Abstract
Opioids, such as morphine, induce potent analgesia and are the gold standard for the treatment of acute pain. However, opioids also activate glia, inducing pro-inflammatory cytokine and chemokine production, which counter-regulates the analgesic properties of classical opioid receptor activation. It is not known how long these adverse pro-inflammatory effects last or whether prior morphine could sensitize the central nervous system (CNS) such that responses to a subsequent injury/inflammation would be exacerbated. Here, multiple models of inflammation or injury were induced 2 d after morphine (5 mg/kg b.i.d., 5 d, s.c.) to test the generality of morphine sensitization of later pain. Prior repeated morphine potentiated the duration of allodynia from peripheral inflammatory challenges (complete Freund’s adjuvant [CFA] into either hind paw skin or masseter muscle) and from peripheral neuropathy (mild chronic constriction injury [CCI] of the sciatic nerve). Spinal cord and trigeminal nucleus caudalis mRNAs were analyzed to identify whether repeated morphine was sufficient to alter CNS expression of pro-inflammatory response genes, measured 2 d after cessation of treatment. Prior morphine elevated IL-1β mRNA at both sites, MHCII and TLR4 in the trigeminal nucleus caudalis but not spinal cord, but not glial activation markers at either site. Finally, in order to identify whether morphine sensitized proinflammatory cytokine release, spinal cord was isolated 2 d after morphine dosing for 5 day, and slices stimulated ex vivo with lipopolysaccharide. The morphine significantly induced TNFα protein release. Therefore, repeated morphine is able to sensitize subsequent CNS responses to immune challenges.
Keywords: opioid, pain, pro-inflammatory cytokine, priming, glia, chronic constriction injury, interleukin-1beta, MHC-II, toll-like receptor 4, priming
1. Introduction
Opioids are the gold standard for treatment of severe acute and chronic pain. Opioids exert their potent analgesic properties via classical opioid receptors. However, opioids also induce a pro-inflammatory response within the central nervous system (CNS). The primary cell type initiating such pro-inflammatory responses is likely microglia, the predominant immunocompetent cell within the CNS. These cells have been implicated in chronic pain and dysregulating the effects of opioids (Hutchinson et al., 2007; Hutchinson et al., 2008a; Hutchinson et al., 2011; Watkins et al., 2007).
Activation of microglia by inflammation or injury can lead to the production of pro-inflammatory mediators (Watkins et al., 2007). Evidence is now emerging that activated microglia can either return to a homeostatic surveying state or become “primed” (sensitized) following the initial immune challenge. If these “primed” microglia are challenged within a certain period with a second immune challenge, the pro-inflammatory response is exacerbated. Most studies that have investigated glial priming have focused on the hippocampus (Frank et al., 2010a; Frank et al., 2010b; Frank et al., 2012; Jurgens and Johnson, 2012). However, microglial priming can also occur in the spinal cord resulting in heightened pain responses to later challenge (Alexander et al., 2009; Hains et al., 2010). Heightened pain responses following later inflammatory challenge from systemic or intrathecal lipopolysaccharide (LPS) are associated with enhanced proinflammatory cytokine levels in spinal cord and are blocked by intrathecal interleukin-1 receptor antagonist (Hains et al., 2011; Loram et al., 2011). Such examples are suggestive that sensitization, likely of microglial origin, can occur in the spinal cord, resulting in exaggerated pain following a subsequent challenge.
Morphine can also induce pro-inflammatory responses within the CNS, likely via the activation of microglia (Hutchinson et al., 2011). To date, most studies that have explored the pro-inflammatory responses to morphine have focused on acute effects soon after morphine administration. The blockade of IL-1 with IL-1 receptor antagonist in the spinal cord increased the intensity and duration of morphine-induced analgesia (Hutchinson et al., 2008a). In addition, repeated morphine enhances the pro-inflammatory response when measured within 2 h of the last dose of morphine (Hutchinson et al., 2008a). However, it is not known how long the morphine-induced pro-inflammatory response lasts or whether morphine exposure sensitizes CNS responses to subsequent peripheral injury/inflammation. If morphine induces, or sensitizes, long duration pro-inflammation, even after termination of morphine administration, this may potentially impact the development of pain to subsequent inflammatory/traumatic events..
Therefore, the aim of this study was to discover whether a multi-day morphine regimen is able to sensitize neuroinflammatory and/or pain responses to a later challenge. Here we explored whether such sensitized responses may occur in response to a variety of later challenges, including peripheral inflammation of both the hind paw and orofacial muscle and peripheral nerve injury.
2. Materials and Methods
2.1 Animals
Pathogen-free male Sprague-Dawley rats (300–350g, Harlan Laboratories, Madison, WI) were housed two per cage with temperature (23±0.3°C) and light (12:12 light:dark cycle; lights on at 07:00) controls. Rats had free access to water and standard chow and acclimated 1 wk before experimentation. All behavioral testing occurred during lights on. The Institutional Animal Care and Use Committee of the University of Colorado at Boulder approved all procedures. In all experiments, behavior and tissue were analyzed using blinded procedures.
2.2 Drugs
Morphine sulfate (gifted by Mallinckrodt, St. Louis, MO, USA) was prepared and reported as free base concentrations. Morphine was dissolved in endotoxin-free sterile saline at 5 mg/ml/kg. Morphine or saline was administered subcutaneously twice daily for 5 d at 08:00–10:00 and at 16:00–18:00. Five days of morphine administration was selected as it has been shown previously to enhance the pro-inflammatory response over acute morphine administration (Hutchinson et al., 2008a; Johnston et al., 2004). A two day recovery period was chosen to ensure morphine had ample time to clear from the circulation and allow for the return of behavioral responses back to pre-morphine values. Complete Freund’s adjuvant (CFA) was purchased from Sigma (F5881; Sigma-Aldrich, St. Louis, MO, USA) and 50 μl of heat killed Mycobacterium tuberculosis suspended in 50 μl of sterile saline to a 1:1 oil: saline emulsion was administered.
2.3 von Frey testing for mechanical allodynia
Hind paw
The von Frey test was performed on the plantar surface of each hind paw within the region of sciatic nerve innervation, as described previously (Milligan et al., 2000). Before testing, rats were habituated to the testing environment for 4 days, 40 min/day. A logarithmic series of 10 calibrated Semmes-Weinstein monofilaments (0.407 – 15.136 g, Stoelting, Wood Dale, IL, USA) was applied randomly to the hind paws, each for 8 s at constant pressure.
Orofacial
The von Frey test was performed on the face within the region of trigeminal nerve innervation, for both the V1 and V3 branches (Ren, 1999; Sugiyo et al., 2005). Before testing, rats were handled and habituated to remain still in a leather glove, unrestrained, while their nose rests between the tester’s thumb and index finger. A logarithmic series of 10 calibrated Semmes-Weinstein monofilaments (2 g – 65 g, Stoelting, Wood Dale, IL, USA) was applied for ~1 s, randomly to the cheek and above the eye. Each rat was tested three times upwards until three consecutive positive responses were identified.
The stimulus intensity threshold to elicit withdrawal responses (face or hind paw) was used to calculate the 50% paw withdrawal threshold (absolute threshold) using the maximum likelihood fit method to fit a Gaussian integral psychometric function (Harvey, 1986) and is described as allodynia or mechanical sensitivity throughout the text.
2.4 RNA isolation and cDNA synthesis
RNA was extracted using phenol: chloroform with TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s guidelines. Total RNA was reverse transcribed using Superscript II First-Strand Synthesis System (Invitrogen, Carlsbad, CA) (Loram et al., 2011). cDNA was diluted 2-fold in nuclease-free water and stored at −80°C until PCR was performed.
2.5 Real-time polymerase chain reaction (PCR)
Primer sequences (Genbank, National Center for Biotechnology Information; www.ncbi.nlm.nih.gov) are displayed in Table 1. cDNA amplification was performed using Quantitect SYBR Green PCR kit (Qiagen, Valenica, CA) in iCycler iQ 96-well PCR plates (Bio-Rad, Hercules, CA) on a MyiQ single Color Real-Time PCR Detection System (Bio-Rad). Each sample was measured in duplicate using the MyiQ single Color Real-Time PCR Detection System (Bio-Rad) (Loram et al., 2011). Threshold for detection of PCR product was set in the log-linear phase of amplification and the threshold cycle (CT) was determined for each reaction. The level of the target mRNA was quantified relative to the housekeeping gene (GAPDH) using the Δ Δ CT method. GAPDH was not significantly different between treatments.
Table 1.
Primer sequences
| Gene | Primer sequence (5′-3′) | GenBank accession No. |
|---|---|---|
| GAPDH | TCTTCCAGGAGCGAGATCCC (forward) TTCAGGTGAGCCCCAGCCTT (reverse) |
NC_005103.2 |
| TLR4 | TCCCTGCATAGAGGTACTTC (forward) CACACCTGGATAAATCCAGC (reverse) |
NM_019178.1 |
| IL-1β | CCTTGTGCAAGTGTCTGAAG (forward) GGGCTTGGAAGCAATCCTTA (reverse) |
NM_0315122.2 |
| MHC-II | AGCACTGGGAGTTTGAAGAG (forward) AAGCCATCACCTCCTGGTAT (reverse) |
NM_019111.4 |
| CD11b | CTGGTACATCGAGACTTCTC (forward) TTGGTCTCTGTCTGAGCCTT (reverse) |
NM_012711.1 |
| GFAP | AGATCCGAGAAACCAGCCTG (forward) CCTTAATGACCTCGCCATCC (reverse) |
NM_017009.2 |
| CCL2 | GTCTCAGCCAGATGCAGTTA (forward) CACAGATCTCTCTCTTGAGC (reverse) |
NM_031530 |
2.6 Enzyme linked immunoassay (ELISA)
Supernatants were collected and assayed for TNF-α using a rat multiplex ELISA (Aushon, CA, USA), according to manufacturer’s instructions. Chemiluminescence was quantified on a Signature PLUS CCD (Aushon, CA, USA) and analyzed using PRO array Analyst Software (Aushon).
2.7 Experimental designs
2.7.1 Effect of prior repeated morphine on CFA-induced hind paw allodynia
In order to identify whether repeated morphine can sensitize later spinally mediated pain responses, CFA or saline was administered into one plantar hind paw (s.c.) with the needle directed between the toes with the needle tip placed subcutaneously into the plantar surface of the foot so to avoid backleak of the injectate, 2 d after the last morphine dose, under brief isoflurane anesthesia. Mechanical thresholds were tested on the ipsilateral and contralateral hind paws before morphine, before CFA, 3 d after CFA and then weekly for 5 wk.
2.7.2 Effect of prior repeated morphine on sciatic injury-induced hind paw allodynia
Two days after the 5 d morphine/saline regimen, a modified chronic constriction injury (CCI) was performed to create mild sciatic nerve injury with subsequent mild allodynia (Grace et al., 2010). Briefly, rats were anesthetized with isoflurane, followed by one loose ligation of the sciatic nerve with sterile chromic gut suture (cuticular 4–0; Ethicon, Somerville, NJ, USA) at mid-thigh level of the left hind leg. Mechanical thresholds were tested on both hind paws before morphine, before CCI, 3, 9, 11 and 14 d, and then weekly after surgery to 5 wk.
2.7.3 Effect of prior repeated morphine on CFA-induced masseter muscle allodynia
To identify whether prior repeated morphine sensitizes orofacial/trigeminal pain, rats received intra-masseter muscle CFA (50 μl CFA in a 1:1 saline emulsion, 100 μl; 26 G needle) under brief isoflurane anesthesia, 2 d or 9 d after the last morphine/saline dose. To minimize animal use, control rats were not included in this study, as no effect was noted in response to morphine alone in the hind paw study. Facial testing occurred before morphine/saline, before CFA, 3 d after CFA and then weekly thereafter for 5 wk. Response thresholds to mechanical stimuli were tested on the ipsilateral side in both the region affected by the CFA (V3) and also in V1 (above the eye).
2.7.4 Effect of prior repeated morphine on pro-inflammatory gene expression in the spinal cord and nucleus caudalis
In order to determine if repeated morphine alters proinflammatory gene expression in the pain-relevant CNS sites after cessation of morphine exposure, mRNA in the spinal cord and nucleus caudalis was collected from a separate group of rats 2 d after cessation of 5 d morphine. Rats were deeply anesthetized with sodium pentobarbital and transcardially perfused with ice-cold saline. The spinal cord was dissected and snap frozen in liquid nitrogen. The brains were dissected out and frozen in isopentane. The hindbrain with attached cervical spinal cord was removed and sliced in a cryostat to reveal the Sp5C nucleus of the trigeminal ganglia. An 18 Gauge needle with a blunted tip kept on dry ice was used to bilaterally punch out the Sp5C nucleus of the trigeminal ganglia. The needle was pushed in ~1–2 mm, just enough so that the tissue filled the very end of the needle. The micropunch was placed directly from the needle into a 1.5 ml tube on dry ice. The tissue was then sliced through at 50 μm until the hole left by the punch was no longer visible. The process was repeated 1–2 more times until the interface between the Sp5C and Sp5I nuclei became apparent. The tissue was then processed for mRNA analysis. mRNA was selected over protein expression as in our hands we are unable to measure TNFα protein expression in CNS tissue.
2.7.5 Effect of prior repeated morphine on pro-inflammatory mediators induced by CFA into the masseter muscle
In a separate group of rats, rats were administered morphine/saline for 5 d, as above. Two days later, rats received either intra-masseter CFA or control procedure, as above. One wk later (9 d after the last morphine dose), rats were transcardially perfused with ice-cold saline. The brains were dissected and snap frozen. Brains were then sliced in a cryostat and the nucleus caudalis micropunched using an 18 G needle. Samples were collected in 2.5 ml Eppendorf tubes on dry ice and processed for mRNA.
2.7.6 Effect of prior repeated morphine on the pro-inflammatory response of spinal cord tissue stimulated with LPS ex vivo
Given the prominent upregulation of TLR4 in the trigeminal nucleus caudalis and a similar trend in the spinal cord (see results), an ex vivo assay was used (Hutchinson et al., 2008a; Johnson et al., 2004) to determine if prior morphine sensitized the spinal neuroinflammatory response to LPS, a classical TLR4 agonist. Two days after cessation of repeated systemic morphine or repeated systemic saline vehicle, rats were lightly anesthetized (isoflurane) and then decapitated. Spinal cords were dissected out and rinsed with 70% ethanol. Dorsal spinal cords were isolated and rinsed with sterile HBSS, and three 1.75 cm sections of the spinal cord were isolated. Tissue was placed, dorsal side up, in 25 μL of incubation medium (DMEM, supplemented with 2 mM L-glutamine, 100 U penicillin, 100 μg streptomycin and 10 mM HEPES buffer, Invitrogen, Carlsbad, CA, USA), inside a sterile modified 500 μl Eppendorf centrifuge tube. 200 μl media and 100 ng/ml LPS or equivolume vehicle were added and tissue incubated for 3 h at 37 °C, 5% CO2. Supernatants were collected and assayed for TNF-α protein levels.
2.8 Statistical analysis
Behavioral measures were normalized as above. Baseline values were compared between groups using a one-way ANOVA. In order to determine the effect of morphine on subsequent injury or inflammation a 2-way repeated measures ANOVA was used to compare morphine+injury vs vehicle+injury in each group with group and time as main effects. RT-PCR was analysed using t-tests. ELISA data was analyzed using a 2-way ANOVA with morphine and second immune challenge as the main effects. Bonferroni post-hoc tests were used where appropriate and P<0.05 was considered statistically significant.
3. Results
3.1 Prior repeated morphine does not potentiate hind paw allodynia induced by subcutaneous CFA
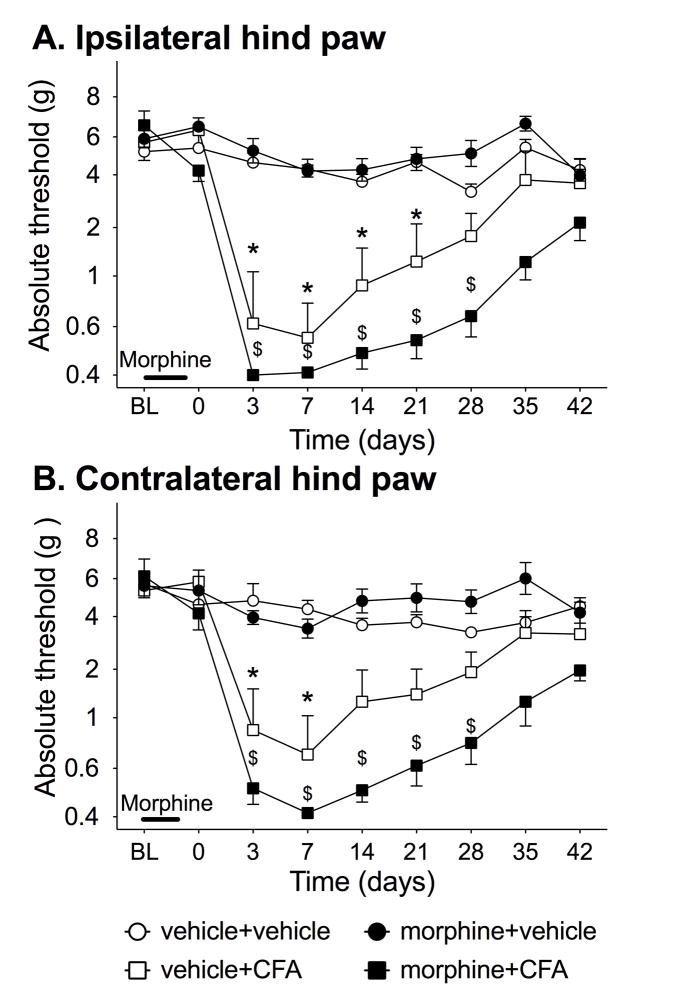
In order to determine if morphine is able to potentiate allodynia from peripheral inflammation, CFA was subcutaneously injected into one hind paw 2 d after the cessation of repeated morphine. Figure 1 shows the response threshold in the ipsilateral (panel A) and contralateral (panel B) hind paws before morphine or vehicle, before and after CFA or vehicle injections into the hind paw. There was no significant difference between groups at baseline or after morphine/saline administration (group effect ipsilateral: F3,20=0.81, P=0.50). There was significant interaction on the ipsilateral paw (time × group: F18,120=6.28, P<0.0001) and contralateral paw (time × group: F18,147=2.05, P<0.05). For the ipsilateral paw, morphine+CFA having greater allodynia compared to morphine alone or saline control groups from 3–35 d (P<0.05). Vehicle+CFA group had greater allodynia compared to morphine alone or saline control groups from 3–21 d (P<0.05). However, there was no significant difference between morphine+CFA and vehicle+CFA at any time point on either hind paw.
Figure 1. Chronic morphine pretreatment potentiates allodynia from hind paw inflammation.
Rats were dosed with morphine (solid) or vehicle (open) at 5mg/kg b.i.d. for 5 days. Two days after the conclusion of dosing, CFA (square) or saline (circle) was injected into the hind paw, and mechanical allodynia characterized in both the (A) ipsilateral and (B) contralateral paws. Significant potentiation of mechanical allodynia by morphine was observed in the ipsilateral and contralateral hind paw. BL denotes baseline pre morphine/saline dosing. Data presented as mean ± SEM (n=6/group). *P<0.05 between vehicle+CFA and saline+saline, $ P<0.05 between morphine+CFA and saline +saline for each time point.
3.2 Prior repeated morphine potentiates peripheral neuropathic allodynia
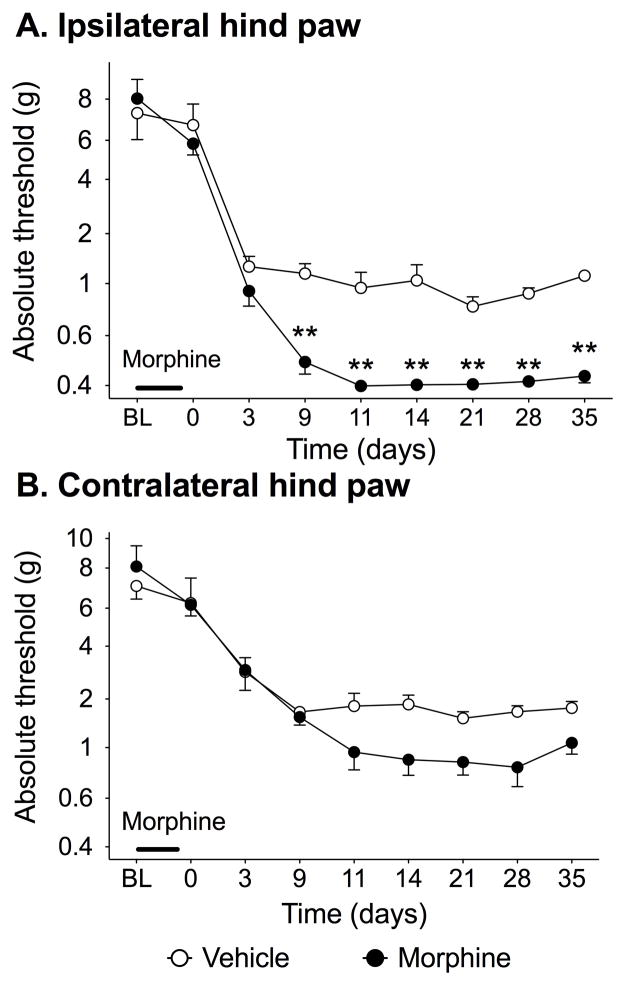
As documented above, prior repeated morphine may lead to a later exaggerated pain response to hindpaw inflammation. As mechanisms underlying inflammatory pain and neuropathic pain differ (Xu and Yaksh, 2011), prior repeated morphine was similarly tested to define whether it would exaggerate allodynia induced by peripheral nerve injury. Here, a mild sciatic CCI model was used to create mild allodynia so to allow observation of pain enhancement, were it to occur. There was significantly greater allodynia in the ipsilateral hind paw induced in rats receiving prior morphine compared to those given vehicle (F8,80 = 7.51 P < 0.0001) from 9–35 d (Fig. 2). There were no significant differences between morphine and vehicle groups at any time in the contralateral paw (F8,80 = 2.23 P > 0.05). Sham control groups were not done, as morphine alone did not affect hind paw response thresholds in the CFA experiment above.
Figure 2. Chronic morphine pretreatment potentiates allodynia resulting from peripheral nerve injury.
Rats were dosed with morphine or vehicle at 5mg/kg b.i.d. for 5 days. Two days after the conclusion of dosing, mild CCI was performed, in which 1 chromic gut suture was tied around the sciatic nerve, and mechanical allodynia characterized in both the (A) ipsilateral and (B) contralateral paws. Significant potentiation of mechanical allodynia was observed in the ipsilateral paw. BL denotes baseline pre morphine/saline dosing. Data presented as mean ± SEM (n=6/group). **P<0.05 compared to vehicle controls.
3.3 Prior repeated morphine potentiates orofacial allodynia induced by masseter inflammation
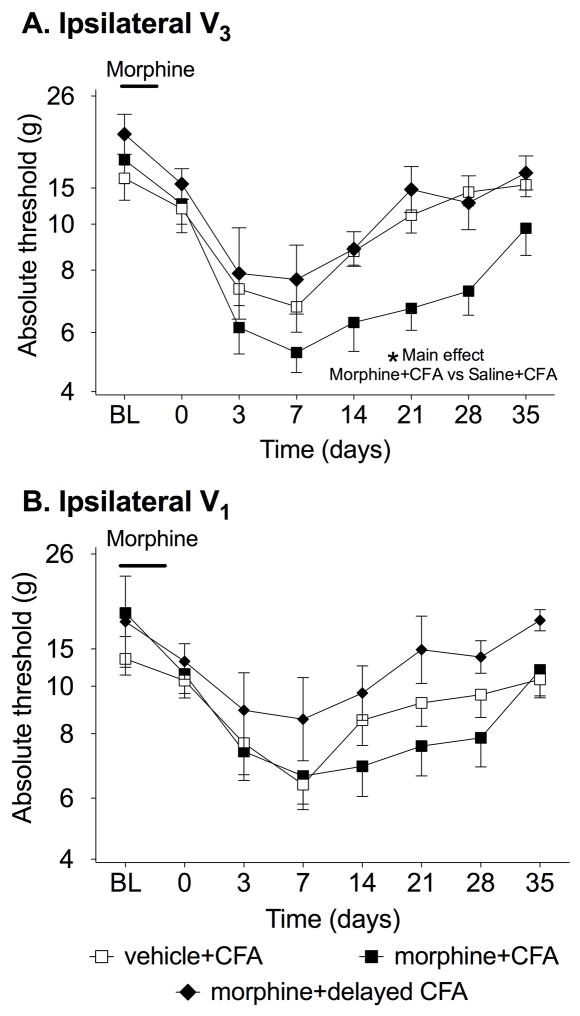
As documented above, prior repeated morphine led to a later exaggerated pain response to both hindpaw inflammation and injury of the hindlimb sciatic nerve. Thus the effect of morphine generalizes across two major types of spinally-mediated pain enhancement. However, it remains an open question whether such effects of prior repeated morphine also generalize to trigeminally-mediated pain. This is because mechanisms underlying spinally- and trigeminally-mediate pain enhancement differ (Bereiter et al., 2000; Chiang et al., 2011; Xu and Yaksh, 2011). Thus, prior repeated morphine was now followed by CFA injected into the masseter muscle so to induce orofacial allodynia. Again, control groups receiving vehicle instead of CFA were not included as experiment one shows no effect of morphine alone. Figure 3 shows the response thresholds to mechanical stimuli applied to the cheek (V3, panel A) and periorbital skin (V1, panel B) before and after repeated systemic saline followed by intra-masseter CFA(vehicle+CFA), and repeated systemic morphine followed by intra-masseter CFA either 2 d (morphine+CFA) or 9 d (morphine+delayed CFA) after cessation of repeated morphine. This morphine+delayed CFA group was included so to define whether pain enhancement was persistent across time. When comparing the three groups in the V3, there was a significant difference between groups (F2,10=18.46, P<0.0001) and a significant allodynia over time (F10,90=9.22, P<0.0001). The significant differences were identified between morphine+CFA and vehicle+CFA (P<0.01). There was no significant difference between delayed morphine+CFA and vehicle+CFA (P>0.05). In the V1 region, there was no significant difference between the three groups (F2,10=1.58, P=0.24). However a significant main effect of time was seen collapsing across groups (F10,80=11.15, P<0.0001). There was no significant interaction between drug and time at either site tested (Ipsilateral: F10,80=0.44, P=0.92, Contralateral: F10,80=0.79, P=0.64). There was also no significant difference between groups either before morphine or before CFA at either site tested (P>0.05).
Figure 3. Chronic morphine pretreatment potentiated allodynia induced by masseter muscle inflammation.
Response thresholds to mechanical stimulation applied on the ipsilateral cheek (V3, A), and above the eye (V1, B) were measured before (BL, baseline) and after morphine/saline and after CFA into the masseter muscle. Morphine was administered for 5 d b.i.d followed by 2 d or 9 d by CFA into the masseter muscle or control procedure. There was significantly greater allodynia in the morphine+CFA group (solid square) vs the saline +CFA (open square) in the V3 but not V1 regions when CFA was given 2 days after the last dose of morphine. In addition, when the CFA was delayed from 2 d to 9 d after the last dose of morphine, there was no significant effect of the morphine (solid diamond). BL denotes baseline pre morphine/saline dosing. Data presented as mean ± SEM (n=6/group) *P<0.05 main effect of morphine+CFA vs saline+CFA.
3.4 Prior repeated morphine enhances pro-inflammatory mediator mRNA in trigeminal and spinal sites in the absence of a second challenge
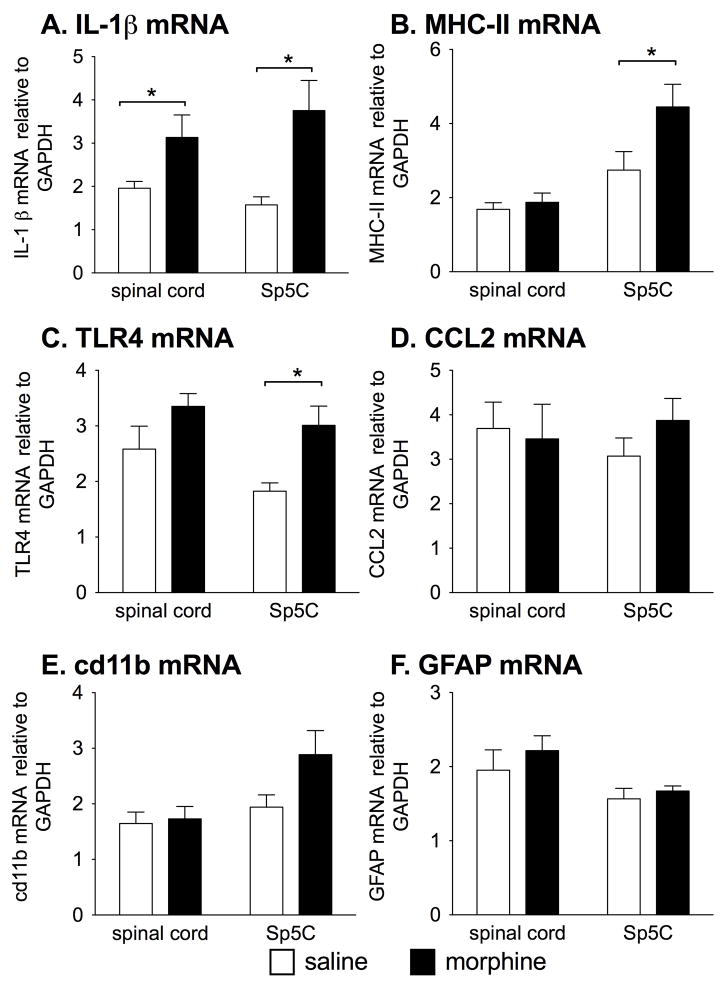
From the prior experiments we have demonstrated that allodynia arising from diverse models of injury or inflammation can be potentiated by 5 d of repeated morphine before inflammation/injury onset. However, it is unknown whether prior repeated morphine altered proinflammatory gene expression the spinal cord or nucleus caudalis (Sp5c) at the time that the later inflammation or nerve injury would occur. Therefore, 2 d after the last dose of morphine, when in the previous experiments the subsequent inflammation/injury was delivered, the lumbar spinal cord and nucleus caudalis (Sp5c) were collected for mRNA analysis. Interleukin-1β (IL-1β) was measured to test for a pro-inflammatory cytokine response. Major histocompatibility class II (MHC-II) and TLR4 mRNA were selected as they have been shown to be upregulated in states where glial “priming” has been identified (Frank et al., 2010b; Loram et al., 2011). Cd11b for microglial activation and glial fibrillary acidic protein (GFAP) for astrocyte activation were assessed. CCL2 was measured to identify whether any changes in the chemokine occurred suggestive of immune cell recruitment. In the trigeminal nucleus caudalis, there was a significant increase in IL-1β (t12=3.01, P<0.05), MHC-II (t14=2.16, P<0.05) and TLR4 (t14=3.15, P<0.01) mRNA in the morphine group compared to saline, whereas CCL2 (P=0.41), cd11b (P=0.07) and GFAP (P=0.51) mRNA showed no significant differences between groups (Fig. 4). For the lumbar spinal cord, there was a significant increase in spinal IL-1β mRNA in the morphine group compared to vehicle (t14=2.17, P<0.05) whereas MHC-II (P=0.55), toll-like receptor 4 (TLR4; P=0.13), CCL2 (P=0.66), cd11b (P=0.79) and GFAP (P=0.45) mRNA showed no significant differences between groups (Fig. 4).
Figure 4. Chronic morphine alters CNS pro-inflammatory markers 2 d after cessation of morphine.
Lumbar spinal cord and the nucleus caudalis (Sp5c) were isolated from rats that were dosed with morphine or vehicle at 5mg/kg b.i.d. for 5 days (n = 8/group, mean±SEM), 2 days after the conclusion of dosing. Significant effect of morphine *P < 0.05.
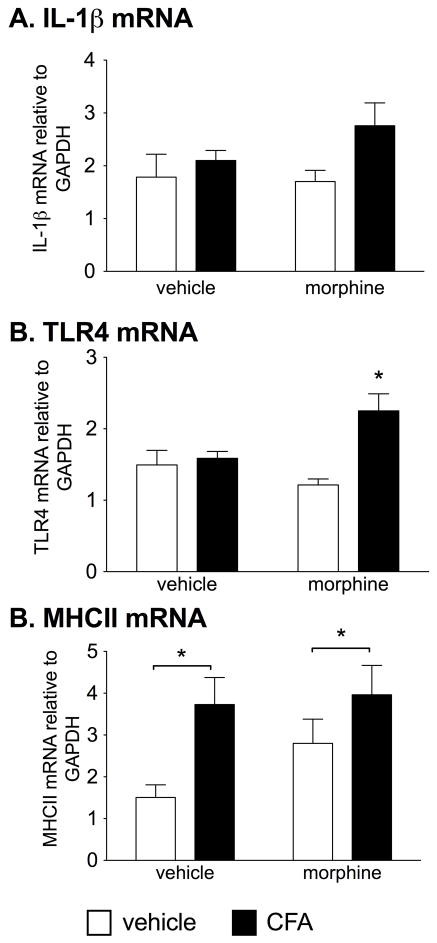
3.5 Prior repeated morphine enhances immune mediators within the ipsilateral nucleus caudalis 1 wk after CFA into the masseter muscle
In order to determine if the potentiated facial allodynia is associated with a potentiated pro-inflammatory response in the trigeminal nucleus as a consequence of prior repeated morphine, nucleus caudalis were collected 1 wk after CFA into the masseter vs. control procedure and analyzed for mRNA. This time point was chosen so to correspond to maximal expression of allodynia observed in Experiment 3. Figure 5 shows the IL-1β, TLR4 and MHC-II mRNA responses in the nucleus caudalis in all groups. Collapsing across prior morphine/saline treatment, there was a significant main effect of CFA with reliable elevations in IL-1β mRNA (F1,23=3.08, P<0.05) and MHC-II mRNA (F1,20=16.34, P<0.05). However, there was no effect of prior morphine (IL-1β: F1,1=0.54, P=0.38, MHC-II: F1,1=3.31, P=0.25). TLR4 mRNA showed a significant interaction (F1,24=1.50, P<0.01) with morphine+CFA showing the greatest elevation.
Figure 5. Chronic morphine potentiates pro-inflammatory and glial response in trigeminal nucleus caudalis subsequent to masseter muscle inflammation.
IL-1β, TLR4 and MHC-II mRNA in the nucleus caudalis 1 wk after CFA/control with or without prior morphine (5 d of 5 mg/kg b.i.d, s.c.). There was a significant elevation in IL-1β and MHC-II in the CFA groups irrespective of prior morphine. TLR4 mRNA was significantly increased in the morphine+CFA group. Data presented as mean ± SEM (n=6–8/group). *P<0.05 between groups where indicated. For TLR4 mRNA, *P<0.05 compared to all other groups.
3.6 Prior repeated morphine increases basal pro-inflammatory responses in spinal cord ex vivo
Acute administration of morphine can induce acute proinflammatory responses in the CNS (Hutchinson et al., 2008a). Here, the aim was to determine whether prior repeated morphine primed a later proinflammatory response by immune and/or glial cells resident in the spinal cord. LPS, a classical TLR4 agonist, was selected due to the prominent upregulation of TLR4 in the trigeminal nucleus caudalis and a similar trend in the spinal cord. The spinal cord was selected over the nucleus caudalis because of availability of tissue allowing for paired analysis of saline versus LPS ex vivo. Ex vivo LPS treatment was chosen instead of in vivo so to focus on spinal cord responses independent of peripheral immune responses to the later inflammatory challenge and to quantify accumulated TNF-α. TNFα was selected as the incubation time was 3 h and may not be sufficient time to induce release of other downstream pro-inflammatory cytokines (Cunha et al., 1992). Therefore, spinal cord sections were isolated from rats 2 d after repeated systemic morphine versus repeated saline vehicle treatment, and incubated for 3 h ex vivo with LPS versus media control, and the release of TNFα quantified (Table 2). Prior repeated morphine significantly increased later release of TNF-α (F1,32 = 8.03 P < 0.01). LPS significantly increased release of TNF-α (F1,32 = 10.68 P < 0.01). However, there was no significant interaction between morphine and LPS at the time point tested. Hence, prior repeated systemic morphine enhanced ex vivo tonic release of TNF-α from acutely isolated dorsal spinal cord as well as enhancing TNF-α release in response to ex vivo LPS.
Table 2.
Chronic morphine pretreatment in vivo increases TNFα release ex vivo in dorsal spinal cord
| Saline in vivo | Morphine in vivo | |
|---|---|---|
| Media | 47.89±15.05 | 71.78±8.73 |
| LPS | 77.70±13.26 | 130.84±16.15 |
Spinal cord sections were isolated from rats that were dosed with morphine or vehicle at 5mg/kg b.i.d. for 5 days (n = 6/group), 2 days after the conclusion of dosing. Tissue was incubated with LPS or media control for 3 h, and the supernatants collected for TNFα protein (mean ±SEM, pg/ml). There was a main effect of morphine (P<0.01) and a main effect of LPS (P<0.01). There were no significant interactions between morphine and LPS. Detection limits were 6.25–1600 pg/ml.
4. Discussion
The present series of studies demonstrate that 5 d of repeated morphine potentiates the duration of mechanical allodynia induced by either subcutaneous CFA inflammation of the hind paw or CFA-induced inflammation of the master muscle, when CFA was delivered 2 d (but not 9 d) after cessation of repeated morphine. Whether the same effect occurs with thermal hyperalgesia remains to be determined. The sensitization by morphine extended to neuropathic allodynia induced by mild sciatic nerve CCI, where nerve damage again occurred 2 d after cessation of repeated morphine. Repeated morphine was sufficient to increase IL-1β mRNA in the spinal cord and trigeminal nucleus caudalis after morphine cessation. MHC-II and TLR4 mRNA were significantly upregulated in trigeminal nucleus caudalis as well. IL-1β, MHC-II, and TLR4 each have established roles in central pain mechanisms (Grace et al., 2011; Milligan and Watkins, 2009; Nicotra et al., 2012). Lastly, ex vivo release of TNF-α protein was enhanced from dorsal spinal cord tissues acutely isolated from rats that had received repeated systemic morphine ending 2 d prior to tissue isolation. This enhanced TNF-α release was observed both basally (potentially stimulated by the acute tissue isolation) and in response to ex vivo LPS. The finding that prior repeated morphine trended toward enhanced TNFα release ex vivo (though no statistical interaction was observed) is consistent with a role of TNFα in pain enhancement. Whether prior repeated morphine enhances peripheral immune responses to inflammation and/or injury remains to be defined.
Prior studies reported that morphine is sufficient to induce IL-1β protein and mRNA within the spinal cord when measured within a few hours after both acute and chronic dosing (Hutchinson et al., 2008a; Johnson et al., 2004). This pro-inflammatory cytokine induction occurred when morphine was administered either systemically or directly over the spinal cord (Hutchinson et al., 2007; Hutchinson et al., 2008a; Muscoli et al., 2010; Tumati et al., 2012). Furthermore, blockade of IL-1β with IL-1 receptor antagonist has been shown to increase the magnitude and duration of morphine-induced analgesia (Hutchinson et al., 2008a). Extending this work, the present study is the first to show that pro-inflammatory cytokines, in this instance IL-1β mRNA in the spinal cord and trigeminal nucleus caudalis, as well as TNFα release from isolated spinal cord, remained elevated at least 2 days after the last dose of morphine. This sustained elevation of pro-inflammatory cytokine gene expression and protein release suggests that morphine induces a classically activated immune response within the spinal cord as well as the trigeminal nucleus caudalis. We did not test whether the biochemical changes caused the change in behavioral outcomes in this series of studies. However, previously studies have tested the causal relationship between pro-inflammatory mediators and behavioral responses after acute and chronic morphine (Hutchinson et al., 2008a). Therefore, it is likely that the same is true here also. Microglia and astrocytes are the major immunocompetent cells within the CNS and are the likely candidates mediating these effects, though future studies to investigate the specific cell types involved and the causal relationship between chemical mediators and behavior are required. They are major contributors of pro-inflammatory mediator release, which critically contributes to the development and maintenance of pain, including in the models studied here (Costigan et al., 2009; Watkins et al., 2007).
Morphine is able to induce a pro-inflammatory response in vitro, in addition to in vivo as noted above (Hutchinson et al., 2008a; Wang et al., 2012). This induction of pro-inflammatory cytokine responses by in vitro and in vivo morphine has now been identified to occur via signaling through the innate immune receptor, TLR4 (Hutchinson et al., 2008a; Wang et al., 2012). Activation of TLR4 by morphine elicits a pain-enhancing neuroinflammatory state, which counter-regulates acute and chronic morphine analgesia (Hutchinson et al., 2011). Thus, morphine has similarities with peripheral nerve injury that induces neuropathic pain in that each can enhance pain via signaling through TLR4 (Hutchinson et al., 2011; Nicotra et al., 2012). Since TLR4 is also expressed on a range of peripheral immune cells (Hennessy et al., 2010), the “sensitizing” effects of systemic morphine, described here, may not be limited to the glial cells. Activated peripheral immune cells may traffic to the CNS (Grace et al., 2011), or their pro-inflammatory products may directly peripherally sensitize nociceptors (Austin and Moalem-Taylor, 2010), vagal afferents or may be transported into the CNS (Dantzer et al., 2008). Finally, the diverse neuronal adaptations that follow repeated morphine exposure (e.g. mu-opioid down-regulation (Christie, 2008)) should be noted. However, such adaptations fail to explain the exacerbated behavioral and neurochemical response to the subsequent non-opioid stimuli examined in the present study.
The observation in the present study that prior repeated systemic morphine can upregulate TLR4 gene expression 2 days later extends the understanding of TLR4 gene regulation in contributing to chronic pain. Prior studies document upregulation of TLR4 mRNA in the dorsal spinal cord shortly (2 hours) after the last dose of repeated intrathecal morphine (Hutchinson et al., 2008a) as well as following exposure to the non-opioid TLR4 agonist (+)-methadone, which induces allodynia (Hutchinson et al., 2010). Further, upregulation of TLR4 mRNA and/or its co-receptors occurs in spinal cord in response to peripheral nerve injury that induces neuropathic pain, leading to its consideration as a glial activation marker (Cao et al., 2009; Hutchinson et al., 2008b; Tanga et al., 2005; Tanga et al., 2004). Upregulation of TLR4 mRNA (as well as allodynia) also occurs in response to low-dose intrathecal LPS, but only if this dose of LPS is preceded by stress levels of systemic glucocorticoids 24 hours prior (Loram et al., 2011). Notably, such studies contribute to the concept that upregulation of TLR4 and/or its co-receptors may be reflective of glial priming or activation (Li et al., 2009) that may contribute to pain enhancement. While TLR4 gene expression was only statistically significant in the trigeminal nucleus caudalis, a similar trend was observed in the spinal cord. Such differences may relate to the glial phenotype heterogeneity that exists between the spinal cord and trigeminal nucleus caudalis (Chiang et al., 2011).
If morphine induced a continued pro-inflammatory state 2 days after the cessation of dosing, one would have anticipated elevations in activation markers for microglia and/or astrocytes (Hutchinson et al., 2007; Song and Zhao, 2001). However, no elevation in either the microglial or astrocyte activation markers were noted at the time the tissue was collected. Therefore, it is possible that morphine can induce a classically activated, pro-inflammatory phenotype while morphine is present (Hanisch and Kettenmann, 2007), but may enduringly sensitize glial cells subsequent to pro-inflammation. In addition, the studies cannot exclude other anatomical regions involved in pain processing including higher order relays or descending pain pathways. Future studies to investigate causality and site involvement are needed.
Microglia that had previously been activated but are not yet fully resolved back to a quiescent, surveying state is reflective of a sensitized phenotype. Primed glial cells, prior to the second immune challenge, usually fail to exhibit elevated expression of glial activation markers (Frank et al., 2010b; Horvath et al., 2010). However, some studies have identified significant elevations in MHC-II in the microglial population (Frank et al., 2010a; Henry et al., 2009). Such elevated MHC-II expression has previously been reported in microglia of aged rats. Aging is described as a priming event with a new immune challenges inducing a significantly greater pro-inflammatory response compared to young rats (Barrientos et al., 2010; Frank et al., 2010a; Jurgens and Johnson, 2012). Aged rats exhibit a greater pro-inflammatory response to a TLR4 challenge both in vivo and upon ex vivo stimulation of their hippocampal microglia. The elevated MHC-II in the trigeminal nucleus caudalis following repeated morphine could occur in both pro-inflammatory, classically activated immune/glial cells and from immune/glial cells in a “primed” state. The lack of increased MHC-II in the spinal cord following morphine requires further investigation, but may relate to the glial phenotype heterogeneity that exists between the spinal cord and trigeminal nucleus caudalis (Chiang et al., 2011).
An additional feature of glial priming is reflected in behavioral responses. To date, studies of the impact of glial priming on later pain responses have all focused on mechanical allodynia as the behavioral response. In all of these reports, while the priming event (i.e., the initial challenge that induces a consequent primed glial state) may have been sufficient to transiently enhance pain responses, pain thresholds were at normal, basal levels prior to the second challenge used to create an enhanced pain state (Alexander et al., 2009; Hains et al., 2010; Hains et al., 2011; Loram et al., 2011). This was true in all the models tested in the present study, as well. Therefore, there appears to be an apparent contradiction in the results of the present study between elevated IL-1β mRNA in the trigeminal and spinal tissues and the absence of enhanced behavioral responses at the time point of tissue collection. Whether this elevated IL-1β mRNA occurs in the absence of elevations in IL-1β protein expression or release remains to be determined, but appears likely given the lack of observed allodynia at this time.
A more defining feature of sensitized glial cells is an exaggerated response to a subsequent challenge (Johnson et al., 2003; Johnson et al., 2002; Perry et al., 2002). In prior studies, such exaggerated responses were reflected in both neuroinflammation and allodynia. For example, prior glucocorticoids, at a dose equivalent to an acute stressor, potentiated spinally mediated neuroinflammation and allodynia induced by intrathecal LPS 24 hours later (Loram et al., 2011). Increased glucocorticoids are not the only priming event to potentiate spinally mediated allodynia and neuroinflammation. A number of different priming events and subsequent second challenges have been used to demonstrate glial priming within the spinal cord resulting in potentiated allodynia both in intensity and duration (Alexander et al., 2009; Hains et al., 2010). Both heightened glial activation and pain responses occurred following incisional pain induced in neonates exacerbating both glial reactivity and pain responses in adults (Beggs et al., 2012). The present series of studies extend these findings by demonstrating that both the duration and the intensity of later allodynia can be potentiated even when the pain-enhancing procedures occur 2 days after termination of repeated morphine.
The putative glial priming markers, identified in the present study, may provide insight into the mechanisms responsible for these exaggerated neuro-inflammatory phenomena. For example, as TLR4 signaling is a powerful initiator of the innate immune response, upregulation in a primed state is indicative of heightened immuno-surveillance, and the capacity for a rapid response to endogenous danger signals (Nicotra et al., 2012). In a similar fashion, upregulation of MHC-II is suggestive of an increased capacity for resident innate immune cells to present antigens to infiltrating T cells, likely causing rapid escalation of a pro-inflammatory environment via direct production of pro-inflammatory mediators and activation of further immune cell populations, such as astrocytes (Grace et al., 2011). It is intriguing that CFA alone was able to induce an upregulation of MHC-II in the nucleus caudalis, but both morphine and CFA were required to sustain an upregulation of TLR4 mRNA in the nucleus caudalis one week after the CFA induction. Whether sustained TLR4 could be used as a marker for chronic pain remains to be determined.
The present study showed differential effects of glial priming on contralateral allodynia. Prior morphine did not significantly potentiate contralateral allodynia induced by mild CCI, in contrast to the significant bilateral allodynia induced by CFA. It has been previously shown that contralateral allodynia is dependent on the degree of inflammation (Chacur et al., 2001). Hence, the discrepancy between models may be due to lower magnitude inflammation induced by mild CCI, compared to that induced by CFA.
Whether prior morphine sensitizes CNS pain-relevant sites with shorter dosing regimens is worthy of further investigation. The aim of this study was to investigate whether prior daily, repeated morphine could potentiate a subsequent pain response. Morphine, administered in the presence of allodynia also exacerbates pre-existing pain, such as that seen following spinal cord injury (Hook et al., 2007; Hook et al., 2009). Morphine is able to activate a pro-inflammatory response following acute morphine administration (Hutchinson et al., 2008a), acutely after repeated administration (Johnston et al., 2004) and potentiate allodynia induced by a second injury induced 2 days subsequent to termination of morphine dosing. Whether the immune cells within the CNS remain activated or resolve to a primed state is worthy of further investigation. Regardless of the glial phenotype, prior morphine potentiates allodynia from a subsequent injury or inflammation.
Research Highlight.
Morphine dosing for 5 days potentiates subsequent neuroinflammation and mechanical allodynia from hindpaw inflammation, hindlimb neuropathy and orofacial inflammation.
Acknowledgments
Financial support for these studies was provided by NIH grants DA024044, DE107782 and DA023132.
Footnotes
Conflict of interest statement: All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander JK, DeVries AC, Kigerl KA, Dahlman JM, Popovich PG. Stress exacerbates neuropathic pain via glucocorticoid and NMDA receptor activation. Brain Behav Immun. 2009;23:851–860. doi: 10.1016/j.bbi.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229:26–50. doi: 10.1016/j.jneuroim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Watkins LR, Maier SF. Memory impairments in healthy aging: Role of aging-induced microglial sensitization. Aging and disease. 2010;1:212–231. [PMC free article] [PubMed] [Google Scholar]
- Beggs S, Currie G, Salter MW, Fitzgerald M, Walker SM. Priming of adult pain responses by neonatal pain experience: maintenance by central neuroimmune activity. Brain. 2012;135:404–417. doi: 10.1093/brain/awr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter DA, Hirata H, Hu JW. Trigeminal subnucleus caudalis: beyond homologies with the spinal dorsal horn. Pain. 2000;88:221–224. doi: 10.1016/S0304-3959(00)00434-6. [DOI] [PubMed] [Google Scholar]
- Cao L, Tanga FY, Deleo JA. The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain. Neuroscience. 2009;158:896–903. doi: 10.1016/j.neuroscience.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–244. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- Chiang CY, Dostrovsky JO, Iwata K, Sessle BJ. Role of glia in orofacial pain. Neuroscientist. 2011;17:303–320. doi: 10.1177/1073858410386801. [DOI] [PubMed] [Google Scholar]
- Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154:384–396. doi: 10.1038/bjp.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Ann Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J Neuroimmunol. 2010a;226:181–184. doi: 10.1016/j.jneuroim.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010b;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun. 2012;26:337–345. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Hutchinson MR, Manavis J, Somogyi AA, Rolan PE. A novel animal model of graded neuropathic pain: utility to investigate mechanisms of population heterogeneity. J Neurosci Methods. 2010;193:47–53. doi: 10.1016/j.jneumeth.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Grace PM, Rolan PE, Hutchinson MR. Peripheral immune contributions to the maintenance of central glial activation underlying neuropathic pain. Brain Behav Immun. 2011;25:1322–1332. doi: 10.1016/j.bbi.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Hains L, Loram L, Weiseler J, Frank M, Bloss E, Sholar P, Taylor P, Harrison J, Martin T, Eisenach J, Maier S, Watkins L. Pain intensity and duration can be enhanced by prior challenge: Initial evidence suggestive of a role of microglial priming. J Pain. 2010;11:1004–1014. doi: 10.1016/j.jpain.2010.01.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains LE, Loram LC, Taylor FR, Strand KA, Wieseler JL, Barrientos RM, Young JJ, Frank MG, Sobesky J, Martin TJ, Eisenach JC, Maier SF, Johnson JD, Fleshner M, Watkins LR. Prior laparotomy or corticosterone potentiates lipopolysaccharide-induced fever and sickness behaviors. J Neuroimmunol. 2011;239:53–60. doi: 10.1016/j.jneuroim.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nature Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Harvey LO. Efficient estimation of sensory thresholds. Behav Res Methods Instrum Comput. 1986;18:623–632. [Google Scholar]
- Hennessy EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook MA, Liu GT, Washburn SN, Ferguson AR, Bopp AC, Huie JR, Grau JW. The impact of morphine after a spinal cord injury. Behav Brain Res. 2007;179:281–293. doi: 10.1016/j.bbr.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook MA, Moreno G, Woller S, Puga D, Hoy K, Jr, Balden R, Grau JW. Intrathecal morphine attenuates recovery of function after a spinal cord injury. Journal of neurotrauma. 2009;26:741–752. doi: 10.1089/neu.2008.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath RJ, Landry RP, Romero-Sandoval EA, DeLeo JA. Morphine tolerance attenuates the resolution of postoperative pain and enhances spinal microglial p38 and extracellular receptor kinase phosphorylation. Neuroscience. 2010;169:843–854. doi: 10.1016/j.neuroscience.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. TheScientificWorldJournal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008a;22:1178–1189. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Rezvani N, Zhang Y, Wieseler JL, Somogyi AA, Yin H, Maier SF, Rice KC, Watkins LR. Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience. 2010;167:880–893. doi: 10.1016/j.neuroscience.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacological reviews. 2011;63:772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008b;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, O’Connor K, Hansen M, Watkins L, Maier S. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol. 2003;284:R422–432. doi: 10.1152/ajpregu.00230.2002. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Watkins LR, Maier SF. The role of IL-1? in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127:569–577. doi: 10.1016/j.neuroscience.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:9353–9365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens HA, Johnson RW. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Experimental neurology. 2012;233:40–48. doi: 10.1016/j.expneurol.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Gao G, Guo Q, Jia D, Wang J, Wang X, He S, Liang Q. Function and phenotype of microglia are determined by toll-like receptor 2/toll-like receptor 4 activation sequence. DNA and cell biology. 2009;28:493–499. doi: 10.1089/dna.2009.0856. [DOI] [PubMed] [Google Scholar]
- Loram LC, Taylor FR, Strand KA, Frank MG, Sholar P, Harrison JA, Maier SF, Watkins LR. Prior exposure to glucocorticoids potentiates lipopolysaccharide induced mechanical allodynia and spinal neuroinflammation. Brain Behav Immun. 2011;25:1408–1415. doi: 10.1016/j.bbi.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscoli C, Doyle T, Dagostino C, Bryant L, Chen Z, Watkins LR, Ryerse J, Bieberich E, Neumman W, Salvemini D. Counter-regulation of opioid analgesia by glial-derived bioactive sphingolipids. J Neurosci. 2010;30:15400–15408. doi: 10.1523/JNEUROSCI.2391-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Experimental neurology. 2012;234:316–329. doi: 10.1016/j.expneurol.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V, Cunningham C, Boche D. Atypical inflammation in the central nervous system in prion disease. Curr Opin Neurol. 2002;15:349–354. doi: 10.1097/00019052-200206000-00020. [DOI] [PubMed] [Google Scholar]
- Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav. 1999;67:711–716. doi: 10.1016/s0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]
- Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neuroscience research. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- Sugiyo S, Takemura M, Dubner R, Ren K. Trigeminal transition zone/rostral ventromedial medulla connections and facilitation of orofacial hyperalgesia after masseter inflammation in rats. The Journal of comparative neurology. 2005;493:510–523. doi: 10.1002/cne.20797. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Tumati S, Largent-Milnes TM, Keresztes A, Ren J, Roeske WR, Vanderah TW, Varga EV. Repeated morphine treatment-mediated hyperalgesia, allodynia and spinal glial activation are blocked by co-administration of a selective cannabinoid receptor type-2 agonist. J Neuroimmunol. 2012;244:23–31. doi: 10.1016/j.jneuroim.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Loram L, Ramos K, de Jesus A, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson M, Watkins L, Yin H. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1200130109. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Yaksh TL. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Current opinion in anaesthesiology. 2011;24:400–407. doi: 10.1097/ACO.0b013e32834871df. [DOI] [PMC free article] [PubMed] [Google Scholar]