Abstract
Natural killer (NK) cells can be engineered to kill resistant B-lymphoid cell lines and primary B-cell chronic lymphocytic leukemia (B-CLL) cells after transfection with chimeric antigen receptors (CARs) recognizing CD19 or CD20. Here we compared mRNA electroporation with lentiviral vector (LV) transduction for both CARs. Transfection efficiency and cytotoxicity of previously NK-92 resistant CLL cells were significantly higher after mRNA electroporation than after LV transduction. Further cell sorting of LV-transduced NK-92 cells resulted in a highly enriched population of transduced cells with significant target cell lysis. Compared to NK-92 cells, peripheral blood and cord blood cells consistently showed < 10% transfection efficiency with mRNA, while LV transduction varied between 8 and 16% for peripheral blood and 12 and 73% for cord blood. These results suggest that LV should be used to achieve sufficient transgene expression if blood NK cells are considered for CAR transduction. Transfection with mRNA results in clinically relevant levels of transfection only in NK-92 cells.
Keywords: CAR, lymphoid malignancies, NK cells, lentiviral vector
INTRODUCTION
Current therapies for lymphoid malignancies generally utilize chemotherapy combined with monoclonal antibodies directed against specific lymphoid surface markers, such as CD20, CD22 and CD23 [1,2]. Cytotoxic cellular therapy, predominantly using T cells and natural killer (NK) cells, is a treatment modality that is increasingly considered as it is non-cross-reactive with chemotherapy and radiation. The recent report on remission induction in patients with advanced chronic lymphocytic leukemia (CLL) using autologous T-lymphocytes transfected with a chimeric antigen receptor (CAR) has focused attention on a new direction of cell therapy [3]. CAR molecules typically comprise an intracellular signaling domain linked to the extracellular scFv region of a monoclonal antibody that binds a specific antigen on the tumor cell surface, thereby activating cytotoxic cells independent of any inhibitory receptors [4 – 6]. Clonogenic malignant B cells constitutively express CD19 and CD20 antigens that are suitable targets for CAR-redirected cytotoxicity.
Lymphoid malignancies are frequently resistant to NK cell-mediated killing [7,8]. In addition, autologous NK cells are generally blocked by self-major histocompatibility complex (MHC) antigens recognized by killer immunoglobulinlike receptors (KIRs) [9]. Although allogeneic NK cells can overcome this inhibition to some extent, this has not been shown consistently for lymphoid malignancies [10,11]. The introduction of CARs recognizing CD19 or CD20 into NK cells is considered one possible pathway to overcome the lack of killing. So far, despite some progress in ex vivo NK cell expansion [12] or transduction [13], it has been challenging to expand sufficient numbers of NK cells and at the same time achieve a level of CAR transfection comparable to that with T cells.
The human natural killer cell line NK-92 is highly cytotoxic against a wide spectrum of malignant cells [14,15], often serves as a model to study the biology of activated NK cells [16] and has completed early stage clinical trials [17,18]. NK-92 cells, like human blood activated NK cells, do not kill malignant lymphoid targets consistently, either cell lines or primary patient cells [17,19]. However, they can become highly cytolytic against previously resistant targets once they express CAR molecules directed against human HER2/neu [6], CD19 [20,21] or CD20 [22]. The introduction of those CAR transgenes was usually achieved by retroviral transduction. Lentiviral vectors (LVs) have recently received some attention as vehicles for transduction with potential for clinical application [23 – 25]. Despite their high transduction efficiency, however, any viral construct carries the risk of insertional mutagenesis and is not preferred for application in humans [26]. Alternative non-viral transfection methods such as mRNA electroporation have been considered, although the duration of expression is limited since the transcript is not incorporated into the genome [27].
The objective of this study was to compare the transfection efficiency of mRNA electroporation versus LV transduction of various sources of NK cells and test cytotoxicity against primary CLL cells and malignant lymphoid cell lines. Our results confirm that whereas transfection efficacy of blood NK cells with CAR mRNA is negligible, cord blood NK cells have reasonable transfection efficiency with LV although it is highly variable. In contrast, NK-92 can be sufficiently transfected with mRNA or LV. The latter provides the advantage of increased purity after cell sorting.
MATERIALS AND METHODS
Primary Effector and Target Cells
Peripheral blood samples were obtained from healthy volunteers. De-identified cord blood samples were obtained through the Division of Maternal Fetal Medicine - Tufts Medical Center, Boston, MA. Primary CLL samples were provided by Dr. Arthur Rabinowitz (Lahey Clinic, Burlington, MA), from thirteen patients with untreated CLL diagnosed according to NCIWorking Group criteria. All patients had stage 0 or I CLL (Rai staging system), and were naïve to previous mAb therapy. Mononuclear cells from all samples (MNCs) were obtained by density gradient centrifugation using Ficoll-Hypaque Plus (Amersham Biosciences, Piscataway, NJ). MNCs were either used for further cell separation (peripheral and cord blood samples), or frozen in 10% DMSO-90% FBS in Liquid Nitrogen and thawed just before the cytotoxicity assays (primary CLL cell samples).
Cells lines and cell culture
NK-92, Raji (Burkitt’s Lymphoma, CD19+/ CD20+, NK-92 sensitive), and SUP-B15 (B-precursor ALL, CD19+/ CD20−, NK-92 resistant) cell lines were purchased from American Type Culture Collection (ATCC, Rockville, MD). TMD5 cell line (B-ALL, CD19+, CD20+, NK-92 resistant) was provided by Dr. Nobuo Nara (Tokyo Medical and Dental University, Japan) (26). HEK-293T packaging cells were provided by Dr. Charlotte Kuperwasser (Molecular Oncology Research Institute, Boston, MA). NK-92.26.5 (“NK-92fc”), an engineered high-affinity FcγRIII-expressing variant of NK-92, was provided by Dr. Kerry Campbell (Fox Chase Cancer Center, Philadelphia, PA). Raji, SUP-B15, and HEK-293T cells were maintained in RPMI-1640 (Mediatech Inc., Manassas, VA) supplemented with 20% FBS (Gibco Invitrogen, Carlsbad, CA) and antibiotics: Penicillin 100 μl ml−1, Streptomycin 10 μg ml−1, and Amphotericin-B 250 μg ml−1 (all from Gibco), and Ciprofloxacin 10 μg ml−1 (Mediatech). TMD-5 was maintained in α-MEM medium (Gibco) supplemented with 10% FBS and the antibiotic cocktail mentioned above. NK-92 cells and variants were maintained in Myelocult (StemCell Technologies, Vancouver, BC) supplemented with Proleukin 500 IU/ml (recombinant human IL-2; Chiron, Emeryville, CA).
MNCs from peripheral or cord blood were either CD3 (T-cell) depleted (CD3depl) or NK-enriched by negative selection (NKenr) using the corresponding immunomagnetic separation kit (MidiMacs system; Miltenyi, Auburn, CA) following the manufacturer’s instructions. MNCs from both sources were used for lentiviral transduction after overnight incubation in RPMI 20% FBS supplemented with IL-2 (1000 units/ml). NK cell purity was determined by flow cytometry (CYAN ABD flow cytometer; DakoCytomation, Carpinteria, CA) using Phycoerythrin (PE)-conjugated anti-CD56 antibody (BD Biosciences Pharmingen, San Jose, CA). The CD3depl fraction contained 8–20% CD56+ cells while the NKenr fraction contained >75% CD56+ cells.
Cloning, mRNA synthesis and lentiviral vector production
The CAR constructs used in this study consisted of a single-chain Fv fragment from a murine antibody specific for human surface antigen CD19 or CD20, linked to the CD3 ζ chain of the T-cell receptor complex. α CD19-CAR and α CD20-CAR cDNAs [22] were subcloned from retroviral vector pLXSN into the plasmid pXT7 (provided by Dr. S. Sokol, Mount Sinai School of Medicine, New York, NY) and used as templates for T7-dependent in vitro mRNA synthesis, as previously published [21] (T7 Ultra mMessage mMachine kit; Ambion Applied Biosystems, Austin, TX). For LV production, both CARs were subcloned into the vector pCL20c-IRES-GFP (provided by Dr. R. Childs, National Heart, Blood and Lung Institute, Bethesda, MD). The CAR constructs were transfected into the HEK-293T packaging cell line alongside helper plasmids, using the Fugene lipofection system (Roche, Indianapolis, IN). Culture supernatants were collected after 48 h, filtered (0.22 μm) and stored at −80 ° C. The supernatants had a titer of LV particles consistently >107 infectious units mL−1.
Electroporation of mRNA and Lentiviral Transduction
NK-92 cells were electroporated as follows: 2×106 cells in 250 μl MEM medium with 40 μg/ml mRNA (GFP, αCD19-CAR, or αCD20-CAR), using a GenePulser II (BioRad, Hercules CA) at 300 mV, 150 μF, 200 W. Electroporated NK-92 cells were maintained in Myelocult + IL-2 and used 20 to 24h post-electroporation. NKenr and CD3depl MNCs were transduced with the lentiviral vector as follows: for “static” transduction cells were incubated for 4 h at 37°C with 450 μl undiluted lentiviral supernatant (CD19-CAR) in a 12-well plate, with either 8 μg ml−1 polybrene or 15 μg ml−1 protamine sulfate (Sigma–Aldrich, Saint Louis, MO). Multiplicity of Infection (MOI) was between 15 and 150. The supernatant was discarded after incubation and replaced with RPMI 20%FBS + IL-2 (1000 UI ml−1). For “spinfection”, cells were prepared as above with the addition of 20 mM Hepes buffer (pH 7.4) and the plates were centrifuged at 2500 x g for 90 min at 32°C, then incubated at 37 °C for 3 h and treated as above. For transduction using Retronectin® (TakaraBio USA, TBU’s Madison, WI), 12-well plates (non-tissue culture treated; Falcon BD Biosciences, Franklin Lakes, NJ) were pre-incubated overnight at 4°C with Retronectin at 40 μg cm−2 according to manufacturer’s instructions. They were then used for transduction as described above, without polybrene or protamine sulfate. NK-92 cells were transduced with the lentiviral vector according to the spinfection protocol, using 2×106 cells in 6-well plates with the corresponding lentiviral supernatant (GFP, CD19-CAR, or CD20-CAR; MOI ~5). Since polybrene is toxic for NK-92 cells, only protamine sulfate was used. Transduced cells were expanded in Myelocult + IL-2 and either used “as is” after 48 h, or GFP-expressing NK-92 cells were further enriched by cell sorting to achieve ~95% purity (MoFlo flow cytometer; DakoCytomation).
Efficiency of electroporation or lentivirus transduction was determined by flow cytometry, measuring either GFP expression directly or CAR expression using α-scFv antibody-biotin (Jackson Immunoresearch, West Grove, PA) and Streptavidin-APC (BD Biosciences Pharmingen). Analysis was performed 24 h after electroporation or 48–72 h after transduction.
Cytotoxicity Assays
To measure cytotoxic killing, targets and effectors were combined at various effector to target (E:T) ratios (2.5:1 to 10:1) and cytotoxicity was measured by flow cytometric assay as published previously (27). For the ADCC assay, CLL targets were incubated with Rituximab (α-CD20 mAb, 0.5 μg ml−1; Biogen/Genentech, South San Francisco, CA) for 30 min before adding the effectors (NK-92 or NK-92fcR expressing the high affinity FcγRIII) at an E:T ratio of 5:1, and treated as previously published (28). These experiments were done in the presence of heat-inactivated FBS to exclude any contribution of complement-mediated cytotoxicity. Samples were analyzed immediately by flow cytometry as above. ADCC was calculated by subtracting the target killing obtained with the parental NK-92 cells (that do not express Fc-receptor) from the target killing obtained with CAR-expressing NK-92 cells.
Statistical Analysis
SPSS 11.5 software (SPSS, Chicago, IL) was used to calculate a one sided Student t-test (independent samples, unpaired). For small sample sizes we also used a Wilcoxon rank-sum test. All P values <0.05 were considered statistically significant. Data are presented as mean ± standard error of the mean (SEM) unless otherwise noted.
RESULTS
Electroporation of mRNA versus Lentiviral transduction of NK-92
We have previously reported that NK-92 cells have high transfection efficiency when electroporated with mRNA, in contrast to electroporation with plasmid DNA [21]. Here we extended these initial observations and compared the efficiency of mRNA electroporation with viral transduction using a LV as a means of introducing CAR transgenes into NK-92 cells. The percentage of NK-92 cells expressing the transgenes (CAR and/or GFP) was significantly higher after mRNA electroporation than after a single round of LV transduction [55.8% ± 5.8 compared to 25.6% ± 4.2, p < 0.001 by Wilcoxon rank-sum test, and Figure 1(A) for individual transgenes data]. Levels of expression of the transgenic proteins in electroporated NK-92 cells, estimated by fluorescence intensity, were similar or superior to the levels detected in transduced cells [Figure 1(B)]. Moreover, both the percentage of expression and the fluorescence intensity measured by flow cytometry were higher for the αCD20-CAR transgene than for α CD19-CAR, suggesting that cells express more αCD20-CAR proteins. However, transduction of NK-92 cells with LV constructs for αCD19-CAR or αCD20-CAR, both of which carry GFP under an internal ribosome entry site (IRES) promoter in the same transcript, gave comparable percentage of expression and fluorescence intensity for GFP, suggesting that rates of transduction were similar for both LV constructs. These observations were confirmed by Western blot analysis (data not shown). Viability after mRNA transfection or LV transduction was consistently over 80%. While mRNA transfection in NK-92 was only transient (2 – 3 days for αCD19-CAR, up to a week for α CD20-CAR) [21], the expression of the exogenous proteins after LV transduction remained unchanged in NK-92 cells for several months in culture (data not shown). This stable expression allowed for enrichment of transgene-positive NK-92 cells by fluorescence activated cell sorting (FACS) using GFP as a marker. Cell sorting yielded a pure population ( > 95%) of high CAR transgene-expressing NK-92 cells that could be maintained in culture and/or kept cryopreserved.
Figure 1. Efficiency of gene transfer into NK-92 cells determined by flow cytometric analysis of transgene expression.
(A) NK-92 cells were electroporated with mRNA (n = 6) or transduced with LV supernatant (n = 4). Transfection yields are expressed as the percentage of transgene- expressing cells within the whole cell population. Error bars correspond to SEM. *p < 0.05; **p < 0.01 (Wilcoxon rank-sum test). (B) Histogram of representative flow cytometric analysis of GFP expression (fluorescein isothiocyanate [FITC], left panels) or CAR expression (allophycocyanin [APC], right panels) in NK-92 cells that were electroporated (mRNA, gray) or lentivirus transduced (LV, light gray) with the indicated transgenes. White: isotype control.
CAR-expressing NK-92 kill previously NK-resistant lymphoid cell lines
NK-92 cells do not spontaneously kill the lymphoid cell lines SUP-B15 or TMD5. After transfection with α CD19-CAR, NK-92 cells killed both cell lines, whether they were electroporated with mRNA (42% ± 6.6 for SUP-B15, 50.5% ± 6.6 for TMD5; E:T of 5:1) or transduced with LV and sorted (78.8% ± 1.6 for SUPB15, 79.3% ± 4.6 for TMD5; E:T of 5:1). NK-92 cells expressing α CD20-CAR killed TMD5 (34.4% ± 6.0 for electroporated cells, 51.4% ± 3.1 for transduced/sorted cells; E:T of 5:1) but not the CD20 negative SUP-B15 (7.1% ± 6.3 for electroporated cells, 5.0% ± 0.1 for transduced/sorted cells; E:T of 5:1). Expression of any transgene (CAR or GFP) by NK-92 did not impair killing of Raji cells when compared to parental NK-92, suggesting that the expression of foreign transgenes did not perturb NK-92 effector function (Figure 2).
Figure 2. In vitro cytotoxicity assay using genetically modified NK-92 against leukemia cell lines.
NK-92 effector cells were transfected with mRNA (56% transgene expression, filled symbols) or lentiviral vector-transduced and sorted (> 95% transgene expression, open symbols). The expressed transgenes were GFP (circle), αCD19-CAR (square) or αCD20-CAR (triangle). Transgene expression did not negatively affect NK-92 killing of Raji, whereas SUP-B15 and TMD5 were resistant to non-CAR-expressing NK-92. αCD19-CAR-expressing NK-92 displayed higher cytotoxicity against TMD5 than αCD20-CAR. The data are representative of 2–5 assays. LV, lentiviral vector.
CAR-expressing NK-92 kill primary CLL cells
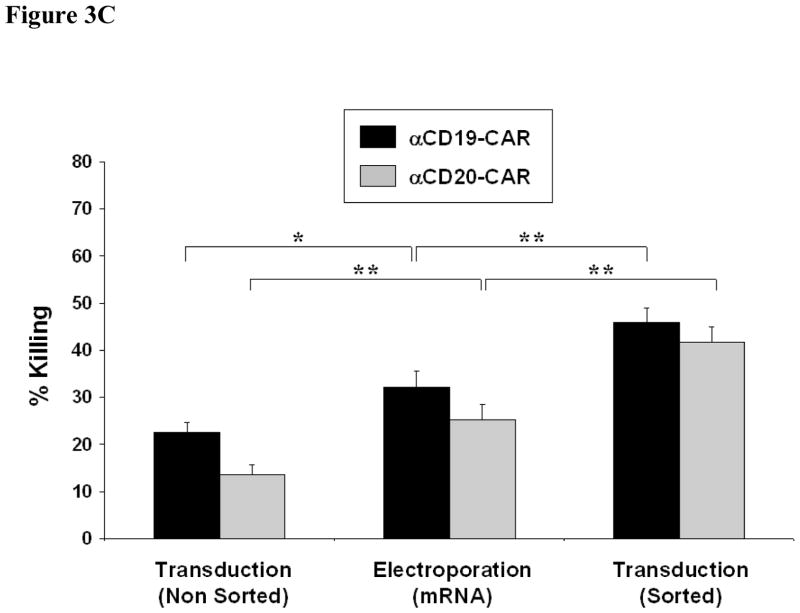
We next compared the cytotoxicity of nine primary CLL cell samples by α CD19-CAR or α CD20-CAR NK-92 cells that had been transfected by mRNA electroporation, single round LV transduction or LV transduction followed by cell sorting [Figs. 3(A) and 3(B), respectively]. Because of the enrichment of CAR-expressing cells, the mean percentage of killing of CLL cells by FACS-sorted LV-transduced NK-92 cells was significantly higher than for the mRNA-transfected NK-92 [Figure 3(C): 45.9% ± 2.8 vs. 32.1% ± 3.4 for α CD19-CAR, p < 0.006; 41.7% ± 2.3 vs. 25.1% ± 3.3 for αCD20-CAR, p < 0.0003; E:T ratio of 5:1). However, non-sorted LV-transduced NK-92 killed less than mRNA-electroporated NK-92 (22.6% ± 2.0 for α CD19-CAR, p < 0.03; 13.6% ± 2.1 for αCD20-CAR, p < 0.009), in line with the observation that target lysis correlated with the percentage of CAR-positive cells in the effector population. Similar to the result with lymphoid cell lines, we found that αCD19-CAR-expressing NK-92 showed consistently superior cytotoxicity against CLL cells over αCD20-CAR-expressing NK-92. There was, however, no correlation between the percentage of killing and the percentage of CD19 or CD20 antigen-expressing cells in the target populations (data not shown). Parental NK-92 cells showed limited or no killing of primary CLL cells.
Figure 3. Comparison of gene transfer methods on the killing efficacy of primary CLL cells by CAR-expressing NK-92 cells.
In vitro cytotoxicity assay using NK-92 effector cells that were electroporated with mRNA [Electroporation (mRNA)], transduced with lentiviral vector [Transduction (Non Sorted)] or cell-sorted following lentiviral transduction [Transduction (Sorted)]. Effectors were tested against nine samples of primary CLL cells at an E:T ratio of 5:1. (A) Individual data for NK-92 cells expressing αCD19-CAR, and (B) individual data for NK-92 cells expressing αCD20-CAR. (C) Comparison of means between the diff erent NK-92 variants (n = 9–13). Error bars correspond to SEM. *p < 0.05; **p < 0.01 (Student’s t-test).
Lentiviral Transduction of blood-derived NK cells
As previously observed [29], even optimized conditions for electroporation of mRNA into blood-derived NK cells yielded a transfection efficiency of consistently less than 10%, with very low cell viability. We therefore determined the efficiency of LV transduction of NK cells derived from either peripheral blood or umbilical cord blood, using CD3 depl or NK enr cells that were activated with IL-2 (1000 IU mL−1) overnight. We compared two protocols of transduction (“ static “ transduction versus spinfection). We also compared two polycationic compounds (polybrene versus protamine sulfate) that facilitate lentiviral transduction but can also affect NK cell viability.
Transduction efficiency of peripheral blood NK cells was low and variable (range 8 – 16% between all conditions, MOI = 150), and there was no difference between the various transduction protocols (Table I). In contrast, NK cells from cord blood consistently showed higher transduction efficiency (range 12 – 73% between all conditions, MOI =150). For cord blood-derived NK cells, spinfection resulted in a higher percentage of transgene-expressing cells within the CD56 + NK cell population (range 19 – 73%) compared to “static “ transduction (range 12 – 30%), regardless of the polycationic adjuvant used. Unlike peripheral blood, cord blood-derived NK cells showed a higher transduction rate when polybrene was used (41.6% ± 8.9) compared to protamine sulfate (26% ± 6.2), regardless of the transduction method used. However, polybrene negatively affected the viability (31% ± 5 survival) of NK cells compared to protamine sulfate (70% ± 7). This adverse eff ect of polybrene on NK cells was still observed at lower concentrations ( < 2 μg mL−1), especially on cord blood-derived NK cells (data not shown). We did not observe any difference in yield between the transfection efficiencies of NKenr or CD3depl blood cells.
Table I.
Transduction* of blood-derived NK cells using the αCD19-CAR lentiviral vector
| Cells | Methods | MOI | Adjuvant | % CD56/GFP+ |
|---|---|---|---|---|
| PB NKenr | Transduction | 150 150 |
Polybrene | 8.0 |
| Prot. Sulfate | 13.0 | |||
| Spinfection | 150 150 |
Polybrene | 11.0 | |
| Prot. Sulfate | 16.5 | |||
| CB NKenr | Spinfection | 150 150 |
Polybrene | 56.0 |
| Prot. Sulfate | 26.0 | |||
| CB CD3depl | Transduction | 150 150 |
Polybrene | 30.0 |
| Prot. Sulfate | 12.0 | |||
| 30 30 |
Polybrene | 29.0 | ||
| Prot. Sulfate | 1.5 – 10.0 | |||
| Spinfection | 150 150 150 |
Polybrene | 19.0 – 68.0 | |
| Prot. Sulfate | 19.0 – 49.0 | |||
| Retronectin | 31.0 – 73.0 | |||
| 30 30 |
Polybrene | 45.0 | ||
| Prot. Sulfate | 6.0 – 16.0 | |||
| 15 15 15 |
Polybrene | 47.0 | ||
| Prot. Sulfate | 9.0 | |||
| Retronectin | 11.0 – 26.0 |
NK, natural killer; PB, peripheral blood; CB, cord blood; MOI, multiplicity of infection.
When available, results of different transduction conditions are presented as a range of values.
We have previously shown that in vitro culture of CD3depl cord blood MNCs results in significantly better expansion of the NK cell population than culture of NKenr MNCs [29], and the former would be the preferred starting cell population for clinical application. To optimize LV transduction conditions for CD3depl cells, we compared spinfection using Retronectin versus spinfection with polybrene or protamine sulfate. Retronectin did not aff ect NK cell survival and was superior to both polybrene and protamine sulfate (52% ± 21 compared to 40.7% ± 14.4 and 30.7% ± 9.3, respectively). When comparing different MOIs (150, 30, 15), the highest transduction rates were obtained for an MOI of 150 using Retronectin or protamine sulfate, while transduction rates using polybrene did not vary. Of note is that transgene expression in NK cells was stable in all cases and could still be detected after 2 weeks of culture in vitro (data not shown).
Comparison of αCD20-CAR-induced killing of primary CLL cells with rituximab-mediated ADCC
We compared the lysis of 13 samples of primary CLL cells by rituximab-mediated ADCC with the cytotoxicity mediated by α CD20-CAR-expressing NK-92 cells (LV transduced and sorted). Cytotoxicity of CLL cells mediated by αCD20-CAR was significantly higher than rituximab-mediated ADCC (Figure 4; 41.7% ± 2.3 for α CD20-CAR compared with 25.6% ± 2.8 for ADCC, p < 0.0001). NK-92 cells did not kill CLL cells spontaneously, whether alone (4.6% ± 1.2) or with rituximab (7.1% ± 1.4).
Figure 4. Comparison of rituximab-mediated ADCC and αCD20-CAR-mediated killing of CLL cells.
In vitro cytotoxicity was determined using cellsorted LV-transduced NK-92 cells expressing αCD20-CAR (dark gray, filled) compared to NK-92 FcR-mediated ADCC using rituximab (light gray, filled). The percentage of killing for NK-92 alone (dark gray, hatched) or NK-92 with rituximab (light gray, hatched) is presented for each sample.
DISCUSSION
Despite progress in the treatment of B-cell malignancies, a significant proportion of patients will eventually relapse. The cytotoxic pathway of cellular therapy is different from chemo-radiotherapy, and potentially targets the cancer stem cells [31]. The development of NK cells for cellular therapy has been lagging behind that of T cells, as NK receptors and their ligands initially were poorly defined [32]. It is now widely accepted that patients ’ own (autologous) NK cells may not lyse transformed cells, as MHC identity can lead to activation of KIRs that inhibit NK cell activation signals. Instead of using allogeneic NK cells, autologous NK cells can be engineered to overcome KIR-mediated inhibition by arming them with activating receptors that override any KIR effects. This is the rationale for introducing CARs into NK cells that can target and lyse specific tumor antigens. This approach has gained some traction for T cells [33,34], which can be more easily expanded and transfected than NK cells. Recent reports have demonstrated tumor control in patients with advanced CLL using autologous T-lymphocytes transduced with a LV expressing αCD19-CAR [3,25,35].
To further develop CAR-directed cytotoxicity of NK cells, we used NK-92 cells that display characteristics of activated NK cells as a platform to test mRNA and LV based modification with αCD19 and αCD20 CARs. Like most blood activated NK cells, NK-92 cells are broadly cytotoxic to malignant cells, but do not kill some lymphoid cell lines and most primary malignant lymphoid cells [19].
We show here that NK-92 cells can be genetically modified to express αCD19-CAR and α CD20-CAR, either by electroporation of mRNA or by transduction with a LV-based construct. The transfection efficiency is about 60% for mRNA electroporation and about 25% for LV transduction, but the latter can be increased to almost 100% after the transduced cells are sorted. When compared to rituximab-mediated ADCC against CLL cells, αCD20-CAR redirected cytotoxicity was significantly higher. The reason for the superior killing effect by αCD20-CAR NK-92 is unknown, but it is possible that the CD3 ζ immunoreceptor tyrosine-based activation motif (ITAM) delivers a stronger activating signal than the FcR ITAM.
A surprising observation was that αCD19-CAR seems to be less expressed or less stable than αCD20-CAR, even though both are encoded by the same LV backbone, yet the former induced stronger killing than the latter. Even though we did not find any correlation between the percentage of antigen expression and killing efficiency, one could speculate that the targets used (either malignant cell lines or primary cells) generally express more CD19 antigen molecules on their surface than CD20 antigen, although conflicting data have been reported [36,37]. It is also possible that the CAR – antigen interaction is stronger in the case of CD19, leading to a more sustained activating signal in the effector or to a longer-lasting immune synapse between target and effector.
We had previously shown [21] that mRNA transfection of peripheral blood or cord blood – even after overnight activation with IL-2 – did not result in clinically meaningful transfection and was consistently < 10%. This is in contrast to LV-mediated transduction, which especially for cord blood-derived NK cells resulted in a median transduction efficiency of 30%. It may be possible to further improve the transduction efficiency by exposing NK cells to multiple rounds of transduction or by stimulating them with different cytokines [13]. In order to use LV-transduced cord blood NK cells for clinical applications their number has to be significantly increased, and investigators are testing different feeder layers and cytokine combinations to achieve this goal [29,38 – 40].
Since large-scale GMP-compatible electroporation devices are commercially available [27] and reportedly result in better transfection efficiency than the one used for this study, mRNA transfection of NK-92 cells with specific CARs could be an option rather than using peripheral blood or cord blood NK cells which only show minimal transfection efficiency. Generally transfection with mRNA may be preferable to LV transduction, as no virus integration into the genome occurs and hence the regulatory pathway to approval for clinical use is less restricted. The disadvantage, though, is that larger amounts of mRNA would be required for clinical applications and that multiple infusions would be necessary to make up for the transient expression of the CAR proteins.
In summary our observations confirm that activated NK cells that are initially unable to kill B-cell malignancies can become highly efficient killer cells for those targets (both primary CLL cells and cell lines) after transfection with specific CAR transgenes that encode receptors for CD19 or CD20. High transduction efficiency was observed with both methods tested (mRNA electroporation and LV transduction) for NK-92 cells, whereas “ meaningful “ (albeit variable) transduction of blood NK cells was only observed after LV transduction. These preclinical studies should assist in designing clinical trials with NK cells as effector cells genetically engineered to express lymphoid cell-specific CARs.
Acknowledgments
We thank Dr. Richard Childs for cloning the αCD19-CAR cDNA into the pCL20c vector and for advice on lentiviral transduction, and Allen Parmelee and Steve Kwok for their help with cell sorting. This work was supported by a grant from the National Heart, Lung and Blood Institute (R01HL093981).
Supported by R01HL093981 from the National Heart Lung and Blood Institute
References
- 1.Cheson BD. Monoclonal antibody therapy of chronic lymphocytic leukaemia. Best Pract Res Clin Haematol. 2010;23:133– 143. doi: 10.1016/j.beha.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Tsimberidou AM. Ofatumumab in the treatment of chronic lymphocytic leukemia. Drugs Today (Barc) 2010;46:451– 461. doi: 10.1358/dot.2010.46.7.1497416. [DOI] [PubMed] [Google Scholar]
- 3.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725– 733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376– 383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruschinski A, Moosmann A, Poschke I, et al. Engineering antigen specific primary human NK cells against HER-2 positive carcinomas. Proc Natl Acad Sci USA. 2008;105:17481– 17486. doi: 10.1073/pnas.0804788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uherek C, Tonn T, Uherek B, et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. 2002;100:1265– 1273. [PubMed] [Google Scholar]
- 7.Mapara MY, Sykes M. Tolerance and cancer: mechanisms of tumor evasion and strategies for breaking tolerance. J Clin Oncol. 2004;22:1136– 1151. doi: 10.1200/JCO.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 8.Verheyden S, Demanet C. NK cell receptors and their ligands in leukemia. Leukemia. 2008;22:249– 257. doi: 10.1038/sj.leu.2405040. [DOI] [PubMed] [Google Scholar]
- 9.Campbell KS, Purdy AK. Structure/function of human killer cell immunoglobulin-like receptors: lessons from polymorphisms, evolution, crystal structures and mutations. Immunology. 2011;132:315– 325. doi: 10.1111/j.1365-2567.2010.03398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051– 3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 11.Ruggeri L, Mancusi A, Capanni M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433– 440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spanholtz J, Preijers F, Tordoir M, et al. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS One. 2011;6:e20740. doi: 10.1371/journal.pone.0020740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Micucci F, Zingoni A, Piccoli M, et al. High-efficient lentiviral vector-mediated gene transfer into primary human NK cells. Exp Hematol. 2006;34:1344– 1352. doi: 10.1016/j.exphem.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–658. [PubMed] [Google Scholar]
- 15.Yan Y, Steinherz P, Klingemann HG, et al. Antileukemia activity of a natural killer cell line against human leukemias. Clin Cancer Res. 1998;4:2859– 2868. [PubMed] [Google Scholar]
- 16.Maki G, Klingemann HG, Martinson JA, et al. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res. 2001;10:369– 383. doi: 10.1089/152581601750288975. [DOI] [PubMed] [Google Scholar]
- 17.Tonn T, Becker S, Esser R, et al. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res. 2001;10:535– 544. doi: 10.1089/15258160152509145. [DOI] [PubMed] [Google Scholar]
- 18.Arai S, Meagher R, Swearingen M, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625– 632. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- 19.Romanski A, Bug G, Becker S, et al. Mechanisms of resistance to natural killer cell-mediated cytotoxicity in acute lymphoblastic leukemia. Exp Hematol. 2005;33:344– 352. doi: 10.1016/j.exphem.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Romanski A, Uherek C, Bug G, et al. Re-targeting of an NK cell line (NK92) with specificity for CD19 efficiently kills human B-precursor leukemia cells. Blood. 2004;104(Suppl 1):Abstract 2747. [Google Scholar]
- 21.Boissel L, Betancur M, Wels WS, et al. Transfection with mRNA for CD19 specific chimeric antigen receptor restores NK cell mediated killing of CLL cells. Leuk Res. 2009;33:1255– 1259. doi: 10.1016/j.leukres.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller T, Uherek C, Maki G, et al. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother. 2008;57:411– 423. doi: 10.1007/s00262-007-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Costa J, Mansfield SG, Humeau LM. Lentiviral vectors in clinical trials: current status. Curr Opin Mol Ther. 2009;11:554– 564. [PubMed] [Google Scholar]
- 24.Matrai J, Chuah MK, VandenDriessche T. Recent advances in lentiviral vector development and applications. Mol Ther. 2010;18:477– 490. doi: 10.1038/mt.2009.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346– 358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Liu LN, Feller S, et al. Expression of chimeric antigen receptors in natural killer cells with a regulatory-compliant non-viral method. Cancer Gene Ther. 2010;17:147– 154. doi: 10.1038/cgt.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tohda S, Sakashita C, Fukuda T, et al. Establishment of a double Philadelphia chromosome-positive acute lymphoblastic leukemia-derived cell line, TMD5: effects of cytokines and differentiation inducers on growth of the cells. Leuk Res. 1999;23:255– 261. doi: 10.1016/s0145-2126(98)00172-6. [DOI] [PubMed] [Google Scholar]
- 29.Boissel L, Tuncer HH, Betancur M, et al. Umbilical cord mesenchymal stem cells increase expansion of cord blood natural killer cells. Biol Blood Marrow Transplant. 2008;14:1031– 1038. doi: 10.1016/j.bbmt.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Weitzman J, Betancur M, Boissel L, et al. Variable contribution of monoclonal antibodies to ADCC in patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50:1361– 1368. doi: 10.1080/10428190903026500. [DOI] [PubMed] [Google Scholar]
- 31.Williams BA, Wang XH, Keating A. Clonogenic assays measure leukemia stem cell killing not detectable by chromium release and flow cytometric cytotoxicity assays. Cytotherapy. 2010;12:951– 960. doi: 10.3109/14653241003628167. [DOI] [PubMed] [Google Scholar]
- 32.Klingemann HG. Natural killer cell-based immunotherapeutic strategies. Cytotherapy. 2005;7:16– 22. doi: 10.1080/14653240510018000. [DOI] [PubMed] [Google Scholar]
- 33.Berry LJ, Moeller M, Darcy PK. Adoptive immunotherapy for cancer: the next generation of gene-engineered immune cells. Tissue Antigens. 2009;74:277– 289. doi: 10.1111/j.1399-0039.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 34.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2011;116:1035– 1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099– 4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Arena G, Dell’ Olio M, Musto P, et al. Morphologically typical and atypical B-cell chronic lymphocytic leukemias display a different pattern of surface antigenic density. Leuk Lymphoma. 2001;42:649– 654. doi: 10.3109/10428190109099325. [DOI] [PubMed] [Google Scholar]
- 37.Ginaldi L, De Martinis M, Matutes E, et al. Levels of expression of CD19 and CD20 in chronic B cell leukaemias. J Clin Pathol. 1998;51:364– 369. doi: 10.1136/jcp.51.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berg M, Lundqvist A, McCoy P, Jr, et al. Clinical-grade ex vivoexpanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy. 2009;11:341– 355. doi: 10.1080/14653240902807034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klingemann HG, Martinson J. Ex vivo expansion of natural killer cells for clinical applications. Cytotherapy. 2004;6:15– 22. doi: 10.1080/14653240310004548. [DOI] [PubMed] [Google Scholar]
- 40.Berg M, Childs R. Ex-vivo expansion of NK cells: what is the priority—high yield or high purity? Cytotherapy. 2010 doi: 10.3109/14653249.2010.536216. [DOI] [PubMed] [Google Scholar]