Abstract
Objective: To investigate the significance of intra-abdominal fat area (IAFA) on new onset of individual components of the metabolic syndrome: high blood pressure, dyslipidemia, or hyperglycemia. Methods: We conducted a longitudinal study using checkup data of a hospital from 1994 to 2010. Of 25,255 subjects, we examined 1,380 Japanese, who underwent computed tomography to measure IAFA and had no metabolic syndrome components at baseline. Results: During 3.6 years of the mean follow-up period, one of metabolic syndrome components occurred in 752 subjects. Of three components, high blood pressure was more prevalent. The multiple Cox regression analysis disclosed that IAFA is significantly associated with onset of metabolic syndrome components (HR: 1.05 per 10 cm2, 95%CI: 1.03–1.07). This finding was independent of BMI, and significant even in non-obese individuals with body mass index <25 kg/m2. Conclusions: MERLOT study demonstrates that IAFA is an independent predictor for new onset of individual components of the metabolic syndrome, even in non-obese healthy Japanese.
Keywords: intra-abdominal fat area, abdominal obesity, metabolic syndrome, cohort study
Introduction
Metabolic syndrome is a cluster of abdominal obesity and metabolic abnormalities including high blood pressure, dyslipidemia, and hyperglycemia.1) This constellation of metabolic disturbances is associated with an increased risk of cardiovascular mortality,2) and the increase in risk begins with the presence of just one metabolic syndrome component.3)
Although there are several definitions for the metabolic syndrome, abdominal or visceral obesity is an essential element in Japanese definition.1) Evidence accumulated indicates that intra-abdominal fat area (IAFA) measured by computed tomography (CT) is the most accurate parameter for assessing abdominal obesity.4) Several cross-sectional analyses suggest a possible association of higher amounts of IAFA with increased prevalence of metabolic syndrome,5,6) even in normal weight men and women.7) To date, however, there are no longitudinal studies to assess the association between IAFA measured by CT and the new onset of one metabolic syndrome component in the population without any metabolic syndrome components. Thus, it is unclear whether abdominal obesity precedes the onset of one of these metabolic syndrome components in Japanese healthy population.
The objective of the present study (MEtabolic syndRome and abdominaL ObesiTy: MERLOT) was to investigate the significance of IAFA measured by CT reflecting abdominal obesity on the new onset of metabolic syndrome components in Japanese. We also studied the role of IAFA as a predictor for the new onset of metabolic syndrome components in non-obese individuals with body mass index (BMI) <25 kg/m2.
Materials and methods
MERLOT study is a single-center, hospital-based non-concurrent prospective cohort study designed to investigate the significance of abdominal obesity on components of the metabolic syndrome, high blood pressure, dyslipidemia, and hyperglycemia.1)
The study included 25,255 subjects, aged 21 and 70 years old, from employees of telecommunication company, NTT West who lived in Kinki area of Japan. The cohort members underwent a corporate subsidized general health check program annually offered to the employees at Health Administration Center, NTT West Kyoto Hospital (Kyoto, Japan) from 1994 to 2010. MERLOT study included a part of subjects who participated in MONK study that we conducted.5)
The checkups were included consulting with physicians, physical examinations and a set of biochemical analyses of blood samples. All subjects voluntarily chose to be examined by CT for the IAFA and subcutaneous fat area (SFA). Registered nurses or physicians recorded information on current medication use and lifestyle using standard questionnaires, from which we identified those taking antihypertensive, lipid-regulating, or glucose-lowering drugs, and smoking status. An average blood pressure was calculated from two consecutive measurements over two days in a sitting position. IAFA and SFA were examined at an umbilical level in the supine position using CT, and were determined using a commercial software (Fat Scan, N2 System, Osaka, Japan). The details of measurements have been described previously.5)
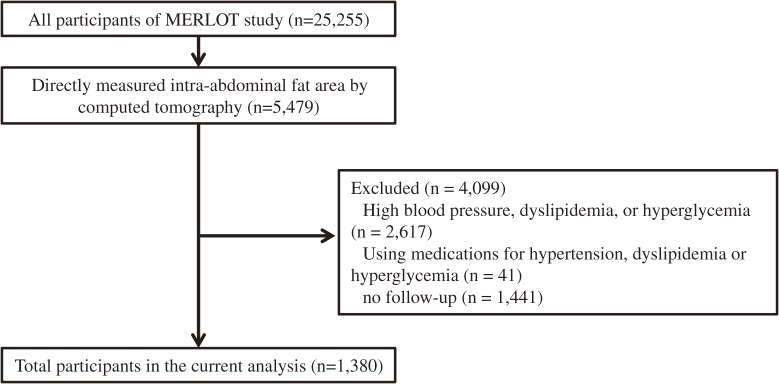
We used data from MERLOT study to assess the new onset of metabolic syndrome components in association with IAFA, as well as potential role of IAFA in non-obese individuals with BMI <25 kg/m2. Of the 25,255 cohort members, 5,479 subjects underwent the measurement of IAFA by CT (Fig. 1). We excluded 4,099 subjects with high blood pressure (blood pressure ≥130/85 mmHg); dyslipidemia (triglycerides ≥150 mg/dl or HDL-cholesterol <40 mg/dl); hyperglycemia (fasting plasma glucose ≥110 mg/dl); medications including antihypertensive, lipid-regulating, or glucose lowering agents; no follow-up. Data from the remaining 1,380 subjects (1,053 men and 327 women) who had no metabolic syndrome components at baseline were analyzed in the present study.
Figure 1.
Study flow chart.
Our endpoint was the new onset of one of three metabolic syndrome components with the definition by the Examination Committee of Criteria for the Metabolic Syndrome in Japan (blood pressure ≥130/85 mmHg; dyslipidemia, triglycerides ≥150 mg/dl or HDL-cholesterol <40 mg/dl; fasting plasma glucose ≥110 mg/dl; new medications for hypertension, dyslipidemia, or hyperglycemia).1) The subjects without event were censored at last clinical follow-up.
MERLOT study was approved by the ethics committee of Kyoto University Graduate School of Medicine and NTT West Kyoto Hospital (E1159).
Statistical analysis.
We assessed IAFA, SFA, and BMI in relation to the endpoint. We generated Kaplan-Meier curves for quartiles of each obesity parameter. We used Cox proportional hazard regression models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Multivariate models were adjusted for age, gender, smoking status, systolic blood pressure, triglyceride, HDL-cholesterol, hemoglobin A1c (HbA1c), BMI, and SFA. We calculated the prediction from a linear regression of relative hazard on IAFA and showed the resulting line along with a 95%CI by BMI categories. A potential effect modification by BMI categories (BMI <25 or ≥25 kg/m2) was evaluated by testing a statistical significance of multiplicative interaction terms in models.8) We used multiple imputation in the validation cohort to replace missing values for smoking status. All statistical analyses were carried out with STATA (version 12.1, STATA, College Station, TX, USA).
Results
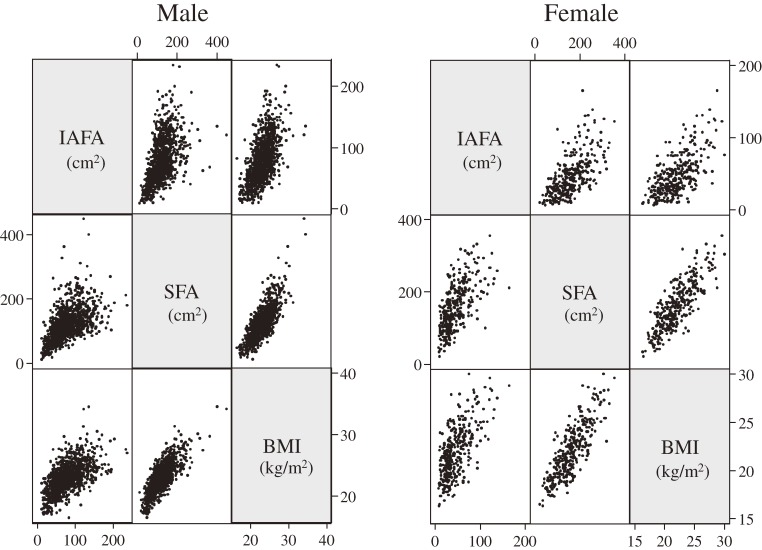
Table 1 showed characteristics of 1,380 subjects (1,053 men and 327 women) at baseline. The mean age was 47.3 ± 7.4 years ranged from 23 to 64 years. The mean BMI was 22.8 ± 2.5 kg/m2 and mean IAFA was 70.6 ± 36.5 cm2. Of the 1,380 subjects, 243 (17.6%) were BMI ≥25 kg/m2. Figure 2 showed the correlation matrix among IAFA, SFA, and BMI. All parameters showed good correlations (p < 0.001), although the range of IAFA levels varied widely among subjects with the same BMI.
Table 1.
Baseline characteristics
| All (n = 1,380) | Male (n = 1,053) | Female (n = 327) | |
|---|---|---|---|
| Age (yr) | 47.3 ± 7.4 | 46.9 ± 7.7 | 48.6 ± 6.3 |
| BMI (kg/m2) | 22.8 ± 2.5 | 23.0 ± 2.4 | 22.0 ± 2.7 |
| IAFA (cm2) | 71.6 ± 36.5 | 79.5 ± 35.5 | 46.4 ± 27.3 |
| SFA (cm2) | 132.0 ± 55.3 | 123.4 ± 49.2 | 159.7 ± 64.3 |
| Obesity (BMI ≥25 kg/m2) | 243 (17.6%) | 196 (18.6%) | 47 (14.4%) |
| Systolic blood pressure (mmHg) | 115.3 ± 8.9 | 116.3 ± 8.6 | 112.0 ± 9.2 |
| Diastolic blood pressure (mmHg) | 72.2 ± 6.4 | 73.1 ± 6.1 | 69.1 ± 6.5 |
| HDL-cholesterol (mg/dl) | 61.0 ± 13.3 | 59.2 ± 12.8 | 66.7 ± 13.3 |
| Triglycerides (mg/dl) | 85.3 ± 29.6 | 89.3 ± 29.4 | 72.6 ± 26.6 |
| Fasting plasma glucose (mg/dl) | 97.1 ± 6.4 | 97.9 ± 6.1 | 94.7 ± 6.5 |
| HbA1c (NGSP, %) | 5.4 ± 0.3 | 5.4 ± 0.3 | 5.4 ± 0.3 |
| Past or current smoker | 50.8% | 62.1% | 17.0% |
BMI: body mass index; IAFA: intra-abdominal fat area; SFA: subcutaneous fat area; NGSP: National Glycohemoglobin Standardization Program.
Figure 2.
Correlation matrix. The Pearson's correlation coefficient between IAFA and SFA (r = 0.536 in male, r = 0.629 in female); IAFA and BMI (r = 0.600 in male, r = 0.658 in female); SFA and BMI (r = 0.792 in male, r = 0.825 in female). IAFA: intra-abdominal fat area; SFA: subcutaneous fat area; BMI: body mass index.
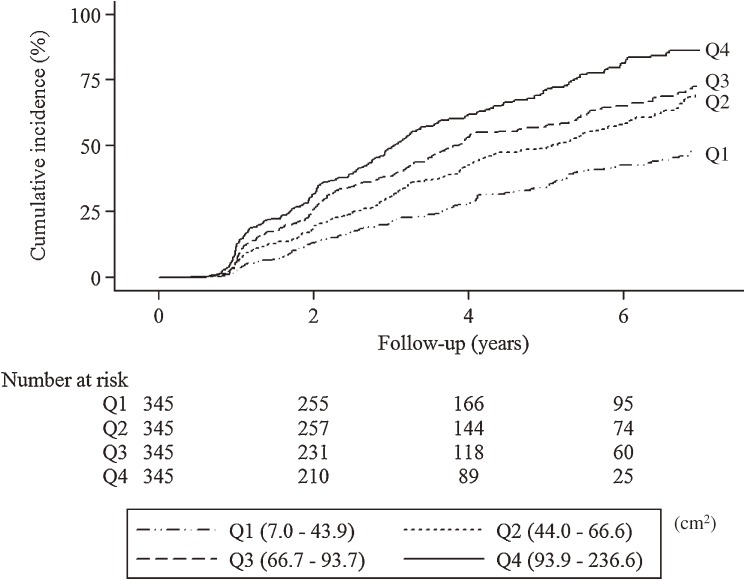
During 3.6 years of the mean follow-up period (maximum 9.7 years), one of metabolic syndrome components occurred in 752 subjects (54.5%) which consisted of 615 men and 137 women. These were 58.4% of men and 41.9% of women, respectively. Of three metabolic syndrome components, high blood pressure was more prevalent (395/615 [64.2%] in men, 79/137 [57.7%] in women) than dyslipidemia (332/615 [54.0%] in men, 59/137 [43.1%] in women) and hyperglycemia (144/615 [23.4%] in men, 36/137 [26.3%] in women). The Kaplan–Meier curves showed the unadjusted incident rate increased in a stepwise fashion across increasing quartiles (Fig. 3 ; Log-rank test, p < 0.001; p for trend, p < 0.001). This pattern of an increased incidence according to quartiles remained significant for other obesity parameters including BMI and SFA, although quartiles 2–4 of BMI and quartiles 2–4 of SFA were nearly overlapping (Fig. 1A in the Supplementary Appendix at http://japanlinkcenter.org/DN/JST.JSTAGE/pjab/88.454).
Figure 3.
Kaplan-Meier curves for new onset of components of metabolic syndrome according to intra-abdominal fat area.
Multivariate Cox proportional hazards analysis for the endpoint showed that IAFA, SFA and BMI were significant predictors after adjusting for age, gender (Table 2 –Model 1). After adjusting for age, gender, and baseline other factors (Model 2), HRs were reduced substantially, but broadly similar (IAFA, HR 1.05 per 10 cm2, 95%CI 1.03–1.07; SFA, HR 1.02 per 10 cm2, 95%CI 1.01–1.04; BMI, HR 1.05 per 1 kg/m2, 95%CI 1.02–1.08). After further adjusting for BMI, IAFA remained an independent predictor for the new onset of metabolic syndrome components (HR 1.04, 95%CI 1.02–1.07; Table 2–Model 3), but SFA did not (HR 1.01, 95%CI 0.99–1.04). In SFA-adjusted model and multivariate model including SFA and BMI, IAFA was still an independent predictor of the endpoint.
Table 2.
Hazard ratios for incidence of metabolic syndrome components according to baseline obesity parameters
| IAFA, per 10 cm2 | SFA, per 10 cm2 | BMI, per 1 kg/m2 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Model 1: Age and gender-adjusted model. | 1.09 (1.07–1.11) | <0.001 | 1.04 (1.03–1.06) | <0.001 | 1.11 (1.08–1.14) | <0.001 |
| Model 2: Multivariate model. (Model 1 + other factors*) | 1.05 (1.03–1.07) | <0.001 | 1.02 (1.01–1.04) | 0.003 | 1.05 (1.02–1.08) | 0.003 |
| Model 3: BMI-adjusted model. (Model 2 + BMI) | 1.04 (1.01–1.07) | 0.003 | 1.01 (0.99–1.04) | 0.275 | — | — |
| Model 4: SFA-adjusted model. (Model 2 + SFA) | 1.04 (1.01–1.07) | 0.002 | — | — | 1.03 (0.98–1.08) | 0.318 |
| Model 5: Multivariate model 2. (Model 2 + BMI + SFA) | 1.04 (1.01–1.07) | 0.004 | — | — | — | — |
HR: hazard ratio; CI: confidence interval; BMI: body mass index; IAFA: intra-abdominal fat area; SFA: subcutaneous fat area. * Smoking status, systolic blood pressure, log-triglyceride, HDL-cholesterol, and HbA1c.
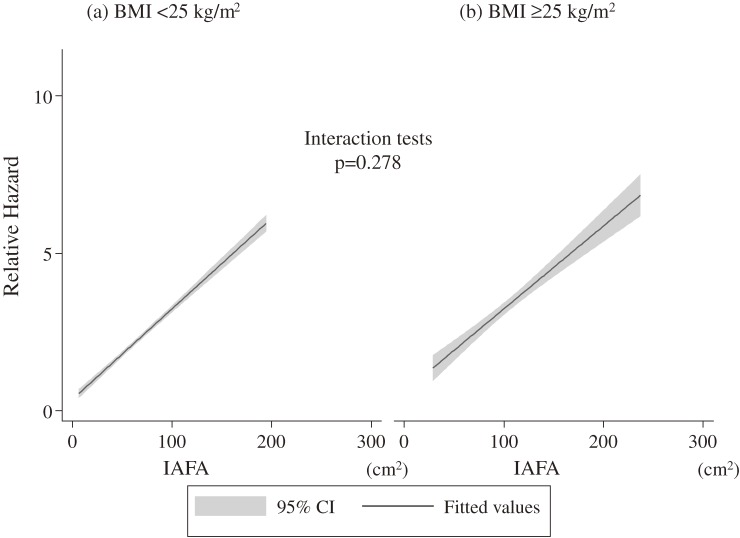
In subgroup analyses, we documented 159 cases of the endpoint in obese subjects (BMI ≥25 kg/m2), while 593 cases in non-obese (BMI <25 kg/m2). In the multiple Cox regression analysis, obese and non-obese subjects showed the same HRs for the endpoint (HR, 1.05; 95% CI, 1.03–1.09 in non-obese: HR 1.05; 95% CI, 1.00–1.10 in obese). There was no significant difference in test for interaction between obese (BMI ≥25 kg/m2) or non-obese (BMI <25 kg/m2) and IAFA in predicting the endpoint (p = 0.278; Fig. 4).
Figure 4.
Relative hazard and intra-abdominal fat area. Relative hazards were calculated on the basis of female gender and baseline data including mean intra-abdominal fat area, mean systolic blood pressure, mean triglyceride, mean HDL-cholesterol, and mean HbA1c in subjects with lower quartile of intra-abdominal fat area. IAFA: intra-abdominal fat area; BMI: body mass index; CI: confidence interval.
Discussion
MERLOT study is the first report of a large-number, long-term follow-up longitudinal analysis on clinical significance of IAFA measured by CT in the metabolic syndrome. MERLOT study demonstrates that IAFA is an independent predictor for the new onset of individual components of the metabolic syndrome in Japanese. In addition, the present study also demonstrates that the finding is significant even in non-obese individuals with BMI <25 kg/m2.
Our findings are in line with previous cross-sectional studies in Japanese that examine the relationship between IAFA measured by CT and metabolic syndrome components.5,6,9,10) Longitudinal studies from a small number of (n = 300–457) Japanese Americans included patients with high blood pressure, dyslipidemia, and high blood glucose showed that greater abdominal adiposity increased the risk of hypertension,11) insulin resistance,12) and coronary heart disease.13) While a substudy from the Diabetes Prevention Program for averaged 3.2 years showed IAFA predicted the development of diabetes,14) this study was limited subjects with BMI ≥24 kg/m2 (≥22 for Asian Americans). MERLOT study includes larger number of subjects with no limits in subjects’ BMI, which ranged from 16.3 to 34.5 kg/m2.
MERLOT study, using a larger cohort with a long-term follow-up period, verified these preliminary findings observed in a limited number of Japanese-Americans11–13) and in a relatively short follow-up subgroup study of the Diabetes Prevention Program14) showing that IAFA is a predictor of the new onset of metabolic syndrome components and further expanded these findings in Japanese without any metabolic syndrome components.
Since another previous study from Japanese Americans showed that the association between IAFA and the future development of the metabolic syndrome appeared to be independent of SFA,15) the important question is whether or not the association is independent of BMI. MERLOT study documented, for the first time, that the new onset of individual components of the metabolic syndrome is predicted by IAFA, independently of BMI, in healthy Japanese. There have been debates and research progress on the relative importance of body weight, BMI and abdominal obesity in predicting cardiometabolic disorders.7,16) Although BMI is widely used to classify obesity and identify population at increased risk of obesity-related adverse health outcomes at population level, BMI is an indirect and imperfect measurement of abnormal or excessive body fat accumulation, because it does not distinguish fat mass and lean body mass components. IAFA reflecting abdominal obesity is proposed to be the essential clinical parameter for the metabolic syndrome.17) MERLOT study demonstrates that IAFA can be a better predictor than BMI for the new onset of individual components of the metabolic syndrome in Japanese.
The results of MERLOT study showed that the important role of IAFA among parameters in rational cardiometabolic risk stratification of Japanese, especially in normal BMI. The “metabolically obese, but normal weight” persons are a subgroup of individuals who have normal weight and BMI but display a cluster of obesity-related abnormalities.18) These individuals can display premature signs of insulin resistance, hyperinsulinemia, and dyslipidemia that may eventually increase their risk for the development of cardiovascular diseases. In general, the presence of these metabolic abnormalities could go undetected for years due to the normal body weight, which may mask the need for early detection and intervention.19) The previous study reported that abdominal obesity is a good indicator of risk for the metabolic syndrome for non-obese individuals in Western countries (BMI <30 kg/m2).20) In other study from Nurses’ Health Study, abdominal obesity speculated by waist circumference and waist-hip ratio which is essentially limited to apply to differentiate the subcutaneous and intra-abdominal fat is strongly associated with increased coronary heart disease risk among women even with BMI <25 kg/m2.21) Our results in MERLOT study using IAFA measured more accurately with CT in Japanese population are consistent with prior findings by using waist circumference in Western population and extended these findings to a large, community-based sample in that we showed that IAFA is associated with a significantly higher prevalence of the metabolic syndrome components in normal-weight subjects (BMI <25 kg/m2). Furthermore, in MERLOT study, the interaction test for the new onset of metabolic syndrome components between BMI <25 kg/m2 and ≥25 kg/m2 was not statistically significant, supporting the risk for the new onset of metabolic syndrome components in non-obese individuals with BMI <25 kg/m2 can increase in a similar way compared in obese subjects with ≥25 kg/m2. Thus, MERLOT study strongly indicates that IAFA can identify persons who are at greater cardiometabolic risk than are those identified by BMI alone.
IAFA measured by CT involves considerable radiation exposure to subjects, indicated the limited clinical use of IAFA measured by CT. Recently, using the dual bio-impedance method, we succeeded in developing the new equipment detecting IAFA without X ray exposure.22,23)
The limitations of our study include the potential selection bias due to inclusion with subjects who voluntarily chose to be examined by CT. In our study, it was seen in only half of population with IAFA ≥100 cm2 (25.6% in men and 5.2% in women) compared with the previous cross-sectional studies, MONK (50.5% in men 11.6% in women)5) and VACATION-J (median IAFA was 115.9 cm2 in men and 74.2 cm2 in women),6) in Japan. It was consistent that our study includes healthier population without any metabolic syndrome components. Another limitations include the potential unaccounted confounding by lifestyle changes during the follow-up. However, we performed routine screening of metabolic risk factors and adjusted for several potential confounders.
In conclusion, MERLOT study demonstrates that IAFA is an independent predictor of the new onset of individual components of the metabolic syndrome and also indicates that this finding can be applied to non-obese subjects with BMI <25 kg/m2.
Abbreviations
- IAFA
intra-abdominal fat area
- CT
computed tomography
- SFA
subcutaneous fat area
- BMI
body mass index
- HRs
hazard ratios
- CIs
confidence intervals
References
- 1).The Examination Committee of the Criteria for Metabolic Syndrome in Japan (2005) Definition and criteria of the metabolic syndrome in Japan. J. Jpn. Society Intern. Med. 94, 188–201 [Google Scholar]
- 2).Mottillo S., Filion K.B., Genest J., Joseph L., Pilote L., Poirier P., Rinfret S., Schiffrin E.L., Eisenberg M.J. (2010) The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 56, 1113–1132 [DOI] [PubMed] [Google Scholar]
- 3).Ho J.S., Cannaday J.J., Barlow C.E., Mitchell T.L., Cooper K.H., FitzGerald S.J. (2008) Relation of the number of metabolic syndrome risk factors with all-cause and cardiovascular mortality. Am. J. Cardiol. 102, 689–692 [DOI] [PubMed] [Google Scholar]
- 4).Ross, R. and Janssen, I. (2005) Human Body Composition. Champaign, IL. [Google Scholar]
- 5).Miyawaki T., Hirata M., Moriyama K., Sasaki Y., Aono H., Saito N., Nakao K. (2005) Metabolic syndrome in Japanese diagnosed with visceral fat measurement by computed tomography. Proc. Jpn. Acad., Ser. B 81, 471–479 [Google Scholar]
- 6).Hiuge-Shimizu A., Kishida K., Funahashi T., Ishizaka Y., Oka R., Okada M., Suzuki S., Takaya N., Nakagawa T., Fukui T., Fukuda H., Watanabe N., Yoshizumi T., Nakamura T., Matsuzawa Y., Yamakado M., Shimomura I. (2012) Absolute value of visceral fat area measured on computed tomography scans and obesity-related cardiovascular risk factors in large-scale Japanese general population (the VACATION-J study). Ann. Med. 44, 82–92 [DOI] [PubMed] [Google Scholar]
- 7).Goodpaster B.H., Krishnaswami S., Harris T.B., Katsiaras A., Kritchevsky S.B., Simonsick E.M., Nevitt M., Holvoet P., Newman A.B. (2005) Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch. Intern. Med. 165, 777–783 [DOI] [PubMed] [Google Scholar]
- 8).Rothman, K.J. (2002) Measuring interactions. In Epidemiology; An Introduction. Oxford University Press: New York, NY, pp. 168–180. [Google Scholar]
- 9).Oka R., Kobayashi J., Yagi K., Tanii H., Miyamoto S., Asano A., Hagishita T., Mori M., Moriuchi T., Kobayashi M., Katsuda S., Kawashiri M., Nohara A., Takeda Y., Hiroshi Mabuchi H., Yamagishi M. (2008) Reassessment of the cutoff values of waist circumference and visceral fat area for identifying Japanese subjects at risk for the metabolic syndrome. Diabetes Res. Clin. Pract. 79, 474–481 [DOI] [PubMed] [Google Scholar]
- 10).Eguchi M., Tsuchihashi K., Saitoh S., Odawara Y., Hirano T., Nakata T., Miura T., Ura N., Hareyama M., Shimamoto K. (2007) Visceral obesity in Japanese patients with metabolic syndrome: reappraisal of diagnostic criteria by CT scan. Hypertens. Res. 30, 315–323 [DOI] [PubMed] [Google Scholar]
- 11).Hayashi T., Boyko E.J., Leonetti D.L., McNeely M.J., Newell-Morris L., Kahn S.E., Fujimoto W.Y. (2004) Visceral adiposity is an independent predictor of incident hypertension in Japanese Americans. Ann. Intern. Med. 140, 992–1000 [DOI] [PubMed] [Google Scholar]
- 12).Hayashi T., Boyko E.J., McNeely M.J., Leonetti D.L., Kahn S.E., Fujimoto W.Y. (2008) Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes 57, 1269–1275 [DOI] [PubMed] [Google Scholar]
- 13).Fujimoto W.Y., Bergstrom R.W., Boyko E.J., Chen K.W., Leonetti D.L., Newell-Morris L., Shofer J.B., Wahl P.W. (1999) Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care 22, 1808–1812 [DOI] [PubMed] [Google Scholar]
- 14).Bray G.A., Jablonski K.A., Fujimoto W.Y., Barrett-Connor E., Haffner S., Hanson R.L., Hill J.O., Hubbard V., Kriska A., Stamm E., Pi-Sunyer F.X., for the Diabetes Prevention Program Research Group (2008) Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. Am. J. Clin. Nutr. 87, 1212–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Tong J., Boyko E.J., Utzschneider K.M., McNeely M.J., Hayashi T., Carr D.B., Wallace T.M., Zraika S., Gerchman F., Leonetti D.L., Fujimoto W.Y., Kahn S.E. (2007) Intra-abdominal fat accumulation predicts the development of the metabolic syndrome in non-diabetic Japanese-Americans. Diabetologia 50, 1156–1160 [DOI] [PubMed] [Google Scholar]
- 16).Klein S., Allison D.B., Heymsfield S.B., Kelley D.E., Leibel R.L., Nonas C., Kahn R. (2007) Waist circumference and cardiometabolic risk: a consensus statement from shaping America's health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care 30, 1647–1652 [DOI] [PubMed] [Google Scholar]
- 17).Matsuzawa Y. (2010) Establishment of a concept of visceral fat syndrome and discovery of adiponectin. Proc. Jpn. Acad., Ser. B 86, 131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Ruderman N.B., Schneider S.H., Berchtold P. (1981) The “metabolically-obese,” normal-weight individual. Am. J. Clin. Nutr. 34, 1617–1621 [DOI] [PubMed] [Google Scholar]
- 19).Karelis A.D., St-Pierre D.H., Conus F., Rabasa-Lhoret R., Poehlman E.T. (2004) Metabolic and body composition factors in subgroups of obesity: what do we know? J. Clin. Endocrinol. Metab. 89, 2569–2575 [DOI] [PubMed] [Google Scholar]
- 20).Ascaso J.F., Romero P., Real J.T., Lorente R.I., Martínez-Valls J., Carmena R. (2003) Abdominal obesity, insulin resistance, and metabolic syndrome in a southern European population. Eur. J. Intern. Med. 14, 101–106 [DOI] [PubMed] [Google Scholar]
- 21).Rexrode K.M., Carey V.J., Hennekens C.H., Walters E.E., Colditz G.A., Stampfer M.J., Willett W.C., Manson J.E. (1998) Abdominal adiposity and coronary heart disease in women. JAMA 280, 1843–1848 [DOI] [PubMed] [Google Scholar]
- 22).Shiga T., Oshima Y., Kanai H., Hirata M., Hosoda K., Nakao K. (2007) A Simple measurement method of visceral fat accumulation by bioelectrical impedance analysis. IFMBE Proceedings 17, 687–690 [Google Scholar]
- 23).Shiga T., Hamaguchi T., Oshima Y., Kanai H., Hirata M., Hosoda K., Nakao K. (2009) A new simple measurement system of visceral fat accumulation by bioelectrical impedance analysis. In World Congress on Medical Physics and Biomedical Engineering 2009. IFMBE Proceedings 25, 338–341 [Google Scholar]