Abstract
Se can be accumulated by plants and volatilized to dimethylselenide, providing an attractive technology for Se phytoremediation. To determine the rate-limiting steps in Se volatilization from selenate and selenite, time- and concentration-dependent kinetics of Se accumulation and volatilization were studied in Indian mustard (Brassica juncea). Time-dependent kinetic studies showed that selenate was taken up 2-fold faster than selenite. Selenate was rapidly translocated to the shoot, away from the root, the site of volatilization, whereas only approximately 10% of the selenite was translocated. For both selenate- and selenite-supplied plants, Se accumulation and volatilization increased linearly with external Se concentration up to 20 μm; volatilization rates were also linearly correlated with root Se concentrations. Se-volatilization rates were 2- to 3-fold higher from plants supplied with selenite compared with selenate. Se speciation by x-ray absorption spectroscopy revealed that selenite-supplied plants accumulated organic Se, most likely selenomethionine, whereas selenate-supplied plants accumulated selenate. Our data suggest that Se volatilization from selenate is limited by the rate of selenate reduction, as well as by the availability of Se in roots, as influenced by uptake and translocation. Se volatilization from selenite may be limited by selenite uptake and by the conversion of selenomethionine to dimethylselenide.
Se is a major pollutant that is present in agricultural drainage water in the Central Valley in California and in effluent from oil refineries and power plants. Selenate is the main chemical species of Se in agricultural drainage water (McNeal and Balisteri, 1989) and power plant wastewater, whereas selenite is the major form of Se in oil refinery effluent (Duda, 1992; Hansen et al., 1998). Phytoremediation, i.e. the use of plants to remove or stabilize pollutants, is an inexpensive, efficient, and environment-friendly technology for the remediation of inorganic Se (Terry and Zayed, 1998). Indian mustard (Brassica juncea) is a good candidate for Se phytoremediation because it produces a large biomass and accumulates high concentrations of Se in its tissues (Wu et al., 1988, 1996; Banuelos and Schrale, 1989; Banuelos et al., 1992, 1995, 1997; Terry et al., 1992). Indian mustard was shown to be a successful remediator of Se-contaminated agricultural soil in the San Joaquin Valley in California (Banuelos and Meek, 1990; Banuelos et al., 1993, 1995). Indian mustard was also identified as a good Se-volatilizing plant species (Terry et al., 1992); Se volatilization is the process by which gaseous forms such as DMSe are produced from other inorganic or organic forms of Se (Lewis et al., 1966; Zieve and Peterson, 1984; Velinsky and Cutter, 1991; Duckart et al., 1992; Terry et al., 1992; Zayed and Terry, 1994). DMSe was reported to be 500 to 700 times less toxic to rats than ionic forms of Se (McConnell and Portman, 1952; Ganther et al., 1966; Wilber, 1980). Thus, Se volatilization ensures that toxic inorganic forms of Se are permanently removed from the contaminated site (Atkinson et al., 1990) and its associated food chain as relatively nontoxic Se.
To optimize Se accumulation and volatilization by plants, it is important to understand the factors that control these processes and to identify the rate-limiting steps. Se accumulation and volatilization are thought to follow the sulfur-assimilation pathway (Anderson, 1993; Lauchli, 1993; Zayed and Terry, 1994). This view was supported by the finding that sulfate inhibited Se volatilization from selenate (Zayed and Terry, 1992). Several plant species volatilized SeMet at higher rates than less-reduced Se forms such as selenite and selenate (Terry and Zayed, 1998; Zayed et al., 1998). The root appeared to be the main site of Se volatilization, since much higher rates of Se volatilization were measured from roots than from shoots (Terry and Zayed, 1994; Zayed and Terry, 1994). However, the kinetics of Se accumulation and volatilization and their relation to each other have yet to be elucidated. To obtain insight into the factors that control uptake, translocation, and volatilization of Se from selenate and selenite, we analyzed time- and concentration-dependent Se volatilization and accumulation, as well as chemical Se speciation in Indian mustard.
MATERIALS AND METHODS
Plant Growth Conditions
Seeds of Indian mustard (Brassica juncea, accession no. 173874) were obtained from the North Central Regional Plant Introduction Station (Ames, IA) and germinated on water-moistened filter paper. After 2 d each seedling was transferred to a 4-inch pot containing coarse sand. The pots were maintained in a greenhouse with a controlled temperature (24°C) and a short-day (9 h) photoperiod. The plants were watered twice a day, once with tap water and once with one-half-strength Hoagland solution (Hoagland and Arnon, 1938). After 6 weeks of growth the plants were gently washed in water to remove the sand adhering to the aerated roots and transferred into plastic boxes containing 3.5 L of hydroponic solution (one-eighth-strength Hoagland solution). After 1 week, the hydroponic solution was replaced with fresh nutrient solution, and the plants were pretreated with Se as indicated below for 7 d (unless stated otherwise). Subsequently, the plants were placed in Magenta boxes (7 × 7 × 9 cm, Sigma) with their roots immersed in 200 mL of deionized water containing Se to measure Se volatilization; details for each experiment are provided below. At the time of harvest, the plants were 20 cm tall and their average dry weight was 4 g; the average root dry weight was 1.2 g.
Experimental Design
To determine the optimum time period for volatile Se collection from selenate and selenite, plants were pretreated with 20 μm Na2SeO4 or Na2SeO3 for 7 d, then placed in deionized water with 20 μm selenate or selenite, and Se volatilization was measured for 4, 8, 12, 24, 28.5, 36, and 49 h. The alkaline peroxide solution used to trap volatile Se (see below) was changed after 24 h for this experiment.
To determine the time-dependent kinetics of Se accumulation and volatilization, different plants were pretreated with 20 μm selenate or selenite for 0, 1, 2, 3, 4, 5, 6, 7, or 14 d, after which they were placed in deionized water containing 20 μm selenate or selenite, and Se volatilization was measured over 24 h.
To study the concentration-dependent kinetics of Se accumulation and volatilization, the plants were pretreated with 0.02, 0.2, 2, 10, 20, 50, 100, or 200 μm Se in the nutrient solution for 7 d, after which they were transferred into deionized water containing Se at the same concentration used for pretreatment, and Se volatilization was measured over 24 h.
To determine the allocation of Se into plant tissues, Indian mustard plants were supplied for 1 d with 20 μm selenate or selenite in one-eighth-strength Hoagland solution. Then the plants were washed and the total Se concentration was determined in all plant parts, as described below.
After the Se-volatilization measurements, all plants were thoroughly washed in running deionized water to remove any Se that was bound to the outside of the roots. The washed plants were dried at 70°C and weighed, and the roots and shoots were ground separately using a Wiley mill (Thomas Scientific). Three replicate plants were used for each treatment in all experiments.
Se Analysis
Se volatilization was measured by placing the plants with their roots in Magenta boxes containing deionized water and Se, with the entire plants in gastight acrylic volatilization chambers (approximately 3 L in volume). A continuous air flow (1.5 L/min) was passed through the chamber by applying suction at the outlet while the incoming air was bubbled into the hydroponic solution. Se volatilization was measured by quantitatively trapping any volatile gases in alkaline peroxide liquid traps, as described previously (Zayed and Terry, 1992). The Se-volatilization chambers were placed in a plant growth chamber with a 24-h photoperiod, and maintained at 25°C and an irradiance of 400 μmol m−2 s−1 photosynthetic photon flux (mainly as fluorescent light with some incandescent light). The connections between the chamber and the glass trap were Teflon tubing. Aliquots of trap solution were kept at 4°C until analysis. The trap-solution samples were heated at 95°C to remove the peroxide. Then, to reduce the Se in the trap solution to selenite, an equal volume of concentrated HCl was added and the solution was heated at 95°C for 30 min. Se concentration was measured by vapor-generation atomic absorption spectroscopy, as described by Mikkelsen (1987). The detection limit of this analytical method was 1.0 μg Se/L. Se dioxide reference solution (Fisher) was diluted in 6 m HCl and used as a standard. All samples were diluted in 6 m HCl to give absorbances in the linear portion of the standard curve. The dried and ground plant tissues were acid digested as described previously (Martin, 1975), after which vapor generation-atomic absorption spectroscopy was used to measure Se concentrations in the acid-digested samples. A wheat flour standard (1.1 mg Se/kg) and a blank were used with all digestions.
Statistical analyses were performed using the JMP IN statistical package (SAS Institute, Cary, NC).
Preparation of Indian Mustard Tissues for XAS Analysis
Leaf and root tissues were collected from Indian mustard plants supplied with 20 μm selenate or selenite for 8 d. The samples were rinsed in deionized water, frozen in liquid nitrogen, ground to a fine texture, then stored at −80°C. Comprehensive XAS analysis of frozen plant tissues was completed at the Stanford Synchrotron Radiation Laboratory on Beam Line 4–1. The electron energy was 3.0 GeV with a current of approximately 50 to 100 mA. X-rays were monochromatized with a Si (111) double-crystal spectrometer detuned 50% for harmonic rejection, with a 1-mm entrance slit that produced a beam of approximately 1-eV band width at the Se K-edge. Frozen tissues were placed in a sample chamber at a 45° angle to the x-ray beam. Fluorescent x-ray spectra of Se in plant tissues and model Se reference compounds, selenate, selenite, and l-SeMet, were collected with a series of replicate scans. Plant tissue Se concentrations ranged well within the resolution range of the XAS technique. The energy positions of all spectra were calibrated against a Se reference foil.
RESULTS
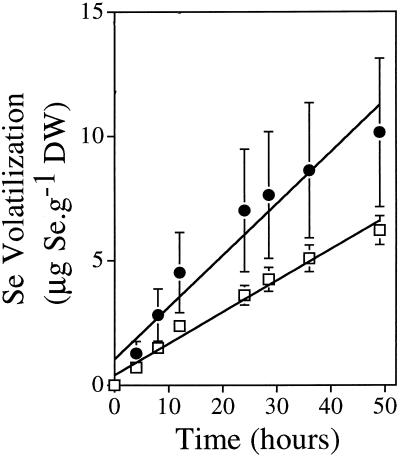
In our first experiment we determined the optimum time period for collection of volatile Se from selenate and selenite. After Indian mustard plants were preincubated for 7 d on 20 μm selenate or selenite, we measured the amounts of Se produced over 4 to 49 h from freshly supplied 20 μm Se. The curves for volatile Se production from selenate and selenite showed similar patterns over time (Fig. 1). The rates of Se volatilization from both Se species were linear over the 49-h time period (r2 = 0.98, P < 0.0001). Therefore, in all of the following experiments volatile Se was collected over the first 24 h.
Figure 1.
Production of volatile Se over 49 h by Indian mustard plants supplied with 20 μm Se as selenate (□) or selenite (•). The plants were pretreated for 7 d with 20 μm of the appropriate Se species. The values shown are the mean of three replicates ±se. DW, Dry weight.
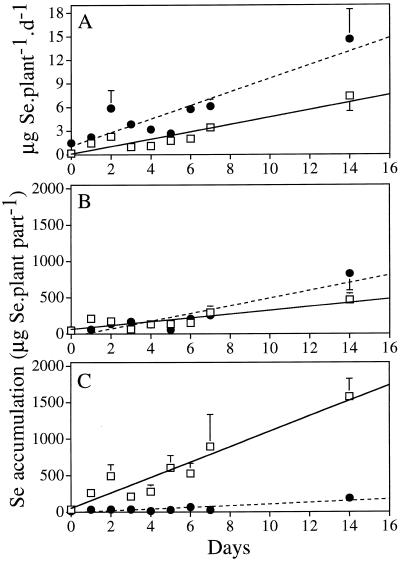
To determine the time-dependent kinetics of Se accumulation and volatilization, Indian mustard plants were preincubated for different time periods (0–14 d) on 20 μm selenate or selenite, after which time Se volatilization was measured over 24 h and the total Se concentration was measured in root and shoot tissues. Se accumulation in tissues and the rate of Se volatilization increased linearly with the length of Se pretreatment for both selenate and selenite (Fig. 2). The rate of Se volatilization from selenite was significantly (approximately 2-fold) higher than from selenate (Fig. 2A); the statistical analyses for the differences between the slopes of the selenate and selenite curves are shown in Table I. Although selenite was volatilized faster than selenate, the rate of uptake was significantly higher for selenate than for selenite (Table I). Consequently, the rate of Se accumulation in the plant (root plus shoot) was significantly higher in plants exposed to selenate compared with selenite (Fig. 2, B and C; Table I). The allocation of Se in the plant differed between selenate and selenite. The total Se accumulated in roots was not significantly different in plants supplied with selenate or selenite (Fig. 2B; Table I); in shoots, however, selenate-supplied plants had a Se content that was significantly (10-fold) higher than selenite-supplied plants (Fig. 2C; Table I).
Figure 2.
Time-dependent kinetics of Se volatilization (A) and amounts of Se accumulated in roots (B) and shoots (C) of Indian mustard plants supplied with 20 μm selenate (□) or selenite (•). The values shown are the mean of three replicates ±se. The average dry weight of plants used in this experiment was 4 g (the average root dry weight was 1.2 g). The results of the statistical analyses for the data presented in this figure may be found in Table I.
Table I.
Statistical comparison of the Se accumulation and volatilization curves shown in Figure 2 for Indian mustard supplied with selenate or selenite
| Se Volatilization and Accumulation | Selenate Slope | Selenite Slope | Selenate versus Selenite |
|---|---|---|---|
| Se volatilization (μg Se plant d−1) | 0.469 (0.0003) | 0.859 (0.0007) | 0.0029 |
| Root Se accumulation (μg Se root d−1) | 25.9 (0.0039) | 52.85 (0.0005) | NSa(0.629) |
| Shoot Se accumulation (μg Se shoot d−1) | 105.3 (<0.0001) | 11.2 (0.0028) | 0.0075 |
| Total plant Se accumulation (μg Se plant d−1) | 131.2 (0.0002) | 64.04 (0.0006) | 0.0035 |
The slopes of the curves are shown with their accompanying P values in parentheses. Significant differences between the slopes of the curves for selenate and selenite are presented in column 4.
NS, Not significant.
In plants supplied with selenate, the Se-volatilization rates were linearly correlated with the Se concentration in roots and in shoots (P < 0.01, data not shown). When plants were supplied with selenite, the Se-volatilization rates were linearly correlated only with the Se concentration in roots (P < 0.01). The tissue Se concentrations in roots and shoots were linearly correlated with each other for all plants (P < 0.01, data not shown).
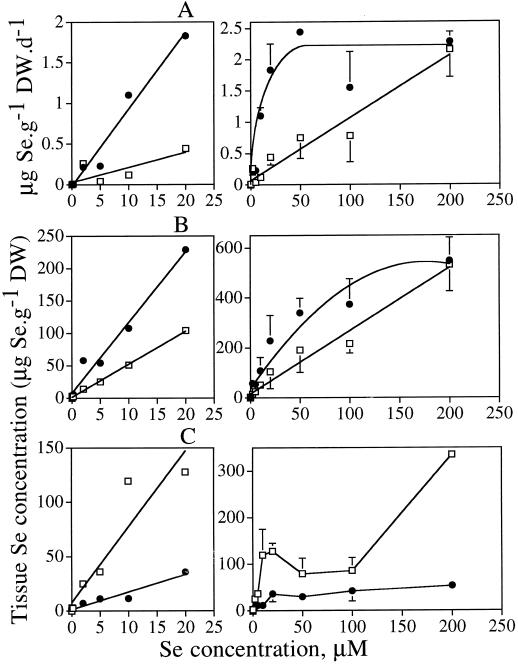
We subsequently investigated the dependence of Se volatilization and accumulation on the concentration of selenate or selenite in the hydroponic medium. Indian mustard plants were preincubated for 7 d on different concentrations of selenate or selenite (0.02–200 μm), after which Se volatilization was measured over 24 h and the total Se concentration was measured in root and shoot tissues. The different concentrations of selenate or selenite did not have any visible detrimental effects on the plants, except at 200 μm, when symptoms of chlorosis were observed. Se volatilization showed different concentration-dependent kinetics curves for selenate and selenite (Fig. 3A). The rate of Se volatilization from selenite-supplied plants showed a Michaelis-Menten-type correlation with Se concentrations (r2 = 0.895, P < 0.0001), increasing linearly with Se concentration up to 20 μm, and then leveling off at higher external selenite concentrations. The rate of Se volatilization from selenate-supplied plants, however, showed a linear concentration dependency (r2 = 0.704, P < 0.0001), and the rate of volatilization did not saturate at the highest selenate concentration tested (200 μm). The change in rate of Se volatilization with external Se concentration, as judged from the slope of the 0 to 20 μm dose-response curve (Fig. 3A, left figure), was about 3 times higher from selenite than from selenate.
Figure 3.
Concentration-dependent kinetics of Se volatilization (A) and Se concentration in roots (B) and shoots (C) of Indian mustard supplied with selenate (□) or selenite (•). The values shown are the mean of three replicates ±se. All selenate versus selenite comparisons gave P values of less than 0.05. The average dry weight (DW) of the plants treated with 0.02, 0.2, 2, 5, 10, 20, 50, 100, or 200 μm selenate were: 2.82, 3.73, 3.19, 5.03, 4.33, 3.67, 3.40, 4.20, and 1.83 g, respectively. For selenite-treated plants these values were: 2.93, 2.57, 3.11, 2.50, 4.05, 2.21, 2.10, 2.32, and 2.43 g, respectively.
The root-tissue Se concentration showed a Michaelis-Menten-type concentration-dependent kinetics curve for selenite-supplied plants (r2 = 0.84; P < 0.0001), but a linear correlation for plants supplied with selenate (r2 = 0.54, P < 0.0001) (Fig. 3B). In shoot tissue the concentration-response curves were more complex (Fig. 3C). Se accumulation in root tissue was comparable in selenate- and selenite-supplied plants (Fig. 3B), whereas Se accumulation in shoot tissue was much higher from selenate than from selenite (Fig. 3C).
In the 0 to 20 μm range, the Se concentrations in roots and shoots of plants treated with selenate or selenite all increased linearly with external Se concentration (P < 0.005). The slopes of these lines were different for selenate and selenite: In roots the rate of accumulation was about 2-fold faster for selenite-supplied plants (Fig. 3B, left figure), whereas in shoots, the rate of accumulation was roughly 3-fold faster for selenate-supplied plants (Fig. 3C, left figure). The Se distribution in roots and shoots was different when plants were supplied with different concentrations of selenate: At 0 to 20 μm external selenate, most of the Se was translocated to the shoot, but at 50 to 200 μm, most of the Se remained in the root. In selenite-supplied plants only approximately 10% of the Se was translocated to the shoot at all external selenite concentrations.
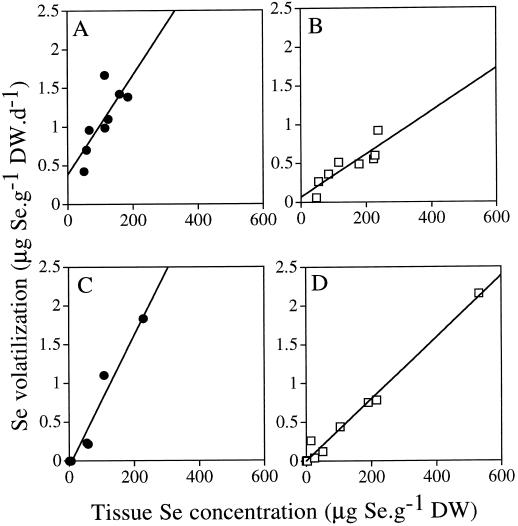
In all plants used for the concentration-dependent kinetics experiment, the rates of Se volatilization were linearly correlated with the Se concentration in both roots and shoots (P < 0.001). The Se concentrations in roots and shoots were also correlated with each other (P < 0.01). The correlations between Se volatilization and root Se concentration from this experiment and the pretreatment experiment are presented in Figure 4 and are discussed below.
Figure 4.
Correlation between Se-volatilization rates and total Se concentrations in roots of plants fed with selenate (□) or selenite (•). A and B, Data from preincubation experiment (Fig. 2); C and D, data from concentration experiment (Fig. 3). In C, only the points from the linear part of the curve were used. Both selenate versus selenite comparisons gave P values of less than 0.05. DW, Dry weight.
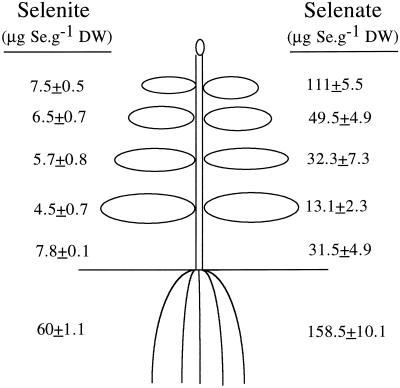
In another approach to investigate the allocation of Se in plants, Indian mustard plants were supplied with 20 μm selenate or selenite for 1 d, after which the Se concentration in different plant parts was measured. As shown in Figure 5, selenate was taken up to a much higher extent than selenite over this 1-d period, and a large part of the selenate was translocated to the shoot, whereas selenite stayed mainly in the root.
Figure 5.
Schematic representation of an Indian mustard plant showing the Se concentrations in leaves of different ages and in stems and roots after 1 d of treatment with 20 μm selenate or selenite. Values shown are the averages ±se of three replicates. DW, Dry weight.
To investigate which chemical form of Se was accumulated in shoots and roots of plants exposed to selenate or selenite, XAS was performed on roots and shoots of Indian mustard plants supplied with 20 μm selenate or selenite for 8 d. Detailed information regarding the electronic structure of Se, i.e. oxidation state and local coordination symmetry (Kutzler et al., 1980), was obtained when the XANES of model Se reference spectra were compared with XANES of Se in leaf and root tissues. In Figure 6A, the K-edge XANES of Se in Indian mustard leaf and root tissues supplied with selenate were very similar to each other and to the selenate reference. Thus, we can conclude that when selenate was supplied in the nutrient solution, Se was also accumulated in leaf and root tissues as selenate.
Figure 6.
A, Se K-edge XANES for leaf and root tissues of Indian mustard supplied with selenate compared with a selenate standard reference. B, Se K-edge XANES for Indian mustard leaf and root tissues supplied with selenite compared with selenite and l-SeMet standard references.
When selenite was supplied, the K-edge XANES of Se in Indian mustard leaf and root tissues were found to be similar to each other but very different from the selenite reference (Fig. 6B). XANES spectra of Se in leaf and root tissues were also compared with XANES of Se in the l-SeMet reference and were found to be quite similar. Apparently, when selenite was supplied in the nutrient solution, Se was accumulated in leaf and root tissues as an organo-Se compound such as l-SeMet.
DISCUSSION
The rate of Se volatilization from selenite was higher than that from selenate in all of the experiments presented here. This finding is in agreement with other reports (Terry and Zayed, 1994; Zayed et al., 1998). To understand the basis for this difference, we focused on the site of volatilization, the root (Zayed and Terry, 1994). Se volatilization depended on the Se concentration in the root, and on the conversion rate of inorganic Se to volatile organo-Se forms such as SeMet. The Se concentration in the root, in turn, was controlled by the rate of uptake into the root and the rate of translocation to the shoot. Our results show that uptake of selenate into roots was much faster than uptake of selenite, since the total amount of Se accumulated in the plant (root plus shoot) was about 10-fold higher from selenate than from selenite (Fig. 2, B and C; Table I). In spite of the faster uptake rate for selenate, the Se accumulation in the root was not statistically different from that of selenite-supplied plants after preincubation with Se for 7 d (Table I). This is because most of the selenate was translocated quickly to the shoot, whereas selenite-derived Se stayed mainly in the root (Fig. 2). This phenomenon has also been observed in bean plants treated with selenate or selenite at 50 μm for 3 h (Arvy, 1993), as well as in detopped tomato plants treated for 4 h (Asher et al., 1967).
Se volatilization was linearly correlated with root-tissue Se concentration, both for selenate and selenite (Fig. 4). This suggests that the uptake of Se limited volatilization for both Se species. However, since the root Se concentrations were similar in selenate- and selenite-supplied plants after a 7-d preincubation with Se, the different volatilization rates from selenate and selenite cannot be attributed to differences in root Se concentration, but instead must be due to different conversion rates of selenate and selenite to volatile forms. Our speciation studies showed that plants supplied with selenite contained a more readily volatilizable form of Se than selenate-supplied plants. When Indian mustard plants were treated with selenate, the predominant form of Se found in the tissue was still selenate. In contrast, when the plants were supplied with selenite, they accumulated a SeMet-like organo-Se species (Fig. 6). Our findings are consistent with other reports. Gissel-Nielson (1979) reported that in maize, selenate-derived Se was transported in xylem as selenate, whereas selenite-derived Se was quickly converted to a Se-amino acid before transport. Asher et al. (1967) reported that selenate-derived Se was translocated in the xylem of tomato plants as selenate, whereas selenite-derived Se was transported as selenate or in an unidentified, organic form. Our other work with broccoli showed similar Se-speciation results, and these plants volatilized SeMet readily at rates that were about 100-fold higher than those from inorganic Se species (Zayed et al., 1998). Similarly, Zhang and Moore (1997) recently showed that Se volatilization depended more on the concentration of dissolved, organic Se (e.g. SeMet) than on inorganic Se. Thus, the higher rates of volatilization that we measured from selenite (compared with selenate) can be explained by the fact that selenite was rapidly converted to easily volatilizable organo-Se, whereas the rate of conversion of selenate to organo-Se was much slower.
Se accumulation and volatilization were linearly correlated with the external Se concentration over the whole concentration range tested for selenate-treated plants, but for selenite-supplied plants they were only linear up to an external Se concentration of 20 μm (approximately 300 μg mL−1 Se in root), above which there was no significant increase (Fig. 3, A and B). Possible explanations for this plateau are: (a) the saturation of a limiting step, (b) a feedback mechanism by which selenite uptake is repressed by the high concentration of Se accumulated inside the plant tissue, or (c) an inhibitory effect caused by the organo-Se that accumulates in selenite-treated plants. Organo-Se is more toxic to plants than selenite, which is more toxic than selenate (Smith and Watkinson, 1984).
Since the total amount of Se accumulated per plant was higher in plants supplied with selenate than with selenite, Indian mustard would be expected to be more efficient for the phytoextraction of selenate than selenite. Indian mustard grown in selenate-treated soil accumulated more Se than when it was grown in selenite-treated soil (Banuelos et al., 1995). Furthermore, Indian mustard has been successfully used as a phytoremediator of selenate from agricultural soils collected from the San Joaquin Valley; in three crops, Indian mustard removed 60% of soil Se (Banuelos and Meek, 1990). Field experiments with Indian mustard showed that in the first year the plants removed 48% of total Se from selenate-contaminated agricultural soil (Banuelos et al., 1993).
If we can pinpoint the rate-limiting factors for Se volatilization from selenate and selenite, we can improve Se volatilization and thus Se phytoremediation efficiency, for instance, through genetic engineering or breeding. Since external and root Se concentrations were correlated with volatilization, uptake appears to be a limiting factor for Se volatilization from both selenate and selenite. Selenate is generally thought to be taken up actively by a sulfate-transporter protein (Legget and Epstein, 1956; Anderson, 1993). Therefore, one approach to enhance Se volatilization from selenate would be to overexpress sulfate permease, thereby increasing selenate uptake and volatilization. The uptake mechanism of selenite is not clear, but has been suggested to be passive (Bange, 1973; Arvy, 1993). However, if a selenite-transporter protein should exist, its overexpression would be expected to increase selenite uptake and volatilization.
Since selenate accumulated in selenate-supplied plants, whereas selenite was readily metabolized to organic Se, a major rate-limiting step for Se volatilization from selenate (in addition to uptake) appears to be the reduction of selenate. The enzyme responsible for the reduction of selenate is ATP sulfurylase (Anderson, 1993), which thus appears to be an important rate-limiting enzyme in the selenate-volatilization pathway. Therefore, a potential genetic engineering approach to increasing Se volatilization from selenate would be to overexpress the ATP sulfurylase enzyme. The root-shoot translocation of selenate probably also limits Se volatilization. As yet, it is not known which enzymes and genes are involved in selenate translocation.
For selenite volatilization, one of the final methylation steps from SeMet to DMSe may be rate limiting (in addition to uptake), since SeMet accumulated in selenite-supplied plants. Thus, another possible approach for improving Se volatilization from selenite would be to overexpress enzymes responsible for the methylation of SeMet (e.g. S-adenosylmethionine:methylmethionine transferase); these genes have not yet been isolated.
In conclusion, we used time- and concentration-dependent kinetics to show that the two Se species, selenate and selenite, show different rates of uptake, translocation, assimilation, and volatilization in Indian mustard. We identified rate-limiting steps in Se accumulation and volatilization, and a difference in Se speciation in selenate- and selenite-supplied plants. This information will be useful for improving the efficiency of Se phytoremediation.
ACKNOWLEDGMENTS
The authors thank Farrel W. Lytle for assistance and support in XAS data collection and analysis, Adel Zayed for a critical review of the manuscript, and the U.S. Department of Energy and the Stanford Synchrotron Radiation Laboratory for beam time and on-line support.
Abbreviations:
- DMSe
dimethylselenide
- SeMet
selenomethionine
- XANES
x-ray absorption near-edge spectra
- XAS
x-ray absorption spectroscopy
Footnotes
This work was supported by the Electric Power Research Institute (grant nos. W08021-30 and W04163 to N.T.) and by the Stanford Synchrotron Radiation Laboratory (grant no. 2413).
LITERATURE CITED
- Anderson JW. Selenium interactions in sulfur metabolism. In: De Kok LJ, editor. Sulfur Nutrition and Assimilation in Higher Plants: Regulatory, Agricultural and Environmental Aspects. The Hague, The Netherlands: SPB Academic Publishing; 1993. pp. 49–60. [Google Scholar]
- Arvy MP. Selenate and selenite uptake and translocation in bean plants (Phaseolus vulgaris) J Exp Bot. 1993;44:1083–1087. [Google Scholar]
- Asher CJ, Evans CS, Johnson CM. Collection and partial characterization of volatile selenium compounds from Medicago sativa L. Aust J Biol Sci. 1967;20:737–748. [Google Scholar]
- Atkinson R, Aschmann SM, Hasegawa D, Thompson-Eagle ET, Frankenberger WT., Jr Kinetics of the atmospherically important reactions of dimethyl selenide. Environ Sci Technol. 1990;24:1326–1332. [Google Scholar]
- Bange GGJ. Diffusion and absorption of ions in plant tissue: the role of the root cortex cells in ion absorption. Acta Bot Neerl. 1973;22:529–542. [Google Scholar]
- Banuelos GS, Cardon G, Mackey B, Ben-Asher J, Wu L, Beuselinck P, Akohoue S, Zambrzuski S. Boron and selenium removal in boron laden soils by four sprinkler irrigated plant species. J Environ Qual. 1993;22:786–792. [Google Scholar]
- Banuelos GS, Mead R, Wu L, Beuselinck P, Akohoue S. Differential selenium accumulation among forage plant species from soils amended with selenium-enriched plant tissue. J Soil Water Conserv. 1992;47:338–342. [Google Scholar]
- Banuelos GS, Meek DW. Accumulation of selenium in plants grown on selenium-treated soil. J Environ Qual. 1990;19:772–777. [Google Scholar]
- Banuelos GS, Schrale G. Crop selection for removing selenium from the soil. Calif Agric. 1989;43:19–20. [Google Scholar]
- Banuelos GS, Terry N, Zayed A, Wu L (1995) Managing high soil Se with phytoremediation. In GE Schuman, GF Vance GF, eds, Selenium, Mining, Reclamation, and Environmental Impact. Proceedings of the 12th Annual Meeting of the American Society for Surface Mining and Reclamation. American Society for Surface Mining and Reclamation, Gillete, WY, pp 394–405
- Banuelos GS, Zayed A, Terry N, Mackey B, Wu L, Akohoue S, Zambrzuski S. Accumulation of selenium by different plant species grown under increasing salt-regimes. Plant Soil. 1997;183:49–59. [Google Scholar]
- Duckart EC, Waldron LJ, Doner HE. Selenium uptake and volatilization from plants growing in soil. Soil Sci. 1992;153:94–99. [Google Scholar]
- Duda PJ (1992) Chevron's Richmond Refinery Water Enhancement Wetland. Technical report submitted to the Regional Water Quality Control Board, Oakland, CA
- Ganther HE, Levander OA, Saumann CA. Dietary control of selenium volatilization in the rat. J Nutr. 1966;88:55–60. doi: 10.1093/jn/88.1.55. [DOI] [PubMed] [Google Scholar]
- Gissel-Nielsen G (1979) Uptake and translocation of selenium-75 in Zea mays. In Isotopes and Radiation in Research on Soil-Plant Relationships. International Atomic Energy Association, Vienna, Austria, pp 427–436
- Hansen D, Duda P, Zayed AM, Terry N. Selenium removal by constructed wetlands: role of biological volatilization. Environ Sci Technol. 1998;32:591–597. doi: 10.1021/es0260216. [DOI] [PubMed] [Google Scholar]
- Hoagland D, Arnon DI (1938) The water culture method for growing plants without soil. Bull Calif Agric Stat 346
- Kutzler FW, Natoli CR, Misemer DK, Doniach S, Hodgson KO. Use of one-electron theory for the interpretation of near edge structure in K-shell x-ray absorption spectra of transition metal complexes. J Chem Phys. 1980;73:3274–3288. [Google Scholar]
- Lauchli A. Selenium in plants: uptake, functions, and environmental toxicity. Bot Acta. 1993;106:455–468. [Google Scholar]
- Leggett JE, Epstein E. Kinetics of sulfate adsorption by barley roots. Plant Physiol. 1956;31:222–226. doi: 10.1104/pp.31.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BG, Johnson CM, Delwiche CC. Release of volatile selenium compounds by plants: collection procedures and preliminary observations. J Agric Food Chem. 1966;14:638–640. [Google Scholar]
- Martin TD. Determining selenium in wastewater sediment and sludge by flameless atomic absorption. Atomic Absorption Newsletter. 1975;14:109–116. [Google Scholar]
- McConnell KP, Portman OW. Toxicity of dimethyl selenide in the rat and mouse. Proc Soc Exp Biol Med. 1952;79:230–231. doi: 10.3181/00379727-79-19333. [DOI] [PubMed] [Google Scholar]
- McNeal JM, Balisteri LS (1989) Geochemistry and occurrence of selenium, an overview: selenium in agriculture and the environment. Soil Science Society of America Special Publication No. 23, Madison, WI, pp 1013
- Mikkelsen R (1987) Materials and methods for determination of selenium in plants and soils. In Workshop on Analytical Methods for Selenium, Other Trace Elements, and on Quality Control and Quality Assurance, Sacramento, CA, pp 63–66
- Smith GS, Watkinson JH. Selenium toxicity in perennial ryegrass and white clover. New Phytol. 1984;97:557–564. [Google Scholar]
- Terry N, Carlson C, Raab TK, Zayed AM. Rates of selenium volatilization among crop species. J Environ Qual. 1992;21:341–344. [Google Scholar]
- Terry N, Zayed AM (1994) Selenium volatilization by plants. In WT Frankenberger Jr, S Benson, eds, Selenium in the Environment. Marcel Dekker, Inc., New York, pp 343–369
- Terry N, Zayed AM (1998) Phytoremediation of selenium. In WT Frankenberger Jr, RA Engberg, eds, Environmental Chemistry of Selenium. Marcel Dekker, Inc., New York, pp 633–657
- Velinsky D, Cutter GA. Geochemistry of selenium in a coastal salt marsh. Geochim Cosmochim Acta. 1991;55:179–191. [Google Scholar]
- Wilber CG. Toxicology of selenium: a review. Clinic Toxicol. 1980;17:171–230. doi: 10.3109/15563658008985076. [DOI] [PubMed] [Google Scholar]
- Wu L, Huang ZH, Burau RG. Selenium accumulation and selenium-salt cotolerance in five grass species. Crop Sci. 1988;28:517–522. [Google Scholar]
- Wu L, Van Mantgem PJ, Guo X. Effects of forage plant and field legume species on soil selenium redistribution, leaching, and bioextraction in soils contaminated by agricultural drainage water. Arch Environ Contam Toxicol. 1996;31:329–338. [Google Scholar]
- Zayed AM, Lytle CM, Terry N (1998) Accumulation and volatilization of different chemical species of selenium by plants. Planta (in press)
- Zayed AM, Terry N. Selenium volatilization in broccoli as influenced by sulfate supply. J Plant Physiol. 1992;140:646–652. [Google Scholar]
- Zayed AM, Terry N. Selenium volatilization in roots and shoots: effects of shoot removal and sulfate level. J Plant Physiol. 1994;143:8–14. [Google Scholar]
- Zhang Y, Moore JN. Environmental conditions controlling selenium volatilization from a wetland system. Environ Sci Technol. 1997;31:511–517. [Google Scholar]
- Zieve R, Peterson PJ. Volatilization of selenium from plants and soil. Science of the Total Environment. 1984;32:197–202. [Google Scholar]