Introduction
γ-Glutamylamines are a series of compounds bearing an amide linkage between the γ-carboxamide of glutaminyl (Q) residues or γ-carboxylate of glutamate and an amine group (Figure 1). An example of such a molecule is γ-glutamylcysteinylglycine or glutathione. This tripeptide is one of the most abundant molecules in the body and indicates that the formation of γ-glutamylamide bonds is a frequent biochemical event. The enzymes responsible for the formation of glutathione are glutamate cysteine ligase and glutathione synthetase. Free γ-glutamylamines can also arise through the combined actions of transglutaminases and proteases (Chung et al. 1972, Abe et al. 1977, Folk et al. 1977, Fink et al. 1980, Folk et al. 1980, Fink et al. 1981, 1983, Folk 1983, Fésüs et al. 1985, Beninati et al. 1988, Beninati et al. 1988, Piacentini et al. 1988, Martinet et al. 1990). In contrast to glutathione, the biology of transglutaminase-derived γ-glutamylamines is poorly understood. There is a compelling case for considering a role for these γ-glutamylamines in neurodegeneration.
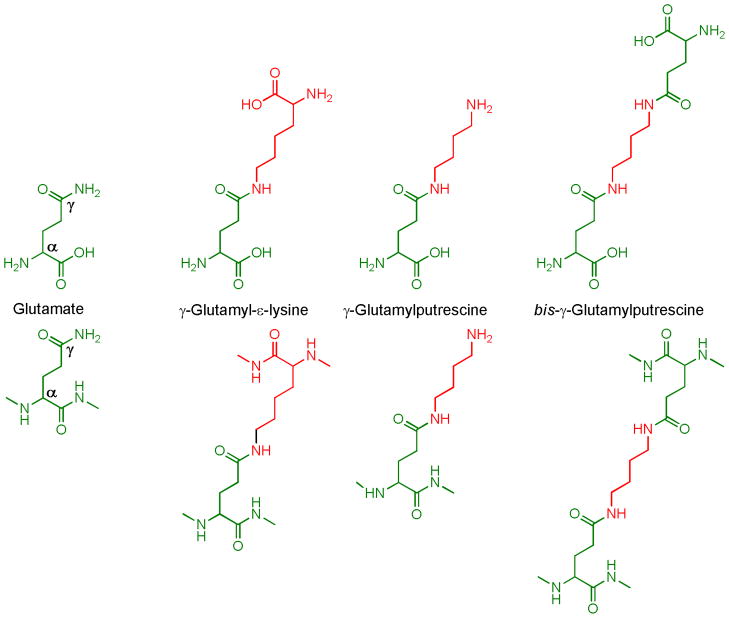
Figure 1. γ-Glutamylamines.
The structures for the free and protein-bound forms of glutamate and some γ-glutamylamines are shown in the upper and lower panels, respectively.
Transglutaminases are thought to contribute to neurodegeneration primarily by cross-linking proteins known to be involved in these diseases (Wilhelmus et al. 2008, Iismaa et al. 2009, Jeitner et al. 2009, Jeitner et al. 2009, Caccamo et al. 2010, Hoffner et al. 2010, Ricotta et al. 2010). These proteins include a-β, α-synuclein and huntingtin in the diseases described by Alzheimer (AD), Parkinson (PD) and Huntington (HD), respectively (Selkoe et al. 1982, Zhang et al. 1998, Kim et al. 1999, Singer et al. 2002, Junn et al. 2003, Nemes et al. 2004, Konno et al. 2005, Zainelli et al. 2005, Wilhelmus et al. 2009). Aggregates containing these proteins, however, are found at sites other than the affected regions in the brain and suggest that other transglutaminase products - namely the free γ-glutamylamines – may contribute to the toxic processes associated with these enzymes. The fact that γ-glutamylamines accumulate in the diseased brain lends support to this notion. The following discussion briefly reviews the current knowledge concerning the metabolism of transglutaminase-derived γ-glutamylamines in the brain and the possible role of these molecules in neurodegenerative disorders.
Cerebral transglutaminases and formation of γ-glutamylamine bonds
Mammals produce eight active transglutaminases, of which transglutaminase 1–3 (Kim et al. 1999, Citron et al. 2001, Wilhelmus et al. 2009) and 6 are currently known to be expressed in the brain (Grenard et al. 2001, Hadjivassiliou et al. 2008). Transglutaminases catalyze various modifications of the carboxamide moiety [-C(O)NH2] of Q residues. These modification include transamidation (Iismaa et al. 2003, Lorand et al. 2003) (Fig. 2), deamination (Molberg et al. 1998, van de Wal et al. 1998, Pinkas et al. 2007, Stamnaes et al. 2008) and esterification (Nemes et al. 1999) which convert the carboxamide group to [-C(O)NHR], [-CO2−] and [-C(O)OR] moieties, respectively. Transamidation is the only reaction attributed thus far to cerebral transglutaminases and results in the formation of γ-glutamylamine isopeptide bonds. To date, all transglutaminase structures studied in detail share a common architecture of four ellipsoidal domains comprised of an N-terminal β sandwich, a catalytic core and two C terminal β barrels as shown for transglutaminase 2 in Figure 2 (Pinkas et al. 2007). In the closed orientation, the C-terminal β barrels drape across the active site to prevent catalysis. The enzyme is subsequently activated by the binding of two calcium ions, which causes the hinge region between catalytic core and β barrels to assume a helical conformation and extend the β barrels away from the catalytic site. This open conformation allows access to the catalytic quartet of Asp358, His335, Cys277 and Trp241 (residue designations as per human transglutaminase 2 (Pinkas et al. 2007)). The conformational changes also juxtapositions Cys277 to Trp241 resulting in the formation of a thiolate-imidazolium ion pair oriented by Asp358 (Figure 3). These changes allow a nucleophilic attack by the thiolate anion on the electron-deficient carbonyl of substrate γ-carboxamide group to generate an oxyanion intermediate (Iismaa et al. 2003). The charge on the oxyanion is stabilized by hydrogen bonding with the backbone nitrogen of Cys277 and the Nε1 nitrogen of Trp241. Subsequent acylation results in the release of NH4+ and the formation of an acyl-enzyme intermediate. This intermediate then undergoes a nucleophilic attack by an amine group and leads to the formation of a second oxyanion. Cys277 and Trp241 again stabilize the oxyanion through hydrogen bonding and deacylation completes the reaction. The key residues and structural motifs described above are conserved among transglutaminases 1 to 3 and suggest that the reaction scheme given in Figure 3 is shared by these enzymes.
Figure 2. Structure of Transglutaminase 2.
The upper illustrations represent the closed (A) and open (B) conformations of transglutaminase 2 as well as the merger of these conformations (C). The lower diagram shows a ribbon diagram of the active site with γ-glutamyl-ε-lysine in place (D). These figures were generated using the program CCP4MG from the PDB coordinates IKV3 (blue) and ZQ3Z (magenta) (McNicholas et al. 2011).
Figure 3. Transamidation as catalyzed by Transglutaminases.
Adapted from IIsmaa et al. (2003).
γ-Glutamyl-ε-lysine crosslinking and neurodegenerative disorders
Monoamines, diamines, polyamines and the ε amino group of lysyl (K) residues all serve as amine-bearing substrates for the transamidation reaction (Lorand et al. 2003). Of the possible reaction products the bond formed between Q and K residues – the γ-glutamyl-ε-lysine or Nε-(γ-L-glutamyl)-L-lysine isopeptide linkage - is the most commonly studied (Figure 1). This bond can be formed within and between polypeptide chains. γ-Glutamyl-ε-lysine linkages between polypeptides can act to stabilize protein aggregates. In many cases, such as the creation of the extracellular matrix or the cornified envelope, this is a crucial event and mutations that affect the activity of the relevant transglutaminases can result in life-threatening disease (Cooper et al. 2002, Kim et al. 2002, Wilhelmus et al. 2008, Herman et al. 2009, Iismaa et al. 2009, Jeitner et al. 2009, Jeitner et al. 2009, Caccamo et al. 2010, Hoffner et al. 2010, Ricotta et al. 2010). The aggregation of particular proteins in the brain, however, is thought to be a contributing factor in a number of important neurodegenerative disorders. Examples include aβ in the amyloid plaques of AD (Zhang et al. 1998, Wilhelmus et al. 2009), α-synuclein in the Lewy bodies of PD (Junn et al. 2003, Nemes et al. 2004, Segers-Nolten et al. 2008, Nemes et al. 2009, Verhaar et al. 2011, Wilhelmus et al. 2011), and huntingtin in the inclusion bodies of HD (Gentile et al. 1998, Kahlem et al. 1998, Cooper et al. 2002, Dedeoglu et al. 2002, Karpuj et al. 2002, Zainelli et al. 2003, Zainelli et al. 2005) (for a comprehensive list of these proteins see (Kim et al. 2002, Hoffner et al. 2010)).
It has been hypothesized that γ-glutamyl-ε-lysine crosslinks help stabilize the aggregates found in neurodegenerative diseases. The following evidence supports this hypothesis: γ-glutamyl-ε-lysine crosslinks colocalize with aggregates of aβ (Wilhelmus et al. 2009), tau-containing paired helical filament (Zemaitaitis et al. 2000, Singer et al. 2002), α-synuclein (Junn et al. 2003) and huntingtin (Dedeoglu et al. 2002, Zainelli et al. 2003, Zainelli et al. 2005), as revealed by immunohistochemical staining of the affected areas of AD, PD, supranuclear palsy, and HD, respectively. The presence of these crosslinks in α-synuclein-containing oligomers isolated from the brains of AD and PD patients, and also from a detergent-insoluble fraction from the diseased regions in AD brains has been confirmed by other methods (Kim et al. 1999, Nemes et al. 2004, Nemes et al. 2009). Moreover, the number of γ-glutamyl-ε-lysine crosslinks present in these fractions is greater in the diseased brains (Table 1). For example, the detergent-insoluble fractions isolated from the cortex and cerebellum of AD patient contains between 30 and 50 γ-glutamyl-ε-lysine crosslinks/10,000 residues as compared to 1 γ-glutamyl-ε-lysine crosslink/10,000 residues found in corresponding regions of age-matched controls (Kim et al. 1999). As revealed in Table 1, there is great disparity in the number of γ-glutamyl-ε-lysine crosslinks reported to be associated with the detergent-insoluble proteins. Even so, the number of these crosslinks is consistently higher in proteins extracted from individuals with neurodegenerative disorders, and correlates with both the deposition of proteinaceous aggregates and the activation of transglutaminases in these diseases.
Table 1.
Frequency of γ-glutamyl-ε-lysine crosslinks in detergent-insoluble protein fractions of AD brains
| Tissue | Group | γ-glutamyl-ε-lysine per 106 residues | Fold change | References |
|---|---|---|---|---|
| Cerebellum/cortex | Control | 0.10 × 103 | (Kim et al. 1999) | |
| AD | 3–5 × 103 | 30–50 | ||
| Occipital lobe | Control | ~2.00 | (Nemes et al. 2004) | |
| AD | ~2.00 | 1.00 | ||
| Frontal Cortex | Control | ~5.83 | ||
| AD | ~21.7 | ~3.71 | ||
| Hippocampus | Control | ~8.33 | ||
| AD | ~31.7 | ~3.80 | ||
| Frontal Cortex | Control | ~0.11 | (Nemes et al. 2009) | |
| AD | ~0.58 | ~5.14 | ||
| Hippocampus | Control | ~0.11 | ||
| AD | ~0.42 | ~3.71 |
bis γ-Glutamylpolyamine residues in neurodegeneration
In addition to γ-glutamyl-ε-lysine crosslinks, transglutaminases catalyze the formation of bis-γ-glutamylpolyamine bridges between polypeptide chains (Folk et al. 1980, Beninati et al. 1988, Piacentini et al. 1988). These bridges are formed by two successive transamidations, the first of which involves the attack of a polyamine on a Q residue to generate a γ-glutamylpolyamine residue (e.g. γ-glutamylputrescine in Figure 1). This moiety has a remaining free terminal amine which allows it to participate in a second transamidation, thereby producing a bis-γ-glutamylpolyamine crosslink (e.g. bis-γ-glutamylputrescine crosslink in Figure 1). The role of bis-γ-glutamylamine crosslinks in the stabilization of oligomerized proteins in plaques, tangles and inclusion bodies has not yet been investigated. Lai et al. (Lai et al. 2004) and Konno et al. (Konno et al. 2005) demonstrated that proteins cross-linked with bis-γ-glutamylputrescine linkages formed insoluble aggregates, whereas those cross-linked with γ-glutamyl-ε-lysine bonds remained in solution. In particular, these researchers discovered that the formation of γ-glutamyl-ε-lysine cross-links by transglutaminases prevented the spontaneous generation of insoluble protein aggregates. The addition to polyamines in these experiments caused the proteins to precipitate implying that the formation of bis-γ-glutamylpolyamine or γ-glutamylpolyamine linkages favored the formation of insoluble aggregates. These findings have so far been restricted to investigations of two polypeptides (Lai et al. 2004, Konno et al. 2005). As discussed below, solubility may be a crucial determinant of the toxicity of protein aggregates, and highlights the importance of future studies on the contribution of bis-γ-glutamylpolyamine bridges to the solubility of proteinaceous aggregates.
Toxicity of plaques, tangles and inclusion bodies
The plaques, tangles, Lewy bodies and inclusions observed in neurodegenerative disorders have long been assumed to be toxic to neurons. This assumption led to the hypothesis that the processes creating these amalgamations - such as the activation of transglutaminases – are similarly detrimental to neurons. Green hypothesized this might particularly be the case in CAG-trinucleotide expansion diseases because novel transglutaminase substrates, specifically stretches of polyQ residues, are produced in this group of neurodegenerative disorders (Green 1993). The CAG nucleotide encodes glutamine and expansion of this sequence in exons results in the insertion of polyQ residues into the expressed protein (Gusella et al. 1993, Rubinsztein et al. 1993). HD is an example of a CAG-expansion disease. This disease is caused by CAG trinucleotide expansions in the huntingtin gene, which encodes huntingtin bearing vary lengths of polyQ (Hoogeveen et al. 1993). The number of CAG repeats, after a certain threshold, is inversely related to the age of onset and the severity of disease (Rubinsztein et al. 1993). Interestingly, the ability of polyQ residues to act as transglutaminase substrates increases as a function of polyQ length (Cooper et al. 1997, Gentile et al. 1998, Kahlem et al. 1998, Cooper et al. 2002, Ruoppolo et al. 2003, Lai et al. 2004). Thus, the transglutaminase substrate activity of polyQ residues parallels the ability of these pathologically-elongated sequences to cause HD, and strengthens the argument that transglutaminases play a role in CAG trinucleotide-expansion diseases. The assumption that insoluble aggregates cause the death of cells, however, is not universally accepted. For example, the distribution of inclusion bodies in the striata of HD patients does not coincide with that of the dead and dying neurons (Kuemmerle et al. 1999, Arrasate et al. 2004). Indeed, aggregates of mutant huntingtin are deposited throughout the body of the HD patients to no apparent ill effect in the early stages of the disease (Choi et al. 2000) 1. Similar observations have been made concerning the insoluble deposits in AD and PD, prompting the hypothesis that these structures are inert and act as a form of storage for cellular debris (Saudou et al. 1998, Arrasate et al. 2004). The possibility also exists that some of these structures may even play a hitherto undiscovered physiological function.
Insoluble aggregates can play beneficial roles in the body. An example of such an insoluble aggregate in the brain is melanin. This aggregate is formed from the oxidation products of dopamine (Bisaglia et al. 2007, Pham et al. 2009, Bisaglia et al. 2010). Melanic polymers occupy approximately 47% of the cytoplasmic volume of the neurons in the substantia nigra and are responsible for the characteristic black or nigra color of this tissue (Fedorow et al. 2006). Substantia nigra neurons do not express ferretin and melanin serves instead to chelate iron and other adventitious metals (Zecca et al. 1996, Zecca et al. 2008). The deposition of melanin outside of these cells, however, is thought to contribute to the pathology of PD (Gibb 1992, Good et al. 1992, Jellinger et al. 1992, Kastner et al. 1992, Lopiano et al. 2000, Zecca et al. 2002, Faucheux et al. 2003, Fasano et al. 2006, Zecca et al. 2008). Other biologically useful precipitates include the extracellular matrix, fibrin clots and the cornified envelope, all of which are stabilized, in part, by γ-glutamyl-ε-lysine and bis-γ-glutamylpolyamine crosslinks (Lorand et al. 2003, Iismaa et al. 2009). Interestingly, in the one tissue where the frequency of these bonds has been compared, namely skin, the number of bis-γ-glutamylpolyamine exceeds that of the γ-glutamyl-ε-lysine bridges (Beninati et al. 1988, Piacentini et al. 1988, Martinet et al. 1990). This observation supports the hypothesis that the insoluble aggregates bearing bis-γ-glutamylpolyamine rather than γ-glutamyl-ε-lysine bridges, are not toxic.
On the other hand, studies performed with cell and animals models suggest that expanded polyQ-containing huntingtin aggregates may indeed be toxic under certain circumstances (Hoffner et al. 2010). This toxicity may occur when the precipitates sequester crucial proteins (Cooper et al. 1997, Ruoppolo et al. 2003) or are sufficiently large enough to physically disrupt the cytoskeleton or organelles (Hoffner et al. 2010). The formation of such precipitates does not necessarily require the activation of transglutaminase (Hoffner et al. 2010). These precipitates are likely to have been formed by a mechanism involving non-covalent polar zippers (Perutz 1995, Hoffner et al. 2010). Many of the studies demonstrating a relationship between aggregate size and toxicity have relied on the explicit or implicit assumption that aggregation of the huntingtin or polyQ domains is by polar zipper formation rather than the actions of transglutaminases. Transglutaminase activity can prevent the generation of polar zippers (Lai et al. 2004, Konno et al. 2005) and polyQ and huntingtin containing polyQ residues act as in vitro and in vivo transglutaminase substrates. As noted above, the propensity of these sequences to act as transglutaminase substrates also increases with the length of polyQ residues (Cooper et al. 1997, Gentile et al. 1998, Kahlem et al. 1998, Cooper et al. 2002, Ruoppolo et al. 2003, Lai et al. 2004). Thus, it is possible that transglutaminases are activated in HD to prevent the formation of aggregates stabilized by polar zippers. This idea is consistent with the observed increase in transglutaminase activity in the diseased tissues of HD, particularly during the early stages of this disease (Lesort et al. 1999), the observation that the number of aggregates increases in mice that express mutant huntingtin but not transglutaminase 2 (Mastroberardino et al. 2002) and the fact that the number of γ-glutamyl-ε-lysine isopeptides per 106 residues thought to be associated with protein aggregates is low (between 0.1 and 100 per 106 residues: Table 1). It may be that in HD, transglutaminases act to cross-link mutant huntingtin with γ-glutamyl-ε-lysine linkages to produce soluble, and presumably, toxic aggregates. If this is the case, then the number of γ-glutamyl-ε-lysine crosslinks would be greater in the soluble rather than the insoluble form of huntingtin in HD brain. A comparable argument could be made for the crosslinking of α-synuclein in PD and αβ in AD. Investigating this possibility is a worthwhile endeavor, but first requires the development and testing of methods for measurement of γ-glutamylamines that do not produce the intra-laboratory variation illustrated in Table 1. One possibility is the LC-EC method we developed and validated with LC-MS (Jeitner et al. 2001), but as yet, has not been tested in other laboratories.
As noted above, total transglutaminase activity is increased in HD. The relevant measurements were of the ex vivo incorporation of polyamines into a Q-bearing transglutaminase substrate rather than an in vivo or in situ assessment of this activity (Lesort et al. 1999, Karpuj et al. 2002). The reported increases in total transglutaminase activity, however, were matched by increases in the amount of transglutaminase 2, as well as, the amounts of bound and free γ-glutamylamines in these tissues (Lesort et al. 1999, Dedeoglu et al. 2002, Karpuj et al. 2002, Zainelli et al. 2005) (Table 1). It was also noted in the preceding paragraph that knocking out transglutaminase 2 in an animal model of HD prolonged the life of these animals. Nonetheless, the animals still die of a neurodegenerative disorder involving the continued deposition of huntingtin aggregates, stabilized in part by polar zippers. The question that arises from these considerations is whether the aggregates crosslinked with bis-γ-glutamylpolyamines are toxic as compared to those stabilized by polar zippers. Answering this question would aid in elucidating the role of transglutaminase in CAG-expansion disease, and also of the significance of bis-γ-glutamylpolyamine and γ-glutamyl-ε-lysine crosslinks in protein aggregation. The implication from these considerations is that the disease process depends to some extent on either relative availability of polyamines or the affinity of the mutant substrates relative to polyamines for the transglutaminases. In this context it is interesting to note the amount of polyamines decreases in the aging brain (Liu et al. 2008) and the number of γ-glutamyl-ε-lysine crosslinks increases (Nemes et al. 2004).
Free γ-glutamylamines and γ-glutamylamine cyclotransferase
γ-Glutamylamines are released intact from proteins during proteolysis because proteases do not hydrolyze isopeptide bonds formed between the γ carboxamide group of a Q residue and an amine. Free γ-glutamylamines can, however, be metabolized to 5-oxo-L-proline and an alkylamine by γ-glutamylamine cyclotransferase (Fig. 3) (Fink et al. 1980, Fink et al. 1981, 1983, Chen 2008, Oakley et al. 2010). The crystal and solution structures of this enzyme were recently resolved and, in combination with site-directed mutagenesis studies, revealed the critical residues required for catalysis by γ-glutamylamine cyclotransferase (Oakley et al. 2010, Serrano et al. 2010). These studies indicate that γ-glutamylamine cyclotransferase possesses a five-strand β-barrel decorated with helices and connecting loops (Figure 4). The barrel formed by this structure appears to be characteristic of cyclotransferases in general and features two β strands -β2a and β3b - that crossover each other (Oakley et al. 2010). A cavity is formed at the bottom of the barrel between the β1 and β5 strands that serves to juxtaposition substrates to the catalytic Glu82 residue. The cavity-forming residues are Tyr7, Gly8, Thr9, Leu10, Glu 82 (Fig. 3), Tyr88 and Tyr119. Access to this cavity is restricted to substrates with extended aliphatic amines bound to a γ-glutamyl group, consistent with the known substrate preferences of this enzyme (Fink et al. 1980, Fink et al. 1981). Substrates enter the cavity and are positioned by hydrogen bonding between the oxygen atoms of the glutamyl portion of the substrate and the amine moieties of Tyr7, Gly8, Thr9, and Leu10 as shown in Figure 5 (for the sake of clarity not all of the possible hydrogen bonds are shown in this figure). The formation of these bonds is accompanied by intra-strand movements that orient the α-amino group of the substrate γ-glutamyl moiety to the carboxyl group of Glu82 [79]. These movements culminate in the abstraction of a proton from the α-amino group by the carboxylate group of Glu82. This proton abstraction accentuates the nucleophilicity of the amine and facilitates its attack on the side chain amide carbon atom to generate an oxyanion intermediate. This tetrahedral intermediate subsequently undergoes an elimination reaction and collapses to form 5-oxo-L-proline and the free amine. During this process, the proton initially abstracted from the amine of the substrate is then donated to the amine of the amino portion of the substrate product (in the case of γ-glutamyl-ε-lysine this would be the ε-linked amine of the lysine product) to complete the catalytic cycle.
Figure 4. Structural Features of γ-Glutamylamine Cyclotransferase.
The enzyme complexed with pyroglutamic acid is shown in A with the relative position of the active site reside in the absence (magenta) and presence (blue) represented in B. Part C depicts the details in B oriented at 180 degrees around the x axis. These figures were generated using the program CCP4MG from the PDB coordinates 3JUB (magenta) and 3JUC (blue) (McNicholas et al. 2011).
Figure 5. Catalytic mechanism of γ-glutamylamine cyclotransferase.
Adapted from Oakley et al. (2010).
It is interesting to compare γ-glutamylamine cyclotransferase to three other enzymes that can cleave or modify a γ-glutamyl peptide bond: γ-glutamylcyclotransferase, γ-glutamyltranspeptidase and transglutaminases. Although γ-glutamylamine cyclotransferase and γ-glutamylcyclotransferase catalyze very similar reactions, there is very little, if any, overlap in specificity of these enzymes. γ-Glutamylcyclotransferase, with rare exceptions, cleaves the isopeptide bond connecting the γ-glutamyl group of glutamate to the α-amino group of an α-amino acid (Meister 1985), whereas, γ-glutamylamine cyclotransferase cleaves the isopeptide bond connecting the γ-glutamyl group of glutamate to an amine that is not bound at the α-carbon of an amino acid (Fink et al. 1980, Danson et al. 2002). Oakley et al. (Oakley et al. 2010) proposed that this discrimination is due to the phenylalanine at position 81 in γ-glutamylamine cyclotransferase, which projects into the barrel made by the cyclotransferase fold and restricts the access of compounds other than extended aliphatic amines to the active site. This group of investigators further demonstrated that the residues responsible for substrate binding in these enzymes differ significantly, including those amino acids that bind the glutamyl moiety (Oakley et al. 2010). γ-Glutamyltranspeptidase catalyzes transformations of the γ-glutamyl bond in glutathione via nucleophilic attack of the amine group of an amino acid substrate, glutathione itself, or water, to generate a γ-glutamyl amino acid plus Cys-Gly in the first case, γ-glutamyl glutathione plus Cys-Gly in the second case, and finally, glutamate plus Cys-Gly (Tate et al. 1985). In contrast to γ-glutamylamine cyclotransferase and γ-glutamylcyclotransferase where the nucleophilic attack is intramolecular, the nucleophilic attack catalyzed by γ-glutamyltranspeptidase is intermolecular. As noted earlier, transglutaminases can catalyze amide hydrolysis of Q residues of polypeptide substrates and this reaction contributes to the generation of the autoimmune forms of gluten in celiac disease (Molberg et al. 1998, van de Wal et al. 1998, Kim et al. 2002, Pinkas et al. 2007, Stamnaes et al. 2008). Transglutaminases can catalyze the breakage of the γ-glutamyl bond of γ-glutamyl amines in the presence of high concentrations of amines such as ammonia (Folk 1969, Parameswaran et al. 1997). This observation suggests that the amidation reaction shown in Figure 3 may be reversible at high ammonia levels. Elevated concentrations of ammonia are toxic to most cells at especially neurons. For this reason, the levels of ammonia are tightly regulated in the body and particularly so in the brain (Cooper et al. 1987). The low concentration of ammonia - normally less than 0.2 mM in most tissues - ensures that the amidations catalyzed by transglutaminase are essentially irreversible events. It is also possible that free γ-glutamylamines can be enzymatically hydrolyzed by transglutaminases. Studies with transglutaminase 2 demonstrated that this enzyme has a minimum size requirement for binding of a Q-containing substrate. For example, glutamine is not a substrate and the Km for the model transglutaminase 2 substrate carbobenzoxy γ-glutamyl glycine is very high (Folk et al. 1966). Even so, it is possible that free γ-glutamylamines, at sufficiently high concentration, may participate in transglutaminase-catalyzed reactions in which the γ-glutamyl bond is hydrolyzed.
γ-Glutamylamine cyclotransferase is expressed throughout the body with highest levels of activity occurring in the kidneys (Fink et al. 1980). Brain tissue contains 12% of the activity present in kidney on a per wet weight basis (Fink et al. 1980). The relatively low specific activity of cerebral γ-glutamylamine cyclotransferase results in an accumulation of γ-glutamylamines in the brain and cerebrospinal fluid (CSF) (Nakajima et al. 1976, Jeitner et al. 2001, Dedeoglu et al. 2002, Liu 2002, Nemes et al. 2004, Hoffner et al. 2008, Jeitner et al. 2008, Hoffner et al. 2009). Moreover the amounts of these γ-glutamylamines correlate with the increases in transglutaminase activity noted with various neurological disorders (Jeitner et al. 2001, Dedeoglu et al. 2002, Nemes et al. 2004, Jeitner et al. 2008). The greatest increase in γ-glutamylamine levels in a neurodegenerative disease measured thus far was for γ-glutamyl-ε-lysine in the caudate nucleus of HD patients, which increased from ~90 to ~760 pmol/mg protein as compared to the healthy individuals (Dedeoglu et al. 2002). This increase is thought to reflect the formation and proteolysis of soluble aggregates containing γ-glutamyl-ε-lysine bonds. It has been argued that such a process may, after time, fatally congest the proteolytic machinery (Cooper et al. 2002, Nemes et al. 2004). Another possibility is that the aforementioned concentrations of γ-glutamyl-ε-lysine are toxic to neurons. This possibility is supported by studies demonstrating the toxicity of γ-glutamyl-ε-lysine to retinoic acid-differentiated SH SY5Y cells (Jeitner, in preparation).
The concentration of γ-glutamyl-ε-lysine or other γ-glutamylamines in peripheral tissues is not known and excludes the possibility of discussing the selective death of neurons in neurodegenerative diseases as a function of γ-glutamylamine content. Some inferences, however, can be made based on our observations of the concentration of γ-glutamylamine in body fluids. CSF contains γ-glutamyl-ε-lysine, γ-glutamylspermidine, γ-glutamylputrescine and bis-γ-glutamylputrescine whereas blood contains γ-glutamyl-εlysine and γ-glutamylspermidine but not γ-glutamylputrescine or bis-γ-glutamylputrescine (Jeitner et al. 2008). This disparity in composition suggests that γ-glutamylamines leave the brain primarily as constituents of the CSF. The lack of γ-glutamylputrescine and bis-γ-glutamylputrescine in blood is not due a lack of synthesis as these compounds are made in skin (Folk et al. 1977, Hennings et al. 1981, Beninati et al. 1988, Piacentini et al. 1988, Martinet et al. 1990). It also unlikely that γ-glutamylputrescine and bis-γ-glutamylputrescine are preferentially cleared from the circulation by renal γ-glutamylamine cyclotransferase activity since this enzyme hydrolyzes γ-glutamylputrescine, γ-glutamyl-ε-lysine or γ-glutamylspermidine at approximately the same rate (~30 mmol/hr/mg) (Fink et al. 1980). γ-Glutamyl-ε-lysine, γ-glutamylspermidine, γ-glutamylputrescine and bis-γ-glutamylputrescine are present in urine (Jeitner et al. 2008) and points to the possibility of specific transporters for the renal clearance of these compounds. These observations suggest the existence of either multiple transporters to transfer γ-glutamylamines to the urine or a single transporter with varying selectivity for the γ-glutamylamines. The identity of these transporters or those regulating the export of γ-glutamylamines from cells is unknown.
CSF and blood contain ~160 and ~860 nM amounts of γ-glutamyl-ε-lysine, respectively (Jeitner et al. 2008). The amount of γ-glutamyl-ε-lysine in blood significantly exceeds that of CSF and indicates that this isodipeptide is actively made by tissues in the periphery and transported to blood for eventual clearance by the kidneys. The existence of γ-glutamylamine cyclotransferase and of clearance mechanisms suggest that the intracellular accumulation of γ-glutamylamines is regulated to a certain threshold concentration above which cellular metabolism may be compromised. This suggestion is supported by the observation that free γ-glutamyl-ε-lysine compromises the viability of differentiated SH SY5Y cells. As in the case of γ-glutamyl-ε-lysine the concentration of γ-glutamylspermidine in the blood significantly exceeds that of CSF. The concentration of γ-glutamylspermidine in blood is 41.0 μM but only 0.7 μM in CSF (Jeitner et al. 2008). This difference coupled with the dissimilarity in γ-glutamylamine composition in blood and CSF (preponderance of γ-glutamyl-ε-lysine and γ-glutamylspermidine versus γ-glutamylspermidine, γ-glutamylputrescine and bis-γ-glutamylputrescine, respectively) argues for a unique metabolism of these compounds in the brain. The difference is unlikely to be due to the activities of γ-glutamylamine cyclotransferase or transglutaminases in the brain as compared to other organs. For example, the specific activity of γ-glutamylamine cyclotransferase in brain is 12% that of the kidneys. This value is on par with the specific activities found in liver (11%), intestine (13%), thyroid (15%) and the adrenal gland (11%) (Fink et al. 1980). The lower amounts of γ-glutamyl-ε-lysine (160 vs. 860 nM) and γ-glutamylspermidine (0.7 vs 41 μM) in CSF relative to blood suggests that these species are rapidly cleared from the brain and CSF. These lower values of γ-glutamylamines in brain also hint that neurons therein may be especially sensitive to excessive concentrations of these molecules.
Conclusions
The observation that γ-glutamylamines are differentially distributed among the various body fluids and tissue suggests that levels of these compounds are regulated in the body. This regulation implies the existence of transport mechanisms for the cellular elimination of γ-glutamylamines. γ-Glutamylamine cyclotransferase is likely to contribute to the maintenance of γ-glutamylamines within certain limits. Since 5-oxo-L-proline is produced as a result of this activity, it is also possible that γ-glutamylamine cyclotransferase acts primarily to provide this important metabolite. Our ability to distinguish between these possibilities is hampered by the current paucity of knowledge concerning γ-glutamylamine cyclotransferase and the metabolism of γ-glutamylamines in general. These considerations and the reports of increased γ-glutamylamines levels in the major neurodegenerative disorders suggest that these metabolites may more indicate about these pathologies than the activation of transglutaminases per se. One important possibility is that the ratio of γ-glutamyl-ε-lysine to γ-glutamylpolyamines may be an important index of the solubility and toxicity of proteins crosslinked by transglutaminases in neurodegenerative disorders. Given this and the other possibilities discussed in this monograph, studies of the metabolism and toxicity of γ-glutamylamines promise to yield important insights into neurodegenerative processes.
Acknowledgments
The authors would like to acknowledge the pertinent comments of Dr. J. Keillor as regards the reversibility of the reactions catalyzed by transglutaminases.
Footnotes
This is probably not the case for sporadic inclusion myosis. The increase in transglutaminase activity observed in this disease is accompanied by remarkable accumulation of protein crosslinked with γ-glutamyl-ε-lysine linkages, in the periphery, which is thought to contribute significantly to the myosis (Choi et al. 2000).
Dedication
This review is dedicated to the memory of the late Dr. Jack Folk. We are indebted to Jack for his willingness to synthesize for us several of the γ-glutamylpolyamines mentioned in the text. Without his help our studies on the levels of γ-glutamylpolyamines in CSF would not have been possible. Jack was always courteous and helpful. With his passing the field has lost a superb chemist and biochemist.
Contributor Information
Thomas M. Jeitner, Email: tjeitner@winthrop.org, Neurosciences, Biomedical Research Core, Winthrop University Hospital, 222 Station Plaza North, Mineola.
Kevin Battaile, Email: battaile@anl.gov, IMCA-CAT, Advanced Photon Source, Argonne National Lab, 9700 S. Cass Ave, Bldg 435A, Argonne, IL 60439.
Arthur J.L. Cooper, Email: arthur_cooper@nymc.edu, Department of Biochemistry and Molecular Biology, New York Medical College, 15 Dana Road, Valhalla, NY 10595
Bibliography
- Abe T, Chung SI, DiAugustine RP, Folk JE. Rabbit liver transglutaminase: physical, chemical, and catalytic properties. Biochemistry. 1977;16:5495–5501. doi: 10.1021/bi00644a016. [DOI] [PubMed] [Google Scholar]
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- Beninati S, Martinet N, Folk JE. High-performance liquid chromatographic method for the determination of ε-(γ-glutamyl)lysine and mono- and bis-γ-glutamyl derivatives of putrescine and spermidine. J Chromatogr. 1988;443:329–335. doi: 10.1016/s0021-9673(00)94804-0. [DOI] [PubMed] [Google Scholar]
- Beninati S, Piacentini M, Cocuzzi ET, Autuori F, Folk JE. Covalent incorporation of polyamines as γ-glutamyl derivatives into CHO cell protein. Biochim Biophys Acta. 1988;952:325–333. doi: 10.1016/0167-4838(88)90134-3. [DOI] [PubMed] [Google Scholar]
- Bisaglia M, Mammi S, Bubacco L. Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with α-synuclein. J Biol Chem. 2007;282:15597–15605. doi: 10.1074/jbc.M610893200. [DOI] [PubMed] [Google Scholar]
- Bisaglia M, Tosatto L, Munari F, Tessari I, de Laureto PP, Mammi S, Bubacco L. Dopamine quinones interact with alpha-synuclein to form unstructured adducts. Biochem Biophys Res Commun. 2010;394:424–428. doi: 10.1016/j.bbrc.2010.03.044. [DOI] [PubMed] [Google Scholar]
- Caccamo D, Curro M, Ientile R. Potential of transglutaminase 2 as a therapeutic target. Expert Opin Ther Targets. 2010;14:989–1003. doi: 10.1517/14728222.2010.510134. [DOI] [PubMed] [Google Scholar]
- Chen S-E. Modeling, design, and development of potential inhibitors of γ-glutamylamine cyclotransferase and inhibitors of cruzain as therapeutic agents for Chagas’ disease. Chemistry and Biochemistry PhD. 2008:392. [Google Scholar]
- Choi YC, Park GT, Kim TS, Sunwoo IN, Steinert PM, Kim SY. Sporadic inclusion body myositis correlates with increased expression and cross-linking by transglutaminases 1 and 2. J Biol Chem. 2000;275:8703–8710. doi: 10.1074/jbc.275.12.8703. [DOI] [PubMed] [Google Scholar]
- Chung SI, Folk JE. Transglutaminase from hair follicle of guinea pig (crosslinking-fibrin-glutamyllysine-isoenzymes-purified enzyme) Proc Natl Acad Sci U S A. 1972;69:303–307. doi: 10.1073/pnas.69.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron BA, SantaCruz KS, Davies PJ, Festoff BW. Intron-exon swapping of transglutaminase mRNA and neuronal Tau aggregation in Alzheimer’s disease. J Biol Chem. 2001;276:3295–3301. doi: 10.1074/jbc.M004776200. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Jeitner TM, Gentile V, Blass JP. Cross linking of polyglutamine domains catalyzed by tissue transglutaminase is greatly favored with pathological-length repeats: does transglutaminase activity play a role in (CAG)n/Qn-expansion diseases? Neurochem Int. 2002;40:53–67. doi: 10.1016/s0197-0186(01)00058-4. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Plum F. Biochemistry and physiology of brain ammonia. Physiol Rev. 1987;67:440–519. doi: 10.1152/physrev.1987.67.2.440. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Sheu KF, Burke JR, Onodera O, Strittmatter WJ, Roses AD, Blass JP. Polyglutamine domains are substrates of tissue transglutaminase: does transglutaminase play a role in expanded CAG/poly-Q neurodegenerative diseases? J Neurochem. 1997;69:431–434. doi: 10.1046/j.1471-4159.1997.69010431.x. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Sheu KR, Burke JR, Onodera O, Strittmatter WJ, Roses AD, Blass JP. Transglutaminase-catalyzed inactivation of glyceraldehyde 3-phosphate dehydrogenase and α-ketoglutarate dehydrogenase complex by polyglutamine domains of pathological length. Proc Natl Acad Sci U S A. 1997;94:12604–12609. doi: 10.1073/pnas.94.23.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danson JW, Trawick ML, Cooper AJL. Spectrophotometric assays for L-lysine α-oxidase and γ-glutamylamine cyclotransferase. Anal Biochem. 2002;303:120–130. doi: 10.1006/abio.2002.5587. [DOI] [PubMed] [Google Scholar]
- Dedeoglu A, Kubilus JK, Jeitner TM, Matson SA, Bogdanov M, Kowall NW, Matson WR, Cooper AJL, Ratan RR, Beal MF, Hersch SM, Ferrante RJ. Therapeutic effects of cystamine in a murine model of Huntington’s disease. J Neurosci. 2002;22:8942–8950. doi: 10.1523/JNEUROSCI.22-20-08942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano M, Bergamasco B, Lopiano L. Is neuromelanin changed in Parkinson’s disease? Investigations by magnetic spectroscopies. J Neural Transm. 2006;113:769–774. doi: 10.1007/s00702-005-0448-4. [DOI] [PubMed] [Google Scholar]
- Faucheux BA, Martin ME, Beaumont C, Hauw JJ, Agid Y, Hirsch EC. Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson’s disease. J Neurochem. 2003;86:1142–1148. doi: 10.1046/j.1471-4159.2003.01923.x. [DOI] [PubMed] [Google Scholar]
- Fedorow H, Halliday GM, Rickert CH, Gerlach M, Riederer P, Double KL. Evidence for specific phases in the development of human neuromelanin. Neurobiol Aging. 2006;27:506–512. doi: 10.1016/j.neurobiolaging.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Fésüs L, Szucs EF, Barrett KE, Metcalfe DD, Folk JE. Activation of transglutaminase and production of protein-bound γ-glutamylhistamine in stimulated mouse mast cells. J Biol Chem. 1985;260:13771–13778. [PubMed] [Google Scholar]
- Fink ML, Chung SI, Folk JE. γ-Glutamylamine cyclotransferase: specificity toward epsilon-(L-gamma-glutamyl)-L-lysine and related compounds. Proc Natl Acad Sci U S A. 1980;77:4564–4568. doi: 10.1073/pnas.77.8.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink ML, Folk JE. γ-Glutamylamine cyclotransferase. An enzyme involved in the catabolism of ε-(γ-glutamyl)lysine and other γ-glutamylamines. Mol Cell Biochem. 1981;38(Spec No):59–67. doi: 10.1007/BF00235688. [DOI] [PubMed] [Google Scholar]
- Fink ML, Folk JE. γ-Glutamylamine cyclotransferase (rabbit kidney) Methods Enzymol. 1983;94:347–351. doi: 10.1016/s0076-6879(83)94063-6. [DOI] [PubMed] [Google Scholar]
- Folk JE. Mechanism of action of guinea pig liver transglutaminase. VI. Order of substrate addition. J Biol Chem. 1969;244:3707–3713. [PubMed] [Google Scholar]
- Folk JE. Mechanism and basis for specificity of transglutaminase-catalyzed ε-(γ-glutamyl) lysine bond formation. Adv Enzymol Relat Areas Mol Biol. 1983;54:1–56. doi: 10.1002/9780470122990.ch1. [DOI] [PubMed] [Google Scholar]
- Folk JE, Cole PW. Transglutaminase: mechanistic features of the active site as detkermined by kinetic and inhibitor studies. Biochim Biophys Acta. 1966;122:244–264. doi: 10.1016/0926-6593(66)90066-x. [DOI] [PubMed] [Google Scholar]
- Folk JE, Finlayson JS. The ε-(γ-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- Folk JE, Park MH, Chung SI, Schrode J, Lester EP, Cooper HL. Polyamines as physiological substrates for transglutaminases. J Biol Chem. 1980;255:3695–3700. [PubMed] [Google Scholar]
- Gentile V, Sepe C, Calvani M, Melone MA, Cotrufo R, Cooper AJL, Blass JP, Peluso G. Tissue transglutaminase-catalyzed formation of high-molecular-weight aggregates in vitro is favored with long polyglutamine domains: a possible mechanism contributing to CAG-triplet diseases. Arch Biochem Biophys. 1998;352:314–321. doi: 10.1006/abbi.1998.0592. [DOI] [PubMed] [Google Scholar]
- Gibb WR. Melanin, tyrosine hydroxylase, calbindin and substance P in the human midbrain and substantia nigra in relation to nigrostriatal projections and differential neuronal susceptibility in Parkinson’s disease. Brain Res. 1992;581:283–291. doi: 10.1016/0006-8993(92)90719-p. [DOI] [PubMed] [Google Scholar]
- Good PF, Olanow CW, Perl DP. Neuromelanin-containing neurons of the substantia nigra accumulate iron and aluminum in Parkinson’s disease: a LAMMA study. Brain Res. 1992;593:343–346. doi: 10.1016/0006-8993(92)91334-b. [DOI] [PubMed] [Google Scholar]
- Green H. Human genetic diseases due to codon reiteration: relationship to an evolutionary mechanism. Cell. 1993;74:955–956. doi: 10.1016/0092-8674(93)90718-6. [DOI] [PubMed] [Google Scholar]
- Grenard P, Bates MK, Aeschlimann D. Evolution of transglutaminase genes: identification of a transglutaminase gene cluster on human chromosome 15q15. Structure of the gene encoding transglutaminase X and a novel gene family member, transglutaminase Z. J Biol Chem. 2001;276:33066–33078. doi: 10.1074/jbc.M102553200. [DOI] [PubMed] [Google Scholar]
- Gusella JF, MacDonald ME, Ambrose CM, Duyao MP. Molecular genetics of Huntington’s disease. Arch Neurol. 1993;50:1157–1163. doi: 10.1001/archneur.1993.00540110037003. [DOI] [PubMed] [Google Scholar]
- Hadjivassiliou M, Aeschlimann P, Strigun A, Sanders DS, Woodroofe N, Aeschlimann D. Autoantibodies in gluten ataxia recognize a novel neuronal transglutaminase. Ann Neurol. 2008;64:332–343. doi: 10.1002/ana.21450. [DOI] [PubMed] [Google Scholar]
- Hennings H, Steinert P, Buxman MM. Calcium induction of transglutaminase and the formation of ε(γ-glutamyl) lysine cross-links in cultured mouse epidermal cells. Biochem Biophys Res Commun. 1981;102:739–745. doi: 10.1016/s0006-291x(81)80194-5. [DOI] [PubMed] [Google Scholar]
- Herman ML, Farasat S, Steinbach PJ, Wei MH, Toure O, Fleckman P, Blake P, Bale SJ, Toro JR. Transglutaminase-1 gene mutations in autosomal recessive congenital ichthyosis: summary of mutations (including 23 novel) and modeling of TGase-1. Hum Mutat. 2009;30:537–547. doi: 10.1002/humu.20952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffner G, Andre W, Vanhoutteghem A, Soues S, Djian P. Transglutaminase-catalyzed crosslinking in neurological disease: from experimental evidence to therapeutic inhibition CNS. Neurol Disord Drug Targets. 2010;9:217–231. doi: 10.2174/187152710791012107. [DOI] [PubMed] [Google Scholar]
- Hoffner G, Hoppilliard Y, van der Rest G, Dansette P, Djian P, Ohanessian G. [Nε-(γ-glutamyl) lysine] as a potential biomarker in neurological diseases: new detection method and fragmentation pathways. J Mass Spectrom. 2008;43:456–469. doi: 10.1002/jms.1331. [DOI] [PubMed] [Google Scholar]
- Hoffner G, van der Rest G, Dansette PM, Djian P. The end product of transglutaminase crosslinking: simultaneous quantitation of [Nε-(γ-glutamyl) lysine] and lysine by HPLC-MS3. Anal Biochem. 2009;384:296–304. doi: 10.1016/j.ab.2008.09.039. [DOI] [PubMed] [Google Scholar]
- Hoogeveen AT, Willemsen R, Meyer N, de Rooij KE, Roos RA, van Ommen GJ, Galjaard H. Characterization and localization of the Huntington disease gene product. Hum Mol Genet. 1993;2:2069–2073. doi: 10.1093/hmg/2.12.2069. [DOI] [PubMed] [Google Scholar]
- Iismaa SE, Holman S, Wouters MA, Lorand L, Graham RM, Husain A. Evolutionary specialization of a tryptophan indole group for transition-state stabilization by eukaryotic transglutaminases. Proc Natl Acad Sci U S A. 2003;100:12636–12641. doi: 10.1073/pnas.1635052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iismaa SE, Mearns BM, Lorand L, Graham RM. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev. 2009;89:991–1023. doi: 10.1152/physrev.00044.2008. [DOI] [PubMed] [Google Scholar]
- Jeitner TM, Bogdanov MB, Matson WR, Daikhin Y, Yudkoff M, Folk JE, Steinman L, Browne SE, Beal MF, Blass JP, Cooper AJL. Nε-(γ-L-glutamyl)-L-lysine (GGEL) is increased in cerebrospinal fluid of patients with Huntington’s disease. J Neurochem. 2001;79:1109–1112. doi: 10.1046/j.1471-4159.2001.00673.x. [DOI] [PubMed] [Google Scholar]
- Jeitner TM, Matson WR, Folk JE, Blass JP, Cooper AJL. Increased levels of γ-glutamylamines in Huntington disease CSF. J Neurochem. 2008;106:37–44. doi: 10.1111/j.1471-4159.2008.05350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeitner TM, Muma NA, Battaile KP, Cooper AJL. Transglutaminase activation in neurodegenerative diseases. Future Neurol. 2009;4:449–467. doi: 10.2217/fnl.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeitner TM, Pinto JT, Krasnikov BF, Horswill M, Cooper AJL. Transglutaminases and neurodegeneration. J Neurochem. 2009;109(Suppl 1):160–166. doi: 10.1111/j.1471-4159.2009.05843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger K, Kienzl E, Rumpelmair G, Riederer P, Stachelberger H, Ben-Shachar D, Youdim MB. Iron-melanin complex in substantia nigra of parkinsonian brains: an x-ray microanalysis. J Neurochem. 1992;59:1168–1171. doi: 10.1111/j.1471-4159.1992.tb08362.x. [DOI] [PubMed] [Google Scholar]
- Junn E, Ronchetti RD, Quezado MM, Kim SY, Mouradian MM. Tissue transglutaminase-induced aggregation of α-synuclein: Implications for Lewy body formation in Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A. 2003;100:2047–2052. doi: 10.1073/pnas.0438021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlem P, Green H, Djian P. Transglutaminase action imitates Huntington’s disease: selective polymerization of Huntingtin containing expanded polyglutamine. Mol Cell. 1998;1:595–601. doi: 10.1016/s1097-2765(00)80059-3. [DOI] [PubMed] [Google Scholar]
- Karpuj MV, Becher MW, Springer JE, Chabas D, Youssef S, Pedotti R, Mitchell D, Steinman L. Prolonged survival and decreased abnormal movements in transgenic model of Huntington disease, with administration of the transglutaminase inhibitor cystamine. Nat Med. 2002;8:143–149. doi: 10.1038/nm0202-143. [DOI] [PubMed] [Google Scholar]
- Kastner A, Hirsch EC, Lejeune O, Javoy-Agid F, Rascol O, Agid Y. Is the vulnerability of neurons in the substantia nigra of patients with Parkinson’s disease related to their neuromelanin content? J Neurochem. 1992;59:1080–1089. doi: 10.1111/j.1471-4159.1992.tb08350.x. [DOI] [PubMed] [Google Scholar]
- Kim SY, Grant P, Lee JH, Pant HC, Steinert PM. Differential expression of multiple transglutaminases in human brain. Increased expression and cross-linking by transglutaminases 1 and 2 in Alzheimer’s disease. J Biol Chem. 1999;274:30715–30721. doi: 10.1074/jbc.274.43.30715. [DOI] [PubMed] [Google Scholar]
- Kim SY, Jeitner TM, Steinert PM. Transglutaminases in disease. Neurochem Int. 2002;40:85–103. doi: 10.1016/s0197-0186(01)00064-x. [DOI] [PubMed] [Google Scholar]
- Konno T, Morii T, Shimizu H, Oiki S, Ikura K. Paradoxical inhibition of protein aggregation and precipitation by transglutaminase-catalyzed intermolecular cross-linking. J Biol Chem. 2005;280:17520–17525. doi: 10.1074/jbc.M413988200. [DOI] [PubMed] [Google Scholar]
- Kuemmerle S, Gutekunst CA, Klein AM, Li XJ, Li SH, Beal MF, Hersch SM, Ferrante RJ. Huntington aggregates may not predict neuronal death in Huntington’s disease. Ann Neurol. 1999;46:842–849. [PubMed] [Google Scholar]
- Lai TS, Tucker T, Burke JR, Strittmatter WJ, Greenberg CS. Effect of tissue transglutaminase on the solubility of proteins containing expanded polyglutamine repeats. J Neurochem. 2004;88:1253–1260. doi: 10.1046/j.1471-4159.2003.02249.x. [DOI] [PubMed] [Google Scholar]
- Lesort M, Chun W, Johnson GV, Ferrante RJ. Tissue transglutaminase is increased in Huntington’s disease brain. J Neurochem. 1999;73:2018–2027. [PubMed] [Google Scholar]
- Liu P, Gupta N, Jing Y, Zhang H. Age-related changes in polyamines in memory-associated brain structures in rats. Neuroscience. 2008;155:789–796. doi: 10.1016/j.neuroscience.2008.06.033. [DOI] [PubMed] [Google Scholar]
- Liu RM. Down-regulation of γ-glutamylcysteine synthetase regulatory subunit gene expression in rat brain tissue during aging. J Neurosci Res. 2002;68:344–351. doi: 10.1002/jnr.10217. [DOI] [PubMed] [Google Scholar]
- Lopiano L, Chiesa M, Digilio G, Giraudo S, Bergamasco B, Torre E, Fasano M. Q-band EPR investigations of neuromelanin in control and Parkinson’s disease patients. Biochim Biophys Acta. 2000;1500:306–312. doi: 10.1016/s0925-4439(99)00116-7. [DOI] [PubMed] [Google Scholar]
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Martinet N, Beninati S, Nigra TP, Folk JE. N1N8-bis(γ-glutamyl)spermidine cross-linking in epidermal-cell envelopes. Comparison of cross-link levels in normal and psoriatic cell envelopes. Biochem J. 1990;271:305–308. doi: 10.1042/bj2710305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroberardino PG, Iannicola C, Nardacci R, Bernassola F, De Laurenzi V, Melino G, Moreno S, Pavone F, Oliverio S, Fésüs L, Piacentini M. ‘Tissue’ transglutaminase ablation reduces neuronal death and prolongs survival in a mouse model of Huntington’s disease. Cell Death Differ. 2002;9:873–880. doi: 10.1038/sj.cdd.4401093. [DOI] [PubMed] [Google Scholar]
- McNicholas S, Potterton E, Wilson KS, Noble ME. Presenting your structures: the CCP4mg molecular-graphics software. Acta Crystallogr D Biol Crystallogr. 2011;67:386–394. doi: 10.1107/S0907444911007281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. γ-Glutamylcyclotransferase from rat kidney. Methods Enzymol. 1985;113:438–445. doi: 10.1016/s0076-6879(85)13055-7. [DOI] [PubMed] [Google Scholar]
- Molberg O, McAdam SN, Korner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Noren O, Roepstorff P, Lundin KE, Sjostrom H, Sollid LM. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;4:713–717. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Kakimoto Y, Tsuji M, Konishi H. Occurrence and formation of γ-glutamylputrescine in mammalian brain. J Neurochem. 1976;26:115–118. [PubMed] [Google Scholar]
- Nemes Z, Devreese B, Steinert PM, Van Beeumen J, Fésüs L. Cross-linking of ubiquitin, HSP27, parkin, and α-synuclein by γ-glutamyl-ε-lysine bonds in Alzheimer’s neurofibrillary tangles. FASEB J. 2004;18:1135–1137. doi: 10.1096/fj.04-1493fje. [DOI] [PubMed] [Google Scholar]
- Nemes Z, Marekov LN, Fésüs L, Steinert PM. A novel function for transglutaminase 1: attachment of long-chain omega-hydroxyceramides to involucrin by ester bond formation. Proc Natl Acad Sci U S A. 1999;96:8402–8407. doi: 10.1073/pnas.96.15.8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemes Z, Petrovski G, Aerts M, Sergeant K, Devreese B, Fésüs L. Transglutaminase-mediated intramolecular cross-linking of membrane-bound α-synuclein promotes amyloid formation in Lewy bodies. J Biol Chem. 2009;284:27252–27264. doi: 10.1074/jbc.M109.033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AJ, Coggan M, Board PG. Identification and characterization of γ-glutamylamine cyclotransferase, an enzyme responsible for γ-glutamyl-ε-lysine catabolism. J Biol Chem. 2010;285:9642–9648. doi: 10.1074/jbc.M109.082099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran KN, Cheng XF, Chen EC, Velasco PT, Wilson JH, Lorand L. Hydrolysis of γ:ε isopeptides by cytosolic transglutaminases and by coagulation factor XIIIa. J Biol Chem. 1997;272:10311–10317. doi: 10.1074/jbc.272.15.10311. [DOI] [PubMed] [Google Scholar]
- Perutz MF. Glutamine repeats as polar zippers: their role in inherited neurodegenerative disease. Mol Med. 1995;1:718–721. [PMC free article] [PubMed] [Google Scholar]
- Pham CL, Leong SL, Ali FE, Kenche VB, Hill AF, Gras SL, Barnham KJ, Cappai R. Dopamine and the dopamine oxidation product 5,6-dihydroxylindole promote distinct on-pathway and off-pathway aggregation of alpha-synuclein in a pH-dependent manner. J Mol Biol. 2009;387:771–785. doi: 10.1016/j.jmb.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Piacentini M, Martinet N, Beninati S, Folk JE. Free and protein-conjugated polyamines in mouse epidermal cells. Effect of high calcium and retinoic acid. J Biol Chem. 1988;263:3790–3794. [PubMed] [Google Scholar]
- Pinkas DM, Strop P, Brunger AT, Khosla C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007;5:e327. doi: 10.1371/journal.pbio.0050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricotta M, Iannuzzi M, Vivo GD, Gentile V. Physio-pathological roles of transglutaminase-catalyzed reactions World. J Biol Chem. 2010;1:181–187. doi: 10.4331/wjbc.v1.i5.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Barton DE, Davison BC, Ferguson-Smith MA. Analysis of the huntingtin gene reveals a trinucleotide-length polymorphism in the region of the gene that contains two CCG-rich stretches and a correlation between decreased age of onset of Huntington’s disease and CAG repeat number. Hum Mol Genet. 1993;2:1713–1715. doi: 10.1093/hmg/2.10.1713. [DOI] [PubMed] [Google Scholar]
- Ruoppolo M, Orru S, Francese S, Caputo I, Esposito C. Structural characterization of transglutaminase-catalyzed cross-linking between glyceraldehyde 3-phosphate dehydrogenase and polyglutamine repeats. Protein Sci. 2003;12:170–179. doi: 10.1110/ps.0216103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudou F, Finkbeiner S, Devys D, Greenberg ME. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell. 1998;95:55–66. doi: 10.1016/s0092-8674(00)81782-1. [DOI] [PubMed] [Google Scholar]
- Segers-Nolten IM, Wilhelmus MM, Veldhuis G, van Rooijen BD, Drukarch B, Subramaniam V. Tissue transglutaminase modulates α-synuclein oligomerization. Protein Sci. 2008;17:1395–1402. doi: 10.1110/ps.036103.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, Abraham C, Ihara Y. Brain transglutaminase: in vitro crosslinking of human neurofilament proteins into insoluble polymers. Proc Natl Acad Sci U S A. 1982;79:6070–6074. doi: 10.1073/pnas.79.19.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Pedrini B, Geralt M, Jaudzems K, Mohanty B, Horst R, Herrmann T, Elsliger MA, Wilson IA, Wuthrich K. Comparison of NMR and crystal structures highlights conformational isomerism in protein active sites. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66:1393–1405. doi: 10.1107/S1744309110033658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer SM, Zainelli GM, Norlund MA, Lee JM, Muma NA. Transglutaminase bonds in neurofibrillary tangles and paired helical filament tau early in Alzheimer’s disease. Neurochem Int. 2002;40:17–30. doi: 10.1016/s0197-0186(01)00061-4. [DOI] [PubMed] [Google Scholar]
- Stamnaes J, Fleckenstein B, Sollid LM. The propensity for deamidation and transamidation of peptides by transglutaminase 2 is dependent on substrate affinity and reaction conditions. Biochim Biophys Acta. 2008;1784:1804–1811. doi: 10.1016/j.bbapap.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate SS, Meister A. γ-Glutamyl transpeptidase from kidney. Methods Enzymol. 1985;113:400–419. doi: 10.1016/s0076-6879(85)13053-3. [DOI] [PubMed] [Google Scholar]
- van de Wal Y, Kooy Y, van Veelen P, Pena S, Mearin L, Papadopoulos G, Koning F. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J Immunol. 1998;161:1585–1588. [PubMed] [Google Scholar]
- Verhaar R, Jongenelen CA, Gerard M, Baekelandt V, Van Dam AM, Wilhelmus MM, Drukarch B. Blockade of enzyme activity inhibits tissue transglutaminase-mediated transamidation of α-synuclein in a cellular model of Parkinson’s disease. Neurochem Int. 2011 doi: 10.1016/j.neuint.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Wilhelmus MM, Grunberg SC, Bol JG, van Dam AM, Hoozemans JJ, Rozemuller AJ, Drukarch B. Transglutaminases and transglutaminase-catalyzed cross-links colocalize with the pathological lesions in Alzheimer’s disease brain. Brain Pathol. 2009;19:612–622. doi: 10.1111/j.1750-3639.2008.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmus MM, van Dam AM, Drukarch B. Tissue transglutaminase: a novel pharmacological target in preventing toxic protein aggregation in neurodegenerative diseases. Eur J Pharmacol. 2008;585:464–472. doi: 10.1016/j.ejphar.2008.01.059. [DOI] [PubMed] [Google Scholar]
- Wilhelmus MM, Verhaar R, Andringa G, Bol JG, Cras P, Shan L, Hoozemans JJ, Drukarch B. Presence of tissue transglutaminase in granular endoplasmic reticulum is characteristic of melanized neurons in Parkinson’s disease brain. Brain Pathol. 2011;21:130–139. doi: 10.1111/j.1750-3639.2010.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainelli GM, Dudek NL, Ross CA, Kim SY, Muma NA. Mutant huntingtin protein: a substrate for transglutaminase 1, 2, and 3. J Neuropathol Exp Neurol. 2005;64:58–65. doi: 10.1093/jnen/64.1.58. [DOI] [PubMed] [Google Scholar]
- Zainelli GM, Ross CA, Troncoso JC, Muma NA. Transglutaminase cross-links in intranuclear inclusions in Huntington disease. J Neuropathol Exp Neurol. 2003;62:14–24. doi: 10.1093/jnen/62.1.14. [DOI] [PubMed] [Google Scholar]
- Zecca L, Bellei C, Costi P, Albertini A, Monzani E, Casella L, Gallorini M, Bergamaschi L, Moscatelli A, Turro NJ, Eisner M, Crippa PR, Ito S, Wakamatsu K, Bush WD, Ward WC, Simon JD, Zucca FA. New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. Proc Natl Acad Sci U S A. 2008;105:17567–17572. doi: 10.1073/pnas.0808768105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L, Fariello R, Riederer P, Sulzer D, Gatti A, Tampellini D. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson’s disease. FEBS Lett. 2002;510:216–220. doi: 10.1016/s0014-5793(01)03269-0. [DOI] [PubMed] [Google Scholar]
- Zecca L, Shima T, Stroppolo A, Goj C, Battiston GA, Gerbasi R, Sarna T, Swartz HM. Interaction of neuromelanin and iron in substantia nigra and other areas of human brain. Neuroscience. 1996;73:407–415. doi: 10.1016/0306-4522(96)00047-4. [DOI] [PubMed] [Google Scholar]
- Zemaitaitis MO, Lee JM, Troncoso JC, Muma NA. Transglutaminase-induced cross-linking of tau proteins in progressive supranuclear palsy. J Neuropathol Exp Neurol. 2000;59:983–989. doi: 10.1093/jnen/59.11.983. [DOI] [PubMed] [Google Scholar]
- Zhang W, Johnson BR, Suri DE, Martinez J, Bjornsson TD. Immunohistochemical demonstration of tissue transglutaminase in amyloid plaques. Acta Neuropathol. 1998;96:395–400. doi: 10.1007/s004010050910. [DOI] [PubMed] [Google Scholar]