Abstract
Overexpression of Transforming Growth Factor Beta1 (TGFβ1) in mouse epidermis causes cutaneous inflammation and keratinocyte hyperproliferation. Here, we examined acute effects of TGFβ1 overproduction by keratinocytes on skin dendritic cells (DCs). TGFβ1 induction for 2 and 4 days increased numbers and CD86 expression of B220+ plasmacytoid DCs (pDCs) and CD207+CD103+, CD207−CD103−CD11b+ and CD207−CD103−CD11b− dermal DCs (dDCs) in skin draining lymph nodes (SDLN). The dermis of TGFβ1-overexpressing mice had significantly more pDCs, CD207+CD103+ dDCs and CD207-CD11b+ dDCs in the absence of increased dermal proliferation. Application of dye, TRITC, in dibutylpthalate (DBP) solution after TGFβ1 induction increased the numbers of TRITC+CD207− dDCs in SDLN, and augmented TRITC/DBP-induced Langerhans cell (LC) migration 72 hrs post-TRITC treatment. Consistent with this, LC migration was increased in vitro by TGFβ1 overexpression in skin explants and by exogenous TGFβ1 in culture media. Transient TGFβ1 induction during DNFB sensitization increased contact hypersensitivity responses by 1.5-fold. Thus, elevated epidermal TGFβ1 alone is sufficient to alter homeostasis of multiple cutaneous DC subsets and enhance DC migration and immune responses to contact sensitizers. These results highlight a role for keratinocyte-derived TGFβ1 in DC trafficking and the initiation of skin inflammation.
Keywords: TGFβ1, DC subsets, dermis, skin-draining lymph nodes, contact hypersensitivity
INTRODUCTION
Skin contains a dense network of dendritic cells (DCs) that are initiators of a wide-range of immune responses and act by bridging innate and adaptive immunity while maintaining tissue homeostasis in steady state (Steinman, 1991). Langerhans cells (LC), which are radio-resistant, self-renewing and characterized by the expression of langerin (CD207), are the primary DC subset in the epidermis of healthy skin (Ginhoux and Merad, 2010). The dermis contains DC subsets broadly classified as CD207+CD103+ and CD207−CD103− with the latter subset further subdivided based on CD11b and other markers (Merad et al., 2008;Henri et al., 2010). Skin DCs acquire and process exogenous antigens, undergo maturation and migrate to skin draining lymph nodes (SDLN) where they induce activation of naïve T cells (Banchereau and Steinman, 1998).
Transforming growth factor beta1 (TGFβ1) is one of the major regulators of DC biology in the skin. In vitro studies show that TGFβ1 is important for promoting LC differentiation from a CD34+ pro-monocyte bone marrow precursor (Strobl and Knapp, 1999) while for both LC and other DC, TGFβ1 inhibits activation, maturation and immunogenicity and promotes tolerogenic function (Geissmann et al., 1999; Fainaru et al., 2007; Ohtani et al., 2009; Torres-Aguilar et al., 2010). In vivo studies have demonstrated a critical requirement of both autocrine and paracrine TGFβ1 signaling for LC development and epidermal residency (Borkowski et al., 1996; Borkowski et al., 1997; Kaplan et al., 2007; Kel et al., 2010; Zahner et al., 2011). However, homeostasis and numbers of CD207+CD103+ dermal DC (dDC) appear to be unaffected in mice with a deletion of the TGFβ1 type 1 receptor in all CD207+ dendritic cells (Kel et al., 2010; Zahner et al., 2011) and in Tgfb1−/− mice (Nagao et al., 2009). In contrast, for CD207−CD103− dDCs and for DC that infiltrate the skin under inflammatory states, there is little information on the in vivo role of TGFβ1.
Significant increases in epidermal and skin TGFβ1 levels occur in response to inflammatory stimuli (Akhurst et al., 1988), following wounding (Kane et al., 1991; Levine et al., 1993; Wang et al., 2006), in chronic skin diseases such as psoriasis (Kane et al., 1990; Flisiak et al., 2002; Flisiak et al., 2003) and in premalignant keratinocytes (Glick et al., 1991). Previous studies have shown that long-term overexpression of active or latent TGFβ1 in mouse epidermis causes a chronic inflammatory phenotype associated with keratinocyte hyperproliferation and T cell infiltration (Liu et al., 2001; Li et al., 2004; Han et al., 2010). However, recent studies have suggested that the TGFβ1-induced inflammation is not solely dependent on T cells (Michaelis et al., 2010), or the IL17/IL23 axis (Fitch et al., 2009), indicating involvement of additional pathways or immune cells.
We previously showed that induction of TGFβ1 in papillomas caused a rapid increase in tumor-infiltrating macrophages and dendritic cells and an increase in the numbers of CD11c+ and CD11b+ cells in skin draining lymph nodes (Mohammed et al., 2010), suggesting that tissue inflammation and migration of DCs may be a primary response to elevated TGFβ1 levels. Here we show that induction of active TGFβ1 in the basal layer of mouse epidermis causes significant and rapid changes in cutaneous dendritic cell migration and influx and enhances contact hypersensitivity responses. These results provide insight into the role of keratinocyte-derived TGFβ1 in skin DC homeostasis and in the initiation of skin inflammation.
RESULTS
Elevated Keratinocyte TGFβ1 increases DC numbers in SDLN
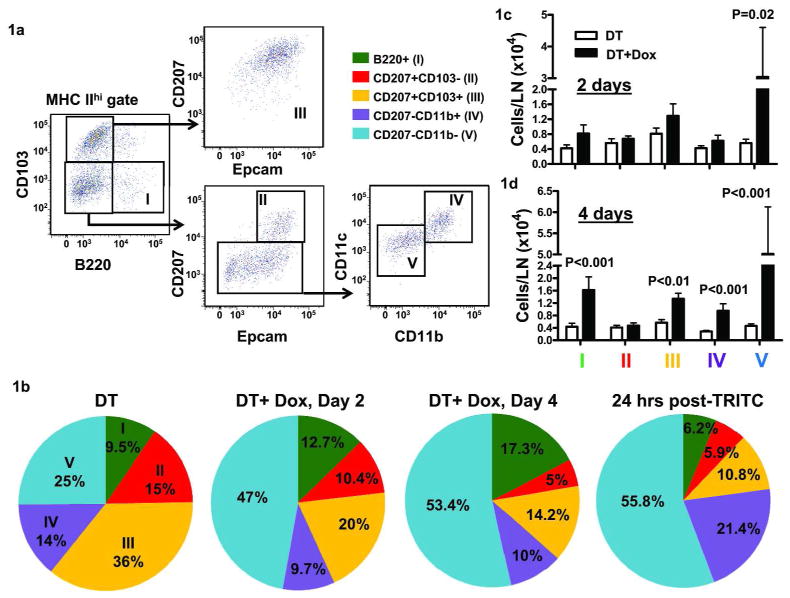
To determine the effect of elevated epidermal TGFβ1 levels on cutaneous DC populations, we placed 7-week old DT (K14rTAxtetOTGFβ1) mice on doxycycline chow to induce active TGFβ1 in keratinocytes and then analyzed migratory DC subset accumulation in the SDLN. We gated on MHCIIhi LN cells to identify DCs migrating from the skin and excluded any LN resident CD8+CD103+CD207+ DC that are MHCIIint (Dakic et al., 2004; Kissenpfennig et al., 2005). We identified five skin derived DC subsets in the SDLN of FVB/n mice (Figure 1a) similar to those in C57BL/6 mice (Henri et al., 2010): B220+ pDCs, CD207+CD103− (LC), CD207+CD103+ dermal DCs (dDCs) and CD207−CD103− dDCs which are either CD11b+ or CD11b− (referred as CD207−CD11b+ dDCs and CD207−CD11b− dDCs, respectively). At steady state, CD207+ dDCs constituted the highest percentage of DCs in the LN (36%). Two days after induction of TGFβ1 expression in keratinocytes, there was a significant expansion of the CD207−CD11b− subset relative to the other DCs, and this persisted through 4 days (Figure 1b). The percentage of pDC also increased after 4 days of TGFβ1 induction. The alteration in skin-derived DC percentages in SDLN at 2 and 4 days after induction was similar to TRITC+ DC subsets 24 hrs post-TRITC/Dibutylpthalate (DBP) application (Figure 1b), confirming their skin origin and suggesting that effects of TGFβ1 overexpression and the irritant DBP on skin CD11b− dDCs were similar. There was also a 4–5 fold increase in absolute numbers of CD207−CD11b− DCs at 2 days which increased to 11-fold relative to steady state at 4 days, a 2-fold increase in number of CD207+CD103+ dDCs, a 3-fold increase in CD207−CD11b+ DC and a 4-fold increase in B220+ pDC in the SDLN at 4 days post-TGFβ1 induction (Figure 1c and d). Thus, TGFβ1 appears to mobilize primarily CD207−CD11b− dDCs but not LC although other DC subsets are significantly affected.
Figure 1. Elevated keratinocyte TGFβ1 alters migratory DC percentages and numbers.
DT mice were given regular or doxycycline (Dox) chow (1 gm/kg) for 2 or 4 days and SDLN harvested for immunophenotypic analysis of MHCIIhi cells. (a) Gating strategy to identify B220+ pDCs, LC, CD207+ and CD207- dDC subsets. (b) Analysis of five DC subsets identified in (a) for their percentages following 2 and 4 days of dox treatment. Additionally, TRITC+ DC percentages were analyzed 24 hrs following TRITC painting in 1:1 solution of acetone and DBP. (c) Analysis of five DC subsets identified in (a) for their numbers following 2 and 4 days of dox treatment. (N=4–8, repeated at least twice with similar results). Error bars = +/− SEM.
Keratinocyte TGFβ1 causes selective influx and proliferation of DCs in the dermis
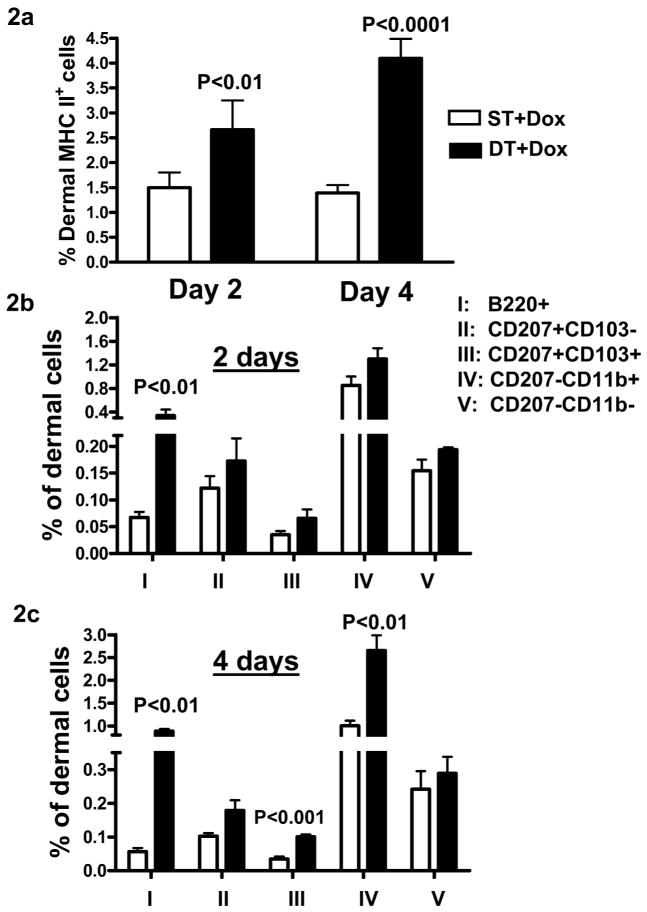
To further examine TGFβ1 induced changes in cutaneous DC homeostasis we analyzed the percentage of MHCII+ cells in the dermis. Two days post-TGFβ1 induction there was a significant increase in the frequency of MHCII+ cells in the dermis that remained high through 4 days (Figure 2a). The increase in MHCII+ cells was primarily due to an increase in B220+ pDCs (Figure 2b and c). Additionally, 4 days after TGFβ1 induction, there was a 2.5-fold higher percentage of CD207+103+ and CD207−CD11b+ dDCs (Figure 2c).
Figure 2. Increase in pDCs and dDCs in dermis following TGFβ1 induction.
ST and DT mice were given dox chow for 2 and 4 days and dermal cells prepared from mouse ears following separation of epidermis and dermis. Percentage of MHCIIhi cells (a) and different dDC subsets of the total dermal cells were analyzed by flow cytometry at day 2 (b) and day 4 (c) of TGFβ1 induction. Error bars = +/− SEM.
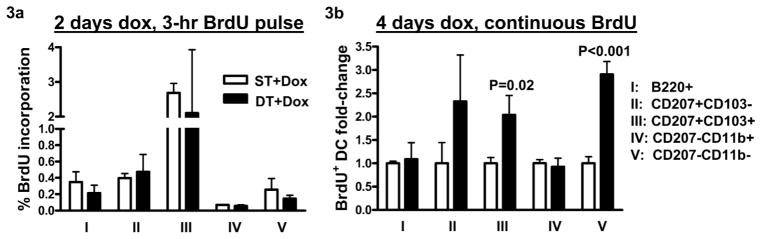
To test whether TGFβ1 induction altered proliferation of resident skin DC subsets we injected mice with 1.5 mg BrdU 3 hours before necropsy following 2 days of TGFβ1 induction and determined the percentage of BrdU positive cells within individual dDC subsets by flow cytometry. Although significantly more MHCII+ and B220+ pDCs were noted in the dermis, no changes in BrdU incorporation in any of the DC subsets was detected (Figure 3a). Alternatively, we dosed mice continuously with BrdU while inducing TGFβ1 at the same time for 4 days. There was a 2- and 3-fold increase in BrdU labeling in CD207+CD103+ and CD207−CD11b− dDCs, respectively, but no difference in other DC subsets including pDCs (Figure 3b). Thus TGFβ1 causes an increase of pDCs in the dermis without affecting proliferation and selectively promotes proliferation of other dermal DC subsets.
Figure 3. TGFβ1 induction does not alter local proliferation of pDCs and dDCs in the dermis.
(a) Dermal cells were prepared from mouse ears following 2 days of dox treatment and 3 hrs after BrdU (1.5 mg/mouse) injection, N=4–6. (b) Dermal cells were prepared following 4 days of dox treatment and continuous BrdU treatment either in drinking water (0.8 mg/ml) or daily i.p. injections (1 mg/mouse), N=4–5. Flow cytometric analysis was done on dermal cells to detect percentage of BrdU+ DC subsets following manufacturer recommended protocols. Error bars = +/− SEM.
CD86 expression of skin-derived DC subsets is increased in SDLN but not dermis
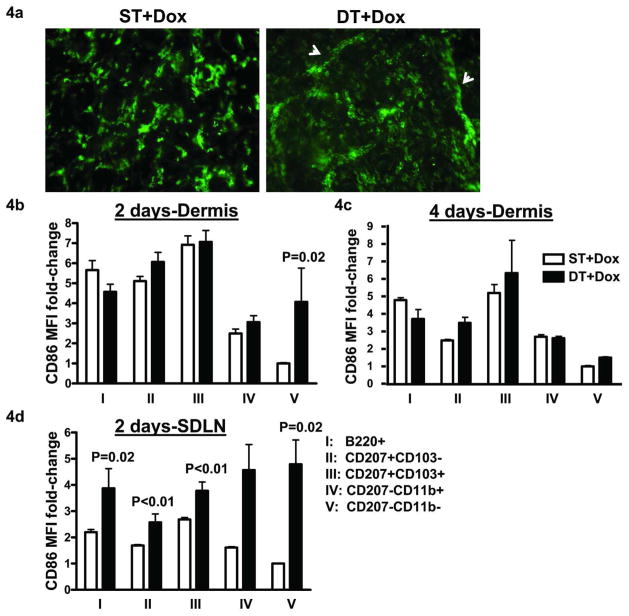
Consistent with increases in numbers of skin-derived DC subsets in SDLN, MHCII+ cells in dermal sheets were detected in numerous distinct dermal cords, a characteristic feature of DCs aligning in the dermal lymphatics prior to migration, following 4 days of TGFβ1 induction (Figure 4a). DCs up regulate CD86 levels upon maturation by antigen encounter and/or inflammatory cytokines that is linked to their migration to SDLNs (Banchereau and Steinman, 1998). We evaluated dermal DC subsets and their counterparts in SDLN for CD86 expression following TGFβ1 induction. In steady state dermis, CD207+103+ dDC had the highest and CD207−CD11b− dDCs had the lowest CD86 levels. TGFβ1 caused a significant increase in CD86 expression only in the CD207−CD11b− dDC subset at day 2 and no change in CD86 could be detected in any subset at day 4 (Figure 4b and c). However, there was an increase in CD86 expression in the SDLN of all of the skin-derived DC subsets (Figure 4d) with the greatest increase occurring in the CD207−CD11b+ and CD207−CD11b− dDC subsets (2.8- and 4.8-fold, respectively), and this was sustained through day 4 post- TGFβ1 induction (data not shown). Figure S1 shows representative histograms for CD86 expression on dDC subsets in dermis and SDLN..
Figure 4. Keratinocyte TGFβ1 promotes migration and increases CD86 expression of migratory DC subsets in SDLN.
(a) Dermal sheets were prepared from mouse ears after 4 days of dox treatment and analyzed for MHCII+ cells. White arrows represent dermal cords. (N=4–6). Dermal cells prepared from mouse ears following 2 (b) and 4 days (c) of dox treatment were analyzed by flow cytometry for CD86 mean fluorescent intensities (MFI) on various DC subsets. The average raw MFI values from controls for DC subset V were normalized to one. Data is represented as fold-change between the groups. (N=5–6). (d) SDLN harvested from ST and DT mice following 2 days of dox treatment and migratory DCs identified in figure 1a were analyzed for CD86 expression. Data represented as fold-change as in (b). (N=5–7). Error bars = +/− SEM.
Keratinocyte TGFβ1 enhances DC migration in response to sensitizer application in skin
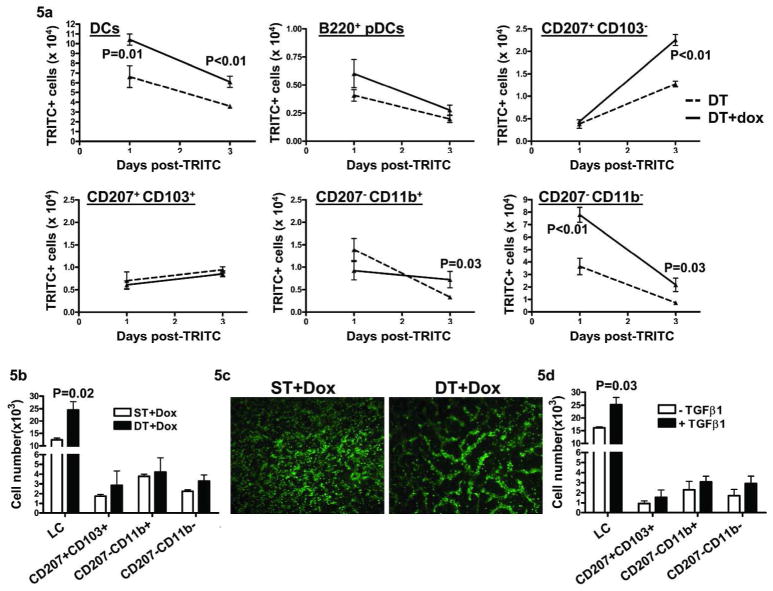
Although TGFβ1 alone altered homeostasis of dDC subsets, it can also suppress action of other inflammatory signals. To determine if elevated epidermal TGFβ1 altered DBP-induced DC migration, mice were given doxycycline chow and 1% TRITC in 1:1 mixture of acetone and DBP was applied to the shaved abdominal skin 18–24 hrs later. At both 24 and 72 hr post-TRITC/DBP treatment, TGFβ1 overexpression significantly enhanced the total number of TRITC+ MHCIIhi cells in SDLN (Figure 5a). At 24 hrs, migration of TRITC+ CD207−CD11b− dDC, that constituted the majority of TRITC+ DC in SDLN, was enhanced by TGFβ1. At 72 hrs, LCs that migrate slower than other DC subsets (Kissenpfennig et al., 2005) were the largest subpopulation of TRITC+ cells in both groups and overexpression of TGFβ1 enhanced DBP-induced LC migration by 1.77-fold. Additionally, TGFβ1 expression also caused a 2-fold increase in CD207−CD11b+ dDC and 3-fold increase in CD207−CD11b− dDC numbers at the 72 hr time point (Figure 5a). A small number of TRITC+B220+ pDCs were detected at 24 and 72 hrs post-TRITC, although their numbers did not change significantly following TGFβ1 induction. Also, there was no effect at any time point on TRITC+CD207+CD103+ dDC migration to SDLN. These results show that, under inflammatory conditions induced by DBP application, TGFβ1 differentially increases numbers of CD207−CD103− dDC and LC in SDLN.
Figure 5. Elevated TGFβ1 enhances LC migration following TRITC/DBP treatment in vivo and in skin explant cultures in vitro.
DT mice were given dox chow 18–24 hrs before painting of shaved belly with 1% TRITC in 1:1 acetone and DBP. SDLN were harvested 24 and 72 hrs post-TRITC painting and analyzed for TRITC+ DC subsets. (a) Kinetics of number of individual TRITC+ DC subsets at 24 and 72 hrs post-TRITC. (N=4–6). Error bars = +/− SEM. (b) ST and DT mice were given dox chow for 24 hrs and ear skin explant cultures initiated. Cells accumulating in the media were harvested after 48 hrs, counted, stained for flow cytometric analysis and represented as number of DCs migrating in to the media per ear. (c) Dermal sheets were prepared from ST and DT ear skin explants following 48 hrs of culture and MHCII immunofluorescence done. (d) FVB/n mouse ears were split and floated in complete RPMI media containing TGFβ1 and cultured for 48 hrs. Cells in the media were analyzed as in (b). For b and c results are the average of 3 independent experiments, Error bars = +/− SEM.
Excess endogenous and exogenous TGFβ1 increases migration of LC in vitro
To further evaluate effects of elevated keratinocyte TGFβ1 on LC migration we used skin explant cultures, an inflammatory setting that causes DC migration (Stoitzner et al., 1999). Explants from ST and DT mice placed on doxycycline chow for twenty-four hrs were cultured and migrated skin DC were analyzed after 48 hrs of culture. Under these conditions, 60–70% of DCs that migrated out of the skin were LC, and TGFβ1 induction in vivo caused a 2-fold increase in their migration in vitro (Figure 5b). Additionally, TGFβ1 caused a 1.5-fold increase in migration of the CD207−CD11b− dDC subset that represented 10% of the migrated DC population. The increase in migration correlated with formation of numerous well organized dermal cords containing DCs in TGFβ1 overexpressing samples as opposed to scattered DCs and fewer dermal cords in ST dermis following 48 hrs of skin culture (Figure 5c). Similarly, treatment of ear explant cultures from FVB/n mice with exogenous TGFβ1 stimulated LC migration (Figure 5d) to nearly the same extent as the DC chemokine CCL21 (Figure S2) (Kissenpfennig et al., 2005).
Elevated Keratinocyte TGFβ1 promotes skin contact hypersensitivity
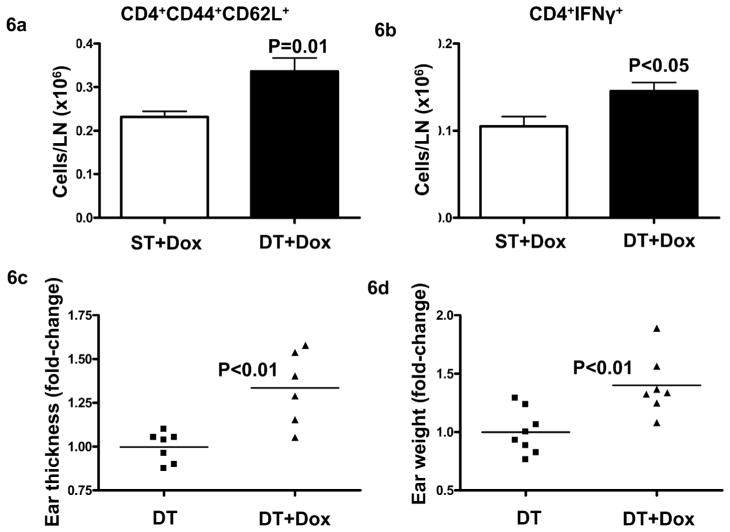
Since overexpression of TGFβ1 in the epidermis enhanced migration of DCs in response to topical application of the contact sensitizer, we hypothesized that TGFβ1 might affect CHS responses. We induced TGFβ1 expression using a one-time i.p. injection of doxycycline 18–24 hrs before sensitization with 0.5% DNFB. TGFβ1 expression caused a significant increase in numbers of CD4+44+62L+ central memory T cells and CD4+IFNγ+ cells in SDLN compared to ST mice (Figure 6a and b) following 5 days of sensitization. When mice sensitized in the presence of TGFβ1 were challenged with 0.3% DNFB, there was a 1.4-fold increase in ear thickness and weight (Figure 6c, d). These results show that the alteration of skin DC homeostasis by elevated keratinocyte TGFβ1 results in enhanced CHS responses.
Figure 6. TGFβ1 induction during sensitization enhances CHS response to DNFB.
ST and DT mice were injected with 500 ng/mouse doxycycline 18–24 hrs prior to sensitization with 0.5% DNFB. SDLN were harvested 5 days post-sensitization and analyzed for CD4 central memory cells (a) and CD4+IFNγ+ cells (b) by flow cytometry. (N=2–3). Error bars = +/− SEM. Alternatively, mice were challenged on both sides of ear at day 5 using 0.3% DNFB and ears thickness (c), ear weight (d) analyzed 24 hrs post-challengess
DISCUSSION
DCs constantly migrate from peripheral tissues to draining LN in steady state at low frequencies and generally promote tolerance and maintain tissue homeostasis (Steinman et al., 2000; Hawiger et al., 2001; Waithman et al., 2007). Peripheral inflammation from infection, injury and autoimmunity can increase the rate of DC migration and numbers in draining LNs and sustain T cell activation (Ohl et al., 2004; Coquerelle and Moser, 2010). Compared to steady state, TGFβ1 overexpression in keratinocytes caused profound changes in composition and increased numbers of skin-derived dDC subsets and B220+ pDCs in the SDLN. The striking resemblance of these TGFβ1-induced changes to those occurring 24 hours following application of skin irritant DBP suggests that increased TGFβ1 and DBP alter the skin microenvironment in a similar way. However, direct effects of TGFβ1 on DCs promoting their migration cannot be ruled out.
TGFβ1 induction also increased DBP-driven migration of CD207−CD11b− and CD207−CD11b+ dDC subsets. While we did not observe TGFβ1 induced mobilization of LC, TGFβ1 expression significantly enhanced DBP-induced LC numbers in SDLN. Consistent with this, ear explant cultures, which mimic an inflammatory environment, from TGFβ1-induced mice had a 2-fold increase in LC migration and LC numbers were comparable between skin explant cultures treated with either exogenous TGFβ1 or the CCR7 ligand, CCL21. Thus, within the context of an inflammatory microenvironment such as that established by DBP or explant culture, elevated TGFβ1 stimulates rather than inhibits LC migration. It is not clear if this is mediated by effects of TGFβ1 on LC within the context of other proinflammatory cytokines, or by indirect activation mediated by TGFβ1-induced upregulation of proinflammatory cytokines along with other changes in the tissue microenvironment. The lack of inhibition of LC homeostatic trafficking to the LN by elevated keratinocyte TGFβ1 contrasts with studies showing that TGFβ1 type I receptor ablation in LC provokes LC maturation and migration (Kel et al., 2010; Zahner et al., 2011). These differences suggest that elevated keratinocyte derived TGFβ1 and LC TGFβ1 signaling may have distinct effects on LC maturation and migration.
While activated DC express elevated levels of the costimulatory molecule CD86 prior to LN migration (Banchereau and Steinman, 1998), we observed no significant change in CD86 expression in dDCs except for the CD207−CD11b− dDC subset. It is possible that coexistance of migrating DCs with higher CD86 and influx of precursors with less CD86 expression results in no apparent change in the mean CD86 levels. However, the CD86 expression was higher in all DC subsets in SDLN as early as 2 days post-TGFβ1 induction and remained high through 4 days. Whether this results from influence of CD207−CD11b− dDC on CD86 levels in other migratory DC population or simply that other dDC subsets increase CD86 levels en route to SDLN remains to be determined. Further analysis of other markers of migration and maturation such as CCR7 and E-cadherin (Kel et al., 2010; Zahner et al., 2011) will also clarify the differential migration of dDC versus LC in response to TGFβ1.
pDCs participate in anti-viral immune responses and may play an important role in pathogenesis of autoimmune diseases such as psoriasis and lupus (Gilliet et al., 2008). pDCs infiltrate the skin after wounding, in tumors, in psoriasis and following imiquimod application (Gilliet et al., 2008; Gregorio et al., 2010; Nestle et al., 2009; Palamara et al., 2004). Here, we report for the first time that TGFβ1 overexpression causes a rapid increase in pDCs in the dermis. The lack of proliferation in these cells suggests that the increase is due to direct infiltration rather than expansion of a skin resident population. While pDCs infiltrating skin during psoriasis or lupus may be activated by complexes of the antimicrobial peptide, LL37, produced by neutrophils, and self-DNA from apoptotic cells in a toll-like receptor (TLR) 9-dependent manner (Nestle et al., 2009; Guiducci et al., 2010), it remains to be determined if this pathway of pDC activation occurs in this mouse model. We have previously reported induction of apoptosis in primary keratinocytes cultured in excess TGFβ1 as well as in telogen follicles of DT mice upon TGFβ1 induction (Liu et al., 2001) suggesting that nucleic acids from the apoptotic cells could trigger TLR7/9-mediated pDC activation. Since pDCs did not migrate in significant numbers following hapten application in TGFβ1 induced mice, it appears that increased pDCs in the skin of TGFβ1 overexpressing mice might modulate or support local activation and maturation of myeloid DCs as in psoriasis (Gilliet et al., 2008). Like pDCs, we also noticed increases in percentages of CD207+ dDCs and CD207−CD11b+ dDCs but not the CD207−CD11b− dDCs following 4 days of induction. Since the predominant dDC subset migrating in response to TGFβ1 induction was CD207−CD11b− (11-fold at 4 days), it appears that a balance is maintained between infiltrating/proliferating and migrating CD207−CD11b− dDC subset.
LC constantly renew in the epidermis throughout life (Merad et al., 2002) and dDCs are maintained by local proliferation with some infiltration of blood-derived precursors in steady state (Bogunovic et al., 2006). Due to increased migration of dDC subsets to SDLN at 4 days of TGFβ1 induction, we predicted an increase in self-renewal potential to maintain their normal homeostatic percentages in the dermis. Despite increases in dermal percentages of most DC subsets, proliferation only occurred in CD207+ and CD207− CD11b− dDC subsets. Hence, it appears that TGFβ1 alters dDC homeostasis by promoting influx of blood-derived pDCs and non-self-renewing DC precursors in the dermis while also influencing proliferation of CD207+CD103+ dDCs and CD207−CD11b− dDCs. The absence of any increases in dermal percentage of CD207−CD11b− cells may reflect the large and rapid migration of this subset to the SDLN.
Our results provide highlight the potential proinflammatory role of TGFβ1 in skin immunity and show that TGFβ1 overexpression by keratinocytes alters DC homeostasis and enhances adaptive immunity in the context of CHS, although the DC subset that mediates this response has not been determined. Nevertheless, the effects of TGFβ1 on dDC migration, pDC influx and enhanced CHS provide insight into the onset of psoriasis-like skin inflammation following chronic TGFβ1 induction in keratinocytes (Li et al., 2004), and suggest that elevated TGFβ1 in human psoriasis and other conditions of elevated cutaneous TGFβ1 may directly impact DC homeostasis, activation and T cell immunity.
MATERIALS AND METHODS
Mice
Single K14rTA or tetoTGFβ1 (ST) and double transgenic (DT) K14rTA-tetoTGFβ1 mice in FVB/n background that were sex and age-matched (7–10 week) were used for all the experiments. To induce keratinocyte TGFβ1, DT mice were given 1 gm/kg doxycycline chow (Bio-serve, Frenchtown, NJ). Animals were treated according to approved Institutional Animal Care and Use protocols.
Antibodies
The following antibodies were purchased from Ebioscience, San Diego, CA: anti-CD16/32 (93), APC eFluor 750-anti-CD45 (30-F11), FITC- and eFluor 450-anti-MHCII (M5/114.15.2), Alexa 700-anti-CD11c (N418), FITC-anti-CD4 (GK1.5), PECy5-anti-CD8α (53-6.7), PE-anti-CD103 (2E7), PECy7-anti-B220 (RA3-6B2), PE-anti-PDCA-1 (eBio-927), PercpCy5.5-anti-CD11b (M1/70), eFluor 450-anti-F4/80 (BM8), PE-anti-CD62L (MEL-14), PECy5-anti-CD44 (IM7). The following antibodies were purchased from BD Pharmingen, San Diego, CA: PE-anti-CD45 (30-F11), Alexa 700-anti-CD86 (GL1) and PECy7-anti-IFNγ (XMG1.2). FITC-anti-BrdU (ABFM18) was purchased from Phoenix flow systems, San Diego, CA. Alexa 568-anti-Epcam (G8.8) and alexa 647-anti-CD207 (L31) antibody conjugates were generated as previously described (Gaiser et al., 2012).
Isolation and flow cytometric analysis of DC
Inguinal lymph nodes were gently disrupted using forceps and incubated in HBSS containing 0.1% collagenase D (Roche, Nutley, NJ) and 0.05% DNase I (Sigma, St Louis, MO) for 30 min at 37 °C. Epidermal and dermal cell suspensions were prepared as described previously with slight modification (Nagao et al., 2009). Dermal components were cut into small pieces and incubated for 45 min in 0.1% collagenase D and 0.05% DNase I for 45 min at 37 °C, filtered using a 70 μm cell strainer and suspended in flow cytometry staining buffer. Single cells were incubated with panels of monoclonal antibodies following CD16/32 pre-incubation. For anti-CD207 staining, cells were fixed and permeabilized using Foxp3 fixation/permeabilization buffers (Ebioscience) and incubated with anti-langerin antibody in 0.2% saponin buffer. Cells were acquired on BD Fortessa LSRII flow cytometer and analyzed using FlowJo software (Tree Star, Ashland, OR).
Analysis of cell proliferation
Mice were initially injected with 1 mg BrdU in sterile PBS and then continuously given 0.8 mg/ml BrdU in driking water, which was changed daily. Alternatively, 1 mg BrdU was injected intraperitoneally (i.p.) daily. Single cells were prepared from inguinal LN, epidermis and dermis as above and were first stained for surface markers and CD207. BrdU staining was performed using the BrdU labeling flow kit (BD Pharmingen) following the manufacturers protocol.
Skin explant cultures
Ears of ST and DT mice previously treated with doxycycline chow for 18–24 hrs were excised, rinsed in 70% ethanol and then in a solution containing 200 IU/ml penicillin and 200 μg/ml streptomycin for 5 min. Ear skin was split into dorsal and ventral halves and at least 4 ears were cultured in complete RPMI media,10% FCS for 48 hrs at 37 °C. FVB/n mouse ears were cultured as above and treated with either 100 ng/ml CCL21 (R&D systems, Minneapolis, MN) or 500 pg/ml TGFβ1 (R&D systems). The migratory cells from the explant were harvested, enumerated and analyzed by flow cytometry.
Epidermal and dermal sheet immunofluorescence
Epidermal and dermal sheets were prepared as described previously (Nagao et al., 2009). For staining, dermal sheets were rehydrated in PBS and incubated overnight with FITC-anti-MHCII antibody at 4 °C. Slides were mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and analyzed on Olympus BX61 microscope.
TRITC painting
ST and DT mice were kept on 1 gm/kg doxycycline chow 18–24 hrs prior to treatment with 1% TRITC (Invitrogen, Eugene, OR) in 1:1 solution of acetone and dibutylpthalate (DBP) on shaved abdominal skin. After 24 and 72 hrs post-TRITC application, inguinal LNs were harvested and single cells prepared as described above. Total viable cells per LN were counted and stained for specific markers and analyzed by flow cytometry.
Contact hypersensitivity
ST and DT mice were injected i.p. with 500 ng doxycycline 18–24 hrs prior to sensitization with 25 μl of 0.5% 2, 4-dintro-1-fluorobenzene (DNFB; Sigma) in 4:1 solution of acetone/olive oil on shaved abdominal skin. Mice were challenged 5 days later with 20 μl of 0.3% DNFB on both sides of the right ear. The left ear was treated with vehicle and served as a control for baseline thickness. Measurements of ear thickness (minus baseline thickness), weight and histological analysis was done 24 hrs following challenge in a blinded fashion.
Statistical analysis
All statistical analysis was done using the GraphPad Prism software. A two-tailed student’s t test was done to compare the groups. Under certain circumstances where the variances were significant, a Mann-Whitney test was done.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Maria Gaiser for suggestions and technical help, and the Huck Institute Flow Cytometry Core Facility for help with flow cytometry. This study was funded by grants CA117957 and 122109 (A.G.) from the NCI and by the Intramural Research Program of the NIH, Center for Cancer Research, NCI (M.C.U).
Abbreviations
- Dox
doxycycline
- ST
single transgenic
- DT
double transgenic
- DBP
Dibutylpthalate
- DNFB
2,4-Dinitro fluorobenzene
Footnotes
CONFLICT OF INTEREST.
The authors state no conflict of interest.
Reference List
- 1.Akhurst RJ, Fee F, Balmain A. Localized production of TGF-beta mRNA in tumour promoter-stimulated mouse epidermis. Nature. 1988;331:363–365. doi: 10.1038/331363a0. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Bogunovic M, Ginhoux F, Wagers A, Loubeau M, Isola LM, Lubrano L, et al. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J Exp Med. 2006;203:2627–2638. doi: 10.1084/jem.20060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184:2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borkowski TA, Letterio JJ, Mackall CL, Saitoh A, Wang XJ, Roop DR, et al. A role for TGFbeta1 in langerhans cell biology. Further characterization of the epidermal Langerhans cell defect in TGFbeta1 null mice. J Clin Invest. 1997;100:575–581. doi: 10.1172/JCI119567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coquerelle C, Moser M. DC subsets in positive and negative regulation of immunity. Immunol Rev. 2010;234:317–334. doi: 10.1111/j.0105-2896.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 7.Dakic A, Shao QX, D’Amico A, O’Keeffe M, Chen WF, Shortman K, et al. Development of the dendritic cell system during mouse ontogeny. J Immunol. 2004;172:1018–1027. doi: 10.4049/jimmunol.172.2.1018. [DOI] [PubMed] [Google Scholar]
- 8.Fainaru O, Shay T, Hantisteanu S, Goldenberg D, Domany E, Groner Y. TGFbeta-dependent gene expression profile during maturation of dendritic cells. Genes Immun. 2007;8:239–244. doi: 10.1038/sj.gene.6364380. [DOI] [PubMed] [Google Scholar]
- 9.Fitch EL, Rizzo HL, Kurtz SE, Wegmann KW, Gao W, Benson JM, et al. Inflammatory skin disease in K5.hTGF-beta1 transgenic mice is not dependent on the IL-23/Th17 inflammatory pathway. J Invest Dermatol. 2009;129:2443–2450. doi: 10.1038/jid.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flisiak I, Chodynicka B, Porebski P, Flisiak R. Association between psoriasis severity and transforming growth factor beta(1) and beta (2) in plasma and scales from psoriatic lesions. Cytokine. 2002;19:121–125. doi: 10.1006/cyto.2002.1953. [DOI] [PubMed] [Google Scholar]
- 11.Flisiak I, Porebski P, Flisiak R, Chodynicka B. Plasma transforming growth factor beta1 as a biomarker of psoriasis activity and treatment efficacy. Biomarkers. 2003;8:437–443. doi: 10.1080/13547500310001599061. [DOI] [PubMed] [Google Scholar]
- 12.Gaiser MR, Lammermann T, Feng X, Igyarto BZ, Kaplan DH, Tessarollo L, et al. Cancer-associated epithelial cell adhesion molecule (EpCAM; CD326) enables epidermal Langerhans cell motility and migration in vivo. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1117674109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geissmann F, Revy P, Regnault A, Lepelletier Y, Dy M, Brousse N, et al. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162:4567–4575. [PubMed] [Google Scholar]
- 14.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 15.Ginhoux F, Merad M. Ontogeny and homeostasis of Langerhans cells. Immunol Cell Biol. 2010;88:387–392. doi: 10.1038/icb.2010.38. [DOI] [PubMed] [Google Scholar]
- 16.Glick AB, Sporn MB, Yuspa SH. Altered regulation of TGF-β 1 and TGF-α in primary keratinocytes and papillomas expressing v-Ha-ras. Mol Carcinog. 1991;4:210–219. doi: 10.1002/mc.2940040308. [DOI] [PubMed] [Google Scholar]
- 17.Gregorio J, Meller S, Conrad C, Di NA, Homey B, Lauerma A, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921–2930. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guiducci C, Tripodo C, Gong M, Sangaletti S, Colombo MP, Coffman RL, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931–2942. doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han G, Williams CA, Salter K, Garl PJ, Li AG, Wang XJ. A role for TGFbeta signaling in the pathogenesis of psoriasis. J Invest Dermatol. 2010;130:371–377. doi: 10.1038/jid.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de BB, et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med. 2010;207:189–206. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kane CJ, Hebda PA, Mansbridge JN, Hanawalt PC. Direct evidence for spatial and temporal regulation of transforming growth factor beta 1 expression during cutaneous wound healing. J Cell Physiol. 1991;148:157–173. doi: 10.1002/jcp.1041480119. [DOI] [PubMed] [Google Scholar]
- 23.Kane CJ, Knapp AM, Mansbridge JN, Hanawalt PC. Transforming growth factor-beta 1 localization in normal and psoriatic epidermal keratinocytes in situ. J Cell Physiol. 1990;144:144–150. doi: 10.1002/jcp.1041440119. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ. Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells. J Exp Med. 2007;204:2545–2552. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kel JM, Girard-Madoux MJ, Reizis B, Clausen BE. TGF-beta is required to maintain the pool of immature Langerhans cells in the epidermis. J Immunol. 2010;185:3248–3255. doi: 10.4049/jimmunol.1000981. [DOI] [PubMed] [Google Scholar]
- 26.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Levine JH, Moses HL, Gold LI, Nanney LB. Spatial and temporal patterns of immunoreactive transforming growth factor beta 1, beta 2, and beta 3 during excisional wound repair. Am J Pathol. 1993;143:368–380. [PMC free article] [PubMed] [Google Scholar]
- 28.Li AG, Wang D, Feng XH, Wang XJ. Latent TGFbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. EMBO J. 2004;23:1770–1781. doi: 10.1038/sj.emboj.7600183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Alexander V, Vijayachandra K, Bhogte E, Diamond I, Glick A. Conditional epidermal expression of TGFbeta 1 blocks neonatal lethality but causes a reversible hyperplasia and alopecia. Proc Natl Acad Sci U S A. 2001;98:9139–9144. doi: 10.1073/pnas.161016098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 31.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michaelis K, Wallbrecht K, Kerstan A, Beyersdorf N, Williams C, Kerkau T, et al. Modulating T cell functions does not alleviate chronic inflammatory skin lesions in K5. TGF beta 1 transgenic mice. Exp Dermatol. 2010;19:406–415. doi: 10.1111/j.1600-0625.2009.01031.x. [DOI] [PubMed] [Google Scholar]
- 33.Mohammed J, Ryscavage A, Perez-Lorenzo R, Gunderson AJ, Blazanin N, Glick AB. TGFbeta1-induced inflammation in premalignant epidermal squamous lesions requires IL-17. J Invest Dermatol. 2010;130:2295–2303. doi: 10.1038/jid.2010.92. [DOI] [PubMed] [Google Scholar]
- 34.Nagao K, Ginhoux F, Leitner WW, Motegi S, Bennett CL, Clausen BE, et al. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci U S A. 2009;106:3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 36.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Ohtani T, Mizuashi M, Nakagawa S, Sasaki Y, Fujimura T, Okuyama R, et al. TGF-beta1 dampens the susceptibility of dendritic cells to environmental stimulation, leading to the requirement for danger signals for activation. Immunology. 2009;126:485–499. doi: 10.1111/j.1365-2567.2008.02919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palamara F, Meindl S, Holcmann M, Luhrs P, Stingl G, Sibilia M. Identification and characterization of pDC-like cells in normal mouse skin and melanomas treated with imiquimod. J Immunol. 2004;173:3051–3061. doi: 10.4049/jimmunol.173.5.3051. [DOI] [PubMed] [Google Scholar]
- 39.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 40.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoitzner P, Zanella M, Ortner U, Lukas M, Tagwerker A, Janke K, et al. Migration of langerhans cells and dermal dendritic cells in skin organ cultures: augmentation by TNF-alpha and IL-1beta. J Leukoc Biol. 1999;66:462–470. [PubMed] [Google Scholar]
- 42.Strobl H, Knapp W. TGF-beta1 regulation of dendritic cells. Microbes Infect. 1999;1:1283–1290. doi: 10.1016/s1286-4579(99)00256-7. [DOI] [PubMed] [Google Scholar]
- 43.Torres-Aguilar H, guilar-Ruiz SR, Gonzalez-Perez G, Munguia R, Bajana S, Meraz-Rios MA, et al. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4+ T cells. J Immunol. 2010;184:1765–1775. doi: 10.4049/jimmunol.0902133. [DOI] [PubMed] [Google Scholar]
- 44.Waithman J, Allan RS, Kosaka H, Azukizawa H, Shortman K, Lutz MB, et al. Skin-derived dendritic cells can mediate deletional tolerance of class I-restricted self-reactive T cells. J Immunol. 2007;179:4535–4541. doi: 10.4049/jimmunol.179.7.4535. [DOI] [PubMed] [Google Scholar]
- 45.Wang XJ, Han G, Owens P, Siddiqui Y, Li AG. Role of TGF beta-mediated inflammation in cutaneous wound healing. J Investig Dermatol Symp Proc. 2006;11:112–117. doi: 10.1038/sj.jidsymp.5650004. [DOI] [PubMed] [Google Scholar]
- 46.Zahner SP, Kel JM, Martina CA, Brouwers-Haspels I, van Roon MA, Clausen BE. Conditional Deletion of TGF-{beta}R1 Using Langerin-Cre Mice Results in Langerhans Cell Deficiency and Reduced Contact Hypersensitivity. J Immunol. 2011;187:5069–5076. doi: 10.4049/jimmunol.1101880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.