Abstract
Tocopherol, a member of the vitamin E family, consists of four forms designated as α, β, γ, and δ. Several large cancer prevention studies with α-tocopherol have reported no beneficial results, but recent laboratory studies have suggested that δ- and γ-tocopherol may be more effective. In two different animal models of breast cancer, the chemopreventive activities of individual tocopherols were assessed using diets containing 0.3% of tocopherol (α-, δ- or γ-) or 0.3% of a γ-tocopherol rich mixture (γ-TmT). While administration of tocopherols did not prevent human epidermal growth factor receptor 2 (HER2/neu)-driven tumorigenesis, δ- and γ-tocopherols inhibited hormone-dependent mammary tumorigenesis in N-methyl-N-nitrosourea (NMU)-treated female Sprague Dawley rats. NMU-treated rats showed an average tumor burden of 10.6 ± 0.8 g in the control group at 11 weeks, whereas dietary administration of δ- and γ-tocopherols significantly decreased tumor burden to 7.2 ± 0.8 g (p<0.01) and 7.1 ± 0.7 g (p<0.01), respectively. Tumor multiplicity was also reduced in δ- and γ-tocopherol treatment groups by 42% (p<0.001) and 32% (p<0.01), respectively. In contrast, α-tocopherol did not decrease tumor burden or multiplicity. In mammary tumors, the protein levels of pro-apoptotic markers (BAX, cleaved-caspase 9, cleaved-caspase 3, cleaved-PARP) were increased, while anti-apoptotic markers (Bcl2, XIAP) were inhibited by δ-tocopherol, γ-tocopherol and γ-TmT. Furthermore, markers of cell proliferation (PCNA, PKC α), survival (PPARγ, PTEN, phospho-Akt) and cell cycle (p53, p21) were affected by δ- and γ-tocopherols. Both δ- and γ-tocopherols, but not α-tocopherol, appear to be promising agents for the prevention of hormone-dependent breast cancer.
Keywords: Breast Cancer, Tocopherols, Apoptosis, Cell Cycle, PPARγ
INTRODUCTION
Breast cancer is the most frequently diagnosed malignancy and a leading cause of cancer death among women (1). Lifestyle risk factors for breast cancer include obesity, lack of exercise, alcohol, and diet high in saturated fat (1, 2). A poor diet is estimated to be responsible for 15% to 35% of all cancer deaths (3). Vitamins and phytochemicals from fruits and vegetables may play a significant role in the prevention of cancer (4). Micronutrients may control intracellular events such as antioxidant activity, anti-inflammatory activity, and induction of apoptosis to reduce carcinogenesis (5).
Vitamin E is a fat-soluble antioxidant which consists of four tocopherols and four tocotrienols and has been suggested to reduce cancer risk (6). Tocopherols have a saturated phytyl tail while tocotrienols have an unsaturated isoprenoid side chain which contains 3 double bonds (6). On the chromanol ring, the four variants (α-, β-, γ-, δ-) are determined by the number and position of methyl groups. Tocopherols are able to efficiently quench lipid free radicals because of the phenolic group in the chromanol ring (7). Structural differences in the chromanol ring may be responsible for the variation in activity of each individual tocopherol form. α-Tocopherol (trimethylated) is expected to be a more potent hydrogen donor and has superior antioxidant activity than either γ-tocopherol (dimethylated) or δ-tocopherol (monomethylated) (8, 9). However, γ- and δ-tocopherols lack a methyl group at the 5-position on the chromanol ring, and are more effective at trapping reactive nitrogen species than α-tocopherol (9).
α-Tocopherol is known as the classic vitamin E because of its important role in the fertility restoration assay (10). However, α-tocopherol is not synonymous with vitamin E, and results from human intervention studies conducted with α-tocopherol are inconclusive. The Alpha-Tocopherol, Beta-Carotene (ATBC) Cancer Prevention Study examined the prevention of lung and other cancers with supplementation of all-racemic-α-tocopherol acetate (50 mg/day) and β-carotene (20 mg/day) daily, and reported no effect of α-tocopherol supplementation on lung or colorectal cancer (11, 12). However, the ATBC study found that males supplemented with α-tocopherol had 32% lower prostate cancer incidence and 41% reduction in prostate cancer deaths (13). The Women’s Health Study (WHS) administered 600 IU of natural-source vitamin E every other day for over 10 years. The WHS observed no overall benefit with supplementation in the prevention of cancer, but showed decreased cardiovascular mortality in healthy women (14). The Selenium and Vitamin E Cancer Prevention Trial (SELECT) using selenium (200 µg/day) and/or all racemic-α-tocopheryl acetate (400 IU/day) did not prevent prostate cancer with either agent alone or in combination (15). Interestingly, it was noted that the high dose of α-tocopherol decreased plasma γ-tocopherol levels and possibly limited cancer preventive and anti-inflammatory effects of γ-tocopherol (15). Previous intervention trials have utilized primarily α-tocopherol, not δ- or γ-tocopherol, for chemoprevention (13–18). More research is needed on each form of tocopherol to determine their respective chemopreventive activity.
We have previously demonstrated that administration of a diet containing 0.1%, 0.3% or 0.5% of a γ-tocopherol rich mixture (γ-TmT) suppressed mammary tumor growth in N-methyl-N-nitrosourea (NMU)-induced rat model (19). In addition, we reported that 0.3% and 0.5% γ-TmT administered to ACI rats with implanted estrogen pellets inhibited cell proliferation in mammary hyperplasia (20). In the present study, we selected a single dose of 0.3% tocopherol in the diet to compare the efficacies of individual forms on two different animal models of mammary tumorigenesis. In the MMTV/ErbB2/neu transgenic mouse model over-expressing HER-2, tocopherols did not have long term protective effects, while in the NMU-treated rat model, representing mainly estrogen-receptor positive breast cancer, δ- and γ-tocopherol, but not α-tocopherol, inhibited mammary growth.
MATERIALS AND METHODS
Animals and experimental procedures
Female MMTV/ErbB2/neu transgenic mice at 6-7 weeks old were purchased from Jackson Laboratory (Bar Harbor, ME). At 12 weeks of age, the mice received AIN-93M control diet or AIN-93M diets containing 0.3% tocopherols (α-, δ-, γ-, or γ-TmT) (n= 28 per group). The body weight and tumor size of each animal were measured weekly. The mice were sacrificed at 55 weeks of age and the tumors were weighed at necropsy. Mammary glands, mammary tumors, and lungs were stored for further analyses. Serum was collected after centrifugation of clotted blood samples.
Female Sprague-Dawley rats were purchased from Taconic Farms (Hudson, NY) and were treated with a single intraperitoneal injection of the carcinogen NMU (50 mg/kg body weight) at 21 ± 1 days of age. One week after NMU injection, rats were fed AIN-93M control diet or AIN-93M diets containing 0.3% tocopherols (α-, δ-, γ-, or γ-TmT) (n=30 per group). Body weight and tumor volume were measured weekly. The rats were sacrificed 11 weeks after NMU injection. The mammary glands and mammary tumors were harvested, fixed in 10% formalin and transferred to 70% ethanol or flash frozen and stored in −80°C. Blood was collected by cardiac puncture immediately prior to necropsy; serum was prepared and stored at −80°C. All animal studies were done in accordance with an institutionally-approved protocol.
Diets
Semipurified modified AIN-93M diet was obtained from Research Diets Laboratory (New Brunswick, NJ) and used as the control diet. The test diets were prepared by Research Diets Laboratory by adding 0.3% α-tocopherol, δ-tocopherol, γ-tocopherol or γ-TmT to the AIN-93M diet. γ-TmT was supplied by the Cognis Corporation (Kankakee, IL) and contained 57% γ-tocopherol, 24% δ-tocopherol, 13% α-tocopherol and 1.5% β-tocopherol. γ-Tocopherol was purified from γ-TmT by silica gel chromatography to a purity of 97%, with no detectable α- and δ-tocopherol. δ-Tocopherol (containing 94% δ-tocopherol, 5.5% γ-tocopherol and 0.5% α-tocopherol) and α-tocopherol (containing 69.7% α-tocopherol, 2.6% γ-tocopherol and 0.2% δ-tocopherol) were purchased from Sigma-Aldrich (St. Louis, MO). The diets were stored at 4°C and the food was replenished with fresh pellets twice weekly.
Serum estradiol levels
Estradiol (E2) levels in the serum were analyzed using an EIA kit from Enzo Life Sciences International, Inc (Plymouth Meeting, PA). Serum samples were purified and the assay was performed according to the manufacturer’s protocol.
Analysis of tocopherols in rat serum, mammary glands and mammary tumors
Serum, mammary glands and mammary tumors were analyzed by high performance liquid chromatography for tocopherol (α-, δ-, γ-) and short chain metabolite, carboxyethyl hydroxychroman (CEHC), levels as previously described (19, 21).
Immunohistochemical analysis
Mammary glands and tumors were processed and stained as previously described (20). Sections were immunostained with antibodies to 8-hydroxy-2'-deoxyguanosine (8-oxo-dG) (JaICA/GENOX Corporation, Baltimore, MD), nitrotyrosine (Millipore, Billerica, MA), proliferating cell nuclear antigen (PCNA) (BD Pharmingen, San Diego, CA) and cleaved-caspase 3 (c-Casp3) (Cell Signaling, Beverly MA). Images were taken randomly with Nikon Eclipse E800 (Melville, N.Y) fitted to Nikon digital sight Ri1. The staining density was determined by using an Aperio® Scan Scope (Vista, CA). Quantification was performed where three mammary glands or tumors from each treatment group were selected randomly and three areas from each gland or tumor were analyzed for over 1000 cells/mammary gland or 4000 cells/mammary tumor.
mRNA expression analysis using quantitative polymerase chain reaction (PCR)
RNA was extracted from mammary tumors, and reverse transcription and quantitative PCR were carried out as previously reported (22). Labeled primers for glyceraldehyde-3-phosphate dehydrogenase, Bcl-2-associated X protein (Bax), B cell lymphoma 2 (Bcl2), x-linked inhibitor of apoptosis (XIAP), PCNA, protein kinase C α (PKCα), phosphatase and tensin homologue (PTEN), Myc, p53, p21, p27, Cyclin D1, estrogen receptor (ER) α, ERβ, peroxisome proliferator-activated receptor γ (PPARγ), nuclear factor (erythroid-derived 2)-like 2 (Nrf2), kelch-like-ECH-associated protein 1 (KEAP1), NAD(P)H dehydrogenase, quinone 1 (NQO1), glutamate cysteine ligase, modifier subunit (GCLm), glutathione s-transferase mu 1 (GSTm1), UDP-glucuronosyltransferase (UGT1A1), catechol-O-methyltransferase (COMT), superoxide dismutase-1 (SOD), heme oxygenase-1 (HO-1), glutathione peroxidase (GPx), thioredoxin (TXN) and catalase were obtained from Applied Biosystems (Carlsbad, CA).
Western blot analysis
Mammary tumors were homogenized and the protein extracts were analyzed by Western blotting as previously described (19). The primary antibodies against apoptotic protease activating factor 1 (Apaf-1), XIAP, cleaved-caspase 9 (c-Casp9), cleaved-caspase 8 (c-Casp8), cleaved-caspase 3 (c-Casp3), cleaved-Poly (ADP-ribose) polymerase (c-PARP), PKCα, phospho-Akt (p-Akt), PTEN, p53, cyclin E, CDK2, CDK4, CDK6, TXN and UGT were from Cell Signaling (Beverly, MA); Bcl-2, ERα, PPARγ, c-Myc, p21, p27, cyclin D1, Nrf2, KEAP1 and NQO1 were from Santa Cruz Biotechnology (Santa Cruz, CA); Bax, SOD, GCLm and GPx were from Abcam (Cambridge, MA); ERβ was from Affinity BioReagents (Golden, CO); PCNA was from BD Pharmingen (San Diego, CA); GSTm1, catalase and HO-1 were from Epitomics (Burlingame, CA); and β-actin was from Sigma-Aldrich (St. Louis, MO). Secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Tumor samples from different animals in each treatment group (n=3 per group) were pooled for analysis by Western blot.
Statistical analysis
Statistical significance was evaluated using. ANOVA with Dunnett’s adjustment, preserving the overall type-1 error at the 5% level. Tumor incidence was calculated by the Log Rank (Mantel-Cox) Test using Graph Pad Prism 4.0 (GraphPad Software Inc.). The data presented represent the mean ± S.E. p-Values of <0.05 were considered significant.
RESULTS
Dietary administration of tocopherols did not inhibit tumorigenesis in MMTV-ErbB2/neu transgenic mice
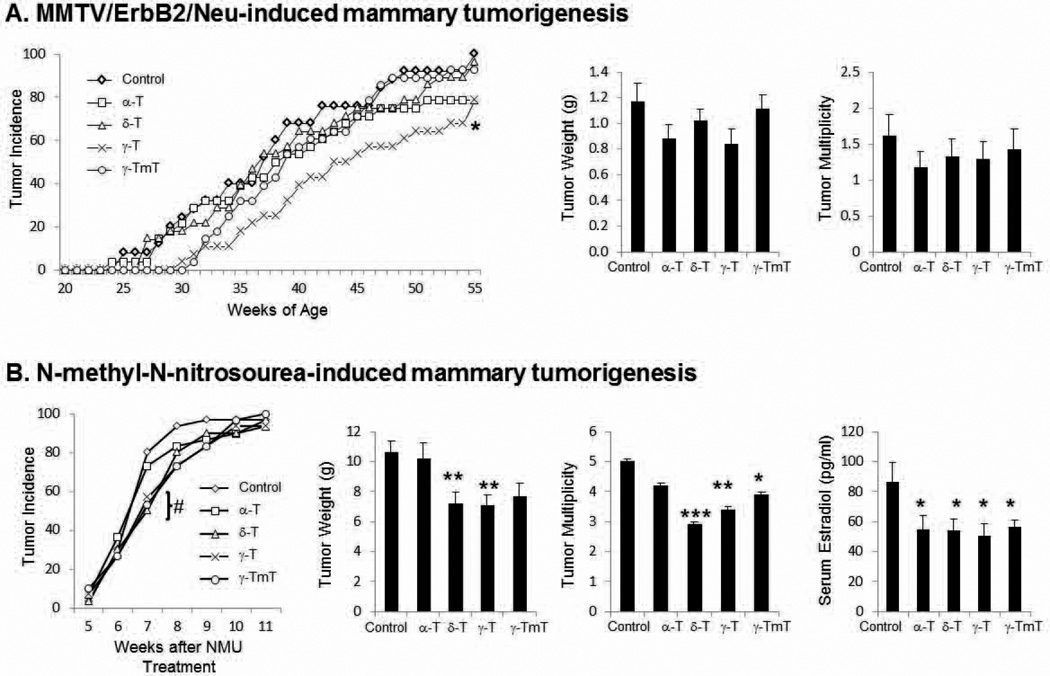
We investigated the effects of 0.3% α-tocopherol, 0.3% δ-tocopherol, 0.3% γ-tocopherol, or 0.3% γ-TmT in the diet on mammary tumor development in MMTV-ErbB2/neu transgenic mice. Body weight was measured weekly and there was no significant difference between treatment groups (data not shown). The median tumor latency was 37 weeks in the control group, and 38, 37, 44 and 39 weeks in mice fed with a diet containing α-, δ-, γ-tocopherol and γ-TmT, respectively (Fig. 1A). Only the diet containing γ-tocopherol significantly increased the median tumor latency (p<0.05). As shown in Fig. 1A, the final mammary tumor weight in the control group was 1.17 ± 0.14 g, compared to α-tocopherol (0.88 ± 0.11 g), δ-tocopherol (1.02 ± 0.09 g), γ-tocopherol (0.84 ± 0.11 g), and γ-TmT (1.11 ± 0.07 g), which corresponds to 24%, 13%, 29%, and 5% inhibition, respectively. The tumor multiplicity was 1.60 ± 0.30 in the control group, as compared to groups treated with α-tocopherol (1.18 ± 0.22), δ-tocopherol (1.32 ± 0.25), γ-tocopherol (1.28 ± 0.25), and γ-TmT (1.42 ± 0.28), which corresponds to 27%, 18%, 20%, and 11% inhibition, respectively (Fig. 1A). Although there was a delay with tumor incidence in the γ-tocopherol fed group, all treatment groups were not effective in reducing the tumor weight at the time of sacrifice and no further analyses were conducted.
Figure 1.
(A) MMTV/ErbB2/neu transgenic mice were administered 0.3% α-, δ-, γ-tocopherol (T), or γ-TmT in the diet. Data are represented as mean ± S.E. (n=28 per group) (B) NMU-treated Sprague-Dawley rats were administered 0.3% α-, δ-, γ-tocopherol, or γ-TmT in the diet. Data are represented as mean ± S.E (n=30 per group). Serum samples were analyzed for E2 (pg/ml) (n=7). Statistical significance was determined by ANOVA with Dunnett’s adjustment, *p<0.05, **p<0.01, ***p<0.001. At 7 weeks of treatment, the tumor incidence for δ-tocopherol and γ-TmT was significant and denoted as #p<0.05.
δ- And γ-tocopherols inhibit tumor growth and multiplicity in NMU-treated mammary tumorigenesis
Female Sprague-Dawley rats were either fed the control diet or the diets containing 0.3% α-tocopherol, 0.3% δ-tocopherol, 0.3% γ-tocopherol, or 0.3% γ-TmT. Body weight was measured weekly and there was no significant difference between treatment groups (data not shown). After 7 weeks of treatment, the tumor incidence for the control group was 80% and similar in the α-tocopherol group (73.3%). However, the corresponding tumor incidence at week 7 for the δ-tocopherol, γ-tocopherol, and γ-TmT groups was markedly lower at 50.0% (p<0.05), 56.7% (p=0.057), and 53.3% (p<0.05), respectively. At the conclusion of the 11-week study, the overall tumor incidence was not significantly different among the groups (Fig. 1B). As compared to the control group, dietary administration of δ- and γ-tocopherol reduced tumor burden by 32% (p<0.01) and 33% (p<0.01), respectively, but α-tocopherol-enriched diet had no effect (Fig. 1B). Tumor multiplicity for the control group was 5.0 ± 0.1 and was decreased by treatment with δ-tocopherol (2.9 ± 0.1), γ-tocopherol (3.4 ± 0.1), and γ-TmT (3.9 ± 0.1) which translates to 42% (p<0.001), 32% (p<0.01), and 22% (p<0.05) inhibition, respectively (Fig. 1B). All subsequent serum and tissue analyses reported are for the activity of individual tocopherols in NMU-treated rats.
Serum levels of estradiol were decreased by the administration of tocopherols
Circulating endogenous serum levels of E2 were analyzed to determine changes between the treatment groups (Fig. 1B). Average E2 serum levels in the control group were 86.6 ± 13.0 pg/ml, whereas average serum E2 levels in the α-, δ-, γ-tocopherol, and γ-TmT groups were significantly decreased by 37% (p<0.05), 38% (p<0.05), 42% (p<0.05), and 35% (p<0.05), respectively.
Levels of tocopherols and metabolites were increased in serum, mammary glands and mammary tumors when treated with α-, γ-, δ-tocopherol and γ-TmT diets
Serum, mammary gland, and mammary tumor samples were collected at necropsy and analyzed for the levels of a-, g-, d-tocopherol and the short chain metabolite, carboxyethyl hydroxychroman (CEHC, Table 1). In general, the levels of individual tocopherols and CEHCs differed among serum, mammary glands and mammary tumors. The highest levels of the hydrophobic parent tocopherols were found in the adipose-rich mammary gland, whereas comparable levels of tocopherols relative to control were found in both serum and mammary tumor. The water-soluble short chain metabolites were more prevalent in serum and mammary tumor compared to mammary gland.
Table 1.
Analysis of tocopherol and short chain metabolite levels in NMU-treated rats fed with tocopherol (α-, δ-, γ-) and γ-TmT-containing diets
| Tocopherol | Short Chain Metabolite | |||||
|---|---|---|---|---|---|---|
| Serum | α-T | δ-T | γ-T | α-CEHC | δ-CEHC | γ-CEHC |
| Control | 18.0 ± 1.0 | 0.1 ± 0.1 | 0.2 ± 0.4 | 0.4 ± 0.1 | 0.1 ± 0.0 | 0.0 ± 0.0 |
| 0.3% α-T | 72.8 ± 5.5d | 0.1 ± 0.0 | 0.1 ± 0.0 | 15.1 ± 2.0c | 0.2 ± 0.0 | 0.0 ± 0.0 |
| 0.3% δ-T | 14.9 ± 1.3 | 10.6 ± 1.3c | 2.4 ± 0.2c | 0.5 ± 0.0 | 12.6 ± 0.7c | 0.3 ± 0.0c |
| 0.3% γ-T | 7.0 ± 0.6a | 0.1 ± 0.0 | 26.8 ± 2.5c | 0.5 ± 0.1 | 0.1 ± 0.0 | 3.7 ± 0.6c |
| 0.3% γ-TmT | 23.1 ± 1.8 | 2.7 ± 0.3a | 4.3 ± 0.5c | 2.9 ± 0.4c | 6.5 ± 0.3c | 2.3 ± 0.1c |
| Mammary Gland | ||||||
| Control | 147.9 ± 3.3 | 1.05 ± 0.3 | 3.2 ± 0.5 | 0.3 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 |
| 0.3% α-T | 215.7 ± 5.5c | 2.1 ± 0.3 | 3.4 ± 0.9 | 9.8 ± 1.1c | 0.2 ± 0.1 | 0.0 ± 0.0 |
| 0.3% δ-T | 131.9 ± 7.5 | 131.3 ± 2.0c | 62.0 ± 6.4c | 0.2 ± 0.0 | 4.2 ± 0.7c | 0.1 ± 0.0 |
| 0.3% γ-T | 95.5 ± 4.3c | 2.8 ± 0.8 | 155.4 ± 1.4c | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.8 ± 0.2c |
| 0.3% γ-TmT | 171.3 ± 4.0a | 96.5 ± 2.8c | 112.8 ± 4.4c | 1.2 ± 0.1a | 1.1 ± 0.1a | 0.5 ± 0.0b |
| Tumor | ||||||
| Control | 7.2 ± 1.3 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.2 | 0.1 ± 0.0 |
| 0.3% α-T | 19.6 ± 3.1c | 0.0 ± 0.0 | 0.1 ± 0.0 | 16.1 ± 7.0b | 1.5 ± 0.6 | 0.0 ± 0.0 |
| 0.3% δ-T | 6.0 ± 0.6 | 22.2 ± 1.3c | 3.2 ± 0.5c | 1.0 ± 0.4 | 7.6 ± 1.1c | 0.1 ± 0.0 |
| 0.3% γ-T | 2.0 ± 0.6b | 0.0 ± 0.0 | 27.8 ± 5.6c | 1.0 ± 0.4 | 0.9 ± 0.5 | 1.2 ± 0.3c |
| 0.3% γ-TmT | 11.8 ± 2.0 | 6.5 ± 0.8c | 6.2 ± 0.7c | 1.4 ± 0.3a | 1.6 ± 0.3c | 0.4 ± 0.1b |
The effects of 0.3% α-, δ-, γ-tocopherol (T), or γ-TmT supplementation on the levels of tocopherols and their metabolites (CEHC) in serum (µmol/L), mammary gland (µmol/kg), and mammary tumor (µmol/kg) in NMU-treated rats. Data are presented as the mean ± S.E (n=6-12 per group);
p<0.05,
p<0.01,
p<0.001.
Due to the selective transport of α-tocopherol by the α-tocopherol transport protein in the liver, α-tocopherol is the major form found in serum. Levels of α-tocopherol and α-CEHC were significantly increased in serum, mammary glands and mammary tumors of rats fed with 0.3% α-tocopherol. Levels of γ-tocopherol and γ-CEHC were significantly increased in serum, mammary glands and mammary tumors of rats fed with 0.3% γ-tocopherol; however the levels of α-tocopherol decreased in the serum (p<0.05), mammary glands (p<0.001) and mammary tumors (p<0.01) by treatment with γ-tocopherol. Administration of 0.3% δ-tocopherol diet increased levels of δ-tocopherol, γ-tocopherol, and δ-CEHC in serum, mammary glands and mammary tumors. Furthermore, 0.3% γ-TmT diet increased levels of δ-tocopherol, γ-tocopherol, α-CEHC, δ-CEHC and γ-CEHC in serum, mammary glands and mammary tumors, while α-tocopherol levels only increased in mammary glands.
Treatment with δ-tocopherol, γ-tocopherol and γ-TmT induced apoptosis and inhibited cell proliferation and cell cycle in mammary tumors
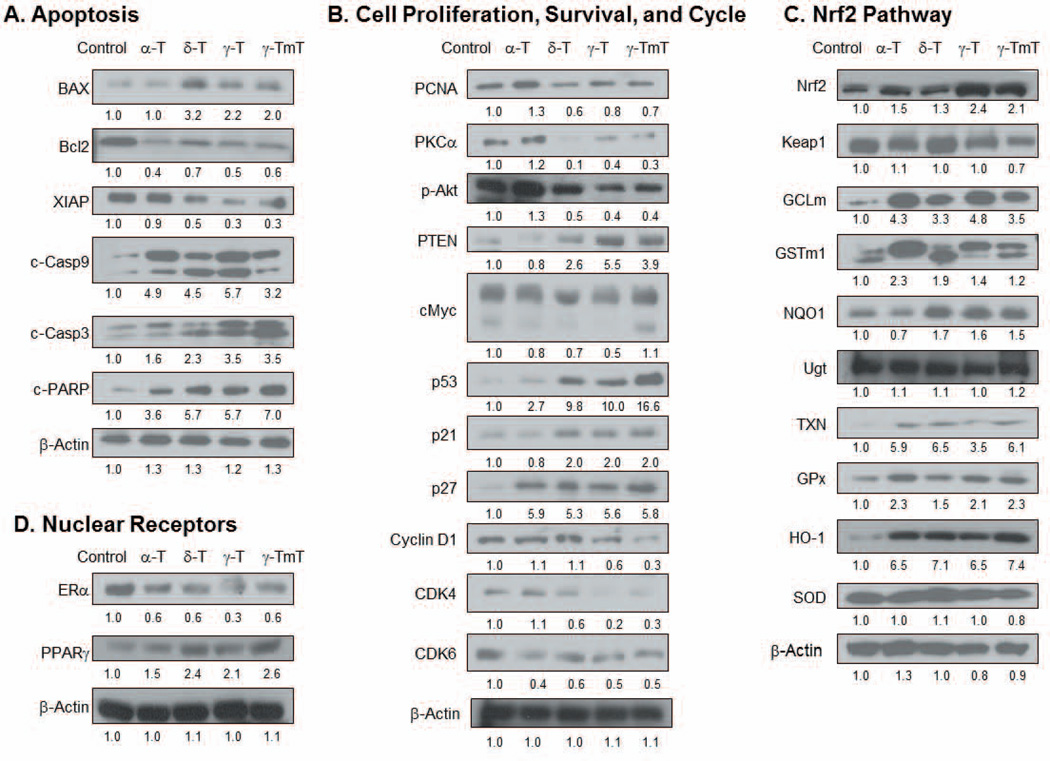
As shown in Fig. 2A, the levels of pro-apoptotic proteins, Bax, c-Casp3 and c-PARP, were increased by δ-tocopherol, γ-tocopherol and γ-TmT-enriched diets. Furthermore, anti-apoptotic proteins, Bcl2 and XIAP, were inhibited by δ-tocopherol, γ-tocopherol and γ-TmT. Levels of c-Casp9 were increased by all tocopherols, whereas c-Casp8 remained unchanged (data not shown). This may indicate that tocopherols induce apoptosis through the extrinsic apoptosis pathway.
Figure 2.
Tocopherol treatment modifies the levels of proteins in the mammary tumor of NMU-treated rats associated with (A) apoptosis, (B) cell proliferation, survival and cell cycle, (C) Nrf2 pathway and (D) nuclear receptors. Mammary tumors were pooled together (n=3 per group). Quantification of Western blot was performed by ImageJ 1.45s (NIH), and the numbers are provided at the bottom of each Western blot.
The cell cycle pathway plays a major role in regulating cancer development for the continuation of cell proliferation and survival. Cell proliferation markers, PCNA and protein kinase C (PKC), were inhibited by treatment with δ-tocopherol, γ-tocopherol and γ-TmT, but not by α-tocopherol (Fig. 2B). More specifically, in the cell survival pathway, PTEN was upregulated whereas p-Akt was down-regulated by δ-tocopherol, γ-tocopherol and γ-TmT. The oncogene cMyc regulates the G1 phase of the cell cycle; dietary δ- and γ-tocopherol decreased cMyc protein levels in mammary tumors. Furthermore, protein levels of tumor suppressor p53, and cyclin dependent kinase (CDK) inhibitors p21 and p27 were increased by δ-tocopherol, γ-tocopherol and γ-TmT. In contrast, α-tocopherol upregulated only p27. The protein level of cyclin D1 was decreased by γ-tocopherol and γ-TmT, CDK4 was reduced by δ-tocopherol, γ-tocopherol and γ-TmT, and CDK6 was down-regulated by each of the tocopherol-containing diets.
All tocopherol diets affected the Nrf2 pathway (Fig. 2C). Although levels of KEAP1 remained unchanged, the treatment increased the protein levels of Nrf2. Furthermore, the levels of down-stream phase II detoxifying and antioxidant enzymes TXN, GCLm, GSTm1, GPx and HO-1 were increased by all tocopherols. NQO1 was induced by δ-tocopherol, γ-tocopherol and γ-TmT, but not by α-tocopherol. Protein levels of SOD and UGT remained unchanged regardless of tocopherol treatment. Levels of nuclear receptors were examined in mammary tumors (Fig. 2D). Protein levels of ERα were decreased in samples from rats treated with α-, δ-, γ-tocopherol and γ-TmT. Interestingly, the protein level of PPARγ was increased by δ-tocopherol, γ-tocopherol and γ-TmT-containing diets, but not by α-tocopherol.
Oxidative and nitrosative stress markers in mammary glands were reduced by δ- and γ-tocopherols
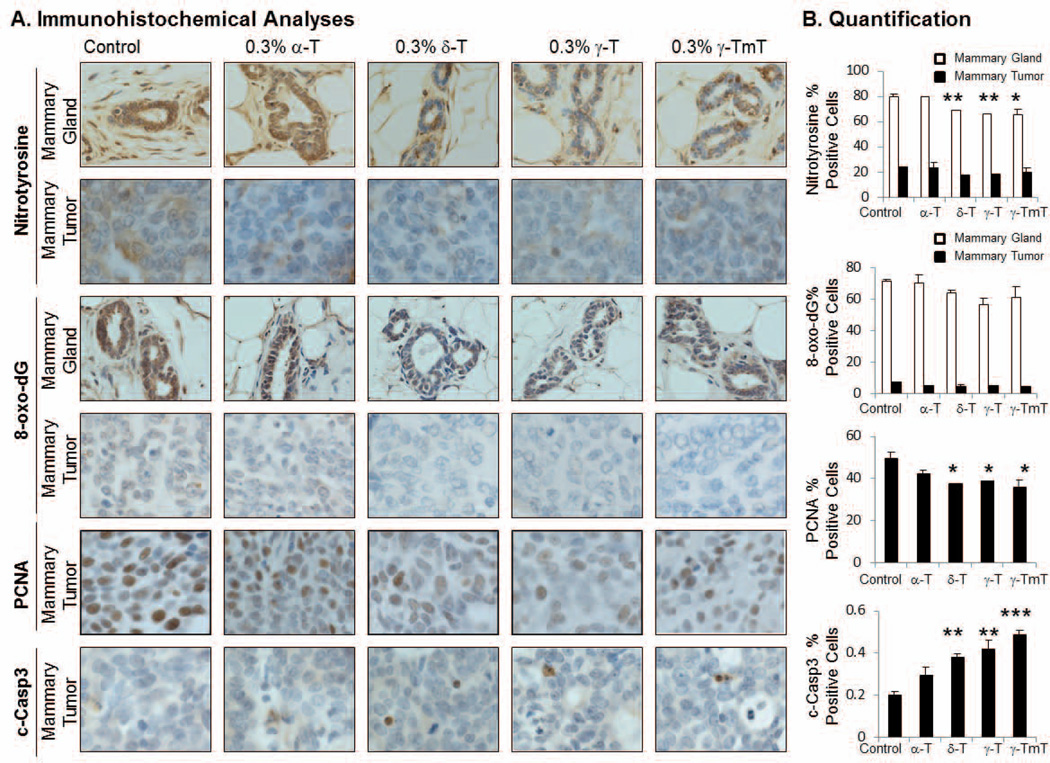
Nitrotyrosine is a known marker for nitrosative stress. In the mammary gland, the dietary administration of δ-tocopherol, γ-tocopherol and γ-TmT resulted in a decrease of nitrotyrosine levels by 14% (p<0.01), 18% (p<0.01), and 19% (p<0.05), respectively (Fig. 3). The level of 8-oxo-dG, a marker of oxidative stress, was also decreased by treatment with δ-tocopherol (10%), γ-tocopherol (21%) and γ-TmT (14%); however the results were not statistically significant (Fig.3). Levels of 8-oxo-dG and nitrotyrosine were not reduced by α-tocopherol in the mammary gland. In mammary tumors, markers of oxidative and nitrosative stress were markedly lower than mammary glands, and tocopherol treatment did not significantly change the levels of 8-oxo-dG and nitrotyrosine in mammary tumors.
Figure 3.
(A) A representative immunostaining of nitrotyrosine, 8-oxo-dG, PCNA and c-Casp3 in the mammary gland or tumor of NMU-treated rats (600x). Positive staining for nitrotyrosine is found in the cytoplasm of the cells. 8-oxo-dG and PCNA show positive staining in the nuclei of the cells. Positive staining for c-Casp3 is shown as a light brown to dark brown precipitate in the cytoplasm and or perinuclei of the cells. (B) Quantification was performed using Aperio® Scan Scope where three mammary glands or tumors from each treatment group were selected and three areas from each gland or tumor were analyzed for over 1000 cells/mammary gland or 4000 cells/mammary tumor. The data are presented as the mean ± S.E (n=3); *p<0.05, **p<0.01, ***p<0.001.
Treatment with δ-tocopherol, γ-tocopherol and γ-TmT reduced PCNA and increased c-Casp3 in mammary tumors
In mammary glands, levels of PCNA and c-Casp3 did not change (data not shown). However, in mammary tumors, PCNA expression was reduced by δ-tocopherol, γ-tocopherol and γ-TmT, but not by α-tocopherol (Fig. 3). Administration of δ-tocopherol, γ-tocopherol and γ-TmT resulted in a 24% (p<0.05), 21% (p<0.05), and 27% (p<0.05) decrease in PCNA level, respectively. Furthermore, treatment by δ-tocopherol, γ-tocopherol and γ-TmT increased c-Casp3 level in the mammary tumor by 89% (p<0.01), 107% (p<0.01), and 141% (p <0.001) above the control group, respectively. α-Tocopherol increased c-Casp3 level by 47% above the control, but it was not statistically significant.
The mRNA levels for apoptotic, cell proliferation, cell survival, and cell cycle markers, nuclear receptors, and Nrf2 pathways are regulated by tocopherols
The mRNA levels of apoptotic markers, Bax, Bcl2, and XIAP, were examined in mammary tumors (Table 2). The mRNA levels of Bcl2 were decreased by α-tocopherol (p<0.01), δ-tocopherol (p<0.05), and γ-tocopherol (p<0.05) and γ-TmT (p<0.01), whereas BAX and XIAP were unchanged. Changes in mRNA levels of proliferation, survival, and cell cycle pathway markers were examined in mammary tumors (Table 2). The tumor mRNA levels of PTEN increased in rats treated with δ-tocopherol (p<0.05), γ-tocopherol (p<0.05) and γ-TmT (p<0.05), while α-tocopherol did not. A CDK inhibitor, p21, was increased with δ-tocopherol (p<0.05), γ-tocopherol (p<0.05) and γ-TmT (p<0.05) treatment; p27 was increased by α-tocopherol (p<0.05), δ-tocopherol (p<0.05), γ-tocopherol (p<0.01) and γ-TmT (p<0.001). However, the mRNA levels of PCNA, PKCα, Myc, p53 and cyclin D1 did not change by tocopherol treatment. mRNA levels for ERα were significantly decreased by δ-tocopherol (p<0.05) and γ-TmT (p<0.05) treatment; levels of ERβ did not decrease. Interestingly, δ-tocopherol, γ-tocopherol and γ-TmT increased mRNA levels of PPARγ (p<0.05, p<0.01, p<0.05, respectively) (Table 2). Both Nrf2 and KEAP1 mRNA levels were unchanged by tocopherol treatment (Table 2). The phase II detoxifying enzymes GCLm, GSTm1 and Ugt1A1 were increased by all tocopherol treatment, while NQO1 and COMT mRNA levels were not affected. The mRNA levels of most of the antioxidant enzymes (SOD-1, HO-1, TXN1 and catalase) were unaltered.
Table 2.
Analysis of mRNA expression levels in the mammary tumor of NMU-treated rats
| Control | α-T | δ-T | γ-T | γ-TmT | |
|---|---|---|---|---|---|
|
Apoptotic Markers | |||||
| BAX | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.2 |
| Bcl2 | 1.0 ± 0.2 | 0.5 ± 0.1b | 0.6 ± 0.1a | 0.6 ± 0.1a | 0.4 ± 0.1b |
| XIAP | 1.0 ± 0.4 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
|
Cell Proliferation, Survival, and Cycle | |||||
| PCNA | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.7± 0.1 |
| PKCα | 1.0 ± 0.3 | 1.1 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.2 |
| PTEN | 1.0 ± 0.1 | 1.4 ± 0.2 | 2.0 ± 0.4a | 2.1 ± 0.3a | 1.6 ± 0.3a |
| Myc | 1.0 ± 0.2 | 0.7 ± 0.2 | 0.9 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 |
| p53 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 | 1.1 ± 0.2 |
| p21 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1a | 1.4 ± 0.1a | 1.5 ± 0.2a |
| p27 | 1.0 ± 0.3 | 1.8 ± 0.2a | 2.0 ± 0.4a | 2.1 ± 0.3b | 2.5 ± 0.3c |
| Cyclin D1 | 1.0 ± 0.2 | 1.2 ± 0.3 | 1.0 ± 0.3 | 0.5 ± 0.1 | 0.7 ± 0.3 |
|
Nuclear Receptors | |||||
| ERα | 1.0 ± 0.1 | 0.9 ± 0.2 | 0.6 ± 0.0a | 0.7 ± 0.1 | 0.5 ± 0.1a |
| ERβ | 1.0 ± 0.1 | 0.7 ± 0.2 | 0.6 ± 0.1 | 0.8 ± 0.2 | 0.7 ± 0.2 |
| PPARγ | 1.0 ± 0.1 | 1.5 ± 0.3 | 1.8 ± 0.2a | 2.1 ± 0.2b | 1.9 ± 0.1a |
|
Nrf2 Pathway | |||||
| Nrf2 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.1 | 0.8 ± 0.1 |
| Keap1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 |
|
Phase II Detoxifying Enzymes | |||||
| NQO1 | 1.0 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.2 | 1.4 ± 0.1 |
| GClm | 1.0 ± 0.3 | 2.8 ± 0.9a | 2.5 ± 0.2a | 2.8 ± 0.5a | 2.9 ± 0.4a |
| GSTm1 | 1.0 ± 0.1 | 1.6 ± 0.2a | 1.5 ± 0.1a | 1.7 ± 0.1a | 2.0 ± 0.5a |
| Ugt1A1 | 1.0 ± 0.1 | 1.8 ± 0.3b | 1.7 ± 0.2a | 1.7 ± 0.2a | 1.8 ± 0.4a |
| COMT | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.3 |
|
Antioxidant Enzymes | |||||
| SOD1 | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.2 ± 0.1 | 1.2 ± 0.2 | 1.4 ± 0.2 |
| HO-1 | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.4 ± 0.3 | 1.3 ± 0.1 | 1.2 ± 0.1 |
| GPx | 1.0 ± 0.1 | 1.4 ± 0.1 | 1.2 ± 0.1 | 1.6 ± 0.2a | 1.5 ± 0.2 |
| TXN1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.3 ± 0.2 |
| Catalase | 1.0 ± 0.1 | 1.2 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 |
NMU-treated Sprague-Dawley rats were administered 0.3% α-, δ-, γ-tocopherol (T), or γ-TmT in the diet. A mammary tumor from each rat was analyzed for mRNA levels by quantitative PCR and normalized by GAPDH. The values (fold-induction) are represented as mean ± S.E (n=6-8 per group);
p<0.05,
p<0.01,
p<0.001.
DISCUSSION
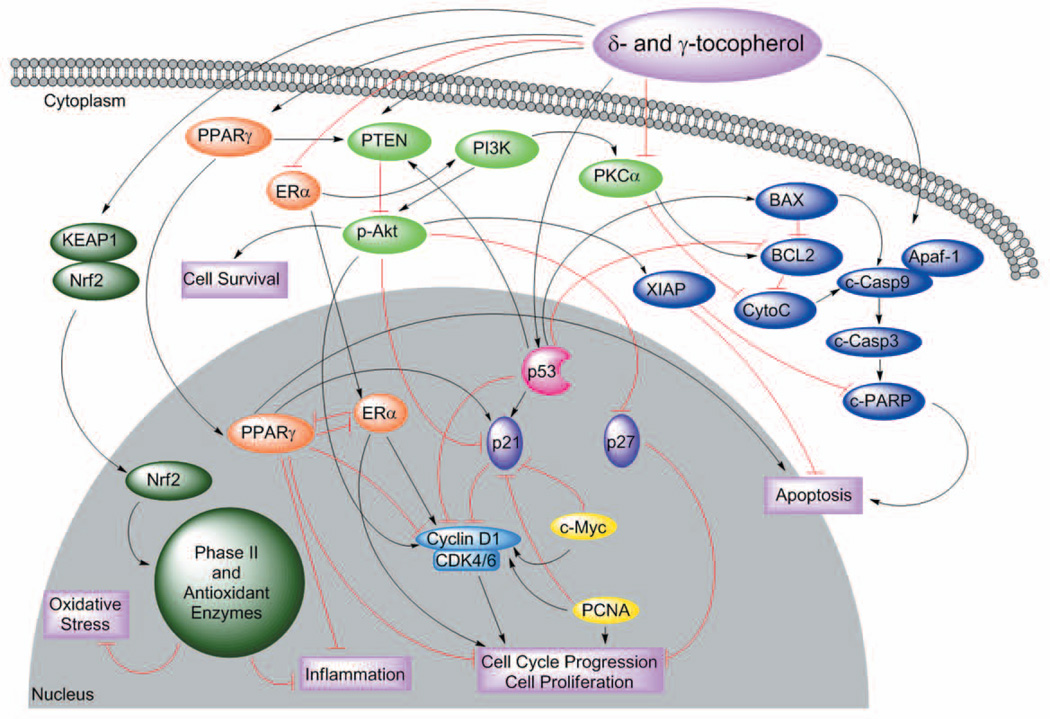
In this study, we examined the effects of individual tocopherols on two different animal models of breast cancer. The over-expression of ErbB2 has been reported in 18-25% of human breast cancers and is hormone independent (23). We found that α-, δ-tocopherol and γ-TmT did not prevent the development of mammary tumorigenesis in transgenic MMTV/ErbB2/neu mice. At the conclusion of the fifty-five week study, tumor weight and multiplicity were not significantly affected by any of the examined tocopherols, suggesting that tocopherols do not affect HER-2 driven mammary tumorigenesis. There were modest effects by γ-tocopherol in reducing tumor incidence at 0.3% dose, and further studies may be needed to determine if higher doses of tocopherols are protective against HER-2 breast cancer. In our second animal model, we utilized the carcinogen NMU to induce mammary tumors in female Sprague-Dawley rats. NMU-induced mammary tumors mainly represent estrogen-dependent and locally invasive phenotypes that are similar to human breast cancer (24, 25). Both δ- and γ-tocopherol, but not α-tocopherol, inhibited mammary tumor development and were examined further for molecular changes. Possible mechanisms of actions involved in the prevention of breast cancer by δ- and γ-tocopherol are represented in Figure 4.
Figure 4.
A schematic representation which shows possible mechanisms of action by δ- and γ-tocopherol in multiple signaling pathways involved in mammary carcinogenesis. Induction of PPARγ by δ- and γ-tocopherol may increase PTEN and p21 and reduce ERα and cyclin D1 resulting in the decrease of inflammation and cell proliferation. The cell cycle (p53 and p21) may be regulated by δ- and γ-tocopherol. Tumor suppressor p53 is able to positively regulate PTEN, p21 and BAX, while inhibiting Bcl2 and cyclin D, which increases apoptosis and reduces cell cycle progression. PPARγ and p53 increase the levels of PTEN, and as a result inhibit the Akt cell survival pathway. δ- And γ-tocopherols reduce c-Myc and PCNA which consequently decrease cell progression and proliferation. Pro-apoptotic proteins BAX, c-Casp9, c-Casp3 and c-PARP are increased while anti-apoptotic proteins XIAP and Bcl2 are decreased by δ- and γ-tocopherol. In summary, δ- and γ-tocopherol may activate PPARγ, PTEN, p53, the apoptotic pathway and Nrf2 and decrease ERα, p-Akt, cyclin D1 and PCNA to inhibit cell cycle progression and cell growth resulting in the inhibition of mammary tumorigenesis.
Tocopherols are known antioxidants and may reduce oxidative and nitrosative stress (RONS) to prevent cellular injury and mutations (26). RONS may promote tumor onset and progression by affecting DNA mutations, cell proliferation, and survival (27). γ-Tocopherol has been shown to be more effective at trapping reactive nitrogen species than α-tocopherol (9, 28–32). Previously, in a lung xenograft tumor model, δ-tocopherol, γ-tocopherol and γ-TmT administration reduced 8-oxo-dG and nitrotyrosine levels, whereas α-tocopherol did not (33). In the current study, treatment with δ-tocopherol, γ-tocopherol and γ-TmT reduced 8-oxo-dG and nitrotyrosine levels in the mammary gland, whereas α-tocopherol did not. Interestingly, very low levels of RONS markers were observed in mammary tumors and were not changed by tocopherols. At the time of the analysis, the damage from the NMU carcinogen leading to mammary tumorigenesis already occurred and may explain why we did not see changes in RONS levels in mammary tumors.
Tocopherols may also function as indirect antioxidants by stimulating the Nrf2 pathway. The down-stream enzymes in the Nrf2 pathway protect cells from neoplastic transformation by maintaining oxidative stress homeostasis (34, 35). More importantly, the loss of Nrf2 may lead to an increase in inflammation and to a decrease in cellular defense against oxidative stress, which may result in tumorigenesis (36). We reported that administration of γ-TmT increased protein levels of Nrf2 and mRNA levels of UGT1A1, GSTm1 and COMT in estrogen-induced mammary hyperplasia (20). In the present study, we showed that all tocopherol diets were able to increase protein levels of Nrf2 and subsequent down-stream phase II and antioxidant enzymes. Previously, δ- and γ-tocopherol were shown to inhibit cell proliferation in ER-positive breast cancer cells (MCF-7 and T47D) in vitro (19), and dietary γ-TmT decreased serum E2 levels and the protein levels of ERα in mammary hyperplasia (20). In the present study, all tocopherol treatment reduced circulating E2 serum levels and ERα protein levels in mammary tumors. Since ERα physically associates with PPARγ and functionally interferes with PPARγ signaling in breast cancer (37), the crosstalk between the nuclear receptors should be taken into account for their different chemopreventive activities by tocopherols. PPARγ has been connected to multiple pathways where it inhibits PI3K/Akt activity, angiogenesis, and inflammatory markers, while inducing CDK inhibitors (p21 and p27), apoptosis and differentiation markers (38). We have reported that administration of γ-TmT induced PPARγ in mammary hyperplasia (20) and in mammary tumors (19). In the present study, δ-tocopherol, γ-tocopherol and γ-TmT, but not α-tocopherol, induced PPARγ and PTEN, and reduced p-Akt levels.
α-Tocopherol has been the primary tocopherol utilized for chemoprevention studies, and the results have been inconclusive (6, 39, 40). Further, the precise mechanism of action of individual tocopherols in cancer prevention is still unknown. We found that all tocopherol treatments had similar effects in modulating Bcl2, c-Casp9, c-PARP, ERα, p27, CDK6, and the Nrf2 pathway (Fig 2). However, δ-tocopherol and γ-tocopherol lack a methyl group at the 5’ position on the chromanol ring while α-tocopherol does not. This structural difference may attribute to the efficacy to remove RNS. δ-Tocopherol and γ-tocopherol, but not α-tocopherol reduced levels of nitrotyrosine in the mammary gland (Fig. 3). δ-Tocopherol and γ-tocopherol may delay tumor onset through the reduction on RNS. In mammary tumors, δ-tocopherol and γ-tocopherol, but not α-tocopherol, increased levels of PTEN, p53 pathway, PPARγ and c-Casp3 while the levels of pAkt and PCNA decreased (Figs. 2 and 3). The relationship between ERα, PPARγ, PTEN, Akt and p53 may be an important mechanism of action for the inhibition of mammary tumorigenesis by δ- and γ-tocopherol in vivo. Our findings indicate that δ- and γ-tocopherol, but not α-tocopherol, work through antioxidant-dependent pathway that decreases cell survival and proliferation, regulating cell cycle and inducing PPARγ and apoptosis, leading to the inhibition of mammary tumorigenesis. This suggests that δ- and γ-tocopherol, but not α-tocopherol, are useful in the prevention of hormone-dependent breast cancer.
ACKNOWLEDGMENTS
We thank the Laboratory of Animal Service at the Department of Chemical Biology for animal care, Samantha Sagot and Taewoo Kim for their assistance in animal handling, Anna Ba Liu for help with the tocopherol diets and Dave Wasniewski for consultation on constructing the graphical design of the pathway figure.
GRANT SUPPORT
This work was supported in part by NIH R03 CA141756, NIEHS Center Grant P30 ES005022, The Charles and Johanna Busch Memorial Fund, and the Trustees Research Fellowship Program at Rutgers, The State University of New Jersey.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Khan N, Afaq F, Mukhtar H. Lifestyle as risk factor for cancer: Evidence from human studies. Cancer Lett. 2010;293:133–143. doi: 10.1016/j.canlet.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Agency for Cancer Research: World Cancer Report 2008. Lyon, France: IARC; 2008. [Google Scholar]
- 4.Tan AC, Konczak I, Sze DM, Ramzan I. Molecular pathways for cancer chemoprevention by dietary phytochemicals. Nutr Cancer. 2011;63:495–505. doi: 10.1080/01635581.2011.538953. [DOI] [PubMed] [Google Scholar]
- 5.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 6.Constantinou C, Papas A, Constantinou AI. Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. Int J Cancer. 2008;123:739–752. doi: 10.1002/ijc.23689. [DOI] [PubMed] [Google Scholar]
- 7.Burton GW, Ingold KU. Vitamin E as an in vitro and in vivo antioxidant. Ann N Y Acad Sci. 1989;570:7–22. doi: 10.1111/j.1749-6632.1989.tb14904.x. [DOI] [PubMed] [Google Scholar]
- 8.Kamal-Eldin A, Appelqvist LA. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31:671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- 9.Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ. Gamma-tocopherol detoxification of nitrogen dioxide: superiority to alpha-tocopherol. Proc Natl Acad Sci U S A. 1993;90:1771–1775. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 11.The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;4:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 12.Albanes D, Malila N, Taylor PR, Huttunen JK, Virtamo J, Edwards BK, et al. Effects of supplemental alpha-tocopherol and beta-carotene on colorectal cancer: results from a controlled trial (Finland) Cancer Causes Control. 2000;11:197–205. doi: 10.1023/a:1008936214087. [DOI] [PubMed] [Google Scholar]
- 13.Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, Hartman AM, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. 1998;90:440–446. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 14.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 15.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hensley K, Benaksas EJ, Bolli R, Comp P, Grammas P, Hamdheydari L, et al. New perspectives on vitamin E: gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic Biol Med. 2004;36:1–15. doi: 10.1016/j.freeradbiomed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Kline K, Lawson KA, Yu W, Sanders BG. Vitamin E and breast cancer prevention: current status and future potential. J Mammary Gland Biol Neoplasia. 2003;8:91–102. doi: 10.1023/a:1025787422466. [DOI] [PubMed] [Google Scholar]
- 18.Bairati I, Meyer F, Gelinas M, Fortin A, Nabid A, Brochet F, et al. A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients. J Natl Cancer Inst. 2005;97:481–488. doi: 10.1093/jnci/dji095. [DOI] [PubMed] [Google Scholar]
- 19.Lee HJ, Ju J, Paul S, So JY, DeCastro A, Smolarek A, et al. Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-gamma. Clin Cancer Res. 2009;15:4242–4249. doi: 10.1158/1078-0432.CCR-08-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smolarek AK, So JY, Thomas PE, Lee HJ, Paul S, Dombrowski A, et al. Dietary tocopherols inhibit cell proliferation, regulate expression of ERalpha, PPARgamma, and Nrf2, and decrease serum inflammatory markers during the development of mammary hyperplasia. Mol Carcinog. 2012 doi: 10.1002/mc.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Lee MJ, Cheung C, Ju JH, Chen YK, Liu B, et al. Analysis of multiple metabolites of tocopherols and tocotrienols in mice and humans. J Agric Food Chem. 2010;58:4844–4852. doi: 10.1021/jf904464u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HJ, So JY, DeCastro A, Smolarek A, Paul S, Maehr H, et al. Gemini vitamin D analog suppresses ErbB2-positive mammary tumor growth via inhibition of ErbB2/AKT/ERK signaling. J Steroid Biochem Mol Biol. 2010;121:408–412. doi: 10.1016/j.jsbmb.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 24.Welsch CW. Host factors affecting the growth of carcinogen-induced rat mammary carcinomas: a review and tribute to Charles Brenton Huggins. Cancer Res. 1985;45:3415–3443. [PubMed] [Google Scholar]
- 25.Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, van Zwieten MJ. Comparative study of human and rat mammary tumorigenesis. Lab Invest. 1990;62:244–278. [PubMed] [Google Scholar]
- 26.Smolarek AK, Suh N. Chemopreventive Activity of Vitamin E in Breast Cancer: A Focus on gamma- and delta-Tocopherol. Nutrients. 2011;3:962–986. doi: 10.3390/nu3110962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiaschi T, Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int J Cell Biol. 2012;2012:762825. doi: 10.1155/2012/762825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Q, Lykkesfeldt J, Shigenaga MK, Shigeno ET, Christen S, Ames BN. Gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic Biol Med. 2002;33:1534–1542. doi: 10.1016/s0891-5849(02)01091-2. [DOI] [PubMed] [Google Scholar]
- 32.Christen S, Woodall AA, Shigenaga MK, Southwell-Keely PT, Duncan MW, Ames BN. gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proc Natl Acad Sci U S A. 1997;94:3217–3222. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li GX, Lee MJ, Liu AB, Yang Z, Lin Y, Shih WJ, et al. delta-tocopherol is more active than alpha - or gamma -tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prev Res (Phila) 2011;4:404–413. doi: 10.1158/1940-6207.CAPR-10-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khor TO, Yu S, Kong AN. Dietary cancer chemopreventive agents - targeting inflammation and Nrf2 signaling pathway. Planta Med. 2008;74:1540–1547. doi: 10.1055/s-0028-1088303. [DOI] [PubMed] [Google Scholar]
- 35.Barve A, Khor TO, Nair S, Reuhl K, Suh N, Reddy B, et al. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int J Cancer. 2009;124:1693–1699. doi: 10.1002/ijc.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen C, Kong AN. Dietary cancer-chemopreventive compounds: from signaling and gene expression to pharmacological effects. Trends Pharmacol Sci. 2005;26:318–326. doi: 10.1016/j.tips.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Bonofiglio D, Gabriele S, Aquila S, Catalano S, Gentile M, Middea E, et al. Estrogen receptor alpha binds to peroxisome proliferator-activated receptor response element and negatively interferes with peroxisome proliferator-activated receptor gamma signaling in breast cancer cells. Clin Cancer Res. 2005;11:6139–6147. doi: 10.1158/1078-0432.CCR-04-2453. [DOI] [PubMed] [Google Scholar]
- 38.Mansure JJ, Nassim R, Kassouf W. Peroxisome proliferator-activated receptor gamma in bladder cancer: a promising therapeutic target. Cancer Biol Ther. 2009;8:6–15. doi: 10.4161/cbt.8.7.7853. [DOI] [PubMed] [Google Scholar]
- 39.Brigelius-Flohe R, Kelly FJ, Salonen JT, Neuzil J, Zingg JM, Azzi A. The European perspective on vitamin E: current knowledge and future research. Am J Clin Nutr. 2002;76:703–716. doi: 10.1093/ajcn/76.4.703. [DOI] [PubMed] [Google Scholar]
- 40.Kline K, Lawson KA, Yu W, Sanders BG. Vitamin E and cancer. Vitam Horm. 2007;76:435–461. doi: 10.1016/S0083-6729(07)76017-X. [DOI] [PubMed] [Google Scholar]