Abstract
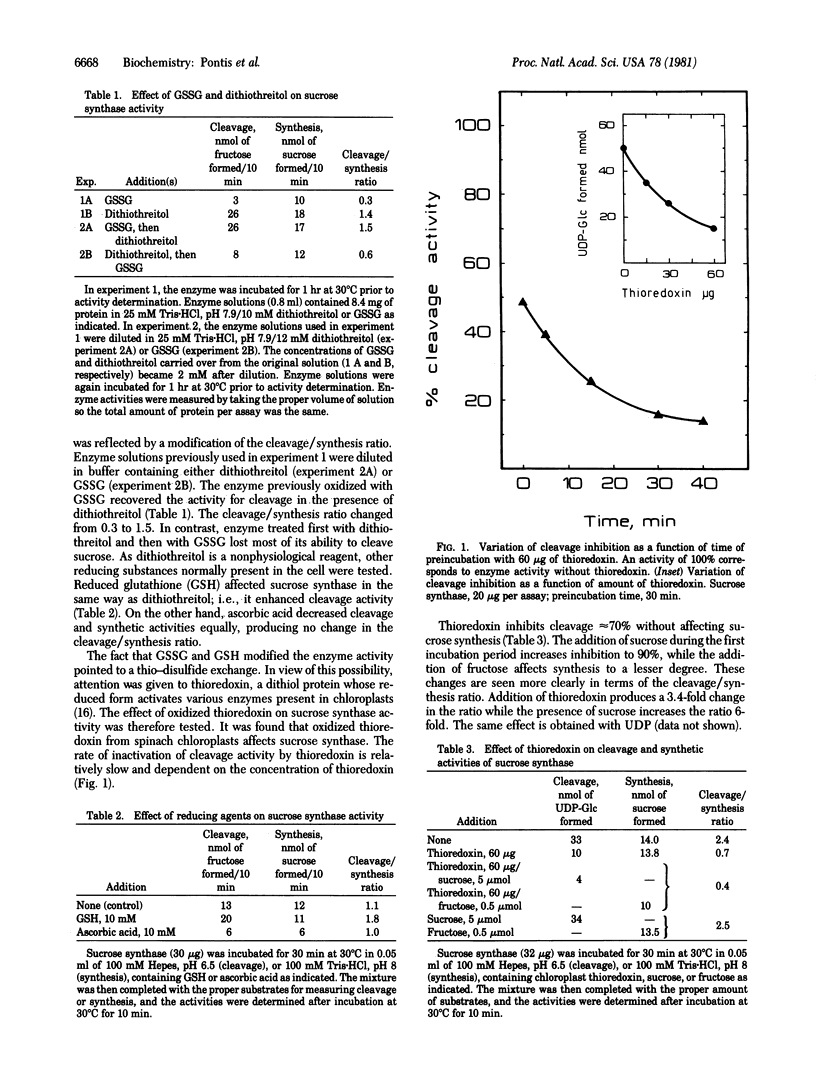
Sucrose synthase (UDPglucose:D-fructose 2-alpha-D-glucosyltransferase, EC2.4.1.13), which catalyzes the synthesis and cleavage of sucrose, exhibits differences in some properties between the two reactions. When enzyme previously incubated with oxidized glutathione or oxidized thioredoxin was used, sucrose cleavage was inhibited whereas sucrose synthesis proceeded at a normal rate. Sucrose cleavage activity could be restored by incubation with dithiothreitol or reduced glutathione. The thioredoxin effect was influenced by the presence of cleavage reaction substrates--i.e., sucrose and UDP. Thioredoxin action was rather slow compared with the catalytic reaction. These findings may have important implications for understanding the metabolic role of sucrose synthase and oxidized thioredoxin. Theoretically, the fact that an enzyme catalyzing a reversible reaction is inhibited in one direction only suggests that a modification in the enzyme affinities for its substrates must have occurred.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Delmer D. P. The Regulatory Properties of Purified Phaseolus aureus Sucrose Synthetase. Plant Physiol. 1972 Oct;50(4):469–472. doi: 10.1104/pp.50.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDEN C. TREATMENT OF ENZYME KINETIC DATA. I. THE EFFECT OF MODIFIERS ON THE KINETIC PARAMETERS OF SINGLE SUBSTRATE ENZYMERS. J Biol Chem. 1964 Oct;239:3522–3531. [PubMed] [Google Scholar]
- Grimes W. J., Jones B. L., Albersheim P. Sucrose synthetase from Phaseolus aureus seedlings. J Biol Chem. 1970 Jan 10;245(1):188–197. [PubMed] [Google Scholar]
- Milner Y., Avigad G. Thymidine diphosphate nucleotides as substrates in the sucrose synthetase reaction. Nature. 1965 May 22;206(4986):825–825. doi: 10.1038/206825a0. [DOI] [PubMed] [Google Scholar]
- Pontis H. G., Wolosiuk R. A. Studies on sucrose synthetase. I. Effect of trypsin on the cleavage activity. FEBS Lett. 1972 Nov 15;28(1):86–88. doi: 10.1016/0014-5793(72)80683-5. [DOI] [PubMed] [Google Scholar]
- Pressey R. Potato sucrose synthetase: purification, properties, and changes in activity associated with maturation. Plant Physiol. 1969 May;44(5):759–764. doi: 10.1104/pp.44.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla R. N., Sanwal G. G. Studies on UDP-glucose: D-fructose 2-glucosyltransferase from tapioca tuber. Arch Biochem Biophys. 1971 Jan;142(1):303–309. doi: 10.1016/0003-9861(71)90288-8. [DOI] [PubMed] [Google Scholar]
- Slabnik E., Frydman R. B., Cardini C. E. Some Properties of Potato Tuber UDPGd-fructose-2-glucosyltransferase (E.C. 2.4.1.14) and UDPGd-fructose-6-phosphate-2-glucosyltransferase (E.C. 2.4.1.13). Plant Physiol. 1968 Jul;43(7):1063–1068. doi: 10.1104/pp.43.7.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosiuk R. A., Pontis H. G. The role of sucrose and sucrose synthetase in carbohydrate plant metabolism. Mol Cell Biochem. 1974 Sep 30;4(2):115–123. doi: 10.1007/BF01770292. [DOI] [PubMed] [Google Scholar]
- Wolosiuk R. W., Pontis H. G. Evidence of the existence of two forms of sucrose synthetase. FEBS Lett. 1971 Sep 1;16(4):237–240. doi: 10.1016/0014-5793(71)80359-9. [DOI] [PubMed] [Google Scholar]


