Abstract
Ascending somatosensory pathways are crossed pathways representing each side of the body in the contralateral neocortex. The principal sensory nucleus of the trigeminal nerve (PrV) relays the facial sensations to the contralateral somatosensory cortex via the ventrobasal thalamus. In the preceding paper we described the normal development of the trigeminal lemniscal pathway in the mouse. In this study we investigated the role of midline axon navigation signals, the netrin and slit proteins. In situ hybridization assays revealed that both netrin and slit mRNAs are expressed along the midline facing the PrV axons and their receptors are expressed in developing PrV neurons. In wild type mouse embryos, PrV axons cross the midline and take a sharp rostral turn heading towards the contralateral thalamus. Examination of trigeminal lemniscal axons in dcc knockout mice revealed absence of midline crossing between E11-E15. However, few axons crossed the midline at E17 and reached the contralateral thalamus, resulting in a bilateral PrV lemniscal pathway at P0. We also found that slit1, 2 or 3 single or double knockout mice have impaired development of the trigeminal-lemniscal pathway. These include axon stalling along the midline, running within the midline and re-crossing of axons back to the site of origin. Collectively, our studies indicate a cooperative role for netrin and slit proteins in midline attraction and crossing behavior of the ascending facial somatosensory projections during development.
INDEXING TERMS: dcc, robo, midline crossing, trigeminal lemniscus
The trigeminothalamic projections (a.k.a. trigeminal lemnniscus) arising from the principal sensory nucleus of the trigeminal nerve (PrV) form the orofacial component of the major ascending somatosensory pathway, the medial lemniscus. This pathway is entirely contralateral and terminates in the ventroposteromedial (VPM) nucleus of the thalamus (Erzurumlu et al., 1980; Ding et al., 2003). The somatosensory map of the body is also conveyed contralateral to the ventroposterolateral (VPL) nucleus of the thalamus via the dorsal column nuclei (reviewed in Tracey and Waite, 1995). The somatotopic face and body maps formed in these two thalamic nuclei are then conveyed to the primary somatosensory cortex via the thalamocortical projections.
In rodents, relatively large volume of neural substrate is devoted to the point-to-point representation of the whiskers on the snout in the VPM (“barreloids”) and in layer IV (“barrels”) of the primary somatosensory cortex; these whisker-specific patterns are first initiated in the PrV and then sequentially relayed to the thalamus and cortex (reviewed in Sehara and Kawasaki, 2011).
Numerous studies have focused on the development of the thalamocortical pathway and formation of the barrels (Erzurumlu and Kind, 2001; Inan and Crair, 2007; Pinon et al., 2009; Wu et al., 2011). In particular studies in mice with targeted gene mutations have revealed many of the underlying molecular mechanisms in thalamocortical axon guidance (Garel and Rubenstein, 2004). However, very little is known about the development of the trigeminal lemniscal pathway and the molecular guidance these axons use as they form a ribbon-shaped (lemniscus) pathway heading towards the contralateral thalamus. In the preceding paper we describe the normal development of the mouse trigeminothalamic pathway and here we address the molecular mechanisms of axon guidance as this tract acquires a contralateral course.
Specialized midline cells of the vertebrate central nervous system secrete a variety of molecular signals, which attract or repel growing axons (Kennedy et al., 1994; Kidd et al., 1999). Commissural pathways and long axonal tracts such as the corticospinal and medial lemniscal pathways all interact with the neuroaxial midline during their establishment. Developmental regulation of the midline cues and specific receptors for them on the growing tips of axons play an essential role in contralateralization of these pathways.
Slit and netrin proteins are abundant along the developing hindbrain and spinal cord midline, and the ascending somatosensory axons are endowed with cognate receptors. Three types of netrins (netrin-1, -3 and -4) have been identified in mammals (Moore et al., 2007). Netrin-1 is essential for proper extension of spinal commissural axons, corpus callosum and hippocampal commissure (Serafini et al., 1996). In the spinal cord, floor plate glial cells secrete netrin-1 and attract commissural axons towards the floor plate (Kennedy et al., 1994; Kennedy et al., 2006). In vertebrates netrin receptors are “deleted in colorectal cancer”(dcc), neogenin and four “uncoordinated” (unc) 5 proteins (unc5A-D). Axon attraction occurs when netrin-1 binds dcc (Moore et al., 2007). In Drosophila, “frazzled” is the name given to the member of the dcc gene family (Kolodziej et al., 1996) and mediates axon attraction upon binding netrin. Genetic and functional blockade of dcc results in loss of attraction towards the midline by commissural axons (Keino-Masu et al., 1996; Fazeli et al., 1997). Similarly, failure in midline crossing of internal arcuate fibers arising from the dorsal column nuclei has been reported for netrin-1 knockout mice (Kubota et al., 2004).
Slit is an axon repellent protein, also secreted by the midline glia (Kidd et al., 1999). Three slit proteins (slit1-3) have been identified in mammals. They bind with roundabout or robo (robo1-4) receptors. Slit2 and slit1/2 double knockout mice display axon pathfinding defects in the corpus callosum, hippocampal commissure and thalamocortical projections (Bagri et al., 2002). Axonal pathfinding defects were also reported in robo1 knockout mice (Andrews et al., 2006; Jaworski et al., 2010). Our in situ hybridization assays indicated that the message for the slit and netrin proteins and their receptors are found at the right time and place during the development of the mouse trigeminal lemniscal pathway. We used dcc and slit 1, slit2, slit3 single or double knockout mice and observed marked path finding errors in trigeminothalamic projection axons of the PrV. Collectively, our results indicate that a cooperative signaling of attractant (netrin1) and repellent (slits) midline cues plays a fundamental role in guiding developing trigeminal lemniscal axons across the midline and contralateralization of the facial somatosensory inputs.
MATERIALS AND METHODS
Animals
Timed pregnant C57BL/6J mice were purchased from Jackson Laboratories; dcc, slit1, slit2 and slit3 knockout mouse lines (strain background C57bl/J6) were obtained from Dr. Z.F. Chen (Washington University, St Louis, MO). The plug date was considered E0.5. Dams were overdosed with pentobarbital (administered at > 100 mg/kg intraperitoneally). The embryos were removed by Cesarean section and placed in ice-cold phosphate buffered saline (PBS, pH 7.4). Animal handling was in accordance with NIH guidelines and a protocol approved by the UMB Animal Use and Care Committee.
Flattened hindbrain wholemount preparations and carbocyanine dye (DiI) labeling
We used a novel approach in which the entire embryonic hindbrain and the diencephalon were flattened to view the PrV lemniscal pathway in its entirety (see Kivrak and Erzurumlu, preceding paper). The flattened preparations were placed on the Millicell membrane inserts (Millipore) and in 6-well culture plates each well filled with 1ml of 4% paraformaldehyde (PFA). The plates were sealed with parafilm and kept at 4°C overnight. On the next day, small crystals of 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI, Invitrogen) were inserted into the PrV region. Trigeminal ganglia were dissected out attached to the brainstem; this helped as a landmark for the dye placement. The trigeminal ganglion fibers bifurcate as they enter the brainstem and the ascending branch terminates in the PrV while the descending branch extends laterally along the spinal trigeminal nucleus. In earlier experiments we placed DiA in the trigeminal ganglion, after visualization of the ascending and descending trigeminal tract we placed DiI next to the ascending tract where the PrV is located. Thus, retrograde labeling of the trigeminal ganglion and the descending trigeminal tract confirms the proper targeting of the PrV with DiI implants. A few cases were cut in the coronal plane using a vibratome.
Images were collected using Nikon AZ100 and Nikon 90i microscopes and NIS Elements software.
In situ hybridization
Mouse embryos were dissected out in RNAse-free condition to prepare diencephalon-hindbrain wholemounts. The cerebral cortex was removed and the rest of the brain was flattened on membrane (as described above). Tissue was fixed with 4% PFA (Electron Microscopy Sciences) for overnight, then gradually dehydrated and stored in 100% methanol at −20°C. All tissues were processed for in situ hybridization within two weeks.
Nucleotide information for RNA probes
Plasmid for drg11 probe preparation was a kind gift from Dr. Zhou-Feng Chen (Washington University, St Louis, MO). Plasmids for probes for slit1, slit2, slit3, robo1, robo2, robo3 and dcc were kindly provided by Dr. Alain Chedotal (Institute de la Vision, Paris, France). Netrin-1 plasmid was constructed in our lab; primer sequences were sense 5`-cctgcaactgcaacctccatgctc-3` and anti-sense 5`-tgattttgggacacttgcagg-3`.
The nucleotide information for plasmid preparation of all RNA probes is as follows:
Mouse drg11 (GenBank Reference: AK039633.1), full-length cDNA.
Mouse slit1 (GenBank Reference: AF144627.1), nucleotide from 1036-2508 bp.
Mouse slit2 (NCBI Reference: NM_178804.3), nucleotide from 361-1687 bp.
Mouse slit3 (NCBI Reference: NM_011412.3), nucleotide from 2710-4642 bp.
Rat robo1 (NCBI Reference: NM_022188.1), nucleotide from 79-1069 bp.
Rat robo2 (NCBI Reference: NM_032106.1), nucleotide from 1184-2884 bp.
Rat robo3 (NCBI Reference: NM_001164767.1), nucleotide from 2126-2596 bp.
Mouse dcc (NCBI Reference: NM_007831.3), nucleotide from 4017-4560 bp.
Mouse netrin1 (GenBank: U65418.1), nucleotide from 1016-1645 bp.
Digoxigenin (DIG)-labeled RNA probes were prepared according to the manufacturer’s instructions (Roche Diagnostic GmbH, Germany). Hybridization was done at 65°C for overnight using about 300ng/ml of DIG labeled RNA probe per sample. RNAse free condition was maintained until hybridization. Unbound probe was removed with RNaseA treatment (Qiagen) and with extensive stringent wash after hybridization. The bound probe was detected with alkaline phosphatase (AP) anti-DIG Fab fragment (Roche Diagnostic GmbH, Germany). Color reaction (purple to blue) was developed with NBT/BCIP tablet (Roche Diagnostic GmbH, Germany). Sense probe was always used to rule out nonspecific probe binding.
Images were collected using Nikon AZ100 and NIS Elements software. The photomicrographs were adjusted for brightness, contrast, sharpness and evenness of illumination.
RESULTS
Expression patterns of netrin-1 and dcc mRNA in the embryonic hindbrain
In the vertebrate embryo, floor plate cells of the ventral midline secrete netrin-1 (Kennedy et al., 1994; Kennedy et al., 2006). Netrin gradient emanating from the midline attracts or repels developing axons depending on the relative abundance of two types of netrin receptors on the approaching growth cones. Dcc receptor binding mediates axon attraction while the UNC5 homologue receptors mediate repulsion (Moore et al., 2007). In the mouse embryo netrin-1 mRNA expression is evident in the ventral midline as early as E11 (Fig. 1 A). Strong expression of netrin-1 message, restricted to the floor plate, continues to be present up to E17 (Fig. 1 B, C, D). The message for the dcc, the receptor that mediates netrin-1 attraction, is developmentally regulated and can be seen along the lateral brainstem where the PrV develops. Dcc mRNA expression in the lateral hindbrain, where PrV forms, is high at E11 (Fig. 1E) and gradually diminishes after E13 (Fig. 1F–H).
Figure 1.
Expression of netrin-1, dcc and drg11 in different embryonic periods with in situ hybridization; are shown with dark purple color deposition. Netrin-1 mRNA is expressed in ventral midline from E11 to E17 (A, B, C, D). Dcc, netrin-1 receptor (mRNA) is expressed significantly up to E13, its expression goes down from E15 onwards (E, F, G, H). Transcription factor drg11 is typically present in the trigeminal ganglion (TG) and the PrV from earliest embryonic periods on. Arrowheads denote the location of the PrV. Scale bar is 200µm.
Trigeminal lemniscal pathway is impaired in dcc knockout mice
In the rat, PrV neurons are born between embryonic days 13–16 (Altman and Bayer, 1980; Al-Ghoul and Miller, 1993; Miller and Muller, 2004). In the mouse, PrV neurogenesis starts at E10.5 (Ding et al., 2003). PrV neurons send their axons across the midline as soon as they differentiate (Kivrak and Erzurumlu, preceding paper). Our in situ labeling studies showed high levels of netrin-1 mRNA expression along the pontine midline, which is an intermediary target for PrV lemniscal axons en route to the contralateral thalamus. In addition, we saw high levels of dcc mRNA expression during a time window when the PrV axons directly head towards the midline, suggesting a strong attraction towards the midline via netrin-1/dcc signaling. Next, we examined the trajectory of PrV axons in dcc knockout mice at different developmental time points.
DiI placement in the PrV of dcc knockout embryos at E11 revealed absence of any midline crossing by trigeminal lemniscal axons (Fig. 2A, B). In some cases a small bundle of axons headed towards the midline but made a sharp turn back as they approached the midline (Fig. 2B). At E13, the descending trigeminal tract was visible, indicating proper placement of the dye in the PrV, however, most axons directed towards the midline were stalled and many bent rostrally or caudally indicating a strong repulsion by the midline. (Fig. 2C, D). A similar picture was obtained from E15 knockout embryos (Fig. 2E, F). By E17, the descending trigeminal tract was more advanced along the caudal extent of the spinal trigeminal nucleus, but other axons emanating from the PrV were deflected by the midline. Only a small proportion of these axons followed a rostral trajectory, while most of the midline deflected axons stalled (Fig. 3A, B). Careful examination of E17 cases by sectioning the flattened brainstem wholemount preparations revealed an ipsilateral projection (Fig. 3C) in addition to a few contralateral axons headed towards the thalamus (Fig. 3D). These crossed and uncrossed pathways were not seen at earlier ages. The midline repellent signal slit2 and 3 mRNA expression is also diminished after E15 (Fig. 5); thus the contralateral projecting axons may arise from late born PrV cells which may not confront strong midline repellent signals during their growth towards the thalamus. At all embryonic ages, the global appearance of the PrV-based trigeminal lemniscal projections in dcc knockouts was “frazzled” (Figs. 2A, C, E, Fig. 3 A), a name given to the gene that encodes a Drosophila member of the dcc family (Kolodziej et al., 1996).
Figure 2.
Trigeminothalamic projection in dcc knockout mice traced with DiI labeling of PrV neurons. Axons did not cross the midline at E11 (A) and a bundle of axons repelled from the midline and turned caudally (B). Marked stalling of axons before reaching the midline was observed at E13 (C). At higher magnification axons are stalled, turning towards different directions away from the midline, thus no midline crossing was observed at E13 (D). Even at E15, axons failed to cross the midline (E) and few axons turned rostrally to reach ipsilateral thalamus (F). B, D and F are higher magnification views of areas boxed in A, C, and E. Double arrow headed lines indicate the midline. PrV: principal sensory nucleus. Scale bar is 200µm.
Figure 3.
Trigeminothalamic projection at E17 in dcc knockout mice, labeled with DiI placed in the PrV. Descending trigeminal tract (black arrows) extended caudally along the side of the spinal trigeminal nucleus (SpV), however PrV axons failed to cross the midline to reach the contralateral side (A). A marked deflection of PrV axons from the midline (double arrow headed lines) can be observed at low (A) and high power views (B). In several cases a bundle of axons turned rostrally and extended towards the midbrain (white arrows) (C). Very few axons at this age crossed the midline and took a rostral turn (white arrows) (D). Scale bars = 200µm.
Figure 5.
Expression of slits in the mouse hindbrain between E11 and E17. Slit1 mRNA is expressed strongly in the ventral midline (marked by asterisks) at all embryonic ages up to E17 (A, B, C, D). Slit2 mRNA is expressed in the ventral midline and in motor neuron columns (arrows) at E 11 and 13 and in the midline at E15 (E, F, G). At E17, slit2 is not detected anymore in the ventral midline of the hindbrain (H). Slit3 mRNA expression is observed in the ventral midline at early embryonic period (I, J) and its expression is down regulated from E15 on (K, L). Although slit1 is expressed in all embryonic age from E11 to E17, both slit2 and slit3 message decrease significantly from E15 (G, H, K, L). Arrowheads indicate the approximate location of the PrV. Scale bar is 200µm A–J and 50µm for K, L.
In a couple of fortuitous cases obtained from P0 dcc knockouts we saw sparse bilateral labeling in the VPM following unilateral DiI placements in the PrV (Fig. 4). Interestingly, the positioning of the labeled PrV lemniscal fibers within the ipsi- and contralateral VPM was not identical but complementary. Contralateral fibers were predominantly distributed in the dorsolateral cap and ventromedial zones of the VPM while the ipsilateral fibers took up a position in between these two loci (Fig. 4B, C).
Figure 4.
Bilateral labeling of VPM following unilateral DiI placement in PrV (right side) at P0 in dcc mutant mice (A). High power view of labeling in the contralateral VPM (B) and ipsilateral VPM (C) are shown. VPM boundaries are marked with asterisks. Pom: posteromedial nucleus, VPL: ventroposterolateral nucleus. B and C are views of the boxed areas shown in A. Scale bar in A = 300µm.
Expression patterns of midline repellent guidance cues slits and their receptors during the development of the trigeminal lemniscus
We studied the expression pattern of slits and robo receptors with in situ hybridization during development of the trigeminal lemniscal pathway. Slit1 mRNA is strongly expressed along the midline at all embryonic ages studied (Fig. 5 A–D). Slit2 and 3 mRNA expression is very high at early embryonic ages (Fig. 5 E, F, I, J) but is diminished on E15-17 (Fig. 5 G,H, K, L). In the wholemount preparations, slit2 was expressed in the floor plate and also in the differentiating somatic motor neurons (Fig. 5 E arrow) as it has been described for the rat embryo (Hammond et al., 2005). The expression pattern of slit3 is almost similar to that of slit2, but expression is weaker as noted previously (Brose et al., 1999).
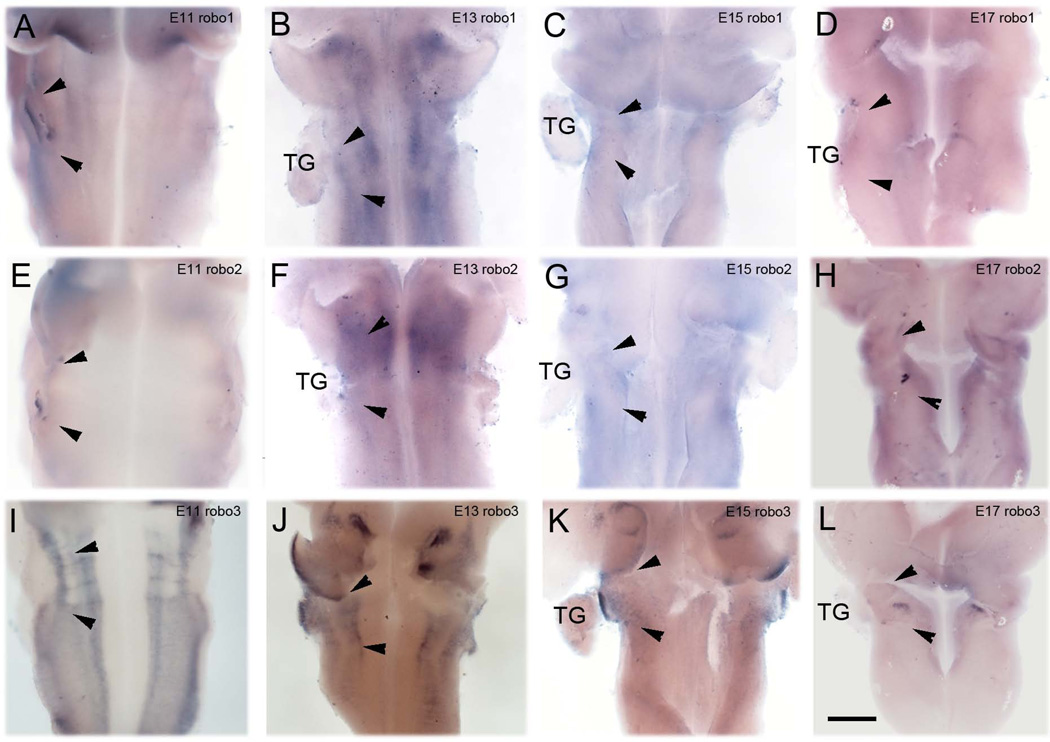
Axon repellent effects of slits are mediated by binding robo receptors. In situ hybridization studies revealed presence of the robo1and 3 mRNA in the developing PrV and expression of robo2 was weaker than others (Fig. 6A–L). Robo1 mRNA was present at E11 and became very prominent by E13, the time point when most trigeminal lemniscal axons have crossed the midline (Fig. 6 A, B, see also Geisen et al., 2008). Robo1 mRNA expression diminished by E15 and was no longer detectable by E17 (Fig. 6 C, D). Robo3 mRNA was also expressed at high levels on E11-13, expression decreased at E15 and was no longer detectable on E17 (Fig. 6 I–L, see also Geisen et al., 2008).
Figure 6.
Slit receptor robo expression pattern in the mouse hindbrain. Robo1 mRNA expression started around the PrV area at E11 and significant expression was found up to E13 (A, B), however expression went down from E15 and was no longer detected in the hind brain at E17 (C, D). (F, G): Robo2 expression started later (E13 and E15) in comparison to robo1, no expression was observed at E11 (E). High expression of robo3 was observed between E11-E13; it started to decrease from E15 (I, J, K, L). All three robos were not detected around PrV area at E17 (D, H, L). Arrowheads indicate the location of the PrV. Scale bar 200µm.
Slit/robo signaling is essential to develop trigeminal-lemniscal pathway
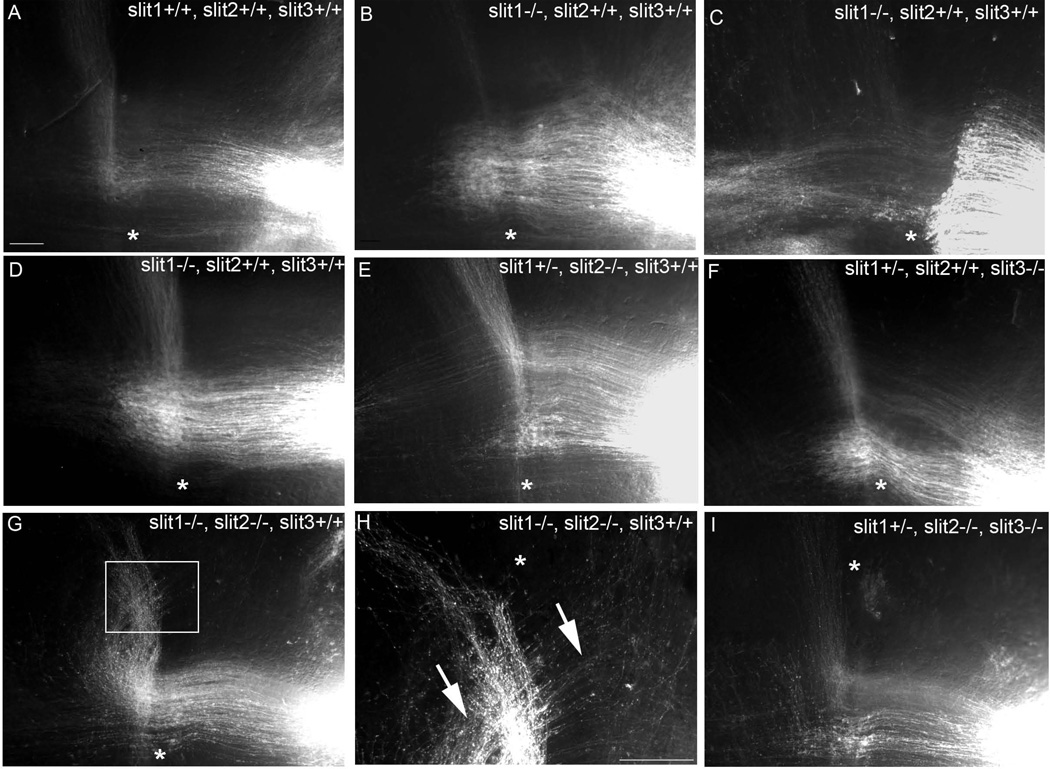
sIn normal mice, axons from the PrV cross the midline between E11-E13 (see Kivrak and Erzurumlu, preceding paper). After crossing the midline, the growth cones take a sharp rostral turn; subsequently, all the crossed axons project rostrally forming a ribbon shaped bundle (Fig. 7A). When slits are genetically blocked, midline-crossing defects are observed. When slit1 gene was knocked out, majority of axons stalled after crossing the ventral midline (Fig. 7B). Partial crossing was also observed in some embryos where axons stalled just before the midline and very few crossed the midline and took a rostral trajectory (Fig. 7C). In some samples, axons stalled near the midline and the rostral projection ran within the midline (Fig. 7D). In slit2 knockouts we observed few axons stalling near the midline (Fig. 7E). Similarly, in slit3 knockout embryos few axons stalled after crossing the midline and the rostral projection ran in close proximity to the midline (Fig. 7F). In double knockout of slit1 and slit2, re-entry of the crossed axons through the midline was observed (Fig. 7G). In four cases one group of lemniscal axons remained crossed while another group recrossed to the ipsilateral side (Fig. 7H). Double knockout of slit2 and slit3 was less severe in phenotype; few axons were stalled in the midline (Fig. 7I).
Figure 7.
Impaired trigeminothalamic pathway in slit knockout mice at E15; DiI crystal was placed in the PrV to trace trigeminothalamic pathway. Axons of PrV neurons crossed the midline and took a sharp rostral turn towards contralateral thalamus at E15 in control embryos (A). Different pathfinding errors including stalling of post-crossing axons, partial crossing and running of trigeminothalamic projection within the midline were observed in slit1 knock out embryos (B, C, D). Few stalling of axons and running of trigeminothalamic projection at close proximity to the midline were observed in slit2 knockout embryo (E). Marked stalling of post-crossed axons was found in slit3 knockout embryos (F). In slit1 and slit2 double knockout embryos, crossed axons reentered the midline (arrows, G, H). H is a higher power view of the boxed region in G. Minor path finding errors with few axon stalling was observed in slit2 and slit3 double knockout embryos (I). Scale bars are 100µm and asterisks mark the midline. Scale bar in A = 50µm.
DISCUSSION
Axonal growth cones navigate through a complex environment by responding to various attractive and repulsive molecular signals as neural connections are laid down. Many of these molecular signals and their receptors on the growth cones are preserved between flies, worms and vertebrates. For example, in Caenorhabditis elegans bifunctional guidance molecule (“uncoordinated”) unc-6 is secreted by midline glial and neuronal cells, and it is a ligand for growth cone receptors unc-5 and unc-40 attracting or repelling different classes of axons to/away from the midline, respectively (Hedgecock et al., 1990; Culotti and Merz, 1998). The mammalian homologues of unc-6, netrin-1, and of receptor unc-40, dcc serve the same function of midline attraction for various classes of commissural axons. Here we show that netrin-1 and dcc regulate midline attraction of trigeminal lemniscal axons, which project primarily to the contralateral thalamus. Slit family of proteins abundant along the hindbrain midline also guide trigeminal lemniscal axons by way of repulsion. As in other axonal pathfinding events, a cooperative action of attraction and repulsion appears to be in place for the midline crossing behavior of trigeminal lemniscal axons.
We found strong expression of netrin-1 mRNA along the hindbrain midline facing the PrV during E11 to E15. Coincident with this, we observed high levels of dcc mRNA in the developing PrV during the time of axonal outgrowth from this nucleus (E11-15) towards the midline. Dcc mRNA expression diminished at E15 and disappeared by E17. Although netrin-1 can bind different receptors, binding to dcc is essential for the axon attractant response (Adler et al., 2006; Moore et al., 2007). The role of netrin-1/dcc signaling in midline attraction of trigeminal lemniscal axons is further underscored by our observations in dcc null embryos. In the absence of functional dcc receptors, PrV axons were no longer directed towards the midline and displayed a strong turning away in their course, starting from the onset of the trigeminal lemniscal development at E11.
Midline expression of slits and robo receptors in the developing PrV indicated that slit/robo signaling is also involved. We found that in slit mutant mouse embryos trigeminal lemniscal development is impaired at the midline with some axons stalling, some remaining ipsilateral and some recrossing the midline.
Studies on commissural axons in Drosophila identified a transmembrane receptor encoded by the roundabout (robo) gene (Seeger et al., 1993; Kidd et al., 1998; Zallen et al., 1998). The ligand for Robo was identified as the members of the slit family of proteins (Wang et.al. 1999, Brose et al., 1999; Kidd et. al. 1999; Li et al., 1999). Slit proteins are expressed at high levels along the midline and act as a repellent guidance cue for those axons that express robo. Slit proteins are known to repel motor, olfactory, and retinal axons, hippocampal dentate gyrus axons, and migrating muscle precursor cells in Drosophila, and postmitotic neurons from the subventricular zone in mammals (Brose et al., 1999; Kidd et al., 1999; Li et al., 1999; Nguyen Ba-Charvet et al., 1999; Wu et al., 1999; Erskine et al., 2000; Ringstedt et al., 2000). Slit proteins and robo receptors show high levels of crosstalk. Slit-mediated repulsion of robo expressing axons or cells and their function in development has been reviewed extensively (Chisholm and Tessier-Lavigne, 1999; Guthrie, 1999; Harris and Holt, 1999; Zinn and Sun, 1999; Brose and Tessier-Lavigne, 2000).
In Drosophila, robo protein is expressed at high levels in growth cones of axons that do not cross the midline. Another protein, commissureless (Comm) down regulates Robo expression in axons that approach and cross the midline, but robo is upregulated again, preventing these axons from recrossing the midline (Kidd et al., 1998a, b). Based on the results of our in situ hybridization studies and observations on dcc and slit mutant mice, we do not think that PrV axons are first attracted to the midline by netrin-1/dcc signaling and after crossing the midline acquire midline repulsion via slit/robo interactions and stay on the contralateral side. Both netrin-1 and slit mRNA is present at high levels during the time of PrV axon crossing of the midline and the levels of both dcc and robo mRNA is quite high. Thus, a balance between the attractant and repulsive forces must exist during the initial phase of the trigeminal tract formation and midline crossing.
It was previously reported that commissural fibers did not cross the midline when either netrin or dcc were knocked out (Serafini et al., 1996; Fazeli et al., 1997). However few axons were still able to reach the midline indicating presence of other midline attractants; later a smaller role for sonic hedgehog (Shh) was identified (Charron et al., 2003; Yam et al., 2009). Although majority axons in trigeminal sensory pathway was stalled in dcc knockout mouse, few could cross the ventral midline and reach to the contralateral thalamus (Fig. 4). This indicates potential involvement of other receptors for netrin-1during the development of trigeminal-lemniscal pathway. DSCAM (Down`s Syndrome Cell Adhesion Molecule) also binds netrin-1 and promotes midline axon crossing (Andrews et al., 2008; Ly et al., 2008; Liu et al., 2009). DSCAM forms a complex with dcc, which dissociate in the presence of netrin-1, suggesting that DSCAM and dcc may work independently (Ly et al., 2008). Neogenin also binds with netrin-1 and it is expressed extensively during development (Keino-Masu et al., 1996; Gad et al., 1997; Wilson et al., 2006). In the chick neogenin works as a substitute for dcc; commissural axons stall and do not cross the midline when neogenin is knocked down with siRNA, but loss of mouse neogenin has no effect on normal trajectory of commissural fibers (Phan et al., 2011).
Bilateral VPM projections in newborn dcc knockout mice also suggest that PrV axons are not uniformly responsive to single or bidirectional molecular guidance cues. On the other hand, it is difficult to explain why the ipsi- and contralateral fibers occupied complementary regions in the VPM. In bilateral input zones of the thalamus, e.g., the lateral geniculate nucleus the ipsi- and contralateral retinogeniculate axons occupy distinct zones and Ephrins and Eph receptors are thought to be involved in this process. Gain- and loss-of-function studies of EphB1 showed that misrouted retinogeniculate axons target the appropriate retinotopic zone in the contralateral lateral geniculate nucleus; however, in EphB1 knockout mice, misrouted retinogeniculate axons segregate into a separate terminal zone (Rebsam et al., 2009). In the PrV lemniscal projections distinct populations of PrV axons might be responsive to netrin/dcc signaling and/or local positional cues in the VPM might be directing the localization of incoming fibers.
It is well known from studies on the developing commissural pathways that growth cones change their responsiveness to midline attractants once they cross this intermediate target; infact, midline repulsion predominates after crossing and prevents growth cones from turning back (Evans et al., 2001; Reeber et al., 2009). Slit proteins and their robo receptors are the major signaling pathways in midline repulsion (Kidd et al., 1999; Simpson et al., 2000; Hammond et al., 2005). We observed strong expression of slit1 in the ventral midline from E11 to E17 in the mouse, brainstem while the expression of slit2 and slit3 decreased in later embryonic periods (Fig. 5 E–P). In commissural axons, dcc expression is upregulated during precrossing and binding with netrin attracts to the ventral midline; at the same time repellent action via slit/ robo1, 2 is suppressed. Robo3 plays an important role in preventing premature repulsion response to slits in mammals (Sabatier et al., 2004). We observed higher expression of robo3 around midline in E11, when axons are either in precrossing or in just crossing state (Fig. 6I). The expression of robo3 is down regulated from E13 to onwards when majority axons already crossed the midline (Fig. 6J–L). After reaching the midline, although dcc is still expressed, netrin-1 attraction is suppressed by binding with robo1 (Stein et al., 2001; Evans et al., 2010). Post-crossing axons become sensitive to repellent action of slits. Our findings are in line with these findings from other systems. Slit and robo mRNA expression patterns are developmentally regulated during the course of PrV lemniscal axon midline crossing and significant midline crossing defects are apparent in slit knockout embryos. In slit1 mutant mice, majority post crossing axons stalled near midline and few made their way to the contralateral thalamus; most PrV axons elongated along the side of the midline or within it (Fig. 7B–D). Slit2 mutant phenotype was less severe than that of other two slits. Marked stalling of post-crossing axons was present found in slit3 mutants. In slit1 and slit2 double knockout, the midline crossing defect was very severe, with re-entry of post-crossed axons back to the midline (Fig. 7 H, I). We did not observe any re-entry of post-crossed axons in the midline in slit1, slit2 or slit3 single mutant mice. Our findings suggest that slit1 and slit2 work synergistically to prevent recrossing of PrV axons back to the ipsilateral side. Presently we do not know to what degree PrV-VPM projections are affected as development proceeds. Future studies on the lemniscal projections of different slit mutants at later embryonic or early postnatal ages should provide answers.
While, in midline crossing of specific classes of axons, slit/robo and netrin-1/dcc signaling mediate opposite effects, more and more new data indicate that they are intertwined signaling pathways regulating each other at many levels (reviewed in Ypsilanti et al., 2010). In Drosophila, combinatorial and opposing actions of robo and frazzled/dcc control target-specific guidance of motor axons (Brierley et al., 2009; Mauss et al., 2009). In the presence of slit, robo1 binds to dcc and blocks netrin-1 attraction (Stein and Tessier-Lavigne, 2001). Robo3 is also expressed by the commissural axons in the spinal cord and hindbrain and genetic invalidation leads to failure in midline crossing (Marillat et al., 2004; Sabatier et al., 2004; Tamada et al., 2008). Functionally antagonistic isoforms of Robo3, arising from alternative splicing, have been found to regulate midline crossing of spinal commissural axons (Chen et al., 2008). Robo3.1 is involved in netrin mediated attraction and silencing slit repulsion during midline crossing of commissural axons and Robo3.2 becomes predominantly expressed postcrossing and switches to slit repulsion, thereby blocking midline recrossing (Chen et al., 2008). It has been suggested that robo3 interacts with dcc and prevents slit/robo1,2-mediated repulsion while enhancing netrin-1 attraction to the midline (Ypsilanti et al., 2010). Our observations from dcc and slit mutants also favor this conclusion that the midline crossing behavior of PrV lemniscal axons is not just a simple attraction followed by repulsion mechanisms but an interplay involving both dcc/netrin and robo/slit signaling pathways.
The hindbrain midline is an intermediate target for PrV lemniscal axons and our results show a strong interplay of slit/robo and netrin/dcc signaling in navigating through the midline. After crossing the ventral midline, PrV axons take a sharp rostral turn observed as early at E13 in mouse (Kivrak preceding article). The sharp rostral turn of post-crossing PrV axons and their well-specified course towards the diencephalon do not appear to involve netrin and slit signaling. Even in slit knockouts, the rostrally directed axons run along the midline or in close proximity to it. Thus while netrin attraction and slit repulsion away from the midline guide the early phase of the pathfinding journey of PrV axons other molecular signals must be in operation in attracting these axons rostrally towards the diencephalon and perhaps repulsing them away from a caudal course towards the spinal cord. It is easy to speculate on the presence of short-range attractant/repellent guidance molecules and long-range attractant, chemotropic signals but at the moment we do not have a clue. Clearly more work is to be done to elucidate specific molecular cues that the PrV axons use once they leave the midline and grow rostrally and invade the VPM nucleus of thalamus with precision.
ACKNOWLEDGMENTS
We are grateful to A. Chedotal for several reagents used in this study and to Z.F. Chen for providing the mutant mice and drg11 plasmid. We thank S. Zhao and C. Bernardelli for their expert technical assistance.
Role of authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: RSE and RM. Acquisition of data: RM, BGK. Analysis and interpretation of data: RM, RSE Drafting of the manuscript: RM. Critical revision of the manuscript for important intellectual content: RSE. Obtained funding: RSE. Technical support: SZ, CB. Study supervision: RSE.
Footnotes
Conflict of interest statement
The authors state that there is no conflict of interest.
LITERATURE CITED
- Adler CE, Fetter RD, Bargmann CI. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat Neurosci. 2006;9:511–518. doi: 10.1038/nn1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghoul WM, Miller MW. Orderly migration of neurons to the principal sensory nucleus of the trigeminal nerve of the rat. J Comp Neurol. 1993;330:464–475. doi: 10.1002/cne.903300403. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the brain stem in the rat. IV. Thymidine-radiographic study of the time of origin of neurons in the pontine region. J Comp Neurol. 1998;194:905–929. doi: 10.1002/cne.901940411. [DOI] [PubMed] [Google Scholar]
- Andrews W, Liapi A, Plachez C, Camurri L, Zhang J, Mori S, Murakami F, Parnavelas JG, Sundaresan V, Richards LJ. Robo 1 regulates the development of major exon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–2252. doi: 10.1242/dev.02379. [DOI] [PubMed] [Google Scholar]
- Andrews GL, Tanglao S, Farmer WT, Morin S, Brotman S, Berberoglu MA, Price H, Fernandez GC, Mastick GS, Charron F, Kidd T. Dscam guides embryonic axons by Netrin-dependent and -independent functions. Development. 2008;135:3839–3348. doi: 10.1242/dev.023739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsal position of major axonal pathway in mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Brierley DJ, Blanc E, Reddy OV, Vijayraghavan K, Williams DW. Dendritic targeting in the leg neuropil of Drosophila: the role of midlinesignalling molecules in generating a myotopic map. PLoS Biol. 2009;7:e1000199. doi: 10.1371/journal.pbio.1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind robo receptor have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Brose K, Tessier-Lavigne M. Slit proteins: key regulators of axon guidance, axonal branching and migration. Curr Opin Neurobiol. 2000;10:95–102. doi: 10.1016/s0959-4388(99)00066-5. [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gore BB, Long H, Ma L, Tessier-Lavigne M. Alternative splicing of the Robo3 axon guidance receptor governs the midlineswitch from attraction to repulsion. Neuron. 2008;58:325–332. doi: 10.1016/j.neuron.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Chisholm A, Tessier-Lavigne M. Conservation and divergence of axon guidance mechanism. Curr Opin Neurobiol. 1999;9:603–615. doi: 10.1016/S0959-4388(99)00021-5. [DOI] [PubMed] [Google Scholar]
- Culotti JG, Merz DC. DCC and netrins. Curr Opin Cell Biol. 1998;10:609–613. doi: 10.1016/s0955-0674(98)80036-7. [DOI] [PubMed] [Google Scholar]
- Ding YK, Yin J, Xu HM, Jacquin MF, Chen JF. Formation of whisker-related principal sensory nucleus-based lemniscal pathway requires a pair domained transcription factor, DRG11. J Neurosci. 2003;23:7246–7254. doi: 10.1523/JNEUROSCI.23-19-07246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine L, Williams SE, Brose K, Kidd T, Rachel RA, Goodman CS, Tessier-Lavigne M, Mason CA. Retinal ganglion cell axon guidance in the mouse optic chiasm: expression and function of robos and slits. J Neurosci. 2000;20:4975–4982. doi: 10.1523/JNEUROSCI.20-13-04975.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Bates CA, Killackey HP. Differential organization of thalamic projection cells in the brain stem trigeminal complex of rat. Brain res. 1980;198:427–433. doi: 10.1016/0006-8993(80)90756-8. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Kind PC. Neural activity: sculptor of 'barrels' in the neocortex. Trends Neurosci. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Bashaw GJ. Axon guidance at the midline: of mice and flies. Curr Opin Neurobiol. 2010;20:79–85. doi: 10.1016/j.conb.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli A, Dickinson SL, Harmiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Raybun H, Simons J, Bronson RT, Gordon JI, Tessier-Lavigne M, Weinberg RA. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- Gad JM, Keeling SL, Wilks AF, Tan SS, Cooper HM. The expression patterns of guidance receptors, DCC and Neogenin, are spatially and temporally distinct throughout mouse embryogenesis. Developmental Biology. 1997;192:258–273. doi: 10.1006/dbio.1997.8756. [DOI] [PubMed] [Google Scholar]
- Garel S, Rubenstein JL. Intermediate targets in formation of topographic projections: inputs from the thalamocortical system. Trends Neurosci. 2004;27:533–539. doi: 10.1016/j.tins.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Geisen MJ, Di Meglio T, Pasqualetti M, Ducret S, Brunet JF, Chedotal A, Rijli FM. Hox paralog group 2 genes control the migration of mouse pontine neurons through slit-robo signaling. PLoS Biol. 2008;6:1178–1194. doi: 10.1371/journal.pbio.0060142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie S. Axon guidance: starting and stopping with slit. Curr Biol. 1999;9:R432–R435. doi: 10.1016/s0960-9822(99)80274-7. [DOI] [PubMed] [Google Scholar]
- Hammond R, Vivancos V, Naeem A, Chilton J, Mambetisaeva E, Andrews W, Sundaresan V, Guthrie S. Slit-mediated repulsion is a key regulator of motor axon pathfinding in the hindbrain. Development. 2005;132:4483–4495. doi: 10.1242/dev.02038. [DOI] [PubMed] [Google Scholar]
- Harris WA, Holt CE. Neurobiology. Slit, the midline repellent. Nature. 1999;398:462–463. doi: 10.1038/18970. [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Culotti JG, Hall DH. The unc-6, and unc-40 genes guide circumferential migration of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- Inan M, Crair MC. Development of cortical maps:perspectives from the barrel cortex. Neuroscientist. 2007;13:49–63. doi: 10.1177/1073858406296257. [DOI] [PubMed] [Google Scholar]
- Jaworski A, Long H, Tessier-Lavigne M. Collaborative and specialized functions of Robo1 and Robo2 in spinal commissural axon guidance. J Neurosci. 2010;30:9445–9453. doi: 10.1523/JNEUROSCI.6290-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotte JG, Tassier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Wang H, Marshall W, Tessier-Lavigne M. Axon guidance by diffusible chemoattractants: A gradient of netrin protein in developing spinal cord. J neurosci. 2006;26:8866–8874. doi: 10.1523/JNEUROSCI.5191-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd T, Russel C, Goodman CS, Tear G. Dosage-sensitive and complementary functions of roundabout and commissureless control axon crossing of the CNS midline. Neuron. 1998;20:25–33. doi: 10.1016/s0896-6273(00)80431-6. [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophilia. Cell. 1999a;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1999a;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Kolodziej PA, Timpe LC, Mitchell KJ, Fried SR, Goodman CS, Jan LY, Jan YN. Frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- Kubota C, Nagano T, Baba H, Sato M. Netrin 1 is crucial for establishment of the dorsal column-medial lemniscal system. J Neurochem. 2004;89:1547–1554. doi: 10.1111/j.1471-4159.2004.02460.x. [DOI] [PubMed] [Google Scholar]
- Li HS, Chen JH, Wu W, Faqaly T, Zhou L, Yuan W, Dupuis S, Jiang ZH, Nash W, Gick C, Ornitz DM, Wu JY, Rao Y. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell. 1999;96:807–818. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Liu G, Li W, Wang L, Kar A, Guan KL, Rao Y, Wu JY. DSCAM functions as a netrin receptor in commissural axon pathfinding. Proc Natl Acad Sci USA. 2009;106:2951–2956. doi: 10.1073/pnas.0811083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- Ly A, Nikolaev A, Suresh G, Zheng Y, Tessier-Lavigne M, Stein E. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell. 2008;133:1241–1254. doi: 10.1016/j.cell.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marillat V, Sabatier C, Failli V, Matsunaga E, Sotelo C, Tessier-Lavign M, Chédotal A. The slit receptor Rig-1/Robo3 controls midlinecrossing by hindbrain precerebellar neurons and axons. Neuron. 2004;43:69–79. doi: 10.1016/j.neuron.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Mauss A, Tripodi M, Evers JF, Landgraf M. Midline signaling systems direct the formation of a neural map by dendritic targeting in the Drosophila motor system. PLoS Biol. 2009;7:e1000200. doi: 10.1371/journal.pbio.1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Muller SJ. Structure and histogenesis of the principal sensory nucleus of the trigeminal nerve: effects of prenatal exposure to ethanol. J Comp Neurol. 1989;282:570–580. doi: 10.1002/cne.902820408. [DOI] [PubMed] [Google Scholar]
- Moore SW, Tessier-Lenigne M, Kennedy TE. Netrins and their receptors. Adv Exp Med Biol. 2007;621:17–31. doi: 10.1007/978-0-387-76715-4_2. [DOI] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, Brose K, Marillat V, Kidd T, Goodman CS, Tessier-Lavigne M, Sotelo C, Chedotal A. Slit2-mediated chemorepulsion and collapse of developing forebrain axons. Neuron. 1999;22:463–473. doi: 10.1016/s0896-6273(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Phan KD, Croteau LP, Kam JW, Kania A, Cloutier JF, Butler SJ. Neogenin may functionally substitute for Dcc in chicken. PLoS One. 2011;6:e22072. doi: 10.1371/journal.pone.0022072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon MC, Jethwa A, Jacobs E, Campagnoni A, Molnar Z. Dynamic integration of subplate neurons into the cortical barrel field circuitry during postnatal development in the Golli-tau-eGFP (GTE) mouse. Physiol. 2009;587:1903–1915. doi: 10.1113/jphysiol.2008.167767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsam A, Petros TJ, Mason CA. Switching retinogeniculate axon laterality leads to normal targeting but abnormal eye-specific segregation that is activity-dependent. J Neurosci. 2009;29:14855–14863. doi: 10.1523/JNEUROSCI.3462-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeber SL and Kaprielian Z. Leaving the midline: how Robo receptors regulate the guidance of post-crossing spinal commissural axons. Cell Adh Migr. 2009;3:300–304. doi: 10.4161/cam.3.3.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstedt T, Braisted JE, Brose K, Kidd T, Goodman CS, Tessier-Lavigne M, O`Leary DD. inhibition of retinal axon growth and its role in retinal axon pathfinding and innervations patterns in the diencephalon. J Neurosci. 2000;20:4983–4991. doi: 10.1523/JNEUROSCI.20-13-04983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier C, Plump AS, Le M, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midlinecrossing by commissural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- Seeger M, Tear G, Ferres-Marco D, Goodman CS. Mutations affecting growth cone guidance in drosophila: genes necessary for guidance toward or away from the midline. Neuron. 1993;10:409–426. doi: 10.1016/0896-6273(93)90330-t. [DOI] [PubMed] [Google Scholar]
- Sehara K, Kawasaki H. Neuronal circuits with whisker-related patterns. Mol Neurobiol. 2011;43:155–162. doi: 10.1007/s12035-011-8170-8. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Simpson JH, Kidd T, Bland KS, Goodman CS. Short-range and long-range guidance by slit and its Robo receptors.Robo and Robo2 play distinct roles in midline guidance. Neuron. 2000;28:753–766. doi: 10.1016/s0896-6273(00)00151-3. [DOI] [PubMed] [Google Scholar]
- Stein E, Tessier-Lavigne M. Hierarchical organization of guidancereceptors: silencing of netrin attraction by slit through a Robo/DCC receptorcomplex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- Tamada A, Kumada T, Zhu Y, Matsumoto T, Hatanaka Y, Muguruma K, Chen Z, Tanabe Y, Torigoe M, Yamauchi K, Oyama H, Nishida K, Murakami F. Crucial roles of Robo proteins in midline crossing of cerebellofugal axons and lack of their upregulation after midline crossing. Neural Dev. 2008;3:29. doi: 10.1186/1749-8104-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey DJ, Waite PME. Somatosensory system. In: Paxinos G, editor. The rat nervous system, 2nd ed. San Diego: Academic Press; 1995. pp. 689–704. [Google Scholar]
- Waite PME, Tracey DJ. Trigeminal sensory system. In: Paxinos G, editor. The rat nervous system. The rat nervous system, 2nd ed. San Diego: Academic Press; 1995. pp. 705–724. [Google Scholar]
- Wilson NH, Key B. Neogenin interacts with RGMa and netrin-1 to guide axons within the embryonic vertebrate forebrain. Dev Biol. 2006;296:485–498. doi: 10.1016/j.ydbio.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Wu CS, Ballester Rosado CJ, Lu HC. What we can get from ‘barrels’: the rodent barrel cortex as a model for studying the establishment of neural circuits. Eur J Neurosci. 2011;34:1663–1676. doi: 10.1111/j.1460-9568.2011.07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Wong K, Chen J, Jiang Z, Dupuis S, Wu JY, Rao Y. Directional guidance of neuronal migration in the olfactory system by protein slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Ypsilanti AR, Zagar Y, Chedotal A. Moving away from the midline: new developments for Slit and Robo. Development. 2010;137:1939–1952. doi: 10.1242/dev.044511. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Yi BA, Bargmann CI. The conserved immunoglobulin superfamily member Sax-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell. 1998;92:217–227. doi: 10.1016/s0092-8674(00)80916-2. [DOI] [PubMed] [Google Scholar]
- Zinn K, Sun Q. Slit branches out: a secreted protein mediates both attractive and repulsive axon guidance. Cell. 1999;97:1–4. doi: 10.1016/s0092-8674(00)80707-2. [DOI] [PubMed] [Google Scholar]