Abstract
Lysophosphatidic acid (LPA), a simple bioactive phospholipid, is present in biological fluids such as plasma and bronchoalveolar lavage (BAL). It appears to have both pro- and anti-inflammatory roles in inflammatory lung diseases. Exogenous LPA promotes inflammatory responses by regulating the expression of chemokines, cytokines, and cytokine receptors in lung epithelial cells. In addition to the modulation of inflammatory responses, LPA regulates cytoskeleton rearrangement and confers protection against lung injury by enhancing lung epithelial cell barrier integrity and remodeling. The biological effects of LPA are mediated through its cell surface G-protein coupled LPA1-7 receptors. The roles of LPA receptors in lung fibrosis, asthma, and acute lung injury have been investigated using genetically engineered LPA receptor deficient mice and there appears to be a definitive role for endogenous LPA and its receptors in the pathogenesis of pulmonary inflammatory diseases. This review summarizes recent reports on the role of LPA and its receptors in the regulation of lung epithelial inflammatory responses and remodeling.
Keywords: Lysophosphatidic acid (LPA), LPA receptors, lung epithelial cells, cytokines, inflammation, cell motility, lung inflammatory diseases
1. Introduction
Lysophosphatidic acid (LPA) is a lysophospholipid, which has the simple structure consisting of a long chain fatty acid linked to sn-1 or sn-2 and a phosphate group at sn-3 position of the glycerol backbone. LPA is a naturally occurring bioactive lysophospholipid present in most tissues and biological fluids at nM to μM concentrations. It plays a critical role in de novo biosynthesis of phospholipids and is also a key intermediate in the biosynthesis of phosphatidic acid (PA). Progress made over the past two decades has characterized LPA as a serum-derived growth factor that is involved in several cellular functions such as proliferation [1, 2], migration [3, 4], cytokine/chemokines secretion [5-7], platelet aggregation [8, 9], smooth muscle cell contraction [10], and neurite retraction [11]. The biological effects of LPA are mediated through binding to G protein-coupled LPA1-7 receptors expressed on the surface of variety of cells [12-14]. The cloning and characterization of LPA receptors have advanced our current understanding of LPA-mediated signal transduction and biological responses in mammalian cells and tissues. Further, development of small molecular antagonists or agonists of LPA receptors and genetically engineered LPA receptor(s) deficient mice have provided direct evidence supporting the various physiological and pathological roles of LPA and LPA receptors. Airway epithelial cells express predominantly LPA1-3 and respond to exogenous LPA challenge by activation of transcriptional factors and cytokine secretion [6, 15, 16]. LPA and LPA receptors have been implicated in a number of human pathologies including cancer, fibrosis, reproductive disorders and bone metabolism [17, 18]. The present review focuses on the role of LPA and LPA receptors in epithelial inflammation and remodeling of the airway under normal and pathological conditions.
2. Metabolism of LPA in airway epithelial cells
LPA synthesis can be intracellular or extracellular. Intracellular LPA synthesis involves at least two pathways: one mediated by phospholipase D (PLD) and phosphatidic acid (PA) specific phospholipase A (PLA) [19]; and the other by acylglycerol kinase (AGK) [20, 21]. The existence of PLD/PLA pathway has been well demonstrated in several cell types; however, there is limited information on LPA generation in lung epithelial cells. LysoPLD or autotaxin contributes to extracellular LPA synthesis [22, 23]. Lipid phosphate phosphatases (LPPs) are localized on the plasma membrane of cells [24, 25] and modulate extracellular LPA catabolism. This review will summarize recent studies regarding the role of AGK, lysoPLD, and LPPs in LPA metabolism in airways and their roles in airway biology.
2.1. Intracellular generation of LPA: role of AGK
While searching for sphingosine kinase isoforms, AGK was identified as a potential lipid kinase that generates intracellular LPA from monoacylglycerol (MAG) [20]. Immunofluorescence staining of lung epithelial cells revealed that AGK is localized to mitochondria [20, 21] and has limited activity on sphingosine or ceramide. Over-expression of AGK increases cellular LPA levels, while down-regulation of AGK by using specific siRNA reduces cellular LPA levels [20, 21]. AGK-mediated intracellular LPA generation modulates the effects of exogenous LPA-induced activation of transcriptional factors and cytokine release [21, 26]. Down-regulation of AGK reduces histone acetylation thereby suggesting a role for AGK in regulating gene expression [26]. Since lung type II epithelial cells are rich in mitochondria, investigating the roles of AGK-mediated LPA and PA generation in the regulation of mitochondria functions in lung epithelial cells is likely to broaden our knowledge of mitochondria in inflammation. Further studies are necessary to delineate physiological role of intracellular LPA in regulating cellular functions such as airway inflammation and remodeling.
2.2 Extracellular generation of LPA: role of lysoPLD
LPA levels in bronchoalveolar lavage (BAL) increase in lung inflammatory diseases, such as asthma [27, 28], fibrosis [29], and acute lung injury [16]. The mechanisms underlying the generation of LPA in BAL are still not clear; however, lysoPLD appears to contribute to BAL LPA generation. LysoPLD, also referred to as autotaxin (ATX) or ectonucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2), is a key enzyme in plasma LPA generation from lysophosphatidylcholine (LPC). LysoPLD deficient mice die at embryonic day E9.5. On the other hand lysoPLD heterozygous mice appear healthy with just half the levels of plasma LPA [30]. LysoPLD is widely expressed in mammalian tissues and abundantly present in blood. LPA production is attenuated in lysoPLD-depleted serum and plasma. In addition to blood, lysoPLD has been detected in other biological fluids such as cerebrospinal [31]. Lung epithelial cells express and release lysoPLD [4]. The lysoPLD substrate, lysoPC, is present in BAL and is a part of the surfactant. Overexpression of lysoPLD or addition of recombinant lysoPLD to lung epithelial cells results in an increase in extracellular LPA levels and enhances cell migration [4]. These findings indicate that 1) BAL LPA is generated from lysoPC by lysoPLD and 2) autotaxin regulates cellular functions through LPA generation. Recent studies also demonstrate that autotaxin regulates cell motility by association with LPA receptor and integrins directly [4]. Investigating the pathological role of lysoPLD in lung inflammatory diseases using lysoPLD transgenic and heterozygous knockout mice should provide the critical information required to develop therapeutic LPA targets to combat various pulmonary disorders.
2.3 Catabolism of LPA
LPA has a relatively short half-life (t1/2 = ~2 h) in the cell culture media due to the presence of LPPs on the cell surface [32]. LPP family is comprised of three major isoforms, termed LPP1-3, which convert LPA to monoacylglycerol (MAG). LPPs are ecto-enzymes, which modulate LPA in circulation [25, 33]. LPPs dephosphorylate various lysophospholipids, including LPA and sphingosine-1-phosphate (S1P). LPP1 has highest activity towards LPA as a substrate. Exogenously added [3H]oleoyl LPA hydrolyzed to [3H]MAG and [3H]oleic acid in lung epithelial cells [32]. LPP1 deficient mice reduce LPA levels in multiple tissues by about 35 – 95% thereby demonstrating that other LPPs may have a role in the breakdown of LPA [34]. Expression of LPPs in lung epithelial cells has been detected by RT-PCR and Western blotting. Over-expression of LPP-1 wild type increased LPA hydrolysis 2-3 fold over control and attenuated LPA-induced intracellular signaling and cytokine release in lung epithelial cells [32]. The relative contributions of this pathway in the regulation of LPA levels in airway epithelium need further investigation.
3. LPA increases IL-8 production in airway epithelium
Airway epithelium, the first line-of-defense to inhaled stimuli, including particulate matter, dust, allergens and various kinds of microbes, plays a protective role by enhancing its barrier integrity and releasing cytokines into the airway that chemo-attract immune cells [35, 36]. Interleukin (IL-8), a major neutrophil chemoattractant, plays an important role in innate immunity. LPA is potent stimulator of airway epithelial cells that triggers IL-8 gene expression [6, 15, 32, 37]. LPA increases IL-8 mRNA and protein release in dose and time dependent manners in human airway epithelial cells [6, 15]. Intratracheal injection of LPA into the mouse lungs stimulates MIP-2 (a murine analog of IL-8) levels in BAL at 3 h and a significant infiltration of neutrophils into the airways at 6 h [6]. In comparison to LPA, LPS is a more powerful agonist and its effect on the induction of MIP-2 can be 1000 fold greater than LPA. The significant differences between the effects of LPA and LPS indicate that exogenous LPA may have a protective effect in endotoxin-induced lung inflammation that was confirmed in later studies using either intratracheal [38] or intravenous [39, 40] routes for LPA administration. Thus, LPA plays a protective role towards infection by increasing transient innate immune responses in the early stage of inflammation.
3.1 LPA activates transcriptional factors
The LPA1 and LPA3 receptors, compared to LPA2, are most efficient in transducing LPA-mediated IL-8 production [15]. Several transcriptional factors are activated in response to LPA treatment in lung epithelial cells. Human IL-8 promoter contains NF-κB and AP-1 binding sites. LPA induces phosphorylation of I-κB that results in its degradation and the translocation of released NF-κB to nucleus. Inhibition of NF-κB pathway attenuates LPA-induced IL-8 production [32]. In addition to NF-κB, JNK-mediated transcriptional factor, AP-1, is also involved in LPA-induced IL-8 production [15]. JNK enhances the transcriptional activity of AP-1 by the phosphorylation of c-Jun on Ser63 and Ser73. LPA treatment stimulates activation of JNK and AP-1 binding to genomic DNA. JNK inhibitor or c-Jun siRNA pretreatment blocks LPA-induced IL-8 release. These results suggest that at least NF-κB and JNK/AP-1 pathways contribute to LPA-induced IL-8 production in lung epithelial cells. The transcriptional factor, C/EBPβ, binding site has been detected on human IL-8 promoter region and LPA activates C/EBPβ mediated transcriptional activity of COX-2 gene expression in lung epithelial cells [41]; however the role of C/EBPβ in LPA-induced IL-8 production is not known. LPA mediated modulation of innate immunity in lungs therefore can be attributed to its ability to promote IL-8 generation.
3.2 Intracellular signaling regulates LPA-induced IL-8 production
LPA stimulates an increase in intracellular calcium levels [32], phosphorylation of PKCδ [6], and p38 MAPK [15, 16], and activation of phospholipase D (PLD) [42]. Activation of these signaling pathways regulates IL-8 production by modulating NF-κB activity [6, 32, 43]. In lung epithelial cells, LPA-induced phosphorylation of I-κB and IL-8 production were abolished by inhibition of these pathways using specific NF-κB inhibitor or siRNA for p65 subunit of NF-κB [6, 32, 42]. Additionally, cross-talk between LPA receptors and receptor tyrosine kinases seems to regulate IL-8 production. In human bronchial epithelial cells, LPA transactivated EGF-R through PKCδ-Lyn kinase signal transduction with subsequent activation of MMP2/9 and proHB-EGF shedding [37]. Further, transactivation of EGF-R-mediated LPA-induced IL-8 production is independent on activation of NF-κB and JNK/AP-1 pathways [37]. LPA-induced ERK1/2 phosphorylation has no effect on IL-8 production as evidenced from studies using ERK inhibitor. Transactivation of EGF-R by LPA regulates C/EBPβ activity, which may contribute to IL-8 production in human bronchial epithelial cells [41]. It will be interesting to determine in vivo the involvement of cross-talk between LPA-Rs and receptor tyrosine kinases in IL-8 secretion and recruitment of neutrophils into alveolar space and the physiological implications of such an interaction in airway diseases.
3.3 LPA metabolizing enzymes regulate LPA-induced IL-8 production
LPA levels in tissues and cells are down-regulated to PA by a long chain fatty acyl CoA: acyltransferase or to MAG by LPPs. LPP1 regulates extracellular LPA levels by converting LPA to MAG [25, 32, 33]. Over-expression of LPP1 enhanced exogenous LPA degradation and attenuated exogenous LPA-induced signaling, such as increases in intracellular calcium, phosphorylation of I-κB, and Erk, and IL-8 production in lung epithelial cells [32]. The mechanism of LPP1 mediated regulation of LPA-induced signaling may be complex, since overexpression of LPP1 (R217K) mutant partially attenuated LPA-induced IL-8 secretion without altering IL-8 mRNA levels [32]. This result suggests that LPP1 partly participates in LPA-induced cytokine secretion mechanism. AGK regulates intracellular LPA levels by converting MAG to LPA [20, 21]. A recent study demonstrates that regulation of intracellular LPA by AGK modulates exogenous LPA-induced signaling and IL-8 production [21]. In addition to LPA receptors, PPARγ is an intracellular receptor of LPA [43]; however, inhibition of PPARγ has no effect on LPA-induced IL-8 production (Zhao, Y. unpublished data) suggesting PPARγ-independent pathways regulate intracellular LPA-mediated IL-8 production in airway epithelial cells. Changes in AGK expression by plasmid overexpression or siRNA treatment modulate exogenous LPA-induced phosphorylation of p38 MAPK, I-κB, and EGF-R, which regulate IL-8 production [21]. However, how intracellular LPA regulates these pathways is still not clear. Inhibition of LPA receptors by PTx (pertussis toxin) attenuated AGK-mediated cell proliferation in PC-3 cells [20]. LPA receptors are not only localized on the cell surface, but also exist in the cytoplasm and the nucleus [44]. The mechanisms of AGK-generated intracellular LPA mediated signal transduction and cellular responses need to be investigated in greater detail using different lung cells.
4. LPA regulates Th2 responses in airway epithelium
The relative balance between Th1 and Th2 responses plays a critical role in pathogenesis of lung inflammatory diseases. In addition to stimulating innate immunity, LPA regulates Th2 responses by increasing Th2 type cytokine decoy receptors [26, 45] and PGE2 release [46] in lung epithelial cells. LPA-activated signaling and transcriptional factors contribute to these effects. Greater understanding of the above roles of LPA in innate and adaptive immunity is therefore crucial for the development of highly efficacious therapeutics with the least side effects.
4.1 LPA modulates IL-13/IL-13 receptor signal transduction
IL-13, a Th2 cytokine, plays a pivotal role in pathogenesis of bronchial asthma via IL-13 receptor alpha1 (IL-13Rα1) and IL-4 receptor alpha (IL-4Rα). Recent studies show that a decoy receptor for IL-13, namely IL-13α2, has higher binding efficiency to IL-13 and mitigates IL-13 signaling [47, 48]. Pretreatment with LPA attenuates IL-13-induced phosphorylation of STAT6 and cytokine release, suggesting an anti-inflammatory effect of LPA via attenuation of IL-13-induced inflammation [45]. LPA treatment induces IL-13Rα2 mRNA expression and protein release, without altering IL-13Rα1 and IL-4Rα expression, in lung epithelial cells. PLD-mediated JNK/AP-1 pathway, but not NF-κB pathway, regulates LPA-induced IL-13Rα2 expression [45]. The above study suggests a novel mechanism of regulation of IL-13Rα2 and IL-13 signaling that may be physiologically relevant to airway inflammation and remodeling.
4.2 LPA increases sST2 release
Soluble ST2 (sST2), an anti-inflammatory mediator, is known to function as a decoy receptor of IL-33 and attenuates IL-33- and endotoxin-induced inflammatory responses [49, 50]. LPA treatment increases sST2 mRNA expression and protein release in a dose and time dependent manner in lung epithelial cells [26]. The above effect of LPA is blocked by inhibition of LPA receptors. Both NF-κB and JNK/AP-1 pathways regulate LPA-induced sST2 release. In addition to activation of transcription factors, LPA treatment decreases histone deacetylase 3 (HDAC3) mass and enhances acetylation of histone H3 at lysine 9 that binds to the sST2 promoter region [26]. Decrease in intracellular LPA level by down-regulation of AGK attenuates exogenous LPA-induced histone H3 acetylation on sST2 promoter region, as well as sST2 gene expression [26]. sST2 treatment reduces LPS-induced cytokine release in macrophages. In lung epithelial cells, treatment with recombinant sST2 protein or sST2-rich cell culture media attenuates LPS-induced lung epithelial barrier disruption [26]. The above study unraveled a novel mechanism of exogenous LPA mediated regulation of Th2 cytokine decoy receptor, sST2, which plays an anti-inflammatory role in lung inflammatory diseases.
4.3 LPA increases COX2 expression and PGE2 release
LPA induces COX2 expression and PGE2 release in various cell types [51, 52], including lung epithelial cells [41]. In the lungs, COX-2 and PGE2 play anti-inflammatory roles, especially in Th2-dominant inflammation [53]. Transcriptional factors, NF-κB, JNK/AP-1, and C/EBPβ pathways regulate the effect of LPA [41]. Activation of C/EBPβ by EGFR is known to regulate expression of a variety of genes [54, 55]. Interestingly, only transcriptional activation of C/EBPβ, not NF-κB and JNK/AP-1, is regulated by the cross-talk between LPA receptors and EGFR in lung epithelial cells [41]. The transactivation of EGF-R by LPA is mediated by PLD2-activated PKCζ [41]. PGE2 induces biological functions through its receptors (EP1-4) on the surface of lung epithelial cells [56]. The released PGE2 regulates particulate matter-induced IL-6 production [46]. In primary airway macrophages, PGE2 blocks IL-4/IL-13-induced eotaxin-2 release [57]. However, the role of PGE2/EP1-4 in IL-13Rα2 and sST2 production and release has not been investigated. It will be interesting to see whether COX-2/PGE2 plays a role in LPA-induced cytokine decoy receptors expression and release.
5. LPA in airway epithelial remodeling
The lung epithelial cells confer protection against environmental toxic injury by enhancing epithelial barrier integrity. LPA promote cellular motility through cytoskeleton rearrangement [4, 38].
5.1 LPA enhances airway epithelial barrier enhancement
Lung epithelial barrier integrity is maintained by cell-cell junctions. The adherens junction plays a critical role in regulating the activity of the entire junctional complex since the formation of the adherens junctions subsequently lead to the generation of other cell-cell junctions. LPA treatment enhances lung epithelial integrity through accumulation of E-cadherin at cell-cell contacts [38, 58]. LPA-activates PKC isoforms, PKCδ and PKCζ, and FAK mediates E-cadherin accumulation at cell-cell contacts [38, 58]. The direct role of these kinases on E-cadherin distribution at cell-cell junctions has not been investigated. However, c-Met, the receptor for HGF, interacts with E-cadherin at cell-cell contacts in response to LPA treatment, and PKCδ promotes c-Met serine phosphorylation and accumulation at cell-cell contacts [58]. These kinases may partly regulate E-cadherin localization through phosphorylation of c-Met, suggesting a potential role of c-Met in lung epithelial barrier integrity. The role of c-Met in lung epithelial barrier integrity and the underlying mechanisms could unravel novel therapeutic approaches to combat inflammatory lung diseases.
5.2 LPA induces lung epithelial cell migration
The migration of healthy lung epithelial cells to the injured site is a major mechanism of lung repair and remodeling that involves various intracellular cytoskeleton related proteins, including cortactin. LPA treatment increases lung epithelial cell migration that is abolished by inhibition of PKCδ or abrogation of cortactin expression [4]. LPA induces tyrosine phosphorylation of cortactin on leading edge of migrating cell through activation of PKCδ [4]. LysoPLD, a key enzyme required for the generation of extracellular LPA, also regulates lung epithelial cell migration through LPA-dependent [4, 59] and independent pathways [4]. LysoPLD associates with LPA receptors and integrins on the cell surface, which induces cell migration. The association between lysoPLD and integrins has been demonstrated in platelets [60]. The mechanisms underlying tyrosine phosphorylation of cortactin by LPA are yet to be delineated, which could serve as a potential therapeutic target using currently available specific tyrosine kinase inhibitors.
6. LPA receptors in murine models of pulmonary inflammatory diseases
In contrast to exogenous LPA, the endogenous LPA appears to be a pro-inflammatory mediator. LPA levels in BAL fluid are elevated in murine models of lung inflammatory diseases, including asthma [27, 28], fibrosis [29], and acute lung injury [16]. The increased LPA levels in these pathologies suggest that the rate of LPA generation is greater than the rate of its degradation. The chronically elevated levels of LPA in BAL fluid may trigger excess cytokine release and cell toxicity [61]. Exogenous LPA on the other hand, has a relatively short half-life and induces a transient increase in neutrophils infiltration that modulates innate immunity and protects endotoxin-induced lung injury.
6.1 Endogenous LPA and LPA receptors in fibrosis
Pulmonary fibrosis is an inflammatory lung disorder characterized by abnormal formation and deposition of fibrous connective tissue proteins such as fibronectin and collagen in the lungs. In idiopathic pulmonary fibrosis subjects, LPA levels in BAL fluid are significantly elevated compared to normal healthy controls [29]. The source of LPA in BAL fluid has not been determined yet. In bleomycin challenged-murine model of fibrosis, LPA levels in BAL fluid significantly increased after 5 - 10 days, while LPA1 knockout mice offered significant resistance to bleomycin induced lung fibrosis [29]. Furthermore, Tager et al. demonstrated that LPA induces fibroblast proliferation, growth, and migration. TGFβ1, a multifunctional cytokine, plays a central role in pathogenesis of pulmonary fibrosis [29]. Recent studies show that LPA enhances TGFβ1 activation and apoptosis in lung epithelial cells [61]. LPA1 is involved in LPA-induced apoptosis [61], while LPA2 contributes to TGFβ1 generation in lung epithelial cells [62]. Epithelial-mesenchymal transition (EMT) plays a critical role in pathogenesis of fibrosis; however the role of LPA and LPA receptors in EMT has not been well investigated.
6.2 Endogenous LPA and LPA receptors in asthma
LPA levels are increased in BAL fluid from challenged asthmatic subjects, compared to unchallenged subjects, indicating that endogenous LPA may contribute to pathogenesis of asthma [27]. In a murine model of asthma, Schistosoma mansoni egg sensitization and challenge increased LPA levels in BAL fluid, eosinophil infiltration into airways and mucus production [28]. LPA1 heterozygous mice however suffered from significantly reduced mucus production but not eosinophil infiltration, while LPA2 heterozygous mice revealed a decrease in both eosinophil infiltration and mucus production [28]. These results suggest a pro-inflammatory role for endogenous LPA and LPA receptors in allergic asthma model. Recent study using OVA-challenged murine model of asthma demonstrated that LPA2 deficient mice develop greater allergen-induced lung inflammation [63]. The opposing role of LPA2 in modulating asthmatic lung inflammation may therefore be dependent on the expression levels of LPA receptors, allergens used to induce asthmatic phenotype in mice and severity of asthma.
6.3 Endogenous LPA and LPA receptors in acute lung injury
Elevated LPA levels in BAL fluid and plasma were detected in LPS-induced murine model of acute lung injury [16]. LPA1 knockout mice show decreased inflammation in LPS-induced acute lung injury; however, protein levels in BAL fluid, which indicate endothelial and epithelial barrier disruption, remain unaltered, compared to LPS challenged-wild type mice [16]. This suggests that genetic knockdown of LPA receptor in mice reduce inflammation, but not injury, mediated by LPS challenge. Furthermore, intratracheally administrated LPA1&3 antagonist ki16425 reduced LPS-induced inflammatory cell infiltration and IL-6 levels in BAL, but had no effect on protein level [16]. These data suggest that the endogenous LPA / LPA1-mediated cytokine release and neutrophil influx contribute to the pathogenesis of acute lung injury [16]. Recent studies demonstrated that LPA1 associates with CD14, a co-receptor of LPS. Inhibition of LPA1 partly blocked LPS-induced signaling and IL-6 release in lung epithelial cells [16]. In addition, in THP-1 cells, either downregulation of lysoPLD or LPA3 attenuated LPS-induced CCL8 release [64]. These studies suggest that LPA receptors are involved in LPS signaling. Future studies on LPS-induced LPA generation and degradation are necessary to understand the role of LPA and LPA receptors in acute lung injury.
7. Conclusion
In recent years, there has been overwhelming evidence in support of LPA and its receptors as key mediators of cell function under normal and pathological conditions. It is also becoming apparent that LPA signals not only extracellularly through its transmembrane G protein-coupled receptors on the cell surface but also intracellularly either directly or via its receptors that are localized on the nuclear membrane. Similar to other bioactive lipids such as S1P, LPA actions can be pro- or anti-inflammatory depending upon the pathology of the disease and the underlying cell type(s). LPA/LPAR mediated signaling also results in the transactivation of receptor tyrosine kinases such as EGFR and c-Met and the cross-talks have profound implication in a variety of cellular functions such as regulation of inflammatory response, angiogenesis and tumorigenesis. In the lung, LPA and LPA receptors have been implicated in the pathophysiology of asthma, pulmonary fibrosis and acute lung injury. The source of LPA and the role of LPA/LPA receptors-mediated signaling in inflammatory cells in response to endotoxin are unclear. In BAL fluid, polyunsaturated LPA species such as 22:6LPA, 22:4LPA and 20:4LPA are elevated after segmental allergen challenge in asthmatics; however, the role of polyunsaturated LPAs and its signaling through its receptors in normal and pathological conditions such as asthma remains to be investigated.
Current studies suggest that LPA and LPA receptors are promising therapeutic targets in cancer, pulmonary and cardiovascular diseases. Development of highly specific small molecule inhibitors towards autotaxin and LPA receptors and their evaluation in clinically relevant in vivo models of human diseases such as pulmonary fibrosis, asthma and sepsis-induced acute lung injury are likely to result in the development of highly efficacious therapies. However, expression of multiple LPA receptors in the same cell type coupled to redundancy in signal transduction pathways poses a formidable challenge in designing specific inhibitors and targeting them to specific organs and cell types in vivo. Formidable challenges faced in achieving the above goal are expression of multiple LPA receptors in the same cell type and the redundancy in signal transduction pathways mediated by LPA/LPA receptors. Despite the above hurdles, significant recent advances in our understanding of various canonical and non-canonical signaling pathways and their overlap and the pathobiology of related diseases project a positive outlook for the near future.
Highlights.
LPA regulates cytokines and cytokine decoy receptors release in lung epithelial cells.
LPA regulates cell-cell adherens junction and cell motility.
LPA-induces its biological responses via LPA receptors and intracellular signaling pathways.
LPA receptors contribute to pathogenesis of lung inflammatory diseases.
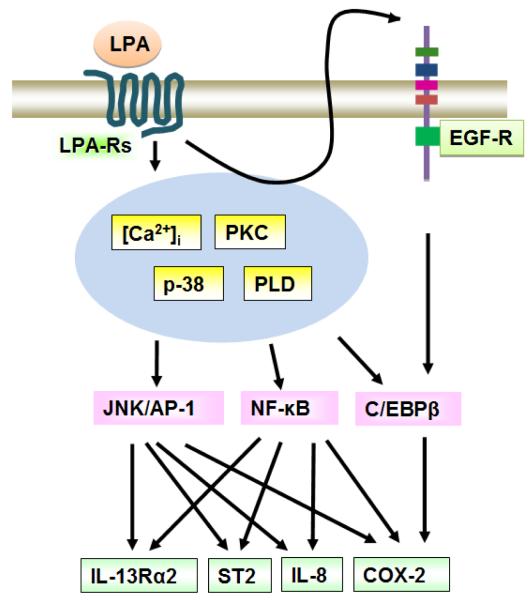
Fig. 1. LPA induces IL-8 production in lung epithelial cells.
LPA binding to LPA receptors on the cell surface induces activation of intracellular signaling pathways and transactivates EGF-R. This transactivation triggers activation of transcriptional factors, thereby regulating IL-8 gene expression.
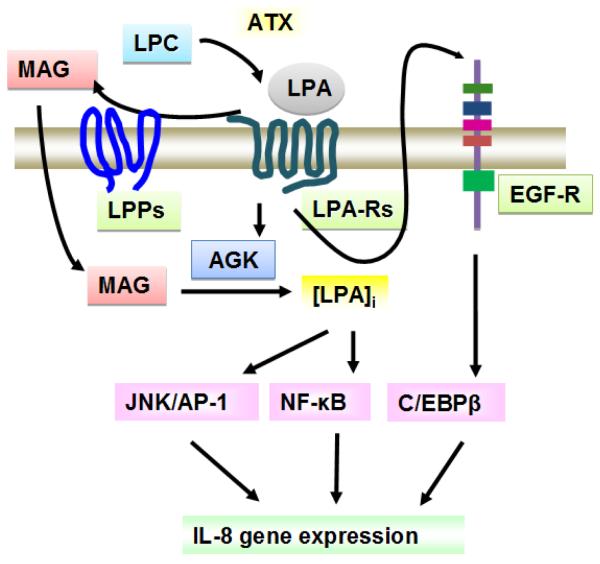
Fig. 2. LPA metabolizing enzymes regulate LPA-induced signal transduction.
Extracellular LPA is generated from LPC by the action of autotaxin (ATX). LPPs on the cell surface degrade LPA to monoacylglycerol (MAG). Extracellular LPA induces LPA signaling through LPA receptors. Intracellular LPA generated from MAG by action of acylglycerol kinase (AGK) regulates activation of transcriptional factors.
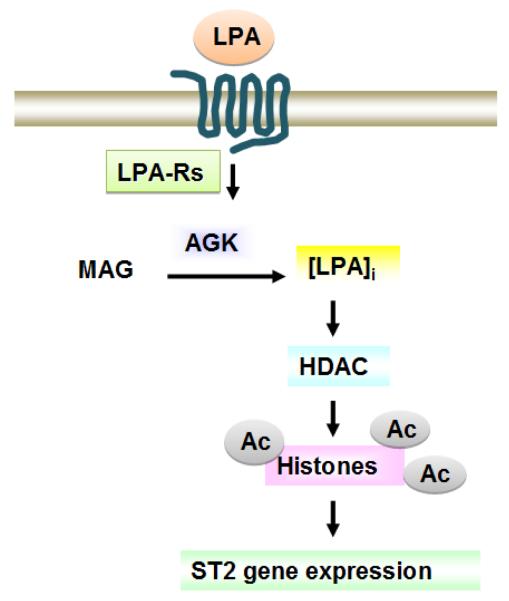
Fig. 3. LPA modulates ST2 expression via histone acetylation.
LPA reduces HDAC expression, thus increasing acetylation of histones and regulating ST2 gene expression. The effect is regulated by intracellular LPA generated by action of AGK.
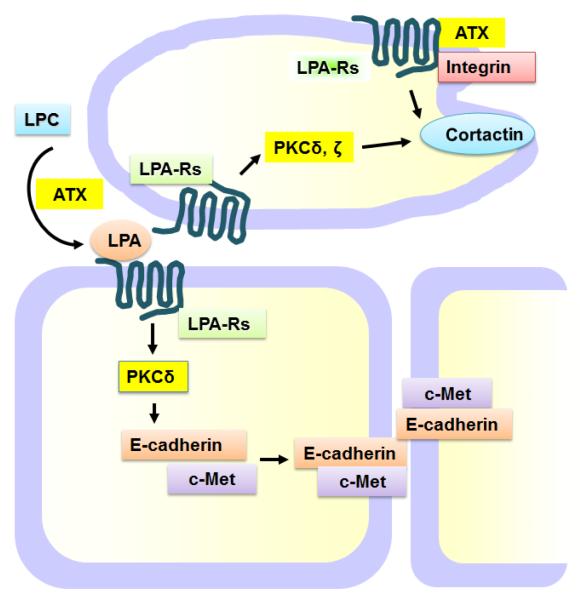
Fig. 4. LPA regulates cell-cell adherens junction and cell motility.
LPA induces PKCδ and ζ-mediated c-Met and E-cadherin accumulation at cell-cell contacts, thus regulating cell-cell adherens junction. ATX or LPA induces and cell migration dependent on LPA receptor-mediated PKCδ activation, phosphorylation of cortactin and interaction with integrins.
Acknowledgements
The study was supported by National Institutes of Health HL091916 to YZ and HL P01 98050 to VN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- [2].Tigyi G, Dyer DL, Miledi R. Lysophosphatidic acid possesses dual action in cell proliferation. Proc Natl Acad Sci U S A. 1994;91:1908–1912. doi: 10.1073/pnas.91.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stahle M, Veit C, Bachfischer U, Schierling K, Skripczynski B, Hall A, Gierschik P, Giehl K. Mechanisms in LPA-induced tumor cell migration: critical role of phosphorylated ERK. J Cell Sci. 2003;116:3835–3846. doi: 10.1242/jcs.00679. [DOI] [PubMed] [Google Scholar]
- [4].Zhao J, He D, Berdyshev E, Zhong M, Salgia R, Morris AJ, Smyth SS, Natarajan V, Zhao Y. Autotaxin induces lung epithelial cell migration through lysoPLD activity-dependent and -independent pathways. Biochem J. 2011;439:45–55. doi: 10.1042/BJ20110274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fang X, Yu S, Bast RC, Liu S, Xu HJ, Hu SX, LaPushin R, Claret FX, Aggarwal BB, Lu Y, Mills GB. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J Biol Chem. 2004;279:9653–9661. doi: 10.1074/jbc.M306662200. [DOI] [PubMed] [Google Scholar]
- [6].Cummings R, Zhao Y, Jacoby D, Spannhake EW, Ohba M, Garcia JG, Watkins T, He D, Saatian B, Natarajan V. Protein kinase Cdelta mediates lysophosphatidic acid-induced NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells. J Biol Chem. 2004;279:41085–41094. doi: 10.1074/jbc.M404045200. [DOI] [PubMed] [Google Scholar]
- [7].Hao F, Tan M, Xu X, Han J, Miller DD, Tigyi G, Cui MZ. Lysophosphatidic acid induces prostate cancer PC3 cell migration via activation of LPA(1), p42 and p38alpha. Biochim Biophys Acta. 2007;1771:883–892. doi: 10.1016/j.bbalip.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schumacher KA, Classen HG, Spath M. Platelet aggregation evoked in vitro and in vivo by phosphatidic acids and lysoderivatives: identity with substances in aged serum (DAS) Thromb Haemost. 1979;42:631–640. [PubMed] [Google Scholar]
- [9].Simon MF, Chap H, Douste-Blazy L. Platelet aggregating activity of lysophosphatidic acids is not related to their calcium ionophore properties. FEBS Lett. 1984;166:115–119. doi: 10.1016/0014-5793(84)80055-1. [DOI] [PubMed] [Google Scholar]
- [10].Toews ML, Ustinova EE, Schultz HD. Lysophosphatidic acid enhances contractility of isolated airway smooth muscle. J Appl Physiol. 1997;83:1216–1222. doi: 10.1152/jappl.1997.83.4.1216. [DOI] [PubMed] [Google Scholar]
- [11].Tigyi G, Fischer DJ, Sebok A, Yang C, Dyer DL, Miledi R. Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+ signaling and Rho. J Neurochem. 1996;66:537–548. doi: 10.1046/j.1471-4159.1996.66020537.x. [DOI] [PubMed] [Google Scholar]
- [12].Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol Pharmacol. 2000;58:1188–1196. doi: 10.1124/mol.58.6.1188. [DOI] [PubMed] [Google Scholar]
- [13].Contos JJ, Chun J. Genomic characterization of the lysophosphatidic acid receptor gene, lp(A2)/Edg4, and identification of a frameshift mutation in a previously characterized cDNA. Genomics. 2000;64:155–169. doi: 10.1006/geno.2000.6122. [DOI] [PubMed] [Google Scholar]
- [14].Chun J, Contos JJ, Munroe D. A growing family of receptor genes for lysophosphatidic acid (LPA) and other lysophospholipids (LPs) Cell Biochem Biophys. 1999;30:213–242. doi: 10.1007/BF02738068. [DOI] [PubMed] [Google Scholar]
- [15].Saatian B, Zhao Y, He D, Georas SN, Watkins T, Spannhake EW, Natarajan V. Transcriptional regulation of lysophosphatidic acid-induced interleukin-8 expression and secretion by p38 MAPK and JNK in human bronchial epithelial cells. Biochem J. 2006;393:657–668. doi: 10.1042/BJ20050791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhao J, He D, Su Y, Berdyshev E, Chun J, Natarajan V, Zhao Y. Lysophosphatidic acid receptor 1 modulates lipopolysaccharide-induced inflammation in alveolar epithelial cells and murine lungs. Am J Physiol Lung Cell Mol Physiol. 2011;301:L547–556. doi: 10.1152/ajplung.00058.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tigyi G. Aiming drug discovery at lysophosphatidic acid targets. British J Pharmacol. 2010;161:241–270. doi: 10.1111/j.1476-5381.2010.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- [19].Eder AM, Sasagawa T, Mao M, Aoki J, Mills GB. Constitutive and lysophosphatidic acid (LPA)-induced LPA production: role of phospholipase D and phospholipase A2. Clin Cancer Res. 2000;6:2482–2491. [PubMed] [Google Scholar]
- [20].Bektas M, Payne SG, Liu H, Goparaju S, Milstien S, Spiegel S. A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells. J Cell Biol. 2005;169:801–811. doi: 10.1083/jcb.200407123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kalari S, Zhao Y, Spannhake EW, Berdyshev EV, Natarajan V. Role of acylglycerol kinase in LPA-induced IL-8 secretion and transactivation of epidermal growth factor-receptor in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L328–336. doi: 10.1152/ajplung.90431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tokumura A, Yamano S, Aono T, Fukuzawa K. Lysophosphatidic acids produced by lysophospholipase D in mammalian serum and body fluid. Ann N Y Acad Sci. 2000;905:347–350. doi: 10.1111/j.1749-6632.2000.tb06576.x. [DOI] [PubMed] [Google Scholar]
- [24].Zhang QX, Pilquil CS, Dewald J, Berthiaume LG, Brindley DN. Identification of structurally important domains of lipid phosphate phosphatase-1: implications for its sites of action. Biochem J. 2000;345(Pt 2):181–184. [PMC free article] [PubMed] [Google Scholar]
- [25].Pyne S, Long JS, Ktistakis NT, Pyne NJ. Lipid phosphate phosphatases and lipid phosphate signalling. Biochem Soc Trans. 2005;33:1370–1374. doi: 10.1042/BST0331370. [DOI] [PubMed] [Google Scholar]
- [26].Zhao J, Chen Q, Li H, Myerburg M, Spannhake EW, Natarajan V, Zhao Y. Lysophosphatidic acid increases soluble ST2 expression in mouse lung and human bronchial epithelial cells. Cell Signal. 2012;24:77–85. doi: 10.1016/j.cellsig.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Georas SN, Berdyshev E, Hubbard W, Gorshkova IA, Usatyuk PV, Saatian B, Myers AC, Williams MA, Xiao HQ, Liu M, Natarajan V. Lysophosphatidic acid is detectable in human bronchoalveolar lavage fluids at baseline and increased after segmental allergen challenge. Clin Exp Allergy. 2007;37:311–322. doi: 10.1111/j.1365-2222.2006.02626.x. [DOI] [PubMed] [Google Scholar]
- [28].Zhao Y, Tong J, He D, Pendyala S, Evgeny B, Chun J, Sperling AI, Natarajan V. Role of lysophosphatidic acid receptor LPA2 in the development of allergic airway inflammation in a murine model of asthma. Respir Res. 2009;10:114. doi: 10.1186/1465-9921-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- [30].van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, Pettit TR, Wakelam MJ, Saulnier-Blache JS, Mummery CL, Moolenaar WH, Jonkers J. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sato K, Malchinkhuu E, Muraki T, Ishikawa K, Hayashi K, Tosaka M, Mochiduki A, Inoue K, Tomura H, Mogi C, Nochi H, Tamoto K, Okajima F. Identification of autotaxin as a neurite retraction-inducing factor of PC12 cells in cerebrospinal fluid and its possible sources. J Neurochem. 2005;92:904–914. doi: 10.1111/j.1471-4159.2004.02933.x. [DOI] [PubMed] [Google Scholar]
- [32].Zhao Y, Usatyuk PV, Cummings R, Saatian B, He D, Watkins T, Morris A, Spannhake EW, Brindley DN, Natarajan V. Lipid phosphate phosphatase-1 regulates lysophosphatidic acid-induced calcium release, NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells. Biochem J. 2005;385:493–502. doi: 10.1042/BJ20041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Brindley DN, Pilquil C, Sariahmetoglu M, Reue K. Phosphatidate degradation: phosphatidate phosphatases (lipins) and lipid phosphate phosphatases. Biochim Biophys Acta. 2009;1791:956–961. doi: 10.1016/j.bbalip.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tomsig JL, Snyder AH, Berdyshev EV, Skobeleva A, Mataya C, Natarajan V, Brindley DN, Lynch KR. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem J. 2009;419:611–618. doi: 10.1042/BJ20081888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23:327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- [36].Hiemstra PS, Bals R. Series introduction: Innate host defense of the respiratory epithelium. J Leukoc Biol. 2004;75:3–4. doi: 10.1189/jlb.0903410. [DOI] [PubMed] [Google Scholar]
- [37].Zhao Y, He D, Saatian B, Watkins T, Spannhake EW, Pyne NJ, Natarajan V. Regulation of lysophosphatidic acid-induced epidermal growth factor receptor transactivation and interleukin-8 secretion in human bronchial epithelial cells by protein kinase Cdelta, Lyn kinase, and matrix metalloproteinases. J Biol Chem. 2006;281:19501–19511. doi: 10.1074/jbc.M511224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].He D, Su Y, Usatyuk PV, Spannhake EW, Kogut P, Solway J, Natarajan V, Zhao Y. Lysophosphatidic acid enhances pulmonary epithelial barrier integrity and protects endotoxin-induced epithelial barrier disruption and lung injury. J Biol Chem. 2009;284:24123–24132. doi: 10.1074/jbc.M109.007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Murch O, Collin M, Thiemermann C. Lysophosphatidic acid reduces the organ injury caused by endotoxemia-a role for G-protein-coupled receptors and peroxisome proliferator-activated receptor-gamma. Shock. 2007;27:48–54. doi: 10.1097/01.shk.0000235086.63723.7e. [DOI] [PubMed] [Google Scholar]
- [40].Fan H, Zingarelli B, Harris V, Tempel GE, Halushka PV, Cook JA. Lysophosphatidic acid inhibits bacterial endotoxin-induced pro-inflammatory response: potential anti-inflammatory signaling pathways. Mol Med. 2008;14:422–428. doi: 10.2119/2007-00106.Fan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].He D, Natarajan V, Stern R, Gorshkova IA, Solway J, Spannhake EW, Zhao Y. Lysophosphatidic acid-induced transactivation of epidermal growth factor receptor regulates cyclo-oxygenase-2 expression and prostaglandin E(2) release via C/EBPbeta in human bronchial epithelial cells. Biochem J. 2008;412:153–162. doi: 10.1042/BJ20071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang L, Cummings R, Zhao Y, Kazlauskas A, Sham JK, Morris A, Georas S, Brindley DN, Natarajan V. Involvement of phospholipase D2 in lysophosphatidate-induced transactivation of platelet-derived growth factor receptor-beta in human bronchial epithelial cells. J Biol Chem. 2003;278:39931–39940. doi: 10.1074/jbc.M302896200. [DOI] [PubMed] [Google Scholar]
- [43].McIntyre TM, Pontsler AV, Silva AR, St Hilaire A, Xu Y, Hinshaw JC, Zimmerman GA, Hama K, Aoki J, Arai H, Prestwich GD. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci U S A. 2003;100:131–136. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Waters CM, Saatian B, Moughal NA, Zhao Y, Tigyi G, Natarajan V, Pyne S, Pyne NJ. Integrin signalling regulates the nuclear localization and function of the lysophosphatidic acid receptor-1 (LPA1) in mammalian cells. Biochem J. 2006;398:55–62. doi: 10.1042/BJ20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhao Y, He D, Zhao J, Wang L, Leff AR, Spannhake EW, Georas S, Natarajan V. Lysophosphatidic acid induces interleukin-13 (IL-13) receptor alpha2 expression and inhibits IL-13 signaling in primary human bronchial epithelial cells. J Biol Chem. 2007;282:10172–10179. doi: 10.1074/jbc.M611210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhao Y, Usatyuk PV, Gorshkova IA, He D, Wang T, Moreno-Vinasco L, Geyh AS, Breysse PN, Samet JM, Spannhake EW, Garcia JG, Natarajan V. Regulation of COX-2 expression and IL-6 release by particulate matter in airway epithelial cells. Am J Respir Cell Mol Biol. 2009;40:19–30. doi: 10.1165/rcmb.2008-0105OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Feng N, Lugli SM, Schnyder B, Gauchat JF, Graber P, Schlagenhauf E, Schnarr B, Wiederkehr-Adam M, Duschl A, Heim MH, Lutz RA, Moser R. The interleukin-4/interleukin-13 receptor of human synovial fibroblasts: overexpression of the nonsignaling interleukin-13 receptor alpha2. Lab Invest. 1998;78:591–602. [PubMed] [Google Scholar]
- [48].Zheng T, Liu W, Oh SY, Zhu Z, Hu B, Homer RJ, Cohn L, Grusby MJ, Elias JA. IL-13 receptor alpha2 selectively inhibits IL-13-induced responses in the murine lung. J Immunol. 2008;180:522–529. doi: 10.4049/jimmunol.180.1.522. [DOI] [PubMed] [Google Scholar]
- [49].Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sweet MJ, Leung BP, Kang D, Sogaard M, Schulz K, Trajkovic V, Campbell CC, Xu D, Liew FY. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J Immunol. 2001;166:6633–6639. doi: 10.4049/jimmunol.166.11.6633. [DOI] [PubMed] [Google Scholar]
- [51].Oyesanya RA, Lee ZP, Wu J, Chen J, Song Y, Mukherjee A, Dent P, Kordula T, Zhou H, Fang X. Transcriptional and post-transcriptional mechanisms for lysophosphatidic acid-induced cyclooxygenase-2 expression in ovarian cancer cells. FASEB J. 2008;22:2639–2651. doi: 10.1096/fj.07-101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kang S, Luo R, Smicun Y, Fishman DA, Meng Y. Selective induction of cyclooxygenase-2 plays a role in lysophosphatidic acid regulated Fas ligand cell surface presentation. FEBS Lett. 2006;580:443–449. doi: 10.1016/j.febslet.2005.12.033. [DOI] [PubMed] [Google Scholar]
- [53].Gauvreau GM, Watson RM, O’Byrne PM. Protective effects of inhaled PGE2 on allergen-induced airway responses and airway inflammation. Am J Respir Crit Care Med. 1999;159:31–36. doi: 10.1164/ajrccm.159.1.9804030. [DOI] [PubMed] [Google Scholar]
- [54].Kagan BL, Henke RT, Cabal-Manzano R, Stoica GE, Nguyen Q, Wellstein A, Riegel AT. Complex regulation of the fibroblast growth factor-binding protein in MDA-MB-468 breast cancer cells by CCAAT/enhancer-binding protein beta. Cancer Res. 2003;63:1696–1705. [PubMed] [Google Scholar]
- [55].Arcidiacono MV, Sato T, Alvarez-Hernandez D, Yang J, Tokumoto M, Gonzalez-Suarez I, Lu Y, Tominaga Y, Cannata-Andia J, Slatopolsky E, Dusso AS. EGFR activation increases parathyroid hyperplasia and calcitriol resistance in kidney disease. J Am Soc Nephrol. 2008;19:310–320. doi: 10.1681/ASN.2007040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Savla U, Appel HJ, Sporn PH, Waters CM. Prostaglandin E(2) regulates wound closure in airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2001;280:L421–431. doi: 10.1152/ajplung.2001.280.3.L421. [DOI] [PubMed] [Google Scholar]
- [57].Wu Q, Martin RJ, LaFasto S, Chu HW. A low dose of Mycoplasma pneumoniae infection enhances an established allergic inflammation in mice: the role of the prostaglandin E2 pathway. Clin Exp Allergy. 2009;39:1754–1763. doi: 10.1111/j.1365-2222.2009.03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhao Y, He D, Stern R, Usatyuk PV, Spannhake EW, Salgia R, Natarajan V. Lysophosphatidic acid modulates c-Met redistribution and hepatocyte growth factor/c-Met signaling in human bronchial epithelial cells through PKC delta and E-cadherin. Cell Signal. 2007;19:2329–2338. doi: 10.1016/j.cellsig.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Xu X, Prestwich GD. Inhibition of tumor growth and angiogenesis by a lysophosphatidic acid antagonist in an engineered three-dimensional lung cancer xenograft model. Cancer. 2010;116:1739–1750. doi: 10.1002/cncr.24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fulkerson Z, Wu T, Sunkara M, Kooi CV, Morris AJ, Smyth SS. Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. J Biol Chem. 2011;286:34654–34663. doi: 10.1074/jbc.M111.276725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Funke M, Zhao Z, Xu Y, Chun J, Tager AM. The lysophosphatidic acid receptor LPA1 promotes epithelial cell apoptosis after lung injury. Am J Respir Cell Mol Biol. 2012;46:355–364. doi: 10.1165/rcmb.2010-0155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Xu MY, Porte J, Knox AJ, Weinreb PH, Maher TM, Violette SM, McAnulty RJ, Sheppard D, Jenkins G. Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q) Am J Pathol. 2009;174:1264–1279. doi: 10.2353/ajpath.2009.080160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Emo J, Meednu N, Chapman TJ, Rezaee F, Balys M, Randall T, Rangasamy T, Georas SN. Lpa2 is a negative regulator of both dendritic cell activation and murine models of allergic lung inflammation. J Immunol. 2012;188:3784–3790. doi: 10.4049/jimmunol.1102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Li S, Xiong C, Zhang J. ATX and LPA receptor 3 are coordinately up-regulated in lipopolysaccharide-stimulated THP-1 cells through PKR and SPK1-mediated pathways. FEBS Lett. 2012;586:792–797. doi: 10.1016/j.febslet.2012.01.044. [DOI] [PubMed] [Google Scholar]