Abstract
Background
ERP studies show reduced P300 amplitudes in alcohol use disorders (AUDs). Alcohol exposure, genetic vulnerability to alcoholism, and comorbid psychopathology may contribute to this reduction. Most previous research has studied treated adult AUD samples, which have more severe alcoholism, a greater family history of AUDs, and more comorbidity than untreated samples. Untreated AUD samples tend to have little or no P300 amplitude reduction. We compared P300 between treatment-naïve alcohol-dependent (TNAD) adolescents with no diagnosable substance abuse or psychiatric comorbidity, and non-substance-abusing control (NSAC) adolescents.
Methods
Individuals between the ages of 13 and 18 years were recruited into either TNAD (n = 45) or NSAC (n = 64) groups. Alcohol-use variables, family history density of alcohol problems, and psychiatric symptom counts were assessed in a clinician-administered evaluation. EEGs were recorded during performance of a 3-condition visual target detection task.
Results
P300 amplitudes were of comparable size in TNAD adolescents and NSAC. Boys demonstrated larger P3a and P3b amplitudes than girls. Within TNAD, P3b amplitude was reduced in those who drank more frequently and P3a latency was more prolonged in subjects with higher internalizing symptom counts.
Conclusions
The P300 deficit was not present in TNAD adolescents without comorbidities. In comparison to results of reduced P300 in treated adolescent AUD samples, this finding likely reflects moderate alcohol exposure, lower genetic vulnerability to alcoholism, and lack of comorbidity in our sample. Further work is needed to determine the relative contributions of these factors to changes in the P300.
Keywords: Event-related potentials, P300, Alcoholism, Treatment-naïve, Adolescents
Introduction
Reduced amplitude of the P3b event-related potential (ERP) is a robust finding in individuals with alcohol use disorders (AUDs), as well as in those with a family history of AUDs (Begleiter and Porjesz, 1999; Begleiter et al., 1984; Fein and Chang, 2006; Iacono et al., 2003). Some literature argues that the P3b reduction is a physiological correlate of chronic alcohol exposure. For instance, animal studies suggest that relatively brief alcohol exposure in adolescence is sufficient to result in P300 changes that endure into adulthood (see, e.g., Criado et al., 2008; Ehlers and Criado, 2010). However, most studies propose that the P3b reduction is an endophenotypic marker for the genetic vulnerability to AUDs (Bauer, 2001; Begleiter and Porjesz, 1988; Hill et al., 1999B, 2009; Pfefferbaum et al., 1991). Furthermore, chronic alcohol use and genetic vulnerability to AUDs may contribute independently to the P3b reduction (Perlman et al., 2009). Because chronic alcohol exposure and genetic risk for AUDs commonly co-occur, it is often difficult to determine the relative contribution of each to the P3b reduction.
Most research on the P3b reduction in AUDs has focused on clinical populations of treated individuals with alcohol dependence. Our group and others have shown repeatedly that treated or treatment-seeking individuals with alcohol dependence, who constitute at most 25% of individuals with an AUD, come from a different population than treatment-naïve alcohol-dependent individuals. The former have more severe alcoholism, a higher family history density of alcohol problems, more psychiatric and polysubstance comorbidity, and more cognitive and structural impairment than the latter (Di Sclafani et al., 2008; Fein et al., 2002; Fein and Landman, 2005; Gazdzinski et al., 2008; Smith and Fein, 2010).
Regarding the P3b reduction specifically, we have shown that treatment-naïve actively drinking alcohol-dependent adults show this reduction to a dramatically smaller degree than do treated alcoholics (Fein and Andrew, 2011). Others have shown that the reduction is no longer detectable when internalizing psychiatric comorbidity is taken into account (Hill et al., 1999a), and, further, that P3b amplitude is negatively correlated with history of externalizing behaviors in individuals with substance use disorders (SUDs) (Bauer, 2001). Ongoing studies of ‘pure’ alcohol dependence (i.e., studies of individuals who are treatment-naïve, and who are negative for SUDs and are without lifetime psychiatric comorbidity) are critical for advancing our understanding of alcoholism.
There is a paucity of data examining possible P3b reductions in treatment-naïve AUD adolescents without substance or psychiatric comorbidity. Published studies on adolescents with AUDs (e.g., Bauer and Hesselbrock, 2003; Carlson et al., 1999) focus on treated samples with childhood externalizing diagnoses and substance (other than alcohol) involvement. Studies of treatment-naïve adolescent AUD samples who are free of psychiatric and other substance comorbidity will help tease out the contribution of alcohol exposure to the P3b reduction because adolescents have much less such exposure than do older individuals who have been drinking for much longer.
We have been studying such a ‘pure’ sample (without substance or psychiatric comorbidity) of alcohol-dependent adolescents in the Cape Town region of South Africa. In the research described here, we hypothesized that they would evidence at most very minimal P3b reductions, given that these subjects have low to moderate lifetime exposure compared to adult samples, little comorbidity, and are likely to have low family history density of alcohol problems.
Although alcoholism research consistently finds a reduction in P3b amplitude, evidence for P3a abnormalities remain equivocal (Biggins et al., 1995; Rodriguez Holguin et al., 1998). In this report, we examine both P3a and P3b responses in ‘pure’ treatment-naïve adolescents with AUDs.
Materials and Methods
Participants
We recruited English- and Afrikaans-speaking adolescents (13–18 years) of low socioeconomic status and mixed ancestry from the Cape Town region in South Africa. The total sample (N = 117) consisted of 52 (34 females) treatment-naïve alcohol-dependent (TNAD) adolescents, and 65 (39 females) non-substance-abusing controls (NSAC). None of the participants had ever been treated for an AUD or SUD. We applied eligibility criteria rigorously to ensure that our samples were free of SUDs and psychiatric comorbidities. Exclusion criteria for study participation were: mental retardation, lifetime DSM-IV Axis I diagnoses other than AUD (including depressive, anxiety, psychotic, post-traumatic stress, elimination, eating, tic, attention-deficit/hyperactivity, oppositional defiant, and conduct disorders); lifetime dosages exceeding 30 cannabis or 4 methamphetamine doses; current use of sedative or psychotropic medication; signs or history of fetal alcohol syndrome or malnutrition; sensory impairment; history of traumatic brain injury with loss of consciousness exceeding 10 minutes; presence of diseases that may affect the CNS (e.g., meningitis, epilepsy, HIV); less than 6 years of formal education; and lack of proficiency in English or Afrikaans.
Measures
History of alcohol use
We used a revised version of the Timeline Followback (TLFB) procedure (Sobell and Sobell, 1992) to assess lifetime history of alcohol use and drinking patterns (such as onset age, drinking and intoxication experiences). For the purposes of this history-taking, a standard drink was defined as one beer or wine cooler, one glass of wine, or one 1.5-oz shot of liquor (alone or in a mixed drink). The Schedule for Affective Disorders and Schizophrenia for School-Aged Children (6–18 years) Lifetime Version (K-SADS-PL; Kaufman et al., 1997), a semi-structured clinician-rated diagnostic scale, was used to determine presence of a DSM-IV-defined AUD.
Group membership was determined thus: Participants in the TNAD group were required to have a lifetime dosage in excess of 100 units, as well as a DSM-IV diagnosis of alcohol abuse or dependence. For these participants, we collected additional alcohol use information, including age at first intoxication and age at which regular use commenced. Participants in the NSAC group were required to have a lifetime dosage of less than 100 units, and no history of an AUD diagnosis.
Psychopathology
Current and past psychiatric symptoms were ascertained during administration of the K-SADS-PL. Although all participants fell below the diagnostic threshold for psychiatric disorders (except AUDs, in the case of the TNAD group), subthreshold symptom counts were collected to examine sub-diagnostic threshold psychopathology. We derived composites for subthreshold internalizing psychopathology (depressive and anxiety disorder symptoms, including posttraumatic stress disorder) and subthreshold externalizing psychopathology (conduct disorder (CD) and oppositional defiant disorder (ODD) symptoms) by summing total subthreshold symptom counts for the specified disorders. We chose to examine subthreshold attention-deficit/hyperactivity disorder (ADHD) symptom counts separately from those of CD and ODD based on evidence suggesting that ADHD is not a socially deviant disruptive behavioral disorder (Kaplan and Sadock, 1998). Similar means of assessing psychopathology in AUD samples have been used in previous studies (Di Sclafani et al., 2008; Fein et al., 2007).
Family history density of alcohol problems
We asked participants whether they were currently cohabiting with, or had ever cohabited with, a first-degree relative (sibling or parent) who had an “alcohol problem”. Responses to this question were coded as 0 if the participant had never cohabited with such an individual; 1 if the participant had cohabited with a single first-degree relative with an alcohol-use problem; and 2 if the participant had cohabited with more than one first-degree relative with an alcohol-use problem. This approach was adapted from the family history drinking questionnaire described by Mann et al. (1985).
Experimental paradigm
We administered a standard three-condition visual target detection task, programmed using E-Prime software (Psychology Software Tools Inc., 2007). The task featured 280 stimuli, presented over approximately 6.5 minutes in a predetermined semi-randomized order. The three different stimulus types were: (a) the standard stimulus (a small hollow white square, accounting for 75% of all stimulus presentations); (b) the target stimulus (a small white X, 12.5% of all stimulus presentations); and (c) the rare non-target stimulus (different shapes of various colors, 12.5% of all stimulus presentations). Participants were instructed to press a response box button only when they saw the target stimulus. Each stimulus appeared on a black screen for 200 ms; the inter-stimulus interval was 1000–1100 ms. Each participant was shown examples of the stimuli and was given a chance to practice the task before data acquisition began. These stimuli were identical those used in our previous work (Fein and Andrew, 2011; Fein and Chang, 2006).
Procedures
The study protocol adhered strictly to the guidelines contained in the Declaration of Helsinki (2008). All procedures were approved by the Western Cape Education Department and the Research Ethics Committee of the Stellenbosch University Faculty of Health Sciences. Written assent and consent were obtained from participants and their parents, respectively. Participant screening included a physical examination (including urine analysis and breathalyzer testing) and assessment of psychiatric and medical histories. After screening was complete, participants completed a demographic questionnaire.
EEGs were recorded for each participant during performance of the visual target detection task. EEG was acquired on a 64-channel Neuroscan SynAmps2 system with Scan 4.3 acquisition software (Compumedics Neuroscan Inc., 2008). A cephalic reference electrode (close to Cz) was used for all recordings. Left and right earlobe electrodes were also used for obtaining signals for re-referencing of data offline. The ground electrode was placed 8 cm above the nasion. Electrode site impedances were kept below 10 kΩ. A vertical electro-oculogram (EOG) was recorded from bipolar electrodes placed above and below the left eye for use in offline reduction of ocular artifacts. The EEG and EOG channels were sampled at 250 Hz and stored for offline analysis.
EEG and Statistical Analysis
We used the BrainVision Analyzer package (BVA; Brain Products, 2008) for ERP processing. Eye movement artifacts were removed using the Gratton and Coles method (Gratton et al., 1983). Data were then band-pass filtered between 0.5 and 15 Hz using a zero-phase lag filter at 48 dB/octave. Data were re-referenced to the right mastoid. Stimulus-locked trials were extracted for all instances where there was a correct behavioral response for the standard, target and rare non-target conditions, with each trial comprising 100 ms of data pre-stimulus and 750 ms post-stimulus. The trials were baseline corrected using the 100 ms pre-stimulus interval. Any trials with out-of-range voltages (± 75 mV) were rejected as artifact and excluded from further processing. If fewer than 21 trials per condition (standard, target, or distracter) remained for any subject following artifact rejection, that subject’s data were eliminated from the analysis. On this basis, data from 1 NSAC and 7 TNAD participants were eliminated, leaving a final sample size of N = 109 (45 TNAD (30 females) and 64 NSAC (38 females)).
ERP waveforms were extracted by averaging across trials for each condition. In addition to the ERP waveforms, the ERP difference waveforms (a) between the target and standard condition, and (b) between the rare non-target and standard condition were also computed. Grand average ERP waveforms for each condition were produced by averaging the ERPs within each group. Grand average topographical maps were also computed to display the spatial distribution of P300 amplitudes. The computation of grand average ERPs and topographical maps, and the extraction of amplitudes and latencies of the P300 components described below, were performed on both the ERP and the ERP difference waveforms.
P3b amplitude for each subject for the target and P3a amplitude for the rare non-target conditions (maximum positive peak in a time window from 300–500 ms) were measured at the midline electrode (Fz, FCz, Cz, CPz, Pz, POz, or Oz) at which the spatial distribution peak was maximal for the ERP and ERP difference waveforms for that subject. The amplitude and latency of the P3b and P3a were then extracted at the selected electrode site. BVA’s semi-automated peak detection algorithm was used to extract the P3b and P3a peaks, with manual adjustment of the peak location as necessary, based on temporal and spatial distribution of the ERP component. Where the P300 was absent or ambiguous for a subject, the component was scored as missing. Five subjects did not show clear P3b peaks and 10 subjects did not show clear P3a peaks, and so these participants were omitted from the relevant statistical analysis. Behavioral data for target (reaction time and accuracy) and rare non-target (accuracy) conditions were extracted over a period of 750 ms following stimulus presentation.
We used SPSS software (SPSS Inc., 2008) for data analyses. The main analyses consisted of 2 (group) × 2 (gender) factorial ANOVAs run separately on (a) demographic variables, (b) alcohol-use (individual and family) variables, (c) psychiatric symptom counts, (d) three behavioral outcome variables (reaction times to target stimuli, and accuracy for both target and rare non-target conditions), and (e) the four major ERP outcome variables (latency and amplitude of P3a and P3b). Effect size is reported as partial η2, the percent of the dependent variable variance independently accounted for by the effect being examined. We examined associations between ERP measures (latency and amplitude of P3a and P3b) and (a) alcohol-use variables, (b) family history density of alcohol problems, and (c) psychiatric symptom counts, using Spearman correlations. The threshold for statistical significance was held at p = .05 for these analyses, despite the fact that we calculated multiple sets of correlations. We took this position because this aspect of the analysis was (a) largely exploratory and (b) conducted on a non-clinical sample that featured low levels of symptom counts and low family history density of alcohol problems, and hence (c) we were more concerned with avoiding Type II errors than Type I errors.
Results
Sample Characteristics
Table 1 shows the demographic, alcohol-use (individual and family), and psychiatric symptom count characteristics of the current sample. Regarding demographic variables and family history density of alcohol problems, there were no significant main effects of group or gender, and no group x gender interaction effects.
Table 1.
Demographic, Alcohol-Use, and Psychiatric Symptom Count Characteristics of the Sample (N = 109)
| Outcome variable | Group
|
Effect size (partial η2)
|
|||||
|---|---|---|---|---|---|---|---|
| NSAC
|
TNAD
|
NSAC vs. TNAD | Girls vs. Boys | Group* Gender | |||
| Girls (n = 38) | Boys (n = 26) | Girls (n = 30) | Boys (n = 15) | ||||
|
|
|
|
|||||
| Demographic variables | |||||||
| Age (years) | 15.24 (0.97) | 15.26 (0.92) | 15.33 (1.24) | 15.35 (1.39) | 0.1 | 0.1 | 0.1 |
| Education (years) | 7.88 (0.65) | 7.91 (0.67) | 7.88 (0.71) | 7.69 (0.48) | 0.6 | 0.3 | 0.7 |
| Language (% Afrikaans-speaking) | 66 | 81 | 53 | 60 | 2.8 | 1.2 | 0.2 |
| Handedness (% Right-handed) | 87 | 85 | 97 | 93 | 2.1 | 0.2 | 0.1 |
| Individual alcohol-use variables | |||||||
| % Never drunk alcohol | 34 | 35 | 0 | 0 | 16.6a | 0.1 | 0.1 |
| % Never been intoxicated | 82 | 96 | 0 | 0 | 74.2a | 1.9 | 1.9 |
| Drinking onset age | 13.80 (2.05) | 15.33 (1.53) | 11.77 (1.79) | 11.33 (2.16) | 24.2a | 1.0 | 3.3 |
| Alcohol lifetime dose | 7.63 (14.91) | 2.73 (4.30) | 1551.77 (1742.41) | 1511.93 (1935.64) | 28.7a | 0.1 | 0.1 |
| Age of first intoxication | 12.67 (1.12) | 12.67 (1.35) | 5.8 | ||||
| Age of onset of regular use | 12.73 (1.05) | 12.73 (1.34) | 0.1 | ||||
| Regular drinking duration | 26.60 (13.55) | 39.13 (32.79) | 4.5* | ||||
| Regular drinking frequency | 4.03 (2.41) | 4.00 (2.33) | 0.1 | ||||
| Regular drinking average monthly quantity | 50.20 (40.30) | 42.08 (29.03) | 1.1 | ||||
| Family history densityb | 0.13 (0.34) | 0.08 (0.27) | 0.30 (0.60) | 0.13 (0.35) | 1.7 | 1.6 | 0.4 |
| Psychiatric symptom counts | |||||||
| Internalizing compositec | 5.66 (6.47) | 3.35 (3.75) | 9.07 (8.55) | 4.53 (5.38) | 2.8 | 6.0* | 0.7 |
| Externalizing composited | 0.42 (1.08) | 1.73 (2.60) | 2.30 (3.56) | 3.93 (3.47) | 11.9*** | 6.6** | 0.1 |
| ADHD symptom counts | 0.58 (1.39) | 1.62 (2.70) | 2.70 (6.39) | 2.60 (2.50) | 3.7* | 0.3 | 0.5 |
Note. Means are presented with standard deviations in parentheses. NSAC = non-substance-abusing control group; TNAD = treatment-naïve alcohol dependent group.
Statistical comparisons on variables relating to selection criteria are not appropriate.
Number of first-degree cohabiting relatives who are problem drinkers. Note these are presented as absolute numbers and not as proportions (as in previous publications by our group), and so they cannot be compared directly with our previously published results on family history density of alcohol problems.
Total symptoms of depressive and anxiety disorders.
Total symptoms of conduct disorder and oppositional defiant disorder.
p < .05.
p < .01.
p < .001.
Regarding alcohol-use variables, we found the expected effects given the study’s selection criteria: TNAD participants, relative to NSAC participants, (a) had earlier onset of alcohol use, (b) had consumed more alcohol, and (c) were more likely to have had a previous intoxication. Of note here is that several NSAC participants had experimented with alcohol, but at a later age than TNAD. Statistical comparison between groups of variables associated with selection criteria are not warranted; however, we note that aside from duration of regular alcohol use (where boys had significant longer drinking histories than girls), there were no other significant main effects of gender, or group x gender interactions, with regard to the alcohol-use variables.
Regarding psychiatric symptom counts, TNAD participants presented with more externalizing symptoms and more ADHD symptoms than did NSAC participants, F(1, 105) = 14.161, p < .001, and F(1, 105) = 4.035, p = .047, respectively. Girls presented with more internalizing symptoms than boys, F(1, 105) = 6.746, p = .011; conversely, boys presented with more externalizing symptoms than girls, F(1, 105) = 7.363, p = .008. There were no other significant main effects or interaction effects with regard to psychiatric symptom counts.
Task Reaction Time and Accuracy
Table 2 presents behavioral data from the three-condition visual target detection task. Regarding the dependent variable of reaction time in response to target stimuli, there was a significant main effect of gender, F(1,105) = 4.102, p = .045, with boys responding more quickly than girls. There were no other significant main or interaction effects with regard to this outcome variable, or with regard to the other two outcome variables. [Insert Table 2 about here]
Table 2.
Three-Condition Visual Target Detection Task: Behavioral outcome data
| Outcome variable | Group
|
Effect size (partial η2)
|
|||||
|---|---|---|---|---|---|---|---|
| NSAC
|
TNAD
|
NSAC vs. TNAD | Girls vs. Boys | Group* Gender | |||
| Girls (n = 38) | Boys (n = 26) | Girls (n = 30) | Boys (n = 15) | ||||
| Target reaction | 407.07 (41.40) | 386.36 | 404.65 | 393.12 (38.00) | 0.1 | 3.8* | 0.3 |
| Accuracy (%) | |||||||
| Target | 96.89 (5.67) | 96.77 (3.89) | 96.61 (4.23) | 95.52 (4.78) | 0.6 | 0.4 | 0.2 |
| Rare non-target | 97.59 (4.01) | 96.92 (4.19) | 98.76 (1.94) | 97.32 (3.14) | 1.1 | 2.1 | 0.3 |
Note. Means are presented with standard deviations in parentheses. NSAC = non-substance-abusing control group; TNAD = treatment-naïve alcohol dependent group.
p < .05.
ERPs
Results obtained for P300 measures derived from the ERP and the ERP difference waveforms for the rare non-target and target were similar. Hence, we present only results for the ERP difference waveforms and measures derived from these.
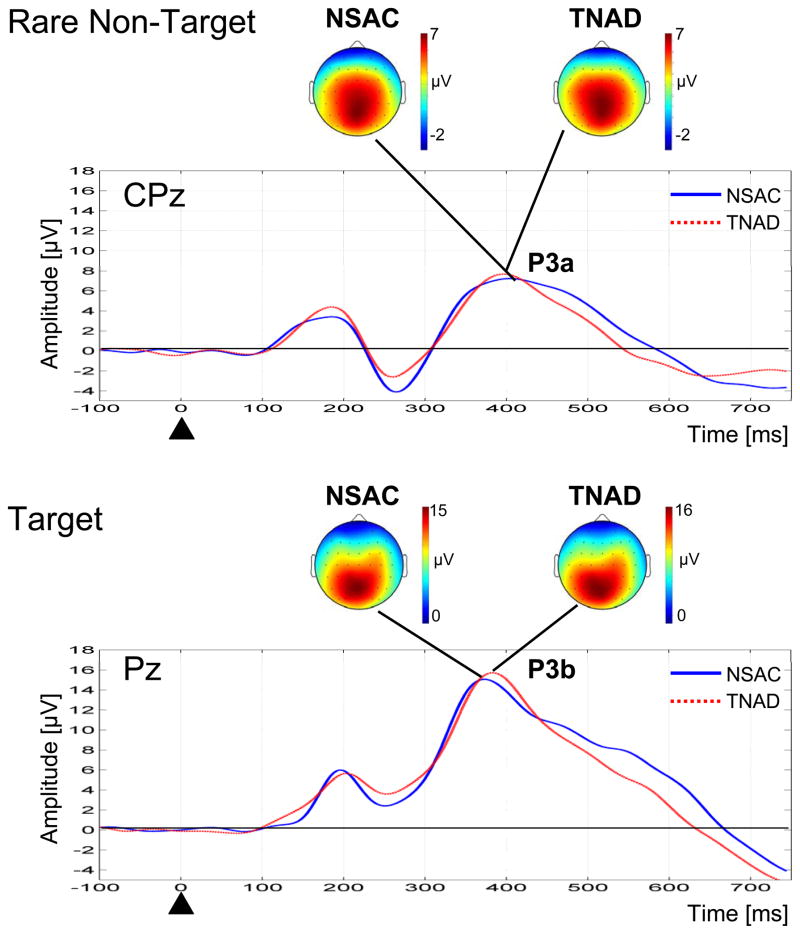
Figure 1 shows the comparison of grand average difference ERP waveforms and topographical maps for the NSAC and TNAD groups for the rare non-target and target conditions. The spatial distributions of the P3a and P3b were similar across groups, with the P3a component being fairly widespread with its maximum over the centro-parietal regions (CPz and Pz), and the P3b showing a more localized spatial distribution at the parietal electrode site (Pz). In accordance with the observed spatial distributions of the P3a and P3b subcomponents, the grand average difference ERP waveforms for the rare non-target and target conditions are displayed at CPz and Pz respectively.
Figure 1.
Grand average ERP difference waveforms for the Rare Non-Target (Rare Non-Target – Standard condition) at electrode site CPz and Target (Target – Standard condition) at electrode site Pz, showing comparison of NSAC and TNAD groups. Grand average spatial maps are shown for the location of the P3 peaks for both the NSAC and TNAD groups.
There was variability across subjects in the location of the maximum of the spatial distribution of the P3a and P3b components across midline electrode sites. Table 3 shows a breakdown by midline electrode site of the location of the P3a and P3b maximal amplitude across subjects. As expected from the spatial distributions of the grand average topographical maps in Figure 1, most subjects had a spatial maximum of the P3b at the parietal electrode site (Pz), while for the P3a there was a more even distribution of subjects across Cz, CPz, Pz, and POz.
Table 3.
Breakdown of Spatial Maxima of the Two P300 ERP Components Across Midline Electrode Sites
| Component | Electrode site
|
||||||
|---|---|---|---|---|---|---|---|
| Fz | FCz | Cz | CPz | Pz | POz | Oz | |
| P3a | 2 | 16 | 16 | 18 | 25 | 20 | 2 |
| P3b | 0 | 3 | 5 | 17 | 46 | 33 | 0 |
Note. Data represent the number of participants whose spatial maxima occurred at each electrode site.
The latencies and amplitudes of the P3a and P3b components were extracted from the electrode site at which the spatial maxima occurred for each subject. Table 4 shows these data. There were no significant main or interaction effects with regard to P3a or P3b latency. There were also no significant main effects of group, or group by gender interaction effects, for P3a or P3b amplitude. There was, however, a significant main effect of gender for both P3a and P3b amplitude, F(1, 95) = 5.175, p = .025, and F(1, 100) = 4.819, p = .030, respectively, with boys having larger amplitudes than girls in both cases.
Table 4.
Three-Condition Visual Target Detection Task: ERP outcome data
| Outcome |
|
||||||
|---|---|---|---|---|---|---|---|
| Group
|
Effect size (partial η2)
|
||||||
| NSAC
|
TNAD
|
NSAC vs. TNAD | Girls vs. Boys | Group* Gender | |||
| Girls | Boys | Girls | Boys | ||||
|
|
|
|
|||||
| P3a | (n = 36) | (n = 24) | (n = 27) | (n = 12) | |||
| Latency | 403.11 (43.06) | 402.83 (47.76) | 409.78 (42.63) | 379.00 (36.55) | 0.9 | 2.8 | 2.7 |
| Amplitude | 11.78 (4.29) | 12.89 (4.59) | 11.35 (4.01) | 14.41 (3.52) | 0.4 | 5.2* | 1.2 |
| P3b | (n = 37) | (n = 24) | (n = 29) | (n = 14) | |||
| Latency | 389.19 (51.29) | 384.33 (31.45) | 388.83 (34.70) | 382.29 (24.69) | 0.1 | 0.5 | 0.1 |
| Amplitude | 18.70 (6.40) | 19.08 (5.55) | 17.90 (6.93) | 23.41 (7.13) | 1.6 | 4.6* | 3.5 |
Note. Means are presented with standard deviations in parentheses. Latency data are presented in ms. Amplitude data are presented in μV. NSAC = non-substance-abusing control group; TNAD = treatment-naïve alcohol dependent group.
p < .05.
ERP Associations with Alcohol-Use and Psychiatric Symptom Counts
Table 5 presents associations, within the TNAD group, between ERP measures and (a) alcohol-use variables, (b) family history density of alcohol problems, and (c) psychiatric symptom counts. There were only two statistically significant correlations: P3a latency was positively associated with internalizing symptom counts (r = .447, p = .004), and P3b amplitude was negatively associated with regular drinking frequency (r = −.330, p = .040).
Table 5.
Associations between ERP outcome variables and alcohol-use and clinical characteristics in TNAD (N = 45)
| ERP components
|
||||
|---|---|---|---|---|
| P3a
|
P3b
|
|||
| Latency | Amplitude | Latency | Amplitude | |
| Individual alcohol-use variables | ||||
| Drinking onset age (years) | −.079 | −.012 | .049 | −.006 |
| Alcohol lifetime dose (units) | .262 | .190 | −.028 | −.150 |
| Age of first intoxication (years) | −.129 | −.029 | .093 | −.133 |
| Age of onset of regular drinking (years) | −.102 | −.008 | .100 | −.157 |
| Regular drinking duration (months) | .278 | .072 | −.060 | −.114 |
| Regular drinking frequency (days/month) | .283 | .012 | .112 | −.330* |
| Regular drinking average monthly quantity (units/month) | .089 | .140 | −.031 | −.232 |
| Family history density of alcohol problemsa | .036 | −.105 | .050 | .005 |
| Clinical variables | ||||
| Internalizing compositeb | .447** | −.046 | .117 | −.196 |
| Externalizing compositec | .027 | .099 | −.207 | −.072 |
| ADHD symptom counts | −.190 | .268 | −.298 | .059 |
Note. Data presented are Spearman correlations.
Number of first-degree cohabiting relatives who are problem drinkers. Note that these are presented as absolute numbers and not proportions (as in previous publications by our group), and so cannot be compared directly with our previously published results on family history drinking scores.
Total symptoms of depressive and anxiety disorders.
Total symptoms of conduct disorder and oppositional defiant disorder.
p < .05.
p < .01
Discussion
The central finding of this study was that, compared to non-substance-abusing controls, there was no reduction of P3b amplitude in treatment-naïve actively drinking adolescents with alcohol dependence, but without other substance use or psychiatric comorbidity. This finding is consistent with our a priori predictions, which were made for two primary reasons. First, treatment-naïve individuals with AUDs tend to have less severe alcoholism, lower family history density of alcohol problems, and less psychiatric and polysubstance comorbidity than their treatment-seeking counterparts (Di Sclafani et al., 2008; Fein and Landman, 2005). Previous research demonstrating the P3b reduction has, by and large, used participants from treatment-seeking or treated populations.
Furthermore, previous studies have shown that alcohol exposure, genetic risk for alcoholism, and comorbid psychopathology may contribute independently to the P3b reduction seen in treatment-seeking populations (Hill et al., 1999a; Perlman et al., 2009). In treatment-naïve AUD samples such as the one studied here, levels of genetic risk for alcoholism and subdiagnostic psychopathology were probably lower than those in treatment-seeking AUD samples used elsewhere (Di Sclafani et al., 2008). Our eligibility criteria specified that those with diagnosable psychopathology were excluded from participation; hence, only those with subdiagnostic symptom counts were eligible, and the ceiling for comorbid psychopathology was set very low. As it turned out, most of our participants had no subdiagnostic psychopathology. Similarly, although we did not empirically evaluate genetic risk for alcoholism, our assessment of family history density of alcohol problems suggested that most had no known family history density of alcohol problems, and so again the ceiling for genetic risk for alcoholism was likely low.
An early COGA (Consortium on the Genetics of Alcoholism) study that compared P300 amplitudes in community-dwelling individuals with AUDs (but with low family history density of alcohol problems) to those in treatment-seeking members of densely affected families reported no P3b reductions in the community-dwelling sample compared to the high-density sample, despite the fact that all participants, regardless of group, met DSM criteria for dependence (Porjesz et al., 1998). Because those findings are consistent with the current study, it is likely that, despite the limitations of our measure of family history density of alcohol problems, the conclusions arising from our assessment of genetic vulnerability to alcoholism in the current TNAD sample, are true.
The second factor that drove our prediction of at most very minimal P3b reduction in the TNAD group was that we examined P300 in an adolescent sample. Hence, the individuals investigated here were at an earlier stage of alcoholism and had comparatively lower lifetime alcohol exposure than more frequently examined adult samples. Alcohol exposure has a cumulative neurotoxic effect on the brain: electrophysiological abnormalities become more marked with increased consumption (Floyd et al., 1997; Polich and Bloom, 1986). If the degree of P3b amplitude reduction is moderated, at least partially, by amount of lifetime alcohol exposure, it is not surprising that this reduction was less marked in a younger sample with relatively low lifetime alcohol exposure.
Because we focused on adolescent participants in this study, it is not possible for us to directly compare the consequences of alcohol use in adolescents and adults. Nevertheless, our findings appear to conflict with predictions of increased susceptibility to P300 reductions in children and adolescents compared to adults (Polich et al., 1994). Such predictions arise from the fact that the developing brain is particularly vulnerable to the neurotoxic effects of alcohol (Gogtay et al., 2004; Sowell et al., 2004). Age-related vulnerability to the deleterious effects of alcohol may, however, be moderated by other factors, including, importantly, genetic vulnerability to alcoholism (Hill and Shen, 2002) and brain differences associated with psychiatric comorbidity (Bauer and Hesselbrock, 1999, 2003). On the other hand, the relative plasticity of the adolescent brain may facilitate recovery from neurotoxic events such as alcohol exposure, thus contributing to limited changes in the P300 following alcohol exposure (Benton and Tranel, 2000). Indeed, our results appear to be consistent with this notion. Taken together, and with regard to age-dependent effects on the P300, it appears that (a) relatively reduced cumulative alcohol exposure in adolescents versus adults, and/or (b) relative plasticity (as opposed to relative vulnerability) of the adolescent brain possibly moderated the relationship between AUDs and P300, thereby contributing to minimal changes in the P300 in the current adolescent sample. Nevertheless, further work is needed to characterize the consequences of alcohol exposure during the adolescent phase.
Regarding associations between P300 and (a) family history density of alcohol problems, and (b) psychiatric symptom counts, we observed only one significant relationship (a positive association between P3a latency and internalizing symptoms). Although genetic risk for alcoholism and the presence of other psychopathology have known effects on the P300 (Hill et al., 1999a, 2000; Perlman et al., 2009), the low levels and limited range of scores on measures of these variables in the current sample probably prevented us from observing other possible significant associations with ERP measures.
Regarding associations between P300 and alcohol-use variables, we again observed only one significant relationship: a negative association between P3b amplitude and regular drinking frequency. Although this association is indeed suggestive of the expected relationship between P300 and alcohol use, it did not generalize to other indices of use, including measures of consumption quantity (i.e., average monthly drinking quantity and alcohol lifetime dose).
The lack of significant association between P3b amplitude and consumption quantity in this sample stands in contrast to previous research reporting that alcohol exposure contributes to changes in the P300 (Criado et al., 2008; Ehlers and Criado, 2010; Perlman et al., 2009), and that these changes become more marked with increased consumption (Floyd et al., 1997; Polich, 1986). Again, we attribute the current lack of significant association to the fact that our sample consisted of treatment-naïve individuals with less alcohol exposure than the treatment-seeking individuals featured in previous studies. Indeed, we found a similar pattern of non-significant associations in treatment-naïve adults (Fein and Andrew, 2011).
Although alcohol use may be characterized in various ways (including frequency of use, age of first use, and various measures of consumption quantity), most other studies restrict alcohol-use measures to indices of consumption quantity (e.g., amount of alcohol consumed in the previous year; maximum amount of alcohol consumed on one occasion), or combine indices of consumption quantity and frequency of use into a single measure (Carlson et al., 2002; Perlman et al., 2009). Emphasis on consumption quantity as a primary index of alcohol use is likely due to the observation that quantity, rather than frequency of use, generally has a larger effect on the P300 (Neville and Schmidt, 1985; Polich, 1984). The observed significant association between P3b amplitude and regular drinking frequency is therefore (a) difficult to contextualize in relation to the literature because frequency of use is rarely examined as an independent measure, and (b) difficult to explain since neither measure of consumption quantity was associated with the P300. Furthermore, the significant association relating to frequency of use alongside the absence of any significant association with consumption quantity is surprising because those in the TNAD group largely endorsed weekend-only binge drinking, and so had a relatively limited range of drinking frequency.
We examined gender differences in P300 following mixed reports of such effects within AUD samples. Overall, boys had larger P300 complexes than girls. These results are consistent with Hill and Steinhauer (1993) and Steinhauer and Hill (1993), who also reported larger P300 amplitudes in boys. Mixed reports of gender-related differences in P300 in the literature, however, may be due to possible interactions between age and gender on the P300. Specifically, it appears that, during adolescence, boys generally exhibit relatively larger P300 complexes; however, from late adolescence onwards, females tend to have relatively larger P300 complexes (Carlson et al., 2002; Hirayasu et al., 2000; Hoffman and Polich, 1999).
Neuroimaging findings suggest that these apparent interactions between age and gender arise due to age-related gender differences in brain maturational processes. Although pubertal development occurs earlier in females, the rates of certain neuromaturational processes during adolescence (notably dendritic pruning, myelination, and increases in corpus callosum size) appear to occur faster in males (De Bellis et al., 2001). Because P300 amplitude may be influenced by both sex steroids (Gould et al., 1990; Woolley et al., 1996) and the volumes of certain brain regions, including the corpus callosum (Alexander and Polich, 1995; Hoffman and Polich, 1999), comparatively early pubertal development in females, alongside comparatively faster rate of neuromaturational processes in males, may explain the noted interactions between age and gender in P300 amplitude. This hypothesis, however, can only be tested within a longitudinal design that spans the period from late childhood to early adulthood.
Regarding the relationship between P300 and AUDs, it is interesting, however, that although TNAD participants (regardless of gender) did not exhibit significant changes in the P300 relative to NSAC participants, gender appeared to moderate the direction of the relationship between P300 and AUDs. Specifically, and relative to corresponding NSAC participants, female TNAD participants showed slight decreases in P3b amplitude (i.e., a change in the expected direction), while male TNAD participants showed slight increases in P3b amplitude (i.e., a change in the opposite direction). These findings contrast with those from a previous study by this laboratory that used an identical task to examine visual P300 in adult treatment-naïve participants and found (non-significant) reductions in P3b amplitude in TNAD relative to NSAC across genders (Fein and Andrew, 2011). These discrepant findings may suggest age-dependent effects of AUDs on P3b amplitude in treatment-naïve males (i.e., young males show slight increases in P3b amplitude, while older males show slight decreases in P3b amplitude). However, because studies rarely examine the P300 in both younger and older AUD populations simultaneously, it is difficult to determine whether age-dependent effects indeed exist. Further work is thus needed to examine gender differences in the P300 in adolescents.
A potential limitation of our findings is that measurement issues may have influenced our examinations of between-group differences in family history density of alcohol problems, as well as of associations between P300 measures and family history density of alcohol problems. Specifically, we measured family history density of alcohol problems by counting the number of first-degree relatives with whom the participant had cohabited and who had an alcohol-use problem; other studies (e.g., Fein and Chang, 2008; Mann et al., 1985) did not restrict their measurement of this variable to cohabiting relatives, but instead calculated the proportion of first-degree relatives with an alcohol-use problem. Nevertheless, we maintain that our findings are sound, as they are consistent with predictions based on the expectation of lower genetic vulnerability to alcoholism in TNAD versus treated samples (Di Sclafani et al., 2008).
Some degree of caution in interpreting the results is warranted given the limited sample sizes of the current study, especially with regards to adolescent TNAD males (n = 15). Nonetheless, the P3b amplitude results for this sample of TNAD males are very supportive of our interpretations that P3b amplitude is not reduced in the AUD population studied. The P3b amplitudes are 22% (although not statistically significantly) larger in the TNAD group than the NSAC group. Longitudinal follow-up of samples such as those studied here could elucidate the stability of the findings over time with continued exposure to hazardous drinking.
In summary, our findings demonstrate an absence of P300 reduction in treatment-naïve adolescents with alcohol-use disorders. We attribute this finding to the fact that treatment-naïve adolescents have comparatively lower alcohol exposure, less genetic vulnerability to alcoholism, and less psychiatric comorbidity than more frequently studied adult treated samples. This rationale is supported by an overall lack of association between ERP and alcohol-use (individual and family) variables, and between ERP and psychiatric symptom counts, within TNAD. These observations contribute to accumulating literature describing numerous and clinically significant differences between treatment-seeking and treatment-naïve samples. Future studies should seek to create models specifying directions of association, and the weight of paths, between psychiatric comorbidity, P300 reductions, and chronic alcohol use.
Acknowledgments
This research was supported by NIH grant RO1 AA016303-01 (PI: G. Fein).
References
- Alexander JE, Polich J. P300 differences between sinistrals and dextrals. Cog Brain Res. 1995;2:277–282. doi: 10.1016/0926-6410(95)90019-5. [DOI] [PubMed] [Google Scholar]
- Bauer LO. CNS recovery from cocaine, cocaine and alcohol, or opioid dependence: a P300 study. Clin Neurophysiol. 2001;112:1508–1515. doi: 10.1016/s1388-2457(01)00583-1. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: implications for substance abuse risk and brain development. Biol Psychiatry. 1999;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Brain maturation and subtypes of conduct disorder: Interactive effects on P300 amplitude and topography in male adolescents. J Am Acad Child Adolesc Psychiatry. 2003;42:106–115. doi: 10.1097/00004583-200301000-00017. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Potential biological markers in individuals at high risk for developing alcoholism. Alcohol Clin Exp Res. 1988;12:488–493. doi: 10.1111/j.1530-0277.1988.tb00231.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin Exp Res. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Benton A, Tranel D. Historical notes on reorganization of function and neuroplasticity. In: Levin HS, Grafman J, editors. Cerebral Reorganization of Function after Brain Damage. Oxford University Press; New York: 2000. pp. 3–23. [Google Scholar]
- Biggins CA, MacKay S, Poole N, Fein G. Delayed P3A in abstinent elderly male chronic alcoholics. Alcohol Clin Exp Res. 1995;19:1032–1042. doi: 10.1111/j.1530-0277.1995.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG, McGue M. P300 amplitude in adolescent twins discordant and concordant for alcohol use disorders. Biol Psychol. 2002;61:203–27. doi: 10.1016/s0301-0511(02)00059-5. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Katsanis J, Iacono WG, Mertz AK. Substance dependence and externalizing psychopathology in adolescent boys with small, average, or large P300 event-related potential amplitude. Psychophysiology. 1999;36:583–590. [PubMed] [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL. Electrophysiological effects of dizocilpine (MK-801) in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2008;32:1752–1762. doi: 10.1111/j.1530-0277.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Finn P, Fein G. Treatment-naïve active alcoholics have greater psychiatric comorbidity than normal controls but less than treated abstinent alcoholics. Drug Alcohol Depend. 2008;98:115–122. doi: 10.1016/j.drugalcdep.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR. Adolescent ethanol exposure: does it produce long-lasting electrophysiological effects? Alcohol. 2010;44:27–37. doi: 10.1016/j.alcohol.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Andrew C. Event-related potentials during visual target detection in treatment-naıïve active alcoholics. Alcohol Clin Exp Res. 2011;35:1171–1179. doi: 10.1111/j.1530-0277.2011.01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Chang M. Visual P300s in long-term abstinent chronic alcoholics. Alcohol Clin Exp Res. 2006;30:2000–2007. doi: 10.1111/j.1530-0277.2006.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Chang M. Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naïve alcoholics. Drug Alcohol Depend. 2008;92:141–148. doi: 10.1016/j.drugalcdep.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Finn P, Scheiner DL. Sub-diagnostic psychiatric comorbidity in alcoholics. Drug Alcohol Depend. 2007;87:139–145. doi: 10.1016/j.drugalcdep.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B. Treated and treatment-naïve alcoholics come from different populations. Alcohol. 2005;36:19–26. [PMC free article] [PubMed] [Google Scholar]
- Floyd EA, Reasor JD, Moore EL, Rucker HK. Effects of chronic ethanol ingestion on mid-latency auditory evoked potentials depend on length of exposure. Alcohol. 1997;14:269–279. doi: 10.1016/s0741-8329(96)00152-8. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Weiner MW, Meyerhoff DJ. Are treated alcoholics representative of the entire population with alcohol use disorders? A magnetic resonance study of brain injury. Alcohol. 2008;42:67–76. doi: 10.1016/j.alcohol.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis C, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. PNAS. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hansenne M. Event-related brain potentials in psychopathology: clinical and cognitive perspectives. Psychologica Belgica. 2006;46:5–36. [Google Scholar]
- Hill SY, Locke J, Steinhauer SR. Absence of visual and auditory P300 reduction in nondepressed male and female alcoholics. Biol Psychiatry. 1999a;46:982–989. doi: 10.1016/s0006-3223(99)00054-2. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S. Neurodevelopmental patterns of visual P3b in association with familial risk for alcohol dependence and childhood diagnosis. Biol Psychiatry. 2002;51:621–631. doi: 10.1016/s0006-3223(01)01301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, Connolly J. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biol Psychiatry. 1999b;46:970–981. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke J. Factors predicting the onset of adolescent drinking in families at high risk for developing alcoholism. Biol Psychiatry. 2000;48:265–275. doi: 10.1016/s0006-3223(00)00841-6. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR. Assessment of prepubertal and postpubertal boys and girls at risk for developing alcoholism with P300 from a visual discrimination task. J Stud Alcohol. 1993;54:350–358. doi: 10.15288/jsa.1993.54.350. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Locke-Wellman J, Ulrich R. Childhood risk factors for young adult substance dependence outcome in offspring from multiplex alcohol dependence families: a prospective study. Biol Psychiatry. 2009;66:750–757. doi: 10.1016/j.biopsych.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayasu Y, Samura M, Ohta H, Ogura C. Sex effects on rate of change of P300 latency with age. Clin Neurophysiol. 2000;111:187–194. doi: 10.1016/s1388-2457(99)00233-3. [DOI] [PubMed] [Google Scholar]
- Hoffman LD, Polich J. P300, handedness, and corpus callosal size: gender, modality, and task. Int J Psychophysiol. 1999;31:163–174. doi: 10.1016/s0167-8760(98)00050-6. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. Int J Psychophysiol. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Kaplan HI, Sadock BJ. Synopsis of Psychiatry. Williams and Wilkins; Baltimore, MD: 1998. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. The Schedule for Affective Disorders and Schizophrenia for School-Aged Children- Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985;15:61–67. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Schmidt AL. Event-related brain potentials in subjects at a risk for alcoholism. In: Chang N, Chao H, editors. Early Identification of Alcohol Abuse. National Institute on Alcohol Abuse and Alcoholism; Rockville MD: 1985. pp. 228–239. [Google Scholar]
- Perlman G, Johnson W, Iacono WG. The heritability of P300 amplitude in 18-year-olds is robust to adolescent alcohol use. Psychophysiology. 2009;46:962–969. doi: 10.1111/j.1469-8986.2009.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Mathalon D. Event-related potentials in alcoholic men: P3 amplitude reflects family history but not alcohol consumption. Alcohol Clin Exp Res. 1991;15:839–850. doi: 10.1111/j.1530-0277.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Polich J. P300 latency reflects personal drinking history. Psychophysiology. 1984;21:592–593. [Google Scholar]
- Polich J. Normal variation of P300 from auditory stimuli. Electroencephalogr Clin Neurophysiol. 1986;65:236–240. doi: 10.1016/0168-5597(86)90059-6. [DOI] [PubMed] [Google Scholar]
- Polich J, Bloom FE. P300 and alcohol consumption in normals and individuals at risk for alcoholism. A preliminary report. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:201–210. doi: 10.1016/0278-5846(86)90074-6. [DOI] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Reich T, Van Eerdewegh P, Edenberg HJ, Foroud T, Goate A, Litke A, Chorlian DB, Stirnus A, Rice J, Blangero J, Almasy L, Sorbell J, Bauer LO, Kuperman S, O’Connor SJ, Rohrbaugh J. Amplitude of visual P3 event-related potential as a phenotypic marker for a predisposition to alcoholism: preliminary results from the COGA project. Alcohol Clin Exp Res. 1998;22:1317–1323. [PubMed] [Google Scholar]
- Rodriguez-Holguin S, Porjesz B, Chorlian DB, Polich J, Begleiter H. Visual P3a in male alcoholics and controls. Alcohol Clin Exp Res. 1999;23:582–91. [PubMed] [Google Scholar]
- Rodriguez Holguin S, Corral M, Cadaveira F. Event-related potentials elicited by infrequent non-target stimuli in young children of alcoholics: family history and gender differences. Alcohol Alcohol. 1998;33:281–290. doi: 10.1093/oxfordjournals.alcalc.a008392. [DOI] [PubMed] [Google Scholar]
- Smith S, Fein G. Cognitive performance in treatment-naive active alcoholics. Alcohol Clin Exp Res. 2010;34:1–9. doi: 10.1111/j.1530-0277.2010.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self reported alcohol consumption. In: Litten A, editor. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa, N.J: 1992. pp. 41–72. [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Hill SY. Auditory event-related potentials in children at high risk for alcoholism. J Stud Alcohol. 1993;54:408–421. doi: 10.15288/jsa.1993.54.408. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Wenzel HJ, Schwartzkroin PA. Estradiol increases the frequency of multiple synapse boutons in the hippocampal CA1 region of the adult female rat. J Comp Neurol. 1996;373:108–117. doi: 10.1002/(SICI)1096-9861(19960909)373:1<108::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- World Medical Association. 59th WMA General Assembly. Seoul, South Korea: 2008. Declaration of Helsinki. Ethical principles for medical research involving human subjects. [Google Scholar]