Abstract
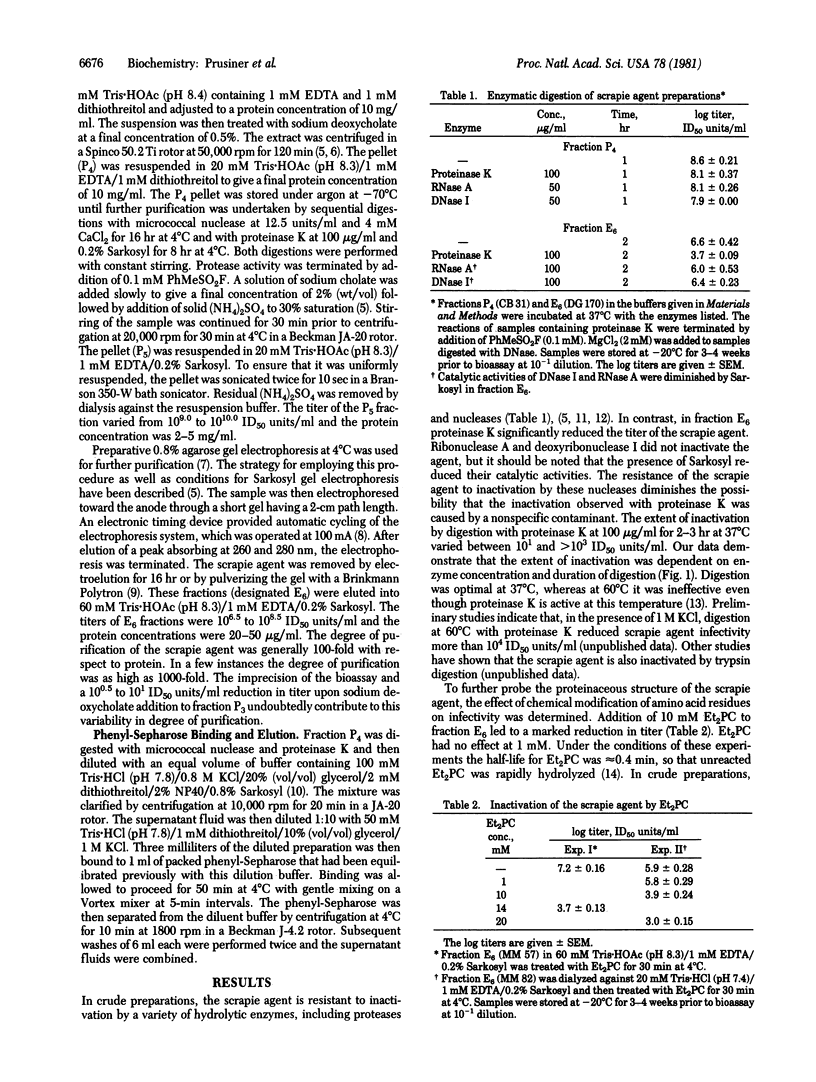
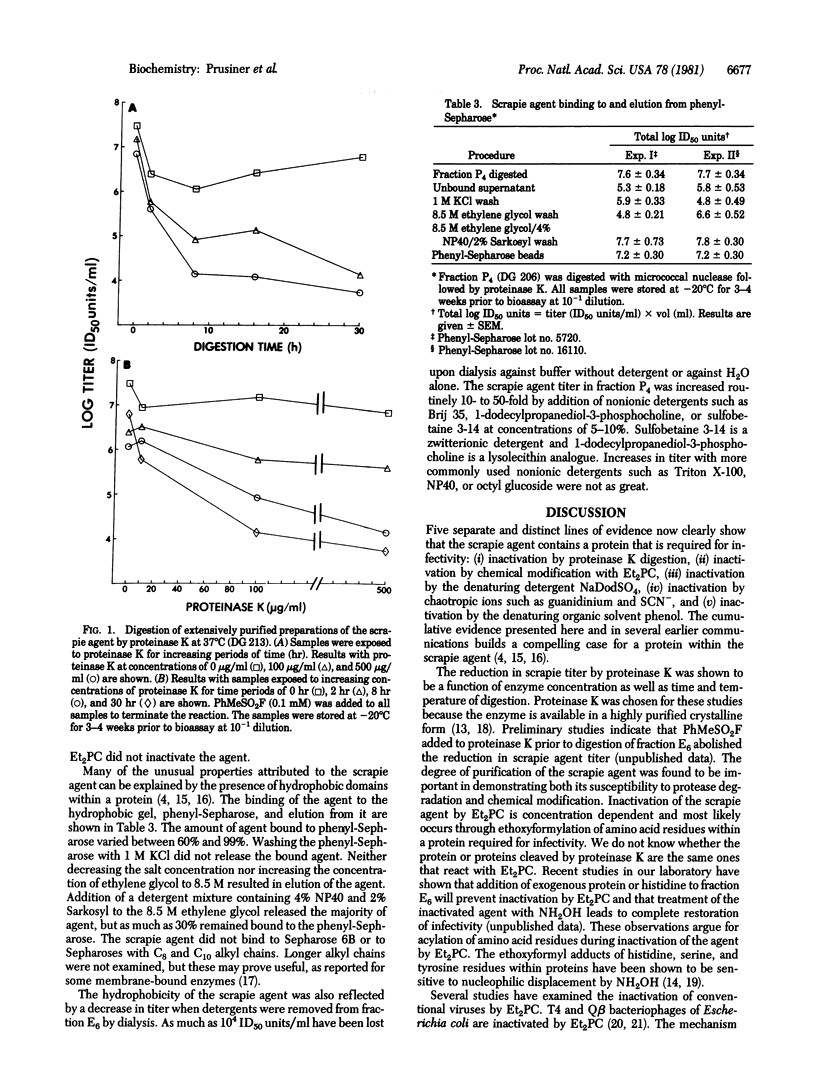
The scrapie agent causes a degenerative nervous system disorder of sheep and goats. Considerable evidence indicates that the scrapie agent contains a protein that is necessary for infectivity [Prusiner, S. B., Groth, D. F., Cochran, S. P., Masiarz, F. R., McKinley, M. P. & Martinez, H. M. (1980) Biochemistry 19, 4883-4891], but direct demonstration of a protein moiety has been hampered by lack of sufficiently purified preparations. Employing preparations of the scrapie agent enriched 100- to 1000-fold with respect to protein, we found that digestion by proteinase K destroyed more than 99.9% of the infectivity. Diethylpyrocarbonate, which chemically modifies amino acid residues in proteins with high efficiency, also inactivated the scrapie agent in these purified preparations. Reductions of infectivity by proteinase K and diethylpyrocarbonate were not observed with less purified preparations. The agent bound to phenyl-Sepharose could not be eluted with 8.5 M ethylene glycol; however, a combination of ethylene glycol and detergents did release the agent. These observations provide good evidence for a protein and for hydrophobic domains within the scrapie agent. Whether the protein required for infectivity is the same protein responsible for the hydrophobic properties of the scrapie agent remains to be established.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper T., Cramp W. A., Haig D. A., Clarke M. C. Does the agent of scrapie replicate without nucleic acid? Nature. 1967 May 20;214(5090):764–766. doi: 10.1038/214764a0. [DOI] [PubMed] [Google Scholar]
- Alper T., Haig D. A., Clarke M. C. The scrapie agent: evidence against its dependence for replication on intrinsic nucleic acid. J Gen Virol. 1978 Dec;41(3):503–516. doi: 10.1099/0022-1317-41-3-503. [DOI] [PubMed] [Google Scholar]
- BAWDEN F. C. Inhibitors and plant viruses. Adv Virus Res. 1954;2:31–57. doi: 10.1016/s0065-3527(08)60528-x. [DOI] [PubMed] [Google Scholar]
- Bartfeld D., Fuchs S. Active acetylcholine receptor fragment obtained by tryptic digestion of acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1979 Jul 27;89(2):512–519. doi: 10.1016/0006-291x(79)90659-4. [DOI] [PubMed] [Google Scholar]
- Carreira L. H., Carlton B. C., Bobbio S. M., Nagao R. T., Meagher R. B. Construction and application of a modified "gene machine": a circular concentrating preparative gel electrophoresis device employing discontinuous elution. Anal Biochem. 1980 Aug;106(2):455–468. doi: 10.1016/0003-2697(80)90548-5. [DOI] [PubMed] [Google Scholar]
- Cho H. J. Requirement of a protein component for scrapie infectivity. Intervirology. 1980;14(3-4):213–216. doi: 10.1159/000149185. [DOI] [PubMed] [Google Scholar]
- DIENER T. O., WEAVER M. L. Reversible and irreversible inhibition of necrotic ringspot virus in cucumbers by pancreatic ribonuclease. Virology. 1959 Apr;7(4):419–427. doi: 10.1016/0042-6822(59)90070-4. [DOI] [PubMed] [Google Scholar]
- Diener T. O., Raymer W. B. Potato spindle tuber virus: a plant virus with properties of a free nucleic acid. II. Characterization and partial purification. Virology. 1969 Mar;37(3):351–366. doi: 10.1016/0042-6822(69)90219-0. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Viroids: structure and function. Science. 1979 Aug 31;205(4409):859–866. doi: 10.1126/science.472709. [DOI] [PubMed] [Google Scholar]
- Ebeling W., Hennrich N., Klockow M., Metz H., Orth H. D., Lang H. Proteinase K from Tritirachium album Limber. Eur J Biochem. 1974 Aug 15;47(1):91–97. doi: 10.1111/j.1432-1033.1974.tb03671.x. [DOI] [PubMed] [Google Scholar]
- Ehrenberg L., Fedorcsak I., Solymosy F. Diethyl pyrocarbonate in nucleic acid research. Prog Nucleic Acid Res Mol Biol. 1976;16:189–262. doi: 10.1016/s0079-6603(08)60758-8. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C. Unconventional viruses and the origin and disappearance of kuru. Science. 1977 Sep 2;197(4307):943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- Gulyás A., Solymosy F. Effect of diethyl pyrocarbonate on the biological activity of intact TMV and TMV-RNA. Acta Biochim Biophys Acad Sci Hung. 1970;5(2):235–238. [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Destabilization of membranes with chaotropic ions. Methods Enzymol. 1974;31:770–790. doi: 10.1016/0076-6879(74)31080-4. [DOI] [PubMed] [Google Scholar]
- Hilz H., Wiegers U., Adamietz P. Stimulation of proteinase K action by denaturing agents: application to the isolation of nucleic acids and the degradation of 'masked' proteins. Eur J Biochem. 1975 Aug 1;56(1):103–108. doi: 10.1111/j.1432-1033.1975.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Homcy C. J., Wrenn S. M., Haber E. Demonstration of the hydrophilic character of adenylate cyclase following hydrophobic resolution on immobilized alkyl residues. Critical role of alkyl chain length. J Biol Chem. 1977 Dec 25;252(24):8957–8964. [PubMed] [Google Scholar]
- Huganir R. L., Racker E. Endogenous and exogenous proteolysis of the acetylcholine receptor from Torpedo californica. J Supramol Struct. 1980;14(1):13–19. doi: 10.1002/jss.400140103. [DOI] [PubMed] [Google Scholar]
- Hunter G. D., Gibbons R. A., Kimberlin R. H., Millson G. C. Further studies of the infectivity and stability of extracts and homogenates derived from scrapie affected mouse brains. J Comp Pathol. 1969 Jan;79(1):101–108. doi: 10.1016/0021-9975(69)90033-4. [DOI] [PubMed] [Google Scholar]
- Hunter G. D., Millson G. C. Attempts to release the scrapie agent from tissue debris. J Comp Pathol. 1967 Jul;77(3):301–307. doi: 10.1016/0021-9975(67)90039-4. [DOI] [PubMed] [Google Scholar]
- Kondorosi A., Fedorcsák I., Solymosy F., Ehrenberg L., Osterman-Golkar S. Inactivation of Q beta RNA by electrophiles. Mutat Res. 1973 Feb;17(2):149–161. doi: 10.1016/0027-5107(73)90162-0. [DOI] [PubMed] [Google Scholar]
- Kondorosi A., Sváb Z., Solymosy F., Fedorcsák I. Effect of diethyl pyrocarbonate on the biological acitivity of deoxyribonucleic acids isolated from bacteriophages. J Gen Virol. 1972 Sep;16(3):373–380. doi: 10.1099/0022-1317-16-3-373. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Gullick W., Conti-Tronconi B., Ellisman M. Proteolytic nicking of the acetylcholine receptor. Biochemistry. 1980 Oct 14;19(21):4791–4795. doi: 10.1021/bi00562a012. [DOI] [PubMed] [Google Scholar]
- Marcus S. L., Smith S. W., Racevskis J., Sarkar N. H. The relative hydrophobicity of oncornaviral structural proteins. Virology. 1978 May 15;86(2):398–412. doi: 10.1016/0042-6822(78)90080-6. [DOI] [PubMed] [Google Scholar]
- McCormick J. J., Larson L. J., Maher V. M. Problems in the extraction DNA when utilizing pancreatic RNAase and pronase. Biochim Biophys Acta. 1974 May 17;349(2):145–147. doi: 10.1016/0005-2787(74)90075-6. [DOI] [PubMed] [Google Scholar]
- Melchior W. B., Jr, Fahrney D. Ethoxyformylation of proteins. Reaction of ethoxyformic anhydride with alpha-chymotrypsin, pepsin, and pancreatic ribonuclease at pH 4. Biochemistry. 1970 Jan 20;9(2):251–258. doi: 10.1021/bi00804a010. [DOI] [PubMed] [Google Scholar]
- Miles E. W. Modification of histidyl residues in proteins by diethylpyrocarbonate. Methods Enzymol. 1977;47:431–442. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- Oberg B. Biochemical and biological characteristics of carbethoxylated poliovirus and viral RNA. Biochim Biophys Acta. 1970 Apr 15;204(2):430–440. [PubMed] [Google Scholar]
- Owens R. A., Erbe E., Hadidi A., Steere R. L., Diener T. O. Separation and infectivity of circular and linear forms of potato spindle tuber viroid. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3859–3863. doi: 10.1073/pnas.74.9.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxelfeldt P., Arstrand K. Effects of diethyl pyrocarbonate on tobacco mosaic virus and its RNA. Biochim Biophys Acta. 1970 Oct 15;217(2):544–547. [PubMed] [Google Scholar]
- Polsky F., Edgell M. H., Seidman J. G., Leder P. High capacity gel preparative electrophoresis for purification of fragments of genomic DNA. Anal Biochem. 1978 Jul 1;87(2):397–410. doi: 10.1016/0003-2697(78)90689-9. [DOI] [PubMed] [Google Scholar]
- Posner I. A new apparatus for the recovery of macromolecules from polyacrylamide gel slabs following preparative vertical gel electrophoresis. Anal Biochem. 1976 May 7;72:491–501. doi: 10.1016/0003-2697(76)90559-5. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Garfin D. E., Cochran S. P., McKinley M. P., Groth D. F., Hadlow W. J., Race R. E., Eklund C. M. Experimental scrapie in the mouse: electrophoretic and sedimentation properties of the partially purified agent. J Neurochem. 1980 Sep;35(3):574–582. doi: 10.1111/j.1471-4159.1980.tb03693.x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Bildstein C., Masiarz F. R., McKinley M. P., Cochran S. P. Electrophoretic properties of the scrapie agent in agarose gels. Proc Natl Acad Sci U S A. 1980 May;77(5):2984–2988. doi: 10.1073/pnas.77.5.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Cochran S. P., Masiarz F. R., McKinley M. P., Martinez H. M. Molecular properties, partial purification, and assay by incubation period measurements of the hamster scrapie agent. Biochemistry. 1980 Oct 14;19(21):4883–4891. doi: 10.1021/bi00562a028. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Cochran S. P., McKinley M. P., Masiarz F. R. Gel electrophoresis and glass permeation chromatography of the hamster scrapie agent after enzymatic digestion and detergent extraction. Biochemistry. 1980 Oct 14;19(21):4892–4898. doi: 10.1021/bi00562a029. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., McKinley M. P., Cochran S. P., Bowman K. A., Kasper K. C. Thiocyanate and hydroxyl ions inactivate the scrapie agent. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4606–4610. doi: 10.1073/pnas.78.7.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B., Hadlow W. J., Eklund C. M., Race R. E., Cochran S. P. Sedimentation characteristics of the scrapie agent from murine spleen and brain. Biochemistry. 1978 Nov 14;17(23):4987–4992. doi: 10.1021/bi00616a020. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Hadlow W. J., Garfin D. E., Cochran S. P., Baringer J. R., Race R. E., Eklund C. M. Partial purification and evidence for multiple molecular forms of the scrapie agent. Biochemistry. 1978 Nov 14;17(23):4993–4999. doi: 10.1021/bi00616a021. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1002–1007. doi: 10.1073/pnas.66.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]


