Abstract
Techniques used to produce partial spinal cord injuries in animal models have the potential for creating variability in lesions. The amount of tissue affected may influence the functional outcomes assessed in the animals. The recording of somatosensory evoked potentials (SSEPs) may be a valuable tool for assessing the extent of lesion applied in animal models of traumatic spinal cord injury (SCI). Intraoperative tibial SSEP recordings were assessed during surgically induced lateral thoracic hemisection SCI in Sprague-Dawley rats. The transmission of SSEPs, or lack thereof, was determined and compared against the integrity of the dosal funiculi on each side of the spinal cord upon histological sectioning. An association was found between the presence of an SSEP signal and presence of intact dorsal funiculus tissue. The relative risk is 4.50 (95% confidence interval: 1.83 to 11.08) for having an intact dorsal funiculus when the ipsilateral SSEP was present compared to when it was absent. Additionally, the amount of spared spinal cord tissue correlates with final functional assessments at nine weeks post injury: BBB (linear regression, R2 = 0.618, p <0.001) and treadmill test (linear regression, R2 = 0.369, p = 0.016). Therefore, we propose intraoperative SSEP monitoring as a valuable tool to assess extent of lesion and reduce variability between animals in experimental studies of SCI.
Keywords: electrophysiology, pathologic correlation, incomplete spinal cord injury, exercise, functional outcome
1. Introduction
Traumatic spinal cord injury (SCI) results from local, abrupt damage to the spinal cord. Of the estimated 12,000 new SCI cases per year in the United States a majority, approximately 61%, of the injuries are classified as incomplete (Center, 2011). Individuals with incomplete injuries maintain some connections between the areas above and below the point of damage. The amount of tissue spared for these connections will influence the affected individual’s ability to recover volitional control below the level of injury (Rossignol and Frigon, 2011). Understanding the mechanisms of an incomplete injury is critical to determine appropriate interventions and future treatments to enhance recovery.
Various animal models of SCI have been developed to study mechanisms of injury and recovery associated with the condition. The most commonly used animal models of SCI are the result of transection, compression, or contusion injury directly to the spinal cord (Rosenzweig and McDonald, 2004; Talac et al., 2004). All of these approaches can be used to produce an anatomically and functionally incomplete injury. The transection injury model utilizes a precise, clean approach by incision to render a lesion in specific target areas (Talac et al., 2004). This incision can be used to apply either complete transection or incomplete transection (hemisection). To date a variety of hemisection-type injuries have been used including the lateral hemisection and dorsal hemisection (Brechtel et al., 2006; Fouad et al., 2000; Goldshmit et al., 2008; Ying et al., 2005). However, due to the technical difficulty of a partial cut, there is the possibility of inconsistencies in the injuries from one animal to the next. It has been noted that the amount of injured cord is related to functional recovery (Basso et al., 1995; Brechtel et al., 2006). Therefore, to ensure consistent injuries and homogenous groups to study, it would be valuable to designate a tool to monitor the extent of injury when it occurs.
Electophysiology is a functional means to assess the integrity of various aspects of the nervous system, including the spinal cord. Clinical applications of electrophysiology, particularly evoked potentials, include diagnosis of peripheral or central nervous system damage and monitoring central nervous system integrity during surgical procedures (Cruccu et al., 2008; Malhotra and Shaffrey, 2010; Wang et al., 2008). Particularly, somatosensory evoked potentials (SSEPs) use a peripherally applied stimulus that is monitored in the central nervous system and can be used to detect the integrity of the dorsal columns in the spinal cord (Cruccu et al., 2008). In the rat spinal cord, the dorsal columns and the corticospinal tract comprise the dorsal funiculi, an area of white matter that lies medial to the dorsal horns (Paxinos, 1995). Stimulation applied to a limb is transmitted via the dorsal root to the spinal cord. Light touch, vibratory, and proprioceptive sensations are localized to the dorsal column tract. This sensory information travels via fast, myelinated fibers in the dorsal column ipsilateral to the side of sensation until decussating at the level of the medulla. The pathway then travels to thalamus and continues via the internal capsule to synapse in the somatosensory cortex contralateral to the side of stimulation. A disruption in the pathway between location of applied stimulus and recording in the cortex would be detectable as an abnormal cortical reading. SSEP monitoring has been applied to detect minor damage, assess ultrastructural damage, and functional recovery in various rat models of peripheral nerve injury and SCI (Agrawal et al., 2009; Hu et al., 2011; Nashmi et al., 1997; Onifer et al., 2007; Onifer et al., 2005; Schlag et al., 2001; Sen and Moller, 1991; Wang et al., 2008; Zhang et al., 2007). Additionally, electrophysiology parameters have found to be associated with function and extent of lesion in both humans and rats (Metz et al., 2000). Based on its prior use, SSEP monitoring may be a useful approach for monitoring the extent of an injury at the time of insult as well.
The purpose of this study was to determine the value of using intraoperative SSEPs for monitoring the extent of tissue damage in a lateral hemisection model of thoracic spinal cord injury in rat. We propose that absence or presence of SSEP will correspond to the integrity of the dorsal funiculi on post-mortem tissue analysis. Here we describe the use of SSEP monitoring in the context of an intervention study assessing the effect of exercise on functional recovery in rats following a right lateral hemisection SCI.
2. Materials and Methods
2.1 Animals
Female Sprague-Dawley rats (n=19, Harlan Laboratories, Indianapolis, Indiana) weighing 250–300 g underwent the lateral hemisection spinal cord injury procedure. Seventeen survived and were randomized to either the exercise (n=9) or control (n=8) group. Of the two rats that did not survive, one was lost during the procedure and one was euthanized four days following the procedure due to complications. One of the 17 surviving rats was excluded after review of histology due to having a nearly complete transection injury. Thus the data from 16 rats (exercise: n =9 and control: n =7) were considered suitable for final functional analysis. An additional animal’s tissue was damaged during the sectioning process. Thus the data from 15 rats (exercise: n =8 and control: n =7) were used for histological analysis. All animal care, handling, and surgical procedures were approved by and conducted in accordance with the guidelines of the institutional animal care committee (Institutional Animal Use and Care Committee, Mayo Clinic).
2.2 Surgical Procedure
2.2.1 Lateral Hemisection Injury
Animals were deeply anesthetized by intraperitoneal injection of Ketamine (80 mg/kg, Fort Dodge Animal Health, Fort Dodge, Iowa) and xylazine (5mg/kg, Lloyd Laboratories, Shenandoah, Iowa). This maintained deep anesthesia for at least two hours, allowing all electrophysiological monitoring to be performed while the rats were under deep anesthesia. Before surgery, animals were weighed and given intramuscular Buprinex (0.05mg/kg, Reckitt Benckiser Pharmaceuticals Inc, Richmond, Virginia), intraperitoneal Baytril (65mg/kg) and 5 ml subcutaneous 0.9% Saline (Baxter Healthcare Corporation, Deerfield, Illinois). Lacrilube gel was applied to both eyes and each animal was maintained on a 37±0.5°C heating pad prior to surgery until waking up after surgery.
The rat’s back was shaved and aseptically prepared using a providone-iodine swabstick (Professional Disposables Inc, Orangeburg, New York). A skin incision was made along the midline of the back and the musculature was bluntly dissected to the spinous processes. The laminae were exposed and a laminectomy was performed at the level of T9 or T10. The midline of the spinal cord was identified and a transverse cut was made laterally from midline to create a right hemisection injury. The accuracy of the lesion was assessed by somatosensory evoked potential (SSEP). SSEP measurements were made between 30 and 70 minutes after induction of anesthesia. After loss of right SSEP was determined the wound was closed in muscle and skin layers using simple interrupted stitches with absorbable vicryl suture.
2.2.2 Intraoperative Somatosensory Evoked Potential (SSEP) Monitoring
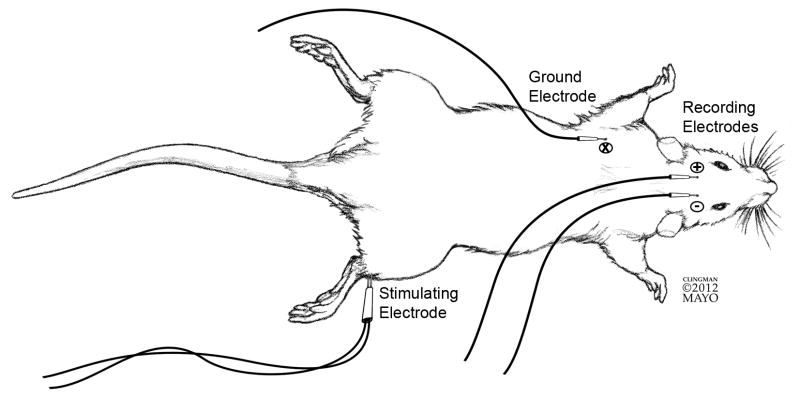
During the surgical procedure tibial SSEPs were monitored bilaterally (Nicolet Viking IV; Viasys Healthcare, Madison, Wisconsin). The tibial nerve was stimulated proximal to the ankle with a dual tip electrode (Figure 1). The stimulation parameters were as follows: 0.05 ms duration, 2.1 Hz frequency, and intensity to elicit a mild muscle twitch (3.9–10.2 mA). Recordings were made via two needle electrodes placed over the somatosensory cortex through the scalp. Waves were odd-even averaged for analysis. SSEP technique has been used and published by our lab (Wang et al., 2008) and was validated via monitoring SSEPs in normal, anesthetized rats. SSEP signals were consistently acquired in the normal animals using the procedure described. These signals had a consistent early negative followed by a positive deflection as shown in Figure 2b.
Figure 1. SSEP set-up.
Set up for detecting right SSEP. Dual tip stimulating electrode applied proximal to right ankle. Recording electrodes applied across the primary somatosensory cortex, with active recording electrode (−) ipsilateral to the stimulated limb and passive recording electrode (+) contralateral to the stimulated limb. Ground electrode placed in soft tissue bulk of the left forelimb.
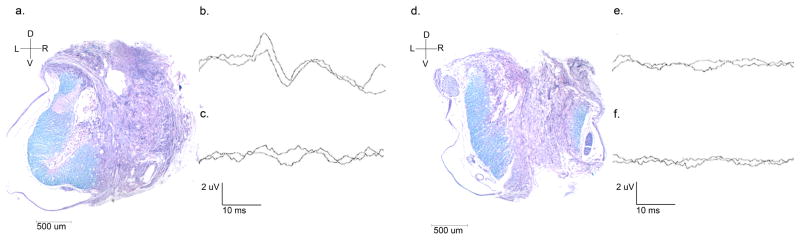
Figure 2. Demonstration of spinal cord tissue and associated somatosensory evoked potential outcomes.
Luxol-fast blue stained spinal cord with left dorsal funiculus present and right dorsal funiculus absent (a) and corresponding SSEPs, present on the left (b) and absent on the right (c). Luxol-fast blue stained spinal cord with both left and right dorsal funiculus absent (d) and corresponding SSEPs, absent on both the left (e) and the right (f).
Dorsal/ventral (D/V) and right/left (R/L) orientation and 500 um scale bar indicated for spinal cord cross sections, taken at 2.5x magnification (a & d)
Wave amplitude and duration scale bars indicated, 2 uV and 10 ms, respectively (b, c, e & f)
Both the right and left SSEP were assessed pre- and post-hemisection cut. At the time of the postoperative recordings, the waves were determined to be absent or present. The response was considered absent when neither negative nor positive deflection was observed. If the right SSEP was considered present following the cut, an additional cut was made to the right side of the spinal cord in the same position as the first. This was done up to three times, until the signal was deemed absent. Five of the rats received these additional cuts. Final assessments used for data analysis of the signal integrity (absent or present) were made by three neurologists blinded to the source of each wave.
2.2.3. Post-operative care
For one week following surgery the animals were kept in low-sided cages. Acetaminophen (Mapap™ Major Pharmaceuticals, Livonia, Michigan) was provided in the drinking water was initiated 24 hours prior to surgery and continued for a week following. For five days post-surgery, a twice daily regimen of intramuscular Buprenex (0.05 mg/kg), intraperitoneal Baytril (65 mg/kg), and subcutaneous 0.9% Saline (5 ml) was administered. Animals were assessed twice daily for health and recovery. During the health checks bladders were manually expressed until the animal regained control of bladder function. If infection was detected in the urine or elsewhere a two-day, twice daily regimen of Baytril (65 mg/kg) was administered to that animal.
2.3 Functional Training – Treadmill
Prior to surgery all animals were trained for a week to run on a treadmill (Animal Treadmill: Exer 3/6, Columbus Instruments, Columbus, Ohio) at speeds up to 20 meters per minute. The animals randomized to the exercise condition returned to using the treadmill one week following injury. At this time, speed was adjusted to animals’ ability starting at speeds as low as 6 m/min. Manual facilitation for offloading weight from the hindlimbs was provided if the animal was unable to maintain consistent stepping. The exercise group completed eight weeks of treadmill exercise at speeds up to 12 m/min. Both pre-operative training and post-operative exercise were performed five days per week, 20 minutes per day (two 10-minute sessions with 10 minutes of rest). During the training sessions speed would be increased or decreased depending on animal performance to allow for continuous exercise at the fastest tolerated speed.
2.4 Functional Assessment
2.4.1 Open Field Gait Assessment – BBB
The BBB open-field gait assessment is a 21-point scale that was developed to assess recovery of function after spinal cord injury in rats (Basso et al., 1995). All animals were assessed at weekly time points following injury by four assessors blinded to their group assignment. Scores were averaged across the assessors for each hindlimb. Combined hindlimb scores were determined by averaging the right and left hindlimb score for each animal.
2.4.2 Ambulation Capacity Assessment
The ambulation capacity assessment was a treadmill based functional assessment adapted from work in a rat model of aging (Groban et al., 2008). The animal’s performance was based on its ability to remain on the treadmill belt with increasing speeds over time. The test started at a speed of 8 m/min and was increased by 2 m/min every two minutes until 20 minutes or failure, whichever came first. Failure was defined as one of two different conditions: (1) loss of hindlimb contact with back of belt lasting for five seconds or (2) loss of hindlimb contact and three sequential unsuccessful attempts to reinitiate and maintain contact. All animals, both in the control and exercise groups, were trained prior to surgery and reacclimated to the test one day before assessment. This training day consisted of a 6-minute increasing speed experience (starting speed of 6 m/min and increasing by 2 m/min every two minutes). The ambulation capacity assessment was preformed a total of five times: pre-operatively and every two weeks following the initiation of the treadmill training protocol (post-operative days 21, 35, 49 and 63).
2.5 Tissue Harvesting and Assessment
2.5.1 Tissue Preparation
At the completion of the nine-week study period all rats were humanely euthanized via deep anesthesia with intraperitoneal injection of pentobarbital (40 mg/kg, Fort Dodge Animal Health, Fort Dodge, Iowa). They were transcardially perfused via the aorta with approximately 40 mL of PBS followed by approximately 40 mL of 4% paraformaldehyde. The spinal column and cord were removed and post-fixed for at least 48 hours in 4% paraformaldehyde at 4°C. The spinal cords were then dissected from the spinal column and post-fixed for at least 48 hours in the same fixative at 4°C, as previously descriebd (Chen et al., 2011; Rooney et al., 2011). Four to seven mm sections containing the lesion were cut and embedded in paraffin. The paraffin blocks were then cut transversely into 10 um serial sections using a microtome (Leica, Bannockburn, IL) and mounted on slides for staining.
2.5.2 Hematoxylin and Eosin (H&E) Staining
Sections from the lesioned and non-lesioned thoracic spinal cord were stained with H&E (Sigma-Aldrich, St. Louis, Missouri) for assessment of lesion size. Sections were chosen at approximately 300 um intervals. Lesion size was assessed on all sections using KS400 image processing software (Zeiss, Oberkochen, Germany). One normal thoracic section (no evidence of lesion) was identified caudal to the lesion and used as a reference for cross-sectional area. Thresholds for all sections were based on color, and each section was traced manually to determine total cross-sectional area and area of spared tissue. Percentage of spared tissue was defined as the spared tissue area from each section divided by the area of the reference section. The section with the lowest percentage of spared tissue was determined to be the epicenter of the lesion and used for data analysis.
2.5.3 Luxol-Fast Blue Staining
Luxol-fast blue (Solvent Blue 38, Sigma-Aldrich, St. Louis, Missouri) staining was used for assessment of myelin. One luxol-fast blue section within 100 um caudal to the section with the largest lesion area, as identified by H&E staining, was assessed. Each section was assessed for integrity of the dorsal funiculi with respect to myelination and absence of scarring. If any portion of the funiculus was myelinated and without scar it was determined to be intact. Assessments of the histological sections were done without awareness of the corresponding SSEP result.
3. Results
3.1 SSEP
A total of 30 final post-operative SSEPs (15 right-sided and 15 left-sided) were assessed by three neurologists blinded to the source of each wave. Ten were determined present and twenty were determined absent (Table 1). The corresponding luxol-fast blue stained sections were assessed for integrity of the right and left dorsal funiculi by an observer blinded to the result of corresponding SSEPs. Fourteen funiculi were determined to be intact and 16 were determined to be absent (Table 1). An association between presence of SSEP and integrity of the ipsilateral dorsal funiculus was detected (Fisher’s Exact, p < 0.001, Figure 2). There was a relative risk of 4.50 (95% confidence interval: 1.83 to 11.08) for having an intact dorsal funiculus when the ipsilateral SSEP was present compared to when the ipsilateral SSEP was absent.
Table 1. SSEP and dorsal funiculi data.
Dorsal funiculus determined to be present or absent based on luxol-fast blue stained cross-section within 100 um of the injury epicenter. SSEP results determined by three blinded assessors. An association between presence of SSEP and integrity of the ipsilateral dorsal funiculus was detected (Fisher’s Exact, p < 0.001), relative risk for detecting an SSEP for when tissue was intact versus absent: 4.50 (95% confidence interval: 1.83 to 11.08)
| SSEP Result | Dorsal funiculus | Total | |
|---|---|---|---|
| Intact (R/L) | Absent (R/L) | ||
| Present (R/L) | 9 (1/8) | 1 (1/0) | 10 (2/8) |
| Absent (R/L) | 5 (1/4) | 15 (12/3) | 20 (13/7) |
|
| |||
| Total | 14 (2/12) | 16 (13/3) | |
3.2 Functional Outcomes
The average combined hindlimb BBB score was determined for each animal at the nine time points (Table 2). At no time point was there a significant difference between the average BBB score of the control group and exercise group (mixed-design repeated measures ANOVA, p>0.05).
Table 2. Average BBB and treadmill test scores by group and time point; mean (standard deviation).
At no time point was there a difference between groups for performance on the BBB or treadmill test, t-test, p <0.05
| Time point | Combined BBB
|
Treadmill Test (time to failure, sec)
|
||
|---|---|---|---|---|
| Control (n=7) | Exercise (n=9) | Control (n=7) | Exercise (n=9) | |
| Pre-op | - | - | 811 (351) | 790 (188) |
|
| ||||
| POD7 | 9.6(2.2) | 9.7(1.8) | - | - |
| POD14 | 12.5(1.3) | 11.9(1.1) | - | - |
| POD21 | 12.0(0.6) | 11.9(0.9) | 415(294) | 393(143) |
| POD28 | 12.4(0.9) | 11.7(1.2) | - | - |
| POD35 | 12.4(0.6) | 12.2(0.8) | 516(207) | 476(171) |
| POD42 | 12.6(0.8) | 12.4(1.4) | - | - |
| POD49 | 12.9(1.0) | 12.3(1.0) | 550(202) | 457(177) |
| POD56 | 12.4(1.1) | 12.2(1.0) | - | - |
| POD63 | 12.5(0.7) | 12.5(0.9) | 505(172) | 599(327) |
Performance on the treadmill test was assessed prior to surgery and at four time points during follow-up (Table 2). At no time point was there a significant difference between the control and exercise groups in performance on the test (mixed-design repeated measures ANOVA, p>0.05).
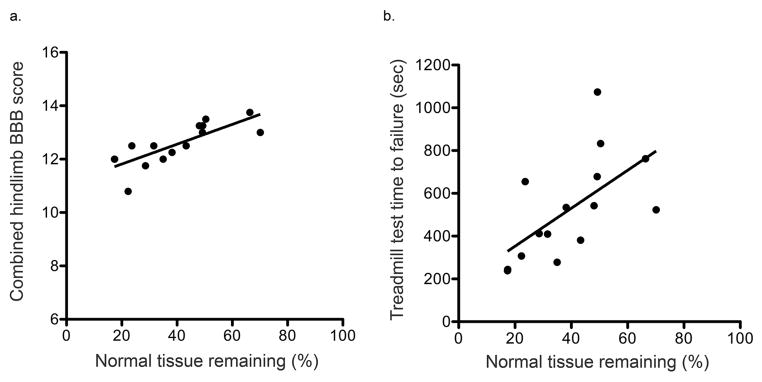
Spared tissue was quantified on H&E spinal cord cross-sections (Figure 3). The amount of spared tissue was expressed as a percentage of the area of a normal thoracic cross-section within the same animal. Average amount of spared tissue was 39.39% (16.44), mean (standard deviation). The percentage of spared spinal cord tissue remaining correlates with final assessments of BBB (linear regression, R2 = 0.618, p <0.001) and treadmill test (linear regression, R2 = 0.369, p = 0.016; Figure 4).
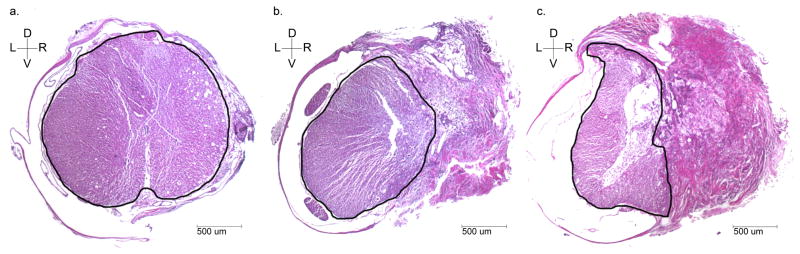
Figure 3. Hematoxylin and Eosin stained spinal cord cross sections.
Selected spinal cord cross sections with H&E staining are shown here to reflect a normal thoracic cross-section caudal to lesion area of an injured rat (a); a spinal cord cross section with approximately 49% (close to goal hemisection injury) spared tissue remaining (b); and a spinal cord cross section with 38% (close to average) spared tissue remaining (c). Spared spinal cord tissue has been outlined to demonstrate full cross-section area (a) and distinguishing between spared and injured tissue (b & c).
For all sections (n=15), spared tissue remaining was 39.39% (16.44), average (standard deviation) Dorsal/ventral (D/V) and right/left (R/L) orientation and 500 um scale bar indicated for spinal cord cross sections, taken at 2.5x magnification
Figure 4. Functional performance vs. spared spinal cord tissue.
Functional performance on the final assessment (post-operative day 63) is plotted against percentage of spared tissue remaining for the BBB combined hindlimb score, linear regression, R2 = 0.618, p <0.001 (a) and treadmill ambulation capacity test, linear regression, R2 = 0.369, p = 0.016 (b)
4. Discussion
SSEPs are routinely used to assess spinal cord integrity during surgical procedures in humans (Cruccu et al., 2008; Malhotra and Shaffrey, 2010). The monitoring is meant to alert surgeons to unintentional compromise of the spinal cord. Similar monitoring procedures have been applied in rodents to assess extent of injury and recovery following injury (Agrawal et al., 2009; Hu et al., 2011; Nashmi et al., 1997; Zhang et al., 2008). The integrity of SSEP recordings under deep anesthesia with ketamine and xylazine has been demonstrated by our laboratory and others (Wang et al., 2008; Zandieh et al., 2003). Here we demonstrate a novel application of SSEP monitoring in rats to assess extent of intended injury intraoperatively. Our results indicate that when an SSEP signal is present it is 4.5 times more likely that the corresponding dorsal funiculus tissue is spared than if the SSEP signal is absent. Our findings are consistent with previously reported results of SSEP recordings following contusion SCI in rat (Zhang et al., 2008). Zhang et al. reported that SSEP signals were absent in injuries with particular depths of impact. The injuries deep enough to result in absent SSEPS also did not have detectible myelinated fibers in the dorsal funiculus on histological cross-section.
In the lateral hemisection model of SCI, the goal is to damage only one side of the spinal cord. However, due to the size of the rat spinal cord it is difficult and unreliable to determine the accuracy of the injury by sight alone. As demonstrated, the use of SSEP monitoring is an effective tool to help address this issue. Bilateral SSEPs can be measured with the goal of having an absent wave on the side of injury and present wave on the uninjured side. As indicated by our data, there is a greater chance of having a spared dorsal funiculus when the SSEP signal at the time of surgery was present than when it was absent. Only one signal was transmitted in a sample where the dorsal funiculus was considered to be absent. However, this approach does not ensure an exact hemisection injury or perfect reflection of tissue integrity. It was noted that more dorsal funiculi were determined to be spared than the amount of SSEPs that remained present. This may be reflective of acute inflammation and edema interfering with the transmission of signals via the dorsal columns at the time of injury despite intact tissue. Thus one can consider presence of an SSEP reflective of spared tissue, whereas absence of SSEP is associated with likely partial or complete damage. This technique can be used as a guide to insure more homogenous injuries with regard to dorsal column sparing though it does not ensure exact lateral hemisections.
One consideration of the present study is that the histological assessment was made nine weeks after the SSEP recording. Though not completed close to one another in time, this comparison does address the goal of the study. Our aim was to determine whether dynamic intraoperative monitoring would be helpful in determining ultimate completeness of a lesion. It would be of interest in the future to examine lesions immediately after surgery and perform axonal tracing to determine functional integrity of tracts.
As noted in prior studies of transection and contusion SCIs, the amount of damaged tissue and pathways in animal models of spinal cord injury is associated with functional outcomes and signal conduction (Basso et al., 1995; Brechtel et al., 2006; Zhang et al., 2008). Our study provides additional evidence for the association between amount of injured tissue and performance on functional outcome measures in rats. Although we were producing a hemisection injury, there was relatively little asymmetry of hindlimb dysfunction. There was a small difference at post-operative day seven (BBB 10.5 left vs. 8.8 right for the controls and 10.2 left vs. 9.1 right for the exercise group). From post-operative day 14 to day 63 there was no difference between hindlimbs in either group. This may reflect the contribution of both crossing and non-crossing fibers to gait control. We demonstrated that higher level performance on both the BBB open field gait assessment and a treadmill-based ambulation capacity assessment was associated with a greater amount of spared spinal cord tissue at the site of injury. When using a partial model of SCI, it is imperative to ensure as much consistency in the injury from animal to animal as possible. Doing so will decrease overall variability between animals and within experimental groups.
In conclusion, the use of intraoperative SSEP monitoring is an effective and valuable tool for assessing extent of dorsal funiculi remaining at the time of injury. SSEPs do not appear to guide the creation of a perfect lateral hemisection injury. However, SSEPs do provide information to confidently estimate the relative extent of dorsal funiculi remaining in a lesion, allowing for extreme or inadequate injuries to be identified when they occur. Therefore we propose the use of SSEP monitoring as a valuable approach for monitoring partial injuries to the spinal cord.
Highlights.
Somatosensory evoked potentials to assess hemisection spinal cord injury in rats
Intraoperative somatosensory evoked potentials relate to post-mortem tissue integrity
Amount of normal tissue after injury correlates with motor function outcomes
Acknowledgments
We thank fellow colleagues at Mayo Clinic, James Abbott, Carl Clingman, Jarred Nesbitt, Jewel Podratz, Ann Schmeichel, Nathan Staff, James Tarara and the Optical Morphology Core, Catalina Vallejo-Giraldo, Huan Wang and Shuya Zhang, for their technical assistance and guidance in this project.
This publication was supported by the Shannon Foundation, the Neilsen Foundation, the Morton Cure Paralysis Fund and an NIH/NCRR CTSA Grant (Number UL1 RR024150). Its contents are solely the responsibility of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal G, Thakor NV, All AH. Evoked potential versus behavior to detect minor insult to the spinal cord in a rat model. J Clin Neurosci. 2009;16:1052–5. doi: 10.1016/j.jocn.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Brechtel K, Tura A, Abdibzadeh M, Hirsch S, Conrad S, Schwab JM. Intrinsic locomotor outcome in dorsal transection of rat spinal cord: predictive value of minimal incision depth. Spinal Cord. 2006;44:605–13. doi: 10.1038/sj.sc.3101894. [DOI] [PubMed] [Google Scholar]
- Center NSCIS. Spinal Cord Injury Facts and Figures at a Glance. Birmingham: 2011. [Google Scholar]
- Chen BK, Knight AM, Madigan NN, Gross L, Dadsetan M, Nesbitt JJ, Rooney GE, Currier BL, Yaszemski MJ, Spinner RJ, Windebank AJ. Comparison of polymer scaffolds in rat spinal cord: a step toward quantitative assessment of combinatorial approaches to spinal cord repair. Biomaterials. 2011;32:8077–86. doi: 10.1016/j.biomaterials.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruccu G, Aminoff MJ, Curio G, Guerit JM, Kakigi R, Mauguiere F, Rossini PM, Treede RD, Garcia-Larrea L. Recommendations for the clinical use of somatosensory-evoked potentials. Clin Neurophysiol. 2008;119:1705–19. doi: 10.1016/j.clinph.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Fouad K, Metz GA, Merkler D, Dietz V, Schwab ME. Treadmill training in incomplete spinal cord injured rats. Behav Brain Res. 2000;115:107–13. doi: 10.1016/s0166-4328(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Lythgo N, Galea MP, Turnley AM. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J Neurotrauma. 2008;25:449–65. doi: 10.1089/neu.2007.0392. [DOI] [PubMed] [Google Scholar]
- Groban L, Jobe H, Lin M, Houle T, Kitzman DA, Sonntag W. Effects of short-term treadmill exercise training or growth hormone supplementation on diastolic function and exercise tolerance in old rats. J Gerontol A Biol Sci Med Sci. 2008;63:911–20. doi: 10.1093/gerona/63.9.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Wen CY, Li TH, Cheung MM, Wu EX, Luk KD. Somatosensory-evoked potentials as an indicator for the extent of ultrastructural damage of the spinal cord after chronic compressive injuries in a rat model. Clin Neurophysiol. 2011;122:1440–7. doi: 10.1016/j.clinph.2010.12.051. [DOI] [PubMed] [Google Scholar]
- Malhotra NR, Shaffrey CI. Intraoperative electrophysiological monitoring in spine surgery. Spine (Phila Pa 1976) 2010;35:2167–79. doi: 10.1097/BRS.0b013e3181f6f0d0. [DOI] [PubMed] [Google Scholar]
- Metz GA, Curt A, van de Meent H, Klusman I, Schwab ME, Dietz V. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma. 2000;17:1–17. doi: 10.1089/neu.2000.17.1. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Imamura H, Tator CH, Fehlings MG. Serial recording of somatosensory and myoelectric motor evoked potentials: role in assessing functional recovery after graded spinal cord injury in the rat. J Neurotrauma. 1997;14:151–9. doi: 10.1089/neu.1997.14.151. [DOI] [PubMed] [Google Scholar]
- Onifer SM, Nunn CD, Decker JA, Payne BN, Wagoner MR, Puckett AH, Massey JM, Armstrong J, Kaddumi EG, Fentress KG, Wells MJ, West RM, Calloway CC, Schnell JT, Whitaker CM, Burke DA, Hubscher CH. Loss and spontaneous recovery of forelimb evoked potentials in both the adult rat cuneate nucleus and somatosensory cortex following contusive cervical spinal cord injury. Exp Neurol. 2007;207:238–47. doi: 10.1016/j.expneurol.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onifer SM, Zhang YP, Burke DA, Brooks DL, Decker JA, McClure NJ, Floyd AR, Hall J, Proffitt BL, Shields CB, Magnuson DS. Adult rat forelimb dysfunction after dorsal cervical spinal cord injury. Exp Neurol. 2005;192:25–38. doi: 10.1016/j.expneurol.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Paxinos G, editor. The Rat Nervous System. 2. Academic Press; San Diego: 1995. [Google Scholar]
- Rooney GE, Knight AM, Madigan NN, Gross L, Chen B, Giraldo CV, Seo S, Nesbitt JJ, Dadsetan M, Yaszemski MJ, Windebank AJ. Sustained delivery of dibutyryl cyclic adenosine monophosphate to the transected spinal cord via oligo [(polyethylene glycol) fumarate] hydrogels. Tissue Eng Part A. 2011;17:1287–302. doi: 10.1089/ten.tea.2010.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, McDonald JW. Rodent models for treatment of spinal cord injury: research trends and progress toward useful repair. Curr Opin Neurol. 2004;17:121–31. doi: 10.1097/00019052-200404000-00007. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Frigon A. Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annu Rev Neurosci. 2011;34:413–40. doi: 10.1146/annurev-neuro-061010-113746. [DOI] [PubMed] [Google Scholar]
- Schlag MG, Hopf R, Redl H. Serial recording of sensory, corticomotor, and brainstem-derived motor evoked potentials in the rat. Somatosens Mot Res. 2001;18:106–16. doi: 10.1080/135578501012006219. [DOI] [PubMed] [Google Scholar]
- Sen CN, Moller AR. Comparison of somatosensory evoked potentials recorded from the scalp and dorsal column nuclei to upper and lower limb stimulation in the rat. Electroencephalogr Clin Neurophysiol. 1991;80:378–83. doi: 10.1016/0168-5597(91)90085-c. [DOI] [PubMed] [Google Scholar]
- Talac R, Friedman JA, Moore MJ, Lu L, Jabbari E, Windebank AJ, Currier BL, Yaszemski MJ. Animal models of spinal cord injury for evaluation of tissue engineering treatment strategies. Biomaterials. 2004;25:1505–10. doi: 10.1016/s0142-9612(03)00497-6. [DOI] [PubMed] [Google Scholar]
- Wang H, Sorenson EJ, Spinner RJ, Windebank AJ. Electrophysiologic findings and grip strength after nerve injuries in the rat forelimb. Muscle Nerve. 2008;38:1254–65. doi: 10.1002/mus.20971. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193:411–9. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Zandieh S, Hopf R, Redl H, Schlag MG. The effect of ketamine/xylazine anesthesia on sensory and motor evoked potentials in the rat. Spinal Cord. 2003;41:16–22. doi: 10.1038/sj.sc.3101400. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Burke DA, Shields LB, Chekmenev SY, Dincman T, Zhang Y, Zheng Y, Smith RR, Benton RL, DeVries WH, Hu X, Magnuson DS, Whittemore SR, Shields CB. Spinal cord contusion based on precise vertebral stabilization and tissue displacement measured by combined assessment to discriminate small functional differences. J Neurotrauma. 2008;25:1227–40. doi: 10.1089/neu.2007.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YP, Shields LB, Zhang Y, Pei J, Xu XM, Hoskins R, Cai J, Qiu MS, Magnuson DS, Burke DA, Shields CB. Use of magnetic stimulation to elicit motor evoked potentials, somatosensory evoked potentials, and H-reflexes in non-sedated rodents. J Neurosci Methods. 2007;165:9–17. doi: 10.1016/j.jneumeth.2007.05.021. [DOI] [PubMed] [Google Scholar]