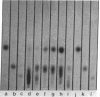
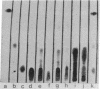
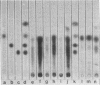
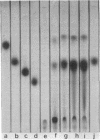
Abstract
The first step in the extension growth of the plant cell is a process in which the cell wall becomes ductile or plastic, after which the actual enlargement takes place passively under the influence of turgor. The nature of this process has not been explained, although much research has been carried out concerning it. In the present report, it is shown that a specific enzyme, which is identical or nearly so with dextranase (α-1,6-D-glucan 6-glucanohydrolase, EC 3.2.1.11) and is associated with the cell walls of growing coleoptiles, plays a prominent role in this process. The action of this enzyme is dependent on the level of growth hormone, auxin, in the tissue. Under its action, certain cell wall components are broken down to yield arabinose and glucose. These sugars are also released during autolysis of cell wall material. The molecular linkages broken in the process are probably the arabinogalactan crosslinks of the hemicellulose matrix, which are the main constituents of the wall containing arabinose. This is substantiated by the finding that dextranase can break down arabinan and compounds containing arabinose chains with the release of arabinose, just as in the action of the enzyme on the wall. The breaking of these crosslinks will impart the necessary plasticity to the wall for cell extension to occur.
Keywords: cell elongation growth, arabinan, dextran
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaiet L., Kempf A. J., Harman R., Kaczka E., Weston R., Nollstadt K., Wolf F. J. Isolation of a pure dextranase from Penicillium funiculosum. Appl Microbiol. 1970 Sep;20(3):421–426. doi: 10.1128/am.20.3.421-426.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. Auxin-induced cell wall loosening in the presence of actinomycin D. Plant Physiol. 1965 Jul;40(4):595–600. doi: 10.1104/pp.40.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn A. N. Dextranase activity and auxin-induced cell elongation in coleoptiles of Avena. Biochem Biophys Res Commun. 1970 Mar 12;38(5):831–837. doi: 10.1016/0006-291x(70)90794-1. [DOI] [PubMed] [Google Scholar]
- Heyn A. N. Dextranase activity in coleoptiles of Avena. Science. 1970 Feb 6;167(3919):874–875. doi: 10.1126/science.167.3919.874. [DOI] [PubMed] [Google Scholar]
- Heyn A. N. Glucanase activity in coleoptiles of Avena. Arch Biochem Biophys. 1969 Jul;132(2):442–449. doi: 10.1016/0003-9861(69)90387-7. [DOI] [PubMed] [Google Scholar]
- Kaji A., Saheki T. Endo-arabinanase from Bacillus subtilis F-11. Biochim Biophys Acta. 1975 Dec 18;410(2):354–360. doi: 10.1016/0005-2744(75)90237-5. [DOI] [PubMed] [Google Scholar]
- Kaji A., Tagawa K. Purification, crystallization and amino acid composition of alpha-L-arabinofuranosidase from Aspergillus niger. Biochim Biophys Acta. 1970 Jun 23;207(3):456–464. doi: 10.1016/s0005-2795(70)80008-3. [DOI] [PubMed] [Google Scholar]
- Kaji A., Yoshihara O. Properties of purified -L-arabinofuranosidase from Corticium rolfsii. Biochim Biophys Acta. 1971 Nov 13;250(2):367–371. doi: 10.1016/0005-2744(71)90193-8. [DOI] [PubMed] [Google Scholar]
- Keegstra K., Talmadge K. W., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: III. A Model of the Walls of Suspension-cultured Sycamore Cells Based on the Interconnections of the Macromolecular Components. Plant Physiol. 1973 Jan;51(1):188–197. doi: 10.1104/pp.51.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Ingle J. REQUIREMENT FOR THE SYNTHESIS OF DNA-LIKE RNA FOR GROWTH OF EXCISED PLANT TISSUE. Proc Natl Acad Sci U S A. 1964 Dec;52(6):1382–1388. doi: 10.1073/pnas.52.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivilaan A., Bandurski R. S., Schulze A. A partial characterization of an autolytically solubilized cell wall glucan. Plant Physiol. 1971 Oct;48(4):389–393. doi: 10.1104/pp.48.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko T. Y., Khouw B. T. A rapid method for the assay of dextranase. Can J Biochem. 1970 Mar;48(3):225–227. doi: 10.1139/o70-041. [DOI] [PubMed] [Google Scholar]
- Labavitch J. M., Freeman L. E., Albersheim P. Structure of plant cell walls. Purification and characterization of a beta-1,4-galactanase which degrades a structural component of the primary cell walls of dicots. J Biol Chem. 1976 Oct 10;251(19):5904–5910. [PubMed] [Google Scholar]
- Lamport D. T., Miller D. H. Hydroxyproline arabinosides in the plant kingdom. Plant Physiol. 1971 Oct;48(4):454–456. doi: 10.1104/pp.48.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport D. T. The isolation and partial characterization of hydroxyproline-rich glycopeptides obtained by enzymic degradation of primary cell walls. Biochemistry. 1969 Mar;8(3):1155–1163. doi: 10.1021/bi00831a049. [DOI] [PubMed] [Google Scholar]
- Masuda Y., Yamamoto R. Effect of auxin on beta-1, 3-glucanase activity in Avena coleoptile. Dev Growth Differ. 1970 Mar;11(4):287–296. doi: 10.1111/j.1440-169x.1970.00287.x. [DOI] [PubMed] [Google Scholar]
- Nevins D. J. Relation of glycosidases to bean hypocotyl growth. Plant Physiol. 1970 Sep;46(3):458–462. doi: 10.1104/pp.46.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooden L. D. Studies on the role of RNA synthesis in auxin induction of cell enlargement. Plant Physiol. 1968 Feb;43(2):140–150. doi: 10.1104/pp.43.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooden L. D. Studies on the role of RNA synthesis in auxin induction of cell enlargement. Plant Physiol. 1968 Feb;43(2):140–150. doi: 10.1104/pp.43.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noodén L. D., Thimann K. V. Action of inhibitors of RNA and protein synthesis on cell enlargement. Plant Physiol. 1966 Jan;41(1):157–164. doi: 10.1104/pp.41.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noodén L. D., Thimann K. V. EVIDENCE FOR A REQUIREMENT FOR PROTEIN SYNTHESIS FOR AUXIN-INDUCED CELL ENLARGEMENT. Proc Natl Acad Sci U S A. 1963 Aug;50(2):194–200. doi: 10.1073/pnas.50.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny P., Galston A. W. The kinetics of inhibition of auxin-induced growth in green pea stem segments by actinomycin D and other substances. Am J Bot. 1966 Jan;53(1):1–7. [PubMed] [Google Scholar]
- Rees D. A., Richardson N. G. Polysaccharides in germination. Occurrence, fine structure, and possible biological role of the pectic araban in white mustard cotyledons. Biochemistry. 1966 Oct;5(10):3099–3107. doi: 10.1021/bi00874a003. [DOI] [PubMed] [Google Scholar]
- Siddiqui I. R., Wood P. J. Structural investigation of oxalate-soluble rapeseed (Brassica campestris) polysaccharides. 3. An arabinan. Carbohydr Res. 1974 Aug;36(1):35–44. doi: 10.1016/s0008-6215(00)81990-4. [DOI] [PubMed] [Google Scholar]
- Uesaka E., Sato M., Raiju M., Kaji A. Alpha-l-arabinofuranosidase from Rhodotorula flava. J Bacteriol. 1978 Mar;133(3):1073–1077. doi: 10.1128/jb.133.3.1073-1077.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein L., Albersheim P. Structure of Plant Cell Walls: IX. Purification and Partial Characterization of a Wall-degrading Endo-Arabanase and an Arabinosidase from Bacillus subtilis. Plant Physiol. 1979 Mar;63(3):425–432. doi: 10.1104/pp.63.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]