Abstract
Background
There is no definite agreement on the better therapy (radiofrequency ablation (RFA) versus surgical resection (SR)) for early hepatocellular carcinoma (HCC) eligible for surgical treatments. The purpose of this study is to evaluate the evidence using meta-analytical techniques.
Methods
A literature search was undertaken until December 2011 to identify comparative studies evaluating survival rates, recurrence rates, and complications. Pooled odds ratios (OR) and 95% confidence intervals (95% CI) were calculated with either the fixed or random effect model.
Results
Thirteen articles, comprising two randomized controlled trials(RCTs), were included in the review, with a total of 2,535 patients (1,233 treated with SR and 1,302 with RFA). The overall survival rates were significantly higher in patients treated with SR than RFA after1, 3, and 5 years (respectively: OR, 0.60 (95% CI, 0.42 to 0.86); OR, 0.49 (95% CI, 0.36 to 0.65); OR, 0.60 (95% CI, 0.43 to 0.84)). In the SR group, the 1, 3, and 5 years recurrence rates were significantly lower than the RFA group (respectively: OR, 1.48 (95% CI, 1.05 to 2.08); OR, 1.76 (95% CI, 1.49 to 2.08); OR, 1.68 (95% CI, 1.21 to 2.34)). However, local recurrence between two groups did not exhibit significant difference. For HCC ≤ 3 cm in diameter, SR was better than RFA at the 1, 3, and 5 years overall survival rates (respectively: OR, 0.34 (95% CI, 0.13 to 0.89); OR, 0.56 (95% CI, 0.37 to 0.84); OR, 0.44 (95% CI, 0.31 to 0.62)). This meta-analysis indicated that the complication of SR was higher than RFA (OR, 6.25 (95%CI, 3.12 to 12.52); P = 0.000).
Conclusion
Although local recurrence between two groups did not exhibit significant difference, SR demonstrated significantly improved survival benefits and lower complications for patients with early HCC, especially for HCC ≤ 3 cm in diameter. These findings should be interpreted carefully, owing to the lower level of evidence.
Keywords: Hepatocellular carcinoma, meta-analysis, radiofrequency ablation, surgery
Review
Background
Hepatocellular carcinoma (HCC) is the seventh most common malignant tumor and the third leading cause of cancer-related deaths worldwide, with an estimated 500,000 deaths per year [1-3]. In past decades, developments of medical devices and interventional techniques have resulted in substantial opportunities for HCC early diagnosis and therapy.
Current options for the treatment of the early HCC conforming to the Milan criteria (single HCC ≤ 5 cm or up to three nodules ≤ 3 cm), that is stage I, consist of liver transplantation, surgical resection, transcatheter arterial chemoembolization (TACE), and percutaneous tumor ablation [4-7]. Theoretically, the best treatment is liver transplantation [8-13]. However, the limited availability of suitable living donors, as well as an increased waiting period, has raised the demand for treatment strategies of early HCC, such as SR and local ablation therapies. Comparison of different local ablative methods has shown that RFA is the most effective in terms of both morbidity and the elimination of tumors locally [14,15].
Some disputes, however, are reported about RFA and SR. Huang et al.[16], Molinari et al.[17], and Takayama et al.[18] reported that SR had more advantages (survival and recurrence rates) regardless of tumor size (larger or smaller than 3 cm; even smaller than 2 cm). However,Chen et al.[19], Hong et al.[20], Vivarelli et al.[21], and Montorsi et al. [22] concluded that RFA was as effective as SR in the treatment of solitary and small HCC. Additionally, Livraghi et al. [23] and Nashikawa et al. [24] considered RFA the first-line treatment for small resectable HCCs.
Whether RFA or SR is the better treatment for early HCC has long been debated. The aim of this review was to examine survival and recurrence rates after RFA and SR for HCC over the past decade.
Materials and methods
Literature search
Electronic searches were accomplished of the MEDLINE, Cochrane Controlled Trial Register (CENTRAL) and EMBASE databases until December 2011. The following MeSH search headings, all in English, were used: surgical resection, hepatic resection or hepatectomy; radiofrequency, radio-frequency or catheter ablation; and liver cancer or hepatocellular carcinoma.
Data extraction and quality assessment
Two reviewers (Gang Xu and Fuzhen Qi) independently extracted the following parameters from each study: (1) first author and year of the publication; (2) patients characteristics, study design, and following-up; (3) clinical outcomes. Discrepancies between the two reviewers were resolved by discussion. The quality of all selected articles was ranked in accordance with Jadad score.
Inclusion and exclusion criteria
Inclusion criteria for this study were as follows:(1) compare the initial therapeutic effects of RFA with or without TACE and SR for the treatment of early HCC, despite the etiology of liver disease, differences in viral hepatitis, or cirrhotic status; (2) report at least one of the outcomes mentioned below; (3) clearly document indications for RFA and HR; (4) If two or more studies were reported by the same authors in the same institution, either the study of higher quality or the most recent publication was included in the analysis. The primary endpoints were overall survival rates at 1, 3, and 5 years. The secondary endpoints were disease-free survival rates at 1, 3, and 5 years.
Criteria for exclusion: case reports, letters, abstracts, editorials, expert opinions, studies lacking control groups and reviews without original data were excluded. The following studies were also excluded: (i) those dealing with liver metastases, recurrence after hepatectomy, or unresectable HCC; (ii) those with no clearly reported outcomes of interest; (iii) those treating patients coupling with cholangiocellular carcinomas.
Subgroup analysis
Subgroup analyses were intended to explore important clinical differences among trials that might be expected to alter the magnitude of treatment effect. A subgroup analysis was performed in this meta-analysis to consider HCC with single nodules of diameter ≤3 cm.
Statistical analysis
We expressed results for dichotomous outcomes as odds ratio (OR) with 95% confidence interval (CI) and continuous outcome as weighted mean difference (WMD) or standard mean difference (SMD). Heterogeneity was explored by χ2 and I2. If the result of the heterogeneity test was P > 0.1 and I2 < 50%, ORs were pooled using the fixed-effect model(Mantel-Haenszel), otherwise, the random-effect model (DerSimonian and Laird) was used. The significance of the pooled ORs was determined by Z-test. P < 0.05 was considered significant.
Publication bias was assessed by visual inspection of funnel plots, in which the standard error of log(OR) of each study was plotted against its log(OR). An asymmetric plot indicates a possible publication bias. The symmetry of the funnel plot was further evaluated by Begg’s and Egger’s test. Statistical analysis was undertaken using the Stata software (version11: StataCorp, Texas, USA).
Results
Study selection and characteristics
After initial screening, 49 potentially relevant clinical trials of HCC were identified. Of these, 16 trials did not analyze the results of RFA separately from the other therapies, while 14 trials only focused on RFA. These 30 studies were excluded. Six trials were also excluded as no information concerning overall survival after three or five years was provided. A total of 13 studies (2 RCT and 11 NRCTs) [16,19-21,24-32] published between 2000 and 2011 were included.
These studies included a total of 2,535 patients: 1,233 treated with RAF and 1,302 with SR. The mean age ranged from 49.2 ± 9.9 to 69.4 ± 9.1 years. The male: female ratio in the pooled data was 2.57: 1. The median or mean tumor size (cm) ranged from 1.8 to 3.8. The median or mean duration of follow-up ranged from 22.7 to 847 months. The quality and characteristics of included studies are shown in Table 1.
Table 1.
Quality and characteristics of included studies
| Reference | Date | Design | Jadad score | Treatment | Number of patients | Sex (M/F) | Mean age (years) | Tumor number (single/multiple) | Mean tumor size (cm) |
|---|---|---|---|---|---|---|---|---|---|
| Nishikawa |
2011 |
NRCT |
1 |
SR |
69 |
50/19 |
67.4 ± 9.7 |
69/0 |
2.68 ± 0.49 |
| |
|
|
|
RFA |
162 |
95/67 |
68.4 ± 8.7 |
162/0 |
1.99 ± 0.62 |
| Tashiro |
2011 |
NRCT |
1 |
SR |
199 |
137/62 |
65.7 ± 9.0 |
132/67 |
2.1 ± 0.63 |
| |
|
|
|
RFA |
87 |
53/34 |
66.3 ± 8.2 |
67/20 |
1.8 ± 0.52 |
| HUNG |
2011 |
NRCT |
1 |
SR |
229 |
184/45 |
60.07 ± 12.56 |
181/48 |
2.88 ± 1.06 |
| |
|
|
|
RFA |
190 |
121/69 |
67.42 ± 11.45 |
152/38 |
2.37 ± 0.92 |
| Nanashima |
2010 |
NRCT |
1 |
SR |
144 |
112/32 |
63.6 ± 8.8 |
128/16 |
NA |
| |
|
|
|
RFA |
56 |
36/20 |
67.7 ± 8.5 |
51/5 |
NA |
| Huang |
2010 |
RCT |
4 |
SR |
115 |
85/30 |
55.91 ± 12.68 |
89/26 |
NA |
| |
|
|
|
RFA |
115 |
79/36 |
56.57 ± 14.30 |
84/31 |
NA |
| Ueno |
2009 |
NRCT |
1 |
SR |
123 |
82/41 |
67 (28 to 85) |
110/13 |
2.7 ± 0.1 |
| |
|
|
|
RFA |
155 |
100/55 |
66 (40 to 79) |
101/54 |
2.0 ± 0.1 |
| Guglielmi |
2008 |
NRCT |
1 |
SR |
91 |
73/18 |
NA |
69/22 |
NA |
| |
|
|
|
RFA |
109 |
88/21 |
NA |
65/44 |
NA |
| Hiraoka |
2008 |
NRCT |
1 |
SR |
59 |
44/15 |
62.4 ± 10.6 |
NA |
22.7 ± 5.5 |
| |
|
|
|
RFA |
105 |
76/29 |
69.4 ± 9.1 |
NA |
19.8 ± 5.2 |
| Abu-Hilal |
2008 |
NRCT |
3 |
SR |
34 |
26/8 |
67 |
NA |
3.8 (1.3 to 5) |
| |
|
|
|
RFA |
34 |
27/7 |
65 |
NA |
3 (2 to 5) |
| Takahashi |
2007 |
NRCT |
1 |
SR |
53 |
39/14 |
66 (41 to 80) |
41/12 |
2.5 (1 to 5) |
| |
|
|
|
RFA |
171 |
120/51 |
69 (44 to 84) |
124/47 |
2.1 (0.7 to 4.8) |
| Chen |
2006 |
RCT |
4 |
SR |
90 |
75/15 |
49.4 ± 10.9 |
NA |
NA |
| |
|
|
|
RFA |
71 |
56/15 |
51.9 ± 11.2 |
NA |
NA |
| Hong |
2005 |
NRCT |
1 |
SR |
93 |
69/24 |
49.2 ± 9.9 |
NA |
2.5 ± 0.8 |
| |
|
|
|
RFA |
55 |
41/14 |
59.1 ± 9.6 |
NA |
2.4 ± 0.6 |
| Vivarell |
2004 |
NRCT |
2 |
SR |
79 |
57/22 |
65.2 ± 8.2 |
66/13 |
NA |
| RFA | 79 | 67/12 | 67.8 ± 8.7 | 46/33 | NA |
NA: Not available; NRCT: non-randomized controlled trial; RCT: randomized controlled trial; RFA: radiofrequency ablation; SR: surgical resection.
Meta-analysis results
Overall survival rates
The meta-analysis showed that there was a significant difference in overall survival between the two groups at one year(12 trials [16,19-21,24-27,29-32], with certain heterogeneity), three years(13 trials [16,19-21,24-32], without heterogeneity) and five years(10 trials [16,24-32], without heterogeneity) and that the SR group was favored (see Table 2).
Table 2.
Main results of the pooled data in the meta-analysis
| Variables | Number of references with data | OR (95% CI) | Q test (Pvalue) | I2(%) | Z test (Pvalue) | Begg’s test (Pvalue) | Egger’s test (Pvalue) |
|---|---|---|---|---|---|---|---|
| Overall survival rates | |||||||
| 1 year |
12 [16,19-21,24-27,29-32] |
0.60(0.42, 0.86) |
0.301 |
14.6 |
0.005 |
0.54 |
0.40 |
| 3 years |
13 [16,19-21,24-32] |
0.49(0.36, 0.65) |
0.036 |
45.8 |
0.000 |
0.2 |
0.15 |
| 5 years |
10 [16,24-32] |
0.60(0.43, 0.84) |
0.003 |
63.7 |
0.003 |
0.37 |
0.57 |
| Recurrence rates | |||||||
| 1 year |
13 [16,19-21,24-32] |
1.48(1.05, 2.08) |
0.001 |
63.4 |
0.025 |
1.00 |
0.61 |
| 3 years |
13 [16,19-21,24-32] |
1.76(1.49, 2.08) |
0.000 |
69.9 |
0.000 |
0.20 |
0.15 |
| 5 years |
10 [16,24-32] |
1.68(1.21, 2.34) |
0.02 |
54.4 |
0.002 |
0.86 |
0.92 |
| Local recurrence |
4 [16,20,24,30] |
0.34(0.09, 1.28) |
0.02 |
68.9 |
0.112 |
NA |
NA |
| Complications | 7 [16,19,24,25,27,30,32] | 6.25(3.12, 12.52) | 0.042 | 54 | 0.000 | 1 | 0.982 |
NA,not available.Q test and I2 were used to evaluate the heterogeneity of the studies; Z test was used to value the combined effect; Begg’s and Egger’s tests were used to assess the publication bias.
Recurrence rates
Our results, as shown in Table 2, indicated that recurrence rates at one year(13 trials [16,19-21,24-32], without heterogeneity), three years(13 trials [16,19-21,24-32], without heterogeneity) and five years(10 trials [16,24-32], without heterogeneity) were significantly higher in the RFA group than in the SR group. However, no differences were found between the two groups (4 trials [16,20,24,30]) with respect to the local intrahepatic recurrence (see Table 2).
Complications
The meta-analysis (7 trials [16,19,24,25,27,30,32] reported these data) showed that there was significant difference between the two groups (OR, 6.25 [95%CI, 3.12 to 12.52]; P = 0.000), without heterogeneity (see Table 2). The RFA group was favored.
Subgroup analysis in HCCs ≤ 3 cm
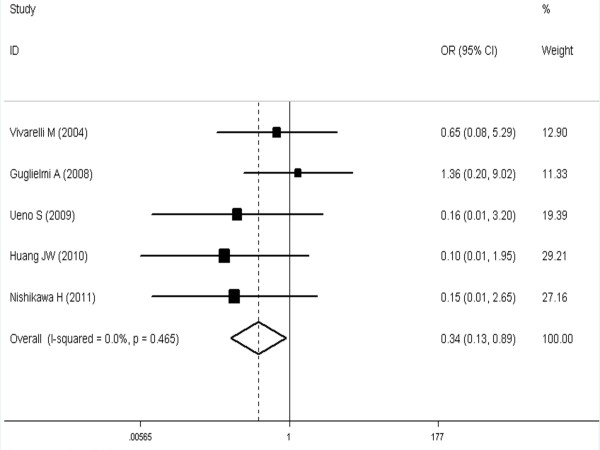
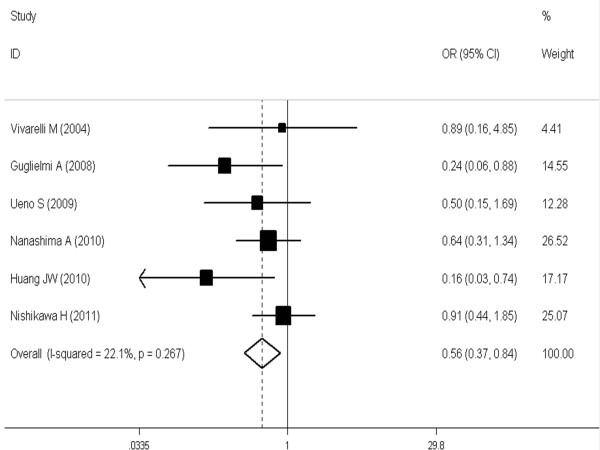
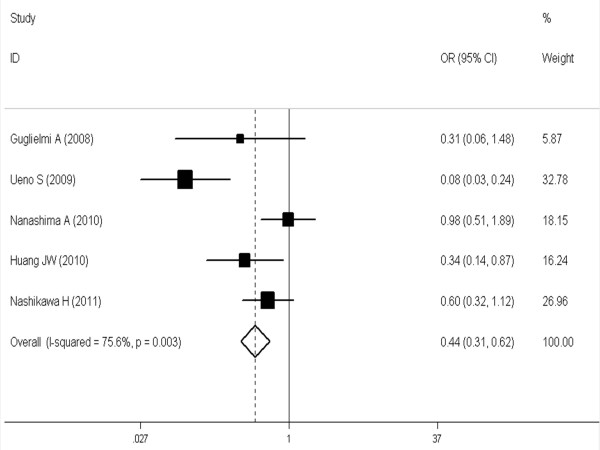
The meta-analysis (6 trials [16,21,24,27-29] reported these data) showed that the difference was significant and favorable to the SR group at 1, 3 and 5 years (respectively, OR, 0.34 [95%CI, 0.13 to 0.89]; OR, 0.56 [95%CI, 0.37 to 0.84]; OR, 0.44 [95%CI, 0.31 to 0.62] (see Figures 1, 2, 3).
Figure 1.
Meta-analysis of one-year overall survival rates after SR versus RFA in HCCs ≤ 3 cm. A fixed model was used. Pooled risk ratios are shown with 95% confidence intervals.
Figure 2.
Meta-analysis of three-year overall survival rates after SR versus RFA in HCCs ≤ 3 cm. A fixed model was used. Pooled risk ratios are shown with 95% confidence intervals.
Figure 3.
Meta-analysis of five-year overall survival rates after SR versus RFA in HCCs ≤ 3 cm. A fixed model was used. Pooled risk ratios are shown with 95% confidence intervals.
Sensitivity analysis
To compare the difference and evaluate the sensitivity of the meta-analysis, we employed one-way sensitivity analysis to evaluate the stability of the meta-analysis. The statistical significance of the results was not altered when any single study was omitted (data not shown). Therefore, results of the sensitivity analysis suggest that the data in this meta-analysis are relatively robust.
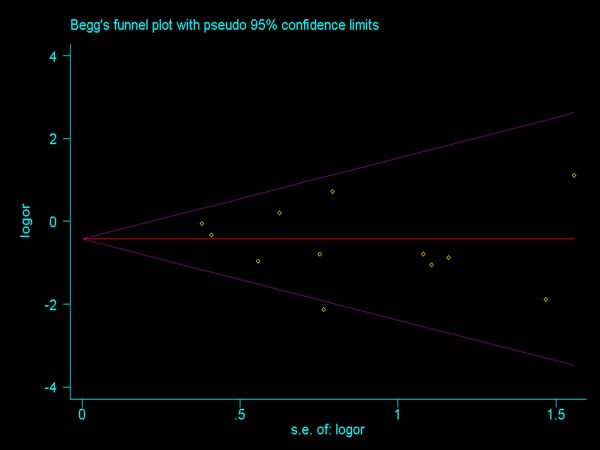
Publication bias
Funnel plots (Figure 4) were created to assess possible publication biases. In addition, Begg’s and Egger’s tests were used to evaluate the symmetry of the plots. As shown in Table 2, the data suggest that the funnel plots were symmetrical, and that publication biases might not have an evident influence on the results of the meta-analyses.
Figure 4.
Funnel plots on one-year overall survival rates following RFA and SR for the treatment of early HCC.
Discussion
There is some dispute whether survival benefits of RFA exist for patients with early HCC compared with SR. This meta-analysis demonstrated that RFA with or without TACE was inferior to SR in terms of overall survival rates and recurrence rates at one, three, and five years, contrary to the opinion of Livraghi [23]. This may be partly explained by advances in surgical and radiological techniques and perioperative care, and by more cautious patient selection [33,34]. This finding may also be adversely impacted by the delay of surveillance in effective treatment using RFA [35,36].
A high rate of recurrence after treatment is the main factor affecting overall survival and late death of patients with HCC [37]. Reportedly, the risk factors for tumor recurrence after treatment include tumor location, tumor size, multinodular tumors, and an insufficient safety margin [38-40]. Additionally, recurrences arise because of pre-existing microscopic tumor foci that were undetected by imaging modalities, or because malignant cells disseminated during operation [41,42]. In this study, recurrence was found to be more frequent after RFA than SR. This may be a result of the safety margin of RFA being narrower than that of SR, as SR usually excises the entire Couinaud segments containing tumors and possible venous tumor thrombus. In addition, high rates of recurrence after RFA may result from insufficient ablation of the primary tumor or the presence of tumor venous invasion in the adjacent liver. As for local intrahepatic recurrence rates, the two groups had no difference. This may be due to the development of techniques of RFA and an accurate evaluation of treatment response via a sufficient safety margin (at least 0.5 cm).
This meta-analysis suggested that the incidence of complications after RFA for HCC was lower than those after the SR group as a result of the microinvasive characterization of RFA. Radiofrequency ablation is a minimally invasive, target-selective technique, which has been applied in clinical studies in the 1990s [43]: it can induce thermal lesions less than 2.5 to 3.5 cm in diameter, using single expandable-tip electrodes, which are handled percutaneously and guided by imaging modalities [44]. This procedure could be performed under conscious sedation and the hospital stay is then shortened.
The subgroup analysis showed marked differences in the overall survival rates between RFA and SR for HCC ≤ 3 cm after one, three, and five years. Considering the fact that patients with single HCC ≤ 3 cm in diameter were at early stage without micro metastases and vascular invasion, SR can achieve better clinic outcomes. However, a lack of sufficient data on RCTs and an unequal constitution of patients may also affect these findings.
The majority of the data in the present study came from non-RCTs, so the overall level of clinical evidence might be low. However, a firm conclusion about bias is difficult to reach as the asymmetry of the funnel plot is minimal. Therefore our pooled OR might be an overestimate of the true effect.
Conclusion
In conclusion, SR demonstrated significantly improved survival benefits for patients with early HCC, especially for HCC ≤ 3 cm in diameter, although local recurrence between two groups did not exhibit significant difference. However, the findings need to be carefully interpreted, owing to the lower level of evidence. Further RCTs are warranted to clarify the exact value of SR and RFA for early HCC, especially for single nodules ≤ 3 cm in diameter.
Abbreviations
CI: confidence interval; HCC: hepatocellular carcinoma; OR: odds ratio; RCT: randomized controlled trial; RFA: radiofrequency ablation; SMD: standard mean difference; SR: surgical resection; TACE: transcatheter arterial chemoembolization; WMD: weighted mean difference.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XG, QFZ, and MY designed the studyand wrote the manuscript.XG, QFZ, ZJH, CGF, and CY performed data acquisition.MY performed quality control of data.XG, QFZ, and MY performed statistical analysis and interpretation. All authors read and approved the final manuscript. XG and QFZ contributed equally to this work.
Contributor Information
Gang Xu, Email: gangxu1365@gmail.com.
Fu-zhen Qi, Email: qi.fuzhen@163.com.
Jian-huai Zhang, Email: jh_zh02@hotmail.com.
Guo-feng Cheng, Email: huaicgf@163.com.
Yong Cai, Email: caiyong1355@163.com.
Yi Miao, Email: miaoyi@njmu.edu.cn.
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Yang JD, Roberts LR. Hepatocellular carcinoma: aglobal view. Nat Rev GastroenterolHepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson AB 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, Covey A, Curley SA, D’Angelica MI, Davila R, Ensminger WD, Gibbs JF, Laheru D, Malafa MP, Marrero J, Meranz SG, Mulvihill SJ, Park JO, Posey JA, Sachdev J, Salem R, Sigurdson ER, Sofocleous C, Vauthey JN, Venook AP, Goff LW, Yen Y, Zhu AX. NCCN clinical practice guidelines in oncology: hepatobiliarycancers. JNatlComprCancNetw. 2009;7:350–391. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(Suppl 1):S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Xia HH. Novel therapeutic approaches for hepatocellular carcinoma: fact and fiction. World J of Gastroenterol. 2008;14:1641–1642. doi: 10.3748/wjg.14.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant N, David CS, Cunningham SC. Early hepatocellular carcinoma: transplantation versus resection: the case for liver resection. Int J Hepatol. 2011;2011:142085. doi: 10.4061/2011/142085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK, Kim DG, Moon IS, Lee MD, Park JH. Liver transplantation versus liver resection for the treatment of hepatocellular carcinoma. JSurgOncol. 2010;101:47–53. doi: 10.1002/jso.21415. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos GC, Druhe N, Sgourakis G, Molmenti EP, Beckebaum S, Baba HA, Antoch G, Hilgard P, Radtke A, Saner FH, Nadalin S, Paul A, Malagó M, Broelsch CE, Lang H. Liver transplantation, liver resection, and transarterial chemoembolization for hepatocellular carcinoma in cirrhosis: which is the best oncological approach? Dig Dis Sci. 2009;54:2264–2273. doi: 10.1007/s10620-008-0604-4. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Kokudo N, Makuuchi M. Surgical management of hepatocellular carcinoma. Liver resection and liver transplantation. Saudi Med J. 2007;28:1171–1179. [PubMed] [Google Scholar]
- Zhou J, Wang Z, Qiu SJ, Huang XW, Sun J, Gu W, Fan J. Surgical treatment for early hepatocellular carcinoma: comparison of resection and liver transplantation. J Cancer Res ClinOncol. 2010;136:1453–1460. doi: 10.1007/s00432-010-0802-2. [DOI] [PubMed] [Google Scholar]
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng ZW, Zhang YJ, Chen MS, Lin XJ, Liang HH, Shi M. Radiofrequency ablation as first-line treatment for small solitary hepatocellular carcinoma: long-term results. Eur J SurgOncol. 2010;36:1054–1060. doi: 10.1016/j.ejso.2010.08.133. [DOI] [PubMed] [Google Scholar]
- Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2011;98:1210–1224. doi: 10.1002/bjs.7669. [DOI] [PubMed] [Google Scholar]
- Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. AnnSurg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- Molinari M, Helton S. Hepatic resection versus radiofrequency ablation for hepatocellular carcinoma in cirrhotic individuals not candidates for liver transplantation: a Markov model decision analysis. Am J Surg. 2009;198:396–406. doi: 10.1016/j.amjsurg.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Takayama T, Makuuchi M, Hasegawa K. Single HCC smaller than 2 cm: surgery or ablation?: surgeon’s perspective. JHepatobiliaryPancreatSci. 2010;17:422–424. doi: 10.1007/s00534-009-0239-7. [DOI] [PubMed] [Google Scholar]
- Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. AnnSurg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SN, Lee SY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC, Rhee JC, Choi D, Lim HK, Lee KW, Joh JW. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. JClinGastroenterol. 2005;39:247–252. doi: 10.1097/01.mcg.0000152746.72149.31. [DOI] [PubMed] [Google Scholar]
- Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, Cavallari A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. AnnSurg. 2004;240:102–107. doi: 10.1097/01.sla.0000129672.51886.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montorsi M, Santambrogio R, Bianchi P, Donadon M, Moroni E, Spinelli A, Costa M. Survival and recurrences after hepatic resection or radiofrequency for hepatocellular carcinoma in cirrhotic patients: a multivariate analysis. JGastrointestSurg. 2005;9:62–67. doi: 10.1016/j.gassur.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- Nishikawa H, Inuzuka T, Takeda H, Nakajima J, Matsuda F, Sakamoto A, Henmi S, Hatamaru K, Ishikawa T, Saito S, Nasu A, Kita R, Kimuar T, Arimoto A, Osaki Y. Comparison of percutaneous radiofrequency thermal ablation and surgical resection for small hepatocellular carcinoma. BMCgastroenterol. 2011;11:143. doi: 10.1186/1471-230X-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka A, Horiike N, Yamashita Y, Koizumi Y, Doi K, Yamamoto Y, Hasebe A, Ichikawa S, Yano M, Miyamoto Y, Ninomiya T, Otomi Y, Kokame M, Iwamura T, Ishimaru Y, Sogabe I, Kashihara K, Nishiura S, Ootani H, Takamura K, Kawasaki H. Efficacy of radiofrequency ablation therapy compared to surgical resection in 164 patients in Japan with single hepatocellular carcinoma smaller than 3 cm, along with report of complications. Hepato-gastroenterology. 2008;55:2171–2174. [PubMed] [Google Scholar]
- Takahashi S, Kudo M, Chung H, Inoue T, Nagashima M, Kitai S, Tatsumi C, Minami Y, Ueshima K, Fukunaga T, Haji S. Outcomes of nontransplant potentially curative therapy for early-stage hepatocellular carcinoma in Child-Pugh stage A cirrhosis is comparable with liver transplantation. Dig Dis. 2007;25:303–309. doi: 10.1159/000106909. [DOI] [PubMed] [Google Scholar]
- Guglielmi A, Ruzzenente A, Valdegamberi A, Pachera S, Campagnaro T, D’Onofrio M, Martone E, Nicoli P, Iacono C. Radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma in cirrhosis. JGastrointestSrug. 2008;12:192–198. doi: 10.1007/s11605-007-0392-8. [DOI] [PubMed] [Google Scholar]
- Nanashima A, Tobinaga S, Masuda J, Miyaaki H, Taura N, Takeshita H, Hidaka S, Sawai T, Nakao K, Nagayasu T. Selecting treatment for hepatocellular carcinoma based on the results of hepatic resection and local ablation therapy. JSurgOncol. 2010;101:481–485. doi: 10.1002/jso.21523. [DOI] [PubMed] [Google Scholar]
- Ueno S, Sakoda M, Kubo F, Hiwatashi K, Tateno T, Baba Y, Hasegawa S, Tsubouchi H. Surgical resection versus radiofrequency ablation for small hepatocellular carcinomas within the Milan criteria. JHepatobiliary Pancreatic Surg. 2009;16:359–366. doi: 10.1007/s00534-009-0069-7. [DOI] [PubMed] [Google Scholar]
- Abu-Hilal M, Primrose JN, Casaril A, McPhail MJ, Pearce NW, Nicoli N. Surgical resection versus radiofrequency ablation in the treatment of small unifocal hepatocellular carcinoma. JGastrointestSurg. 2008;12:1521–1526. doi: 10.1007/s11605-008-0553-4. [DOI] [PubMed] [Google Scholar]
- Hung HH, Chiou YY, Hsia CY, Su CW, Chou YH, Chiang JH, Kao WY, Huo TI, Huang YH, Su YH, Lin HC, Lee SD, Wu JC. Survival rates are comparable after radiofrequency ablation or surgery in patients with small hepatocellular carcinomas. ClinGastroenterolHepatol. 2011;9:79–86. doi: 10.1016/j.cgh.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Tashiro H, Aikata H, Waki K, Amano H, Oshita A, Kobayashi T, Tanimoto Y, Kuroda S, Tazawa H, Chayama K. Treatment strategy for early hepatocellular carcinomas: comparison of radiofrequency ablation with or without transcatheter arterial chemoembolization and surgical resection. JSurgOncol. 2011;104:3–9. doi: 10.1002/jso.21745. [DOI] [PubMed] [Google Scholar]
- Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology. 2010;78(Suppl 1):113–124. doi: 10.1159/000315239. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Mehrabi A, Mollberg NM, Muller SA, Koch M, Buchler MW, Weitz J. Hepatocellular carcinoma: current management and perspectives for the future. AnnSurg. 2011;253:453–469. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- Chen WT, Fernandes ML, Lin CC, Lin SM. Delay in treatment of early-stage hepatocellular carcinoma using radiofrequency ablation may impact survival of cirrhotic patients in a surveillance program. JSurgOncol. 2011;103:133–139. doi: 10.1002/jso.21797. [DOI] [PubMed] [Google Scholar]
- Wong GL, Wong VW, Tan GM, Ip KI, Lai WK, Li YW, Mak MS, Lai PB, Sung JJ, Chan HL. Surveillance programme for hepatocellular carcinoma improves the survival of patients with chronic viral hepatitis. LiverInt. 2008;28:79–87. doi: 10.1111/j.1478-3231.2007.01576.x. [DOI] [PubMed] [Google Scholar]
- Blum HE, Spangenberg HC. Hepatocellular carcinoma: an update. Arch Iran Med. 2007;10:361–371. [PubMed] [Google Scholar]
- Liu L, Miao R, Yang H, Lu X, Zhao Y, Mao Y, Zhong S, Huang J, Sang X, Zhao H. Prognostic factors after liver resection for hepatocellular carcinoma: a single-center experience from China. Am J Surg. 2012;203:741–750. doi: 10.1016/j.amjsurg.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Ko CJ, Chien SY, Chou CT, Chen LS, Chen ML, Chen YL. Factors affecting prognosis of small hepatocellular carcinoma in Taiwanese patients following hepatic resection. Can J Gastroenterol. 2011;25:485–491. doi: 10.1155/2011/790528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grat M, Holowko W, Grzegorczyk K, Skalski M, Krawczyk M. Long-term results of liver resection in the treatment of patients with hepatocellular carcinoma. PolPrzeglChir. 2011;83:319–324. doi: 10.2478/v10035-011-0049-x. [DOI] [PubMed] [Google Scholar]
- Ohashi M, Wakai T, Korita PV, Ajioka Y, Shirai Y, Hatakeyama K. Histological evaluation of intracapsular venous invasion for discrimination between portal and hepatic venous invasion in hepatocellular carcinoma. JGastroenterolHepatol. 2010;25:143–149. doi: 10.1111/j.1440-1746.2009.06008.x. [DOI] [PubMed] [Google Scholar]
- Park YN. Pathology of hepatocellular carcinoma: recent update. Korean J Gastroenterol. 2005;45:227–233. [PubMed] [Google Scholar]
- Bangard C. Radiofrequency of the liver - an update. RoFo. 2011;183:704–713. doi: 10.1055/s-0029-1246082. [DOI] [PubMed] [Google Scholar]
- Rossi S, Ravetta V, Rosa L, Ghittoni G, Viera FT, Garbagnati F, Silini EM, Dionigi P, Calliada F, Quaretti P, Tinelli C. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011;53:136–147. doi: 10.1002/hep.23965. [DOI] [PubMed] [Google Scholar]