Promega, Madison, WI), 100 μM concentrations of mixture of dNTPs, and 2 μM MgCl2 was amplified by 40 cycles of 95°C for 30 sec, 42°C for 30 sec, and 60°C for 45 s. One microliter of the first PCR product was added to a second PCR for semi-nested amplification. Fifty microliters of a reaction mix containing 0.2 μM of primers AN89 and AN88, 2.5 U of Taq DNA polymerase (Promega), 100 μM concentrations of a mixture of dNTPs, and 2 μM MgCl2 was incubated at 95°C for 6 min prior to 40 amplification cycles of 95°C for 30 s, 60°C for 20 s, and 72°C for 15 s. PCR products were purified using the QIA quick PCR purification kit (Qiagen, Valencia, CA). Purified DNA was added in a reaction mixture containing 2 μL of Big Dye terminator reaction mix (Applied Biosystems, Foster City, CA) and 2 pmoles of AN88 and AN89 primers. Sequencing reactions were subjected to initial denaturation at 96°C for 1 min and 25 cycles consisting of 96°C for 10 s, 50°C for 5 s, and 60°C for 4 min in a Gene Amp PCR system 2700 (Applied Biosystems, Foster City, CA). The products were purified by precipitation with 100% cold ethanol and 3 M sodium-acetate (pH 5.8), and then loaded on a model 3100 automated genetic analyzer (Applied Biosystems). The molecular type of each isolates was determined by the serotype of the highest scoring strain in Genbank using the Basic Local Alignment Search Tool (BLAST); that is, the sequence of the HEV strain that gave the highest nucleotide similarity value with the query sequence [24].
Sequence analysis
Sequence analysis was carried out for five strains of CVA 2, 16 strains of CVA 5, 18 strains of CVA 16, two strains of CVB 1, and six strains of EV71 (Table 1). Nucleotide and deduced amino acid sequences of all HEV isolates were compared with the reference strains using CLUSTAL W (version 1.81) and Megalign (DNASTAR) [25]. The phylogenetic relationships among the VP1 sequences of each virus isolate were inferred using MEGA software v. 5.05. Maximum Composite Likelihood was used as the substitution method, while the neighbor-joining method was used to reconstruct the phylogenetic tree [26]. The reliability of the phylogenetic tree was determined by bootstrap re-sampling of 1,000 replicates.
Table 1.
Numbers of enterovirus serotype isolated from patients with HFMD and herpangina
| Serotypes |
HFMD |
Herpangina |
||
|---|---|---|---|---|
| Isolate numbers | Percentage of subtotal | Isolate numbers | Percentage of subtotal | |
| Coxsakievirus A2 |
0 |
- |
5 |
20.0 |
| Coxsakievirus A5 |
3 |
11.5 |
13 |
52.0 |
| Coxsakievirus A16 |
16 |
61.5 |
2 |
8.0 |
| Coxsakievirus B1 |
1 |
3.8 |
1 |
4.0 |
| Enterovirus 71 |
4 |
15.4 |
2 |
8.0 |
| Untypable |
2 |
7.7 |
2 |
8.0 |
| Total | 26 | 100 | 25 | 100 |
Nucleotide sequence accession numbers
The HEV sequences reported here were deposited in the GenBank sequence database under accession numbers JF773151 to JF773197.
Results
Enterovirus detection and molecular typing
Among stool specimens from 29 HFMD and 32 herpangina patients, the approach detected 26 HEV isolates (89.7%) in HFMD and 25 HEV isolates (78.1%) in herpangina. The types in the 26 isolates from HFMD patients were mainly identified as CVA 16 (16 isolates, 61.5%), EV 71 (four isolates, 15.4%), CVA 5 (three isolates, 11.5%). The types in the 25 isolates from herpangina patients were mainly identified as CVA 5 (13 isolates, 52.0%), CVA 2 (five isolates, 20.0%) (Table 1).
Epidemiological features
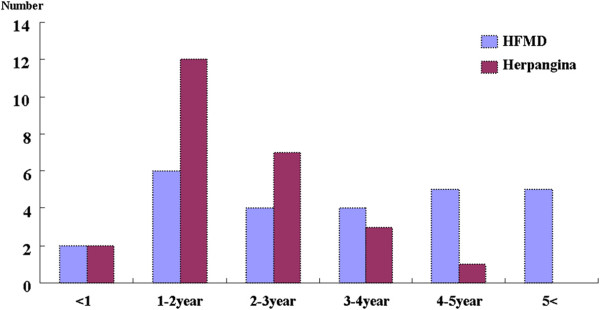
In temporal distribution, HEV positive samples from HFMD patients comprised three isolates (11.5%) in May, 18 isolates (69.2%) in June, one isolate (3.8%) in July, two isolates (7.7%) in August, one isolate (3.8%) in September, and one isolate (3.8%) in October. The HEV positive samples from herpangina patients comprised five isolates (20.0%) in May, 10 isolates (40.0%) in June, six isolates (24.0%) in July, three isolates (12.0%) in August, and one isolate (4.0%) in September (Figures 1). Concerning the age of HFMD patients with enterovirus infection, two isolates (7.7%) were recovered from patients <1-year-of-age, six isolates (23.1%) at 1-year-of-age, four isolates (15.4%) at 2-years-of-age, four isolates (15.4%) at 3-years-of-age, five isolates (19.2%) at 4-years-of-age, and five isolates (19.2%) at >5-years-of-age. Concerning the age of herpangina patients with enterovirus infection, two isolates (8.0%) were recovered at <1-year-of-age, 12 isolates (48.0%) at 1-year-of-age, seven isolates (28.0%) at 2-years-of-age, three isolates (12.0%) at 3-years-of-age, and one isolate (4.0%) at 4-years-of-age (Figures 2).
Figure 1.
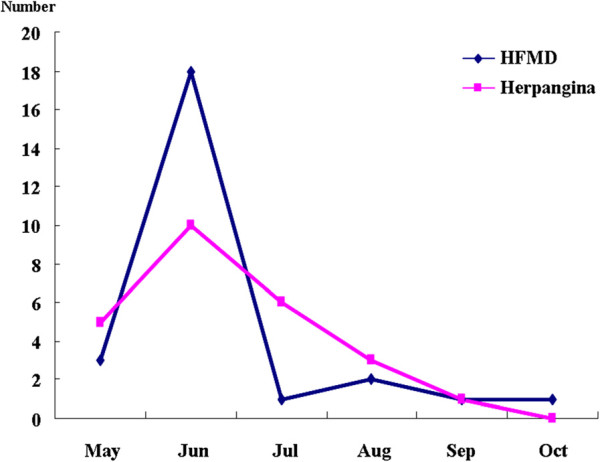
Temporal distribution of enteroviruses isolated from patients with HFMD and herpangina.
Figure 2.
Age distribution of enteroviruses isolated from patients with HFMD and herpangina.
Sequence analysis of enteroviruses
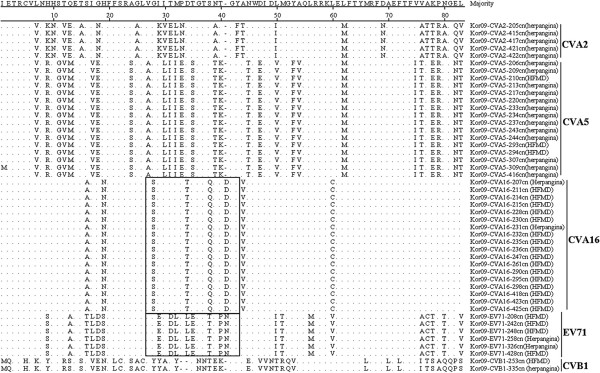
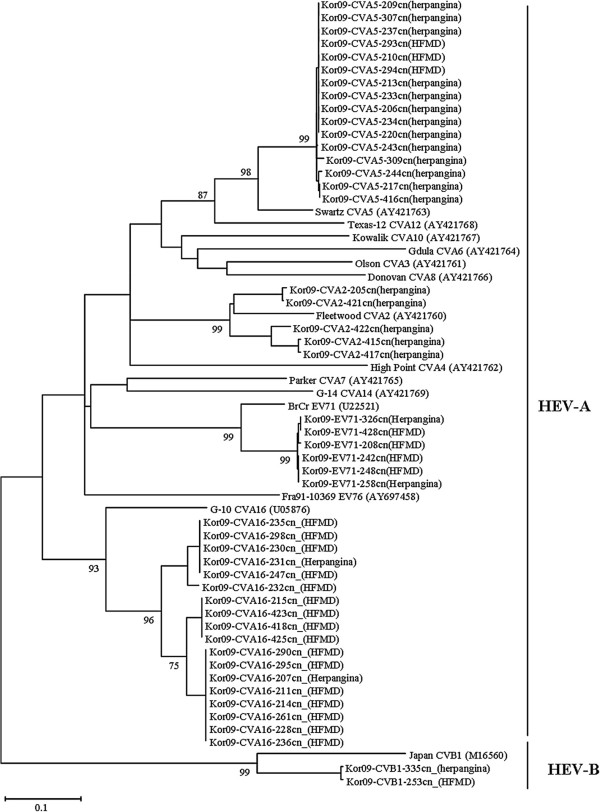
Based on the VP1 amino acid sequence comparison for all isolates, all sequences represented the same intra-serotype, except for the Kor09-CVA5-309cn (herpangina) isolate, regardless of syndrome (Figures 3). The pairwise VP1 nucleotide sequence alignment of all isolates was compared within the same serotypes; CVA2 isolates displayed 84.6–99.3% nucleotide identity within the intra-type. CVA5, 16, CVB1, and EV71 isolates showed 98.7–100%, 89.6–100%, 99.4%, and 98.9–100% identity, respectively. The phylogenetic relationships of Korean isolates and reference strains are shown in Figures 4.
Figure 3.
Comparison of the deduced amino acid sequences of VP1 region of 47 enteroviruses isolated from patients with HFMD and herpangina. The rectangular parts are BC loop previously reported.
Figure 4.
Phylogenetic analysis based on VP1 region of enteroviruses isolated from patients with HFMD and herpangina. Nucleotide sequences were analyzed by the neighbor-joining method. The numbers at the branches indicate the bootstrap values for 1,000 replicates.
Discussion
HFMD and herpangina were epidemic in Korea in 2009 [13,14] and are commonly prevalent illness in young children. They are similarly characterized by lesions on the skin and oral mucosa. Also, these two diseases are associated with various enterovirus serotypes. In HFMD, it has been reported that the major causative HEV types are CVA16 and EV71; these types were also more prevalent serotypes in Korea in 2009 [27,28]. Generally, CVA6 and CVA10 are the more prevalent serotypes in herpangina; however, CVA5 and CVA2 were the prevalent herpangina serotypes in Korea in 2009 [29]. In multiple comparison of partial VP1 gene in the intratypes, no notable variation in amino acid sequences by clinical syndrome was evident. The VP1 region containing the BC loop is associated with viral antigenicity and substitutions of amino acid sequences in this region are believed to be important in host infection of HEVs [20,21,30]. No significant clustered relationship was found according to the clinical syndromes. When the pairwise VP1 nucleotide sequence alignment of all isolates was compared within the same serotypes, CVA2 isolates showed an appreciable variation in nucleotide identity (84.6–99.3%) within the intra-type. Deduced amino acid sequences of all strains were in the same intra-serotype, except for one isolate, regardless of syndromes.
There was no significant difference in age distribution between herpengina (3.74 ± 2.48 years) and HFMD (3.54 ± 2.05 years) in a previous report [31]. But, in this study, age distribution of HEV positive in herpangina patients was relatively lower than in HFMD patients. In the 2009 Korean epidemic, enterovirus infections were most prevalent in July [32]. However, temporal distribution of HEV positive in herpangina and HFMD peaked in June, with herpangina gradually decreased thereafter while HFMD decreased rapidly.
In conclusion, HFMD and herpangina showed differences in demographic data and serotypes of isolated HEVs, but there was no notable difference in amino acid sequences by clinical syndromes in multiple comparison of the partial VP1 gene sequence.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KSP, SGY, KAB, JWS, HKC and DSC performed molecular diagnosis and sequence analysis. BHL and JSP contributed to specimen collections and clinical diagnosis. KAY and YJC designed the study and critically revised the manuscript. All of the authors read and approved the final version of the manuscript.
Contributor Information
KwiSung Park, Email: kwisungp@korea.kr.
BaeckHee Lee, Email: baeckhee@chol.com.
KyoungAh Baek, Email: micbaek@korea.kr.
DooSung Cheon, Email: Cheonds@hanmail.net.
SangGu Yeo, Email: 3910003@hanmail.net.
JoonSoo Park, Email: allergist@korea.com.
JaeWan Soh, Email: armada@schmc.ac.kr.
HaeKyung Cheon, Email: hgcheon@bscu.ac.kr.
KyungAh Yoon, Email: kayun@hit.ac.kr.
YoungJin Choi, Email: clinpath@sch.ac.kr.
References
- Bending WA, Fleming DM. Epidemiological, virological and clinical features of an epidemic of hand, food and mouth disease in England and Wales. Commun Dis Rep CDR Rev. 1996;6:R81–R86. [PubMed] [Google Scholar]
- Chang LY, Lin TY, Huang YC. Fulminant neurogenic pulmonary edema with hand, foot and mouth disease. Lancet. 1998;352:367–368. doi: 10.1016/S0140-6736(98)24031-1. [DOI] [PubMed] [Google Scholar]
- Rabenau HF, Richter M, Doerr HW. Hand, foot and mouth disease: seroprevalence of Coxsackie A16 and Enterovirus 71 in Germany. Med Microbiol Immunol. 2009;199:45–51. doi: 10.1007/s00430-009-0133-6. [DOI] [PubMed] [Google Scholar]
- Flewett TH, Warrin RP, Clarke SKR. Hand, foot and mouth disease associated with Coxsackie A5 virus. J Clin Pathol. 1963;16:53–55. doi: 10.1136/jcp.16.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MF. Hand-foot-and-mouth syndrome in humans: coxsackie A10 infections in New Zealand. Br Med J. 1968;2:661–664. doi: 10.1136/bmj.2.5606.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbaum JE, Van Dyck PC, Allen RG. Hand, foot and mouth disease associated with coksackievirus group B. Scand J Infect Dis. 1975;7:161–163. doi: 10.3109/inf.1975.7.issue-3.01. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Nakano S, Yamaoka K, Takami S. Outbreaks of hand, foot, and mouth disease by enterovirus 71. Arch Dis Child. 1980;55:583–588. doi: 10.1136/adc.55.8.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterback R, Vuorinen T, Linna M, Susi P, Hyypiä T, Waris M. Coksackievirus A6 and hand, foot and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485–1488. doi: 10.3201/eid1509.090438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich S, Weinbrecht A, Schreier E. Seroprevalence and molecular epidemiology of enterovirus 71 in Germany. Arch Virol. 2009;154:1139–1142. doi: 10.1007/s00705-009-0413-x. [DOI] [PubMed] [Google Scholar]
- Jacques J, Moret H, Minette D, Leveque N, Jovenin N, Deslee G, Lebargy F, Motte J, Andreoletti L. Epidemiological, molecular, and clinical features of enterovirus respiratory infections in French children between 1999 and 2005. J Clin Microbiol. 2008;46:206–213. doi: 10.1128/JCM.01414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee YM, Cheon DS, Choi WY, Ahn JB, Kim KS, Chung YS, Lee JW, Lee KB, Noh HS, Park KS, Lee SH, Kim SH, Cho KS, Kim ES, Jung JK, Yoon JD, Cho HW. Updates on enterovirus surveillance in Korea. Inf Chemotherapy. 2004;36:294–303. [Google Scholar]
- Baek K, Park K, Jung E, Chung E, Park J, Choi H, Baek S, Jee Y, Cheon D, Ahn G. Molecular and epidemiological characterization of enteroviruses isolated in Chungnam, Korea from 2005 to 2006. J Microbiol Biotechnol. 2009;19:1055–1064. doi: 10.4014/jmb.0810.584. [DOI] [PubMed] [Google Scholar]
- Choi CS, Choi YJ, Choi UY, Han JW, Jeong DC, Kim HH, Kim JH, Kang JH. Clinical manifestations of CNS infections caused by enterovirus type 71. Korean J Pediatr. 2011;54:11–16. doi: 10.3345/kjp.2011.54.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim SJ, Cheon DS. Hand-Foot-Mouth Disease Related to Enterovirus 71. J Korean Med Assoc. 2009;52:886–894. doi: 10.5124/jkma.2009.52.9.886. [DOI] [Google Scholar]
- Romero JR. Reverse-transcription polymerase chain reaction detection of the enteroviruses. Arch Pathol Lab Med. 1999;123:1161–1169. doi: 10.5858/1999-123-1161-RTPCRD. [DOI] [PubMed] [Google Scholar]
- Thoelen I, Moes E, Lemey P, Mostmans S, Wollants E, Lindberg AM, Vandamme AM, Van Ranst M. Analysis of the serotype and genotype correlation of VP1 and the 5' noncoding region in an epidemiological survey of the human enterovirus B species. J Clin Microbiol. 2004;42:963–971. doi: 10.1128/JCM.42.3.963-971.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste MS, Maher K, Kilpatrick DR, Pallansch MA. Molecular evolution of the human enteroviruses: Correlation of serotype with VP1 sequence and application to picornavirus classification. J Virol. 1999;73:1941–1948. doi: 10.1128/jvi.73.3.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006;44:2698–2704. doi: 10.1128/JCM.00542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste MS, Maher K, Williams AJ, Dybdahl-Sissoko N, Brown BA, Gookin MS, Peñaranda S, Mishrik N, Uddin M, Pallansch MA. Species-specific RT-PCR amplification of human enteroviruses: A tool for rapid species identification of uncharacterized enteroviruses. J Gen Virol. 2006;87:119–128. doi: 10.1099/vir.0.81179-0. [DOI] [PubMed] [Google Scholar]
- Stirk HJ, Thornton JM. The BC loop in poliovirus coat protein VP1: An ideal acceptor site for major insertions. Protein Eng. 1994;7:47–56. doi: 10.1093/protein/7.1.47. [DOI] [PubMed] [Google Scholar]
- Norder H, Bjerregaard L, Magnius L, Lina B, Aymard M, Chomel JJ. Sequencing of ‘untypable’ enteroviruses reveals two new types, EV-77 and EV-78, within human enterovirus type B and substitutions in the BC loop of the VP1 protein for known types. J Gen Virol. 2003;84:827–836. doi: 10.1099/vir.0.18647-0. [DOI] [PubMed] [Google Scholar]
- Chen SP, Huang YC, Li WC, Chiu CH, Huang CG, Tsao KC, Lin TY. Comparison of clinical features between coxsackievirus A2 and enterovirus 71 during the enterovirus outbreak in Taiwan, 2008: a children's hospital experience. J Microbiol Immunol Infect. 2010;43:99–104. doi: 10.1016/S1684-1182(10)60016-3. [DOI] [PubMed] [Google Scholar]
- Zoll GJ, Melchers WJ, Kopecka H, Jambroes G, Van der Poel HJ, Galama JM. General primer-mediated polymerase chain reaction for detection of enteroviruses: Application for diagnostic routine and persistent infections. J Clin Microbiol. 1992;30:160–165. doi: 10.1128/jcm.30.1.160-165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste MS, Maher K, Flemister MR, Marchetti G, Kilpatrick DR, Pallansch MA. Comparison of classic and molecular approaches for the identification of untypeable enteroviruses. J Clin Microbiol. 2000;38:1170–1174. doi: 10.1128/jcm.38.3.1170-1174.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. Clustal W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;8:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ito M, Taniguchi A, Sakae K. Prevalence of coxsackievirus A5, A6, and A10 in patients with herpangina in Aichi Prefecture, 2005. Jpn J Infect Dis. 2005;58:390–391. [PubMed] [Google Scholar]
- Zong W, He Y, Yu S, Yang H, Xian H, Liao Y, Hu G. Molecular phylogeny of Coxsackievirus A16 in Shenzhen, China, from 2005 to 2009. J Clin Microbiol. 2011;49:1659–1661. doi: 10.1128/JCM.00010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Masaki A, Hasegawa S, Obara M, Horimoto E, Nakamura K, Tanaka Y, Endo K, Tanaka K, Ueda J, Shiraki K, Kurata T, Takizawa T. Genetic changes of coxsackievirus A16 and enterovirus 71 isolated from hand, foot, and mouth disease patients in Toyama, Japan between 1981 and 2007. Jpn J Infect Dis. 2009;62:254–259. [PubMed] [Google Scholar]
- Lee ST, Ki CS, Lee NY. Molecular characterization of enteroviruses isolated from patients with aseptic meningitis in Korea, 2005. Arch Virol. 2007;152:963–970. doi: 10.1007/s00705-006-0901-1. [DOI] [PubMed] [Google Scholar]
- Lee MH, Huang LM, Wong WW, Wu TZ, Chiu TF, Chang LY. Molecular diagnosis and clinical presentations of enteroviral infections in Taipei during the 2008 epidemic. J Microbiol Immunol Infect. 2011;44:178–183. doi: 10.1016/j.jmii.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Baek K, Yeo S, Lee B, Park K, Song J, Yu J, Rheem I, Kim J, Hwang S, Choi Y, Cheon D, Park J. Epidemics of enterovirus infection in Chungnam Korea, 2008 and 2009. Virol J. 2011;8:297. doi: 10.1186/1743-422X-8-297. [DOI] [PMC free article] [PubMed] [Google Scholar]