Abstract
Objective
Vasoactive medications improve hemodynamics after cardiac surgery but are associated with high metabolic and arrhythmic burdens. The vasoactive-inotropic score was developed to quantify vasoactive and inotropic support after cardiac surgery in pediatric patients but might similarly be useful in adults. Accordingly, we examined the time course of this score in a substudy of the Biventricular Pacing After Cardiac Surgery trial. We hypothesized that the score would be lower in patients randomized to biventricular pacing.
Methods
Fifty patients selected for increased risk of left ventricular dysfunction after cardiac surgery and randomized to temporary biventricular pacing or standard of care (no pacing) after cardiopulmonary bypass were studied in a clinical trial between April 2007 and June 2011. Vasoactive agents were assessed after cardiopulmonary bypass, after sternal closure, and 0–7 hours after admission to the intensive care unit.
Results
Over the initial three collection points after cardiopulmonary bypass (mean duration 131 minutes), mean vasoactive-inotropic score decreased in the biventricular pacing group from 12.0±1.5 to 10.5±2.0 and increased in the standard of care group from 12.5±1.9 to 15.5±2.9. Using a linear mixed effects model, this slopes of the time courses were statistically significant (p=0.02) and remained so for the first hour in the intensive care unit. However, the difference was no longer significant beyond this point (p=0.26).
Conclusions
Vasoactive-inotropic score decreases in patients undergoing temporary biventricular pacing in the early postoperative period. Future studies are needed to assess the impact of this effect on arrhythmogenesis, morbidity, mortality, and hospital costs.
INTRODUCTION
The inotropic score was introduced by Wernovsky et al. as a quantitative measure of cardiovascular support received by neonates after the arterial switch operation (1). The score has since been used to assess illness severity in infants and neonates undergoing cardiothoracic surgery (2–8). Recently Gaies et al. introduced the vasoactive-inotropic score (VIS), which expanded on the inotrope score to include other vasoactive agents commonly used in intensive care units (ICUs) (9). The authors showed that VIS was superior to the older inotrope score as a surrogate outcome measure for infants undergoing cardiac surgery. However, VIS has not been evaluated in the adult population as a measure of cardiovascular support or severity of illness after cardiopulmonary bypass (CPB).
Currently, vasoactive and inotropic medications are useful for the treatment of hypotension and low output states after cardiac surgery. These agents may improve cardiac output but at the significant costs of increases in myocardial oxygen consumption, arrhythmias, systemic hypoperfusion, and risk of myocardial ischemia and necrosis (10–13).
Biventricular pacing (BiVP) is an established therapy for select patients with chronic congestive heart failure, improving clinical end points (symptoms, exercise capacity, quality of life, survival) and echocardiographic end points (systolic function, left ventricular size, mitral regurgitation) (14–17). Temporary BiVP has also been shown to improve intraoperative cardiac output in patients undergoing cardiac surgery (18–22). One unexplored benefit of BiVP is its potential to decrease the use of inotropes and vasoactive medications after cardiac surgery. BiVP does not increase myocardial oxygen consumption and, thus, may provide a safer alternative to inotropic medications (11).
The Biventricular Pacing After Cardiac Surgery (BiPACS) trial is a randomized clinical trial studying the effect of optimized temporary BiVP in selected patients undergoing cardiac surgery (18–21). Patients undergo BiVP optimization at three time points in the postoperative period and are randomized to continuous optimized BiVP or standard of care (no pacing). The central hypothesis is that cardiac index will increase an average of 15% in the optimized BiVP group.
In this BiPACS substudy, we hypothesized that temporary BiVP would lower the perioperative requirements for vasoactive medication support, as measured by VIS. Although temporary BiVP has been shown to improve postoperative hemodynamics, there have not been any studies investigating whether it reduces the need to administer perioperative inotropic agents.
MATERIALS AND METHODS
Study Design
The BiPACS protocol was approved by the Institutional Review Board for Human Subject Research at Columbia University Medical Center and conducted under an Investigational Device Exemption from the Food and Drug Administration. Attending surgeon permission was obtained before approaching patients for enrollment. Written consent was obtained from all patients.
This unblinded observational study was conducted using a subgroup analysis for eligible patients enrolled in the BiPACS trial between October 1, 2007 and June 30, 2011. Patient flow for the BiPACS trial and this substudy cover recruitment during this time period. The number of patients undergoing cardiac surgery at Columbia University Medical Center and screened for the BiPACS trial was 5101. Of these, 764 were eligible for the BiPACS protocol. One hundred and five were enrolled. The study was initiated in 57 patients, who were allocated to testing in Phase I and were subsequently randomized to either BiVP (experimental group) or SOC (control group) as per the BiPACS protocol.
Patients were randomized after Phase I testing confirmed that BiVP was feasible. To avoid imbalances using simple randomization, patients were randomized to the two arms using randomly permuted blocks of four, six, and eight. A treatment allocation ratio of one was used, expecting each group to be of equal size. Phase 1 testing was done in all patients prior to randomization and has been described elsewhere (21). Group assignment was determined by forms in sealed envelopes opened at the randomization point. All required forms were prepared prior to the enrollment of the first patient. 7 patients were eliminated from this substudy because they were removed from the BiPACS trial before the start of Phase III. In the end, 24 patients randomized to the BiVP group and 26 patients randomized to the SOC group were available and included in this substudy. The baseline clinical characteristics of these 50 patients are displayed in Table 1.
Table 1.
Baseline characteristics
| Characteristic | Randomized to BiVP (n=24) | Randomized to NoP (n=26) | p-value | ||
|---|---|---|---|---|---|
| Mean age (range) | 69.0 | (49–85) | 66.8 | (48–87) | 0.46 |
| Female, % | 17% | 23% | |||
| Median BMI (range) | 25.3 | (19–46) | 26.4 | (16–41) | 0.77 |
| Mean Weight (kg) | 78.5 | (48–123) | 76.4 | (50–104) | 0.66 |
| Mean CPB time (minutes) | 133. 0 | (57–325) | 132. 5 | (68–239) | 0.83 |
| Mean X-Clamp time (minutes) | 90.9 | (0–235) | 77.8 | (0–210) | 0.35 |
| Type of Surgery1 | |||||
| CABG (32) | 13 | 19 | 0.17 | ||
| Aortic Valve Replacement (27) | 15 | 12 | 0.26 | ||
| Mitral Valve Replacement (21) | 11 | 10 | 0.61 | ||
Several patients underwent combined surgery
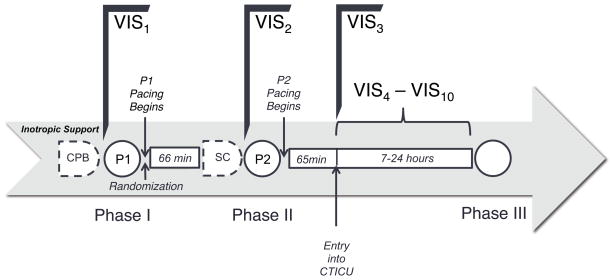
BiPACS Optimization Protocol (Figure 1)
Figure 1. Time Course of BiPACS Protocol.
During Phase I, after weaning from cardiopulmonary bypass (CPB), the protocol maximizing cardiac output was performed. The optimal paced setting, as determined by Phase I optimization, was designated P1. Patients were randomized after Phase I into either the BiVP arm or the standard of care arm. Patients in the BiVP arm were paced under P1 until Phase II. During Phase II, after sternal closure (SC), the protocol maximizing mean arterial pressure was determined and designated P2. Pacing was then resumed using P2 in the BiVP arm until Phase III optimization, at which point the study period was concluded. VIS was calculated before randomization (VIS1), before Phase II (VIS2), upon relocation from the operating room to the ICU (VIS3), and hourly when the patient was in the ICU (VIS4 – VIS10).
The BIPACS trial is delineated by optimizations at three distinct time points (21) to determine the optimal pacing site, AVD and VVD. Phase I optimization is performed after the patient is separated from cardiopulmonary bypass and deemed hemodynamically stable by the clinical team. Phase II optimization is performed during sternal closure at the end of the surgery. Finally, Phase III optimization is performed 8–24 hours after Phase II optimization). Patients are randomized to either the optimized BiVP group or the standard of care (SOC) group at the end of Phase I optimization. Phase I optimization is performed using cardiac output by aortic flow probe, Phase II is performed using mean arterial pressure, and Phase III optimization is performed using a combination of mean arterial pressure and cardiac output via thermodilution. During periods of BiVP optimization (eight minutes during Phase I and 16 minutes during Phase II), the rates of administration of fluids, blood products, anesthetics, and vasoactive medications are held constant, except for windows for adjustment between optimization of individual parameters.
Anesthesia Protocol
General endotracheal anesthesia is utilized for all patients, and dosing is at the discretion of the attending anesthesiologist. Fentanyl and midazolam are used as adjunct agents to attenuate autonomic responses. Inotropes, vasopressors, and vasodilators are administered at the discretion of the attending anesthesiologist, based on hemodynamics and realtime transesophageal echocardiography. In general, norepinephrine infusion is the first line vasoconstrictor, and vasopressin infusion is added if additional vasoconstriction is desirable. The primary inotropic agent is milrinone. The majority of patients undergoing coronary artery bypass grafting with the use of bilateral internal mammary arteries receive a low dose (5mcg/min) nitroglycerin infusion at the conclusion of cardiopulmonary bypass. This infusion is maintained for at least 24 hours postoperatively.
Vasoactive-Inotropic Score
We used a modification of the VIS described by Gaies et al. (9). The score at each time point was based on the concurrent doses and types of inotropic and vasopressor medication being administered. We expanded this formula to include the vasopressor phenylephrine and chose its coefficient in the same manner as originally described by Wernovsky et al. (1). VIS is calculated as follows:
Specific vasoactive agents used are presented in Table 2. We normalized dosages for patient weight as described previously (2,7,8).
Table 2.
Vasoactive Agents used by Phase
| Time | Vasoactive Agent | Randomized to BiVP (n=24) | Randomized to NoP (n=26) | ||
|---|---|---|---|---|---|
| Entering Phase I (OR) | Dopamine | 0 | (0%) | 1 | (4%) |
| Dobutamine | 1 | (4%) | 2 | (8%) | |
| Epinephrine | 2 | (8%) | 3 | (12%) | |
| Milrinone | 14 | (58%) | 17 | (65%) | |
| Norepinephrine | 21 | (88%) | 22 | (85%) | |
| Vasopressin | 18 | (75%) | 21 | (81%) | |
| Phenylephrine | 2 | (8%) | 1 | (4%) | |
| Entering Phase II (OR) | Dopamine | 0 | (0%) | 1 | (4%) |
| Dobutamine | 1 | (4%) | 2 | (8%) | |
| Epinephrine | 2 | (8%) | 3 | (12%) | |
| Milrinone | 14 | (58%) | 17 | (65%) | |
| Norepinephrine | 21 | (88%) | 23 | (88%) | |
| Vasopressin | 17 | (71%) | 22 | (85%) | |
| Phenylephrine | 2 | (8%) | 1 | (4%) | |
| Before Phase III (ICU) | Dopamine | 0 | (0%) | 2 | (8%) |
| Dobutamine | 3 | (13%) | 2 | (8%) | |
| Epinephrine | 3 | (13%) | 5 | (19%) | |
| Milrinone | 14 | (58%) | 17 | (65%) | |
| Norepinephrine | 22 | (92%) | 22 | (85%) | |
| Vasopressin | 19 | (79%) | 23 | (88%) | |
| Phenylephrine | 1 | (4%) | 3 | (12%) | |
VIS1 is calculated on all patients immediately preceding Phase I optimization. VIS2 is calculated immediately preceding Phase II optimization and VIS3 is calculated upon entry into the ICU. VIS4 – VIS10 is then calculated hourly until the end of the study period (immediately preceding Phase III optimization) (Figure 1). The average time between the onset of Phase I and the onset of Phase II was 66 ± 30 minutes, and the average time between the onset of Phase II and entry into the ICU was 65 ± 35 minutes.
VISmax
VIS was calculated for each hour the patient spent in the ICU. VISmax is defined as the highest VIS prior to Phase III.
Other variables
Urine output produced for each patient during cardiac bypass was determined by measuring the volume of urine in the catheter bag upon admission to ICU. The patient’s serum glucose levels were measured hourly in the ICU.
Statistical Analysis
VIS was compared between the BiVP group and the SoC group under linear mixed effects models where VIS was the outcome and pacing group (BiVP versus SoC) and time (since the start of Phase I) were the two main predictors. Pacing group by time interaction was also included in the initial model but excluded from the final model if not significant. The analysis also included the subject random effects to account for within subject correlation on VIS. In addition, the maximum VIS (VISmax) is calculated for each patient after arrival into the ICU and before the start of Phase III. VISmax was compared between the BiVP group and the SoC group using an independent 2-sample T-test. All the analyses were conducted in SAS version 9.2 software (SAS Institute Inc., Cary, NC). A p-value of < 0.05 was considered as statistically significant.
RESULTS
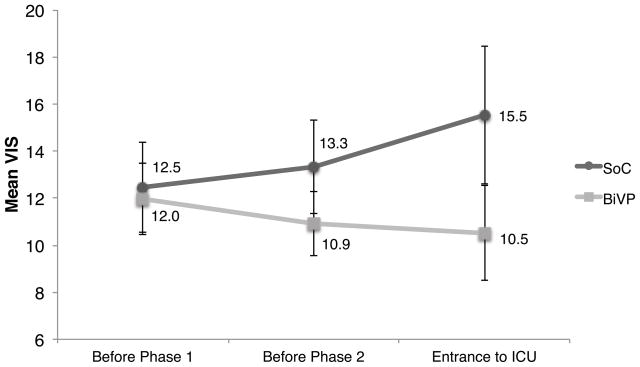
Figure 2 illustrates variation of mean VIS for patients in the temporary BiVP group and in the SOC group from the end of CPB to admission into the ICU. Both groups entered Phase I with similar VIS, but the scores subsequently diverge, increasing in the SOC group and decreasing in the BiVP group. The difference in mean VIS between the SOC and BiVP groups is statistically significant (p=0.02) from the beginning of Phase I until entry into the ICU (mean duration 131 minutes). This represents a significant interaction between pacing group and time. There were no significant differences between the two groups, as shown in Table 1. In particular, patient weight was the same, which was important given the per-weight dosing of medications.
Figure 2. Diverging time course from randomization to ICU admission in the standard of care (SOC, n=26) and biventricular pacing groups (BiVP, n=24).
VIS increased from 12.5±1.9 at randomization to 15.5±2.9 at ICU entry in the SOC group but decreased from 12.0±1.5 to 10.5±2.0 in the BiVP group. The slopes of these VIS-time relations are significantly different by linear mixed effects analysis (p=0.02). The time between Phase I and Phase II averaged 66±30 minutes and 65±35 minutes between Phase II and ICU entry. Pacing was optimized at time points VIS1 and VIS2.
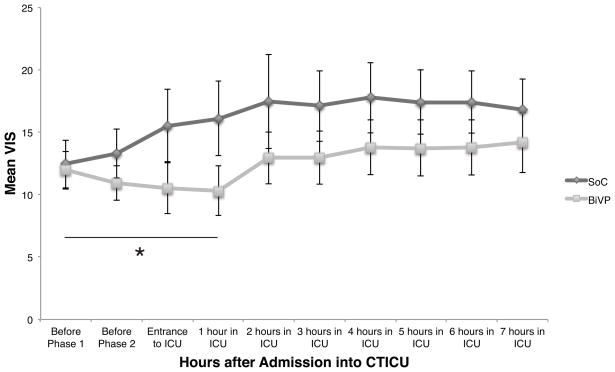
Figure 3 illustrates the time course of mean VIS of the two groups from point of randomization until seven hours after entry into the ICU. Pacing optimization for patients in the BiVP group was not repeated after sternal closure. The observation period in the ICU was based on the protocol of the BiPACS trial which defined the end of the study period as the time point at which the Swann catheter was removed or 24 hours, whichever came first. To standardize the data, VIS was computed only through the first seven hours, which all patients had in common. The data were analyzed under a piecewise linear mixed effects model with the focal time period being one hour after entrance into the ICU. The difference in the change of VIS between the BiVP group and SOC, measured each hour, remains significant up until the first hour in the ICU, which extends the therapeutic effect of BiVP as measured by VIS to a total of three hours after randomization (Figure 3, p=0.0015).
Figure 3. VIS from randomization until 7 hours after ICU entry.
Hourly averages of VIS are shown for the standard of care (SoC, n=26) and biventricular pacing (BiVP, n=24) groups. The slopes of the ViS-time relations are significantly different through the first hour in the ICU by linear mixed effects analysis (p=0.0015). Pacing was optimized at time points VIS1 and VIS2. Data collection was limited to 7 hours because premature Swann-Ganz catheter removal disqualified one patient from continuing study.
Group differences in VIS after one hour in the ICU are no longer statistically significant (p=0.26). This p-value represents an insignificant difference in pacing group effect, although the time effect was significant (coefficient=0.28, p=0.02).
When the mean VISmax is compared between the two groups, there is a trend towards lower VISmax in the BiVP group, but the difference is not significant (BiVP mean 17.6 ± 2.5 versus 22.4 ± 4.3 for SOC, p=0.33).
Urine output from the conclusion of CPB until ICU admission was significantly higher in patients randomized to BiVP than in patients randomized to SOC (7.0±1.7 mL/hour/kg vs. 2.6± 0.5 mL/hour/kg, p=0.012). The concurrent maximal glucose levels were similar in both groups (168±8 mg/dL vs. 181±13 mg/dL respectively, p=0.42).
DISCUSSION
Previous data from the BiPACS trial have demonstrated hemodynamic superiority of temporary BiVP vs, SOC, as judged by increases in cardiac output or mean arterial pressure (21). However, possible decreases in inotrope and vasoactive agent usage associated with temporary BiVP were not examined.
The present results demonstrate decreasing VIS in the temporary BiVP vs. the SOC group over time, suggesting that superior hemodynamics result in the decreased administration of inotropes and vasoactive agents. We have not defined a mechanism for feedback of hemodynamics in these patients on vasoactive drug administration, and use of these agents is not regulated by protocol in our institution. Since weaning of vasoactive drugs is generally based on the time course of arterial pressure, urine output, and cardiac output, we speculate that differences in VIS between the two groups reflect differences in these parameters.
While several previous studies have demonstrated hemodynamic benefits related to temporary BiVP, demonstration of benefits in outcome has been elusive. However, as Gaies et al. reported that VISmax in the ICU can predict morbidity and mortality (9), the present results suggest that such benefits might be demonstrable in a larger study. Inotropes and vasopressors in the perioperative period can support cardiac output, regulate myocardial contractility, improve hemodynamics, and reduce left and right ventricular filling pressures (10). However, inotropes are pro-arrhythmic and also increase MVO2. The latter can induce myocardial ischemia and adversely affect reperfusion injury after CPB (10–13). Under well-controlled conditions, BiVP can increase ventricular function without increasing MVO2 (11), an appealing aspect of this therapy.
The present results demonstrate that adults who receive optimized temporary BiVP require lower levels of vasoactive and inotropic support during the early postoperative period (Figures 2 and 3). This could reflect increased cardiac output in temporary BiVP vs. SOC patients. Since inotropes are linked to arrhythmogenesis, reduced usage during the early postoperative period could decrease the patient’s risk of a complicating arrhythmia. The reduced use of inotropes is further beneficial by reducing MVO2 and thus lowing the chance of reperfusion injury after CPB.
Our results suggest that the benefit of temporary BiVP pacing begins immediately and remains significant as long as the pacing protocol is optimized hourly. We find a statistically significant reduction in vasoactive and inotropic support in the BiVP group for two hours after the last optimization of settings. This suggests that deterioration of the effects of temporary BiVP may be due to the lack of continued pacing optimization, and further indicates that effects of temporary BiVP would be maximized by continuous optimization during the postoperative period.
In addition, a significant increase in urine output was observed for the BiVP patients versus the SOC group from the beginning of Phase I until entry into the ICU. While this study was not designed for a multifactorial assessment of factors affecting urine output, this result, which mirrored our VIS analysis, further supports the postoperative cardiovascular benefit of optimized temporary BiVP.
VIS was validated in pediatric patients undergoing cardiac surgery as a marker of inotropic support as well as an established predictor of outcome (9,23). However, until now, there have been no studies using this updated vasoactive inotrope score in the adult population. VIS scores in adults are expected to differ from those in infants since the response of cardiac muscle and vasculature to inotropic and vasopressor mediations differs between pediatric and adult patients (24). VIS clearly merits further evaluation as an adjunct to management of adult patients after cardiopulmonary bypass. The present results also support the value of temporary BiVP as an inotrope replacement. Further studies are needed of both VIS in adults and hemodynamics of temporary BiVP after cardiopulmonary bypass.
It is important to note that VIS focuses on the amount, not the specific types, of vasoactive and inotropic support. Specific agents are used at the individual clinician’s discretion as to improve outcome and severity of illness. This non-standardized protocol cannot be controlled for in a retrospective study such as ours, so prospective randomized trials are necessary to account for selection bias and optimize the strategy for implementing specific medications.
There are several additional limitations to our study. This study is part of the larger BiPACS trial. An independent justification of sample size for this substudy would be based on anticipated differences in VIS between the two groups as well as the variance of VIS. This would define an ideal sample size larger than presented here. Termination of the parent trial precludes additional data collection. However, statistical significance was achieved by fitting linear effects models over time and comparing the slope. Anesthesiologists were not blinded to the two arms of the study; however, they were also not aware that VIS would be evaluated. Additionally, this is a single-center study, and thus our findings may not be generalizable to other patient populations.
CONCLUSIONS
Our results show that optimized temporary BiVP after CPB lowers the requirement for inotropic support in the early postoperative period, therefore decreasing the exposure to inotrope-associated risks. VIS can be useful in post- CPB management and pacing intervention as a tool to quantify vasoactive and inotropic support as well as a potential measure of cardiovascular function. VIS has the potential to be a component of a multivariable severity of illness scoring system, which would be particularly useful to practitioners trying to decide between various diagnostic and therapeutic options for patients recovering from cardiac surgery with BiVP. Additional studies are required to show a correlation between VIS and outcomes such as arrhythmias, morbidity, or mortality.
Acknowledgments
This study was funded by a grant from the National Institutes of Health (RO1 HL080152 to Dr Spotnitz). Dr Spotnitz is the George H. Humphreys, II, Professor of Surgery. Mr. Nguyen is supported by funds provided to Columbia University by the Weinstein Foundation.
Footnotes
No reprints will be ordered.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wernovsky G, Wypij D, Jonas RA, Mayer JE, Hanley FL, Hickey PR, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92(8):2226–2235. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 2.Talwar S, Sandeep JA, Choudhary SK, Velayoudham D, Lakshmy R, Kasthuri JM, et al. Effect of preoperative administration of allopurinol in patients undergoing surgery for valvular heart diseases. Eur J Cardiothorac Surg. 2010;38(1):86–90. doi: 10.1016/j.ejcts.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes JF, Blaufox AD, Seiden HS, Asnes JD, Gross RP, Rhodes JP, et al. Cardiac arrest in infants after congenital heart surgery. Circulation. 1999;100(19 Suppl):II194–9. doi: 10.1161/01.cir.100.suppl_2.ii-194. [DOI] [PubMed] [Google Scholar]
- 4.Gruenwald CE, McCrindle BW, Crawford-Lean L, Holtby H, Parshuram C, Massicotte P, et al. Reconstituted fresh whole blood improves clinical outcomes compared with stored component blood therapy for neonates undergoing cardiopulmonary bypass for cardiac surgery: a randomized controlled trial. J Thorac Cardiovasc Surg. 2008;136(6):1442–1449. doi: 10.1016/j.jtcvs.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulik TJ, Moler FW, Palmisano JM, Custer JR, Mosca RS, Bove EL, et al. Outcome-associated factors in pediatric patients treated with extracorporeal membrane oxygenator after cardiac surgery. Circulation. 1996;94(9 Suppl):II63–8. [PubMed] [Google Scholar]
- 6.Rosenzweig EB, Starc TJ, Chen JM, Cullinane S, Timchak DM, Gersony WM, et al. Intravenous arginine-vasopressin in children with vasodilatory shock after cardiac surgery. Circulation. 1999;100(19 Suppl):II182–6. doi: 10.1161/01.cir.100.suppl_2.ii-182. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, Yang J, Wang Q, Chen M, Lu Z, Chen S, et al. Cardioprotective effects of electroacupuncture pretreatment on patients undergoing heart valve replacement surgery. a randomized controlled trial. Ann Thorac Surg. 2010;89(3):781–786. doi: 10.1016/j.athoracsur.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Ricci Z, Morelli S, Ronco C, Polito A, Stazi GV, Giorni C, et al. Inotropic support and peritoneal dialysis adequacy in neonates after cardiac surgery. Interact Cardiovasc Thorac Surg. 2008;7(1):116–120. doi: 10.1510/icvts.2007.165118. [DOI] [PubMed] [Google Scholar]
- 9.Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11(2):234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 10.Parissis JT, Rafouli-Stergiou P, Stasinos V, Psarogiannakopoulos P, Mebazaa A. Inotropes in cardiac patients: update 2011. Current Opinion in Critical Care. 2010;16(5):432–441. doi: 10.1097/MCC.0b013e32833e10fb. [DOI] [PubMed] [Google Scholar]
- 11.Nelson GS, Berger RD, Fetics BJ, Talbot M, Spinelli JC, Hare JM, et al. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation. 2000;102(25):3053–3059. doi: 10.1161/01.cir.102.25.3053. [DOI] [PubMed] [Google Scholar]
- 12.Lewis KP. Early intervention of inotropic support in facilitating weaning from cardiopulmonary bypass: the New England Deaconess Hospital experience. J Cardiothorac Vasc Anesth. 1993;7(4 Suppl 2):40–45. doi: 10.1016/1053-0770(93)90096-4. [DOI] [PubMed] [Google Scholar]
- 13.Cuffe MS, Califf RM, Adams KF, Jr, Benza R, Bourge R, Colucci WS, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287(12):1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 14.Auricchio A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P, et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. 2002;39(12):2026–2033. doi: 10.1016/s0735-1097(02)01895-8. [DOI] [PubMed] [Google Scholar]
- 15.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346(24):1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 16.Bax JJ, Abraham T, Barold SS, Breithardt OA, Fung JW, Garrigue S, et al. Cardiac resynchronization therapy: Part 1--issues before device implantation. J Am Coll Cardiol. 2005;46(12):2153–2167. doi: 10.1016/j.jacc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Bax JJ, Abraham T, Barold SS, Breithardt OA, Fung JW, Garrigue S, et al. Cardiac resynchronization therapy: Part 2--issues during and after device implantation and unresolved questions. J Am Coll Cardiol. 2005;46(12):2168–2182. doi: 10.1016/j.jacc.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Spotnitz ME, Wang DY, Quinn TA, Cheng B, Richmond ME, Rusanov A, et al. Hemodynamic stability during biventricular pacing after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2011;25(2):238–242. doi: 10.1053/j.jvca.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berberian G, Quinn TA, Kanter JP, Curtis LJ, Cabreriza SE, Phillips AB, et al. Optimized biventricular pacing in atrioventricular block after cardiac surgery. Ann Thorac Surg. 2005;80(3):870–875. doi: 10.1016/j.athoracsur.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 20.Quinn TA, Berberian G, Cabreriza SE, Maskin LJ, Weinberg AD, Holmes JW, et al. Effects of sequential biventricular pacing during acute right ventricular pressure overload. Am J Physiol Heart Circ Physiol. 2006;291(5):H2380–7. doi: 10.1152/ajpheart.00446.2006. [DOI] [PubMed] [Google Scholar]
- 21.Wang DY, Richmond ME, Quinn TA, Mirani AJ, Rusanov A, Yalamanchi V, et al. Optimized temporary biventricular pacing acutely improves intraoperative cardiac output after weaning from cardiopulmonary bypass: a substudy of a randomized clinical trial. J Thorac Cardiovasc Surg. 2011;141(4):1002–8. 1008.e1. doi: 10.1016/j.jtcvs.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisse U, Isgro F, Werling C, Lehmann A, Saggau W. Impact of atrio-biventricular pacing to poor left-ventricular function after CABG. Thorac Cardiovasc Surg. 2002;50(3):131–135. doi: 10.1055/s-2002-32403. [DOI] [PubMed] [Google Scholar]
- 23.Zahorec M, Hrubsova Z, Skrak P, Poruban R, Mosal M, Kovacikova L, et al. A Comparison of Blalock-Taussig Shunts With and Without Closure of the Ductus Arteriosus in Neonates With Pulmonary Atresia. Ann Thorac Surg. 2011;92(2):653–8. doi: 10.1016/j.athoracsur.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Rao V, Ivanov J, Weisel RD, Ikonomidis JS, Christakis GT, David TE. Predictors of low cardiac output syndrome after coronary artery bypass. J Thorac Cardiovasc Surg. 1996;112(1):38–51. doi: 10.1016/s0022-5223(96)70176-9. [DOI] [PubMed] [Google Scholar]
- 25.Butterworth JF, 4th, Legault C, Royster RL, Hammon JW., Jr Factors that predict the use of positive inotropic drug support after cardiac valve surgery. Anesth Analg. 1998;86(3):461–7. doi: 10.1097/00000539-199803000-00002. [DOI] [PubMed] [Google Scholar]